Abstract
The effects of isavuconazole (active moiety of isavuconazonium sulfate) on cardiac ion channels in vitro and cardiac repolarization clinically were assessed in a phase I, randomized, double‐blind study in healthy individuals who received isavuconazole (after 2‐day loading dose), at therapeutic or supratherapeutic doses daily for 11 days, moxifloxacin (400 mg q.d.), or placebo. A post‐hoc analysis of the phase III SECURE trial assessed effects on cardiac safety. L‐type Ca2+ channels were most sensitive to inhibition by isavuconazole. The 50% inhibitory concentrations for ion channels were higher than maximum serum concentrations of nonprotein‐bound isavuconazole in vivo. In the phase I study (n = 161), isavuconazole shortened the QT interval in a dose‐ and plasma concentration‐related manner. There were no serious treatment‐emergent adverse events; palpitations and tachycardia were observed in placebo and supratherapeutic isavuconazole groups; no cardiac safety signals were detected in the SECURE study (n = 257). Isavuconazole was associated with a shortened cardiac QT interval.
Study Highlights.
WHAT IS THE CURRENT KNOWLEDGE ON THE TOPIC?
☑ Although a rare occurrence, studies have shown that various noncardiac drugs can precipitate abnormalities in the cardiac waveform such as QT interval prolongation, which is associated with ventricular arrhythmias including potentially fatal Torsades de Pointes. Isavuconazole is a recently approved antifungal triazole delivered by the prodrug isavuconazonium sulfate; however, triazoles can be associated with QT interval prolongation.
WHAT QUESTION DID THIS STUDY ADDRESS?
☑ Given the known effects of some triazole antifungal agents on the QT interval, the potential effects of isavuconazole on cardiac repolarization were assessed.
WHAT THIS STUDY ADDS TO OUR KNOWLEDGE
☑ Isavuconazole shortens the cardiac QT interval in a dose‐related manner, with no evidence of an associated cardiac risk.
HOW THIS MIGHT CHANGE CLINICAL PHARMACOLOGY OR TRANSLATIONAL SCIENCE
☑ The QT‐shortening observed with isavuconazole is unique among triazole antifungal agents and was not associated with any obvious untoward cardiovascular events. Nevertheless, this effect underlies the contraindication of isavuconazole in patients with congenital SQTS and suggests a need for caution when using concomitant medications that also shorten the QT interval.
Previous studies have shown that various noncardiac drugs can, in rare instances, precipitate abnormalities in the cardiac waveform such as QT interval prolongation, which is associated with ventricular arrhythmias including potentially fatal Torsades de Pointes.1, 2 Although congenital forms of long QT syndrome (LQTS) are caused by mutations in various ion channels, drug‐induced QT interval prolongation is predominantly caused by inhibition of human ether‐a‐go‐go (hERG; encoded by KCNH2 gene), which underlies the rapid component of the delayed rectifier K+ current (IKr).1 Some triazole antifungal agents have been associated with QT interval prolongation.3, 4, 5, 6, 7, 8, 9, 10 Other noncardiac drugs have been found to be associated with shortening of the QT interval, but the clinical implications are not clear.11
Isavuconazonium sulfate is a novel antifungal prodrug approved by the US Food and Drug Administration (FDA) for the treatment of adults with invasive aspergillosis (IA) and invasive mucormycosis12 and by the European Medicines Agency (EMA) for the treatment of adults with IA and with invasive mucormycosis when amphotericin B is inappropriate.13 Following either oral or intravenous (i.v.) administration, the water‐soluble prodrug is rapidly hydrolyzed to release its broad‐spectrum triazole component, isavuconazole14 (hereafter, the drug is referred to as isavuconazole), and an inactive moiety. In the phase III SECURE study, isavuconazole was shown to be noninferior to voriconazole for the primary treatment of IA and invasive fungal disease caused by other filamentous fungi.15 In the phase III VITAL study, isavuconazole also was found to be effective and well tolerated in patients with invasive mucormycosis or other rare fungal diseases, as well as in patients with IA and renal impairment.16, 17 Isavuconazole has been reported to be both a substrate as well as a moderate inhibitor of the CYP3A4 isoenzyme.18, 19 This is important to consider when isavuconazole is coadministered with concomitant medications that may prolong the corrected QT (QTc) interval for other underlying conditions in patients receiving antifungal treatment.
Given the known effects of some triazole antifungal agents on the QT interval,3, 4, 5, 6, 7, 8, 9, 10 the potential effects of isavuconazole on cardiac repolarization were assessed. In vitro studies were performed to assess the effects of isavuconazole on a panel of cardiac ion channels. A phase I clinical study was performed to assess effects of repeated administration of isavuconazole at therapeutic and supratherapeutic doses on electrocardiogram (ECG) parameters. Finally, data from the phase III SECURE study were reassessed specifically to evaluate cardiac safety signals.
RESULTS
In vitro studies
To determine whether isavuconazole affects cardiac ion channels, cell lines expressing individual cardiac Ca2+, K+, or Na+ channels were subjected to automated patch clamping in the presence of isavuconazole at concentrations of 0, 1, 3, 10, or 30 μM. The L‐type Ca2+ channels (hCav1.2) were most sensitive to inhibition by isavuconazole in vitro (Supplementary Table 1). The hNav1.5 Na+ channels also were inhibited, albeit to a lesser extent, by isavuconazole under tonic or phasic conditions within the tested drug concentration range. Isavuconazole inhibited the different K+ channels to variable degrees. The 50% inhibitory concentration (IC50) values for hERG and hKvLQT1/hminK channels were within the tested drug concentration range. The IC50 for the hKv4.3/KChiP2.2 K+ channel was outside the tested drug concentration range but an estimate could be extrapolated. The IC50 for the hKIR2.1, hKIR3.1/hKIR3.4, KIR6.2/SUR2A, and hKv1.5 could not be calculated as there was no discernable inhibition over the concentration range tested.
Phase I clinical study
A total of 161 subjects were randomized in this phase I thorough QT clinical study and 148 subjects completed the study (Figure 1). Four subjects withdrew (placebo, n = 1; isavuconazole 200 mg, n = 3), one subject was discontinued for noncompliance (isavuconazole 200 mg), one subject was randomized but did not receive the study drug (isavuconazole 600 mg), and seven subjects discontinued because of a treatment‐emergent adverse event (TEAE; all from the isavuconazole 600‐mg group). The demographics of the subjects are shown in Table 1.
Figure 1.
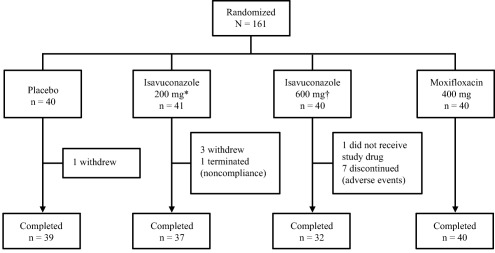
Subject disposition in the phase I clinical study. *Equivalent to the therapeutic dose of isavuconazonium sulfate 372 mg. †Equivalent to supratherapeutic dose isavuconazonium sulfate 1,116 mg.
Table 1.
Demographics of all subjects in the safety analysis set of the phase I clinical study
| Parameter | Placebo (n = 40) | Isavuconazole | Moxifloxacin (n = 40) | |
|---|---|---|---|---|
| 200 mg (n = 41) | 600 mg (n = 39) | |||
| Age [years], mean ± SD | 33.4 ± 9.8 | 33.0 ± 10.6 | 31.5 ± 9.2 | 32.2 ± 9.5 |
| Sex, n (%) | ||||
| Male | 22 (55.0) | 17 (41.5) | 20 (51.3) | 22 (55.0) |
| Female | 18 (45.0) | 24 (58.5) | 19 (48.7) | 18 (45.0) |
| Race, n (%) | ||||
| White | 22 (55.0) | 20 (48.8) | 29 (74.4) | 16 (40.0) |
| Black or African American | 10 (25.0) | 17 (41.5) | 7 (17.9) | 14 (35.0) |
| Asian | 4 (10.0) | 2 (4.9) | 2 (5.1) | 4 (10.0) |
| American Indian/Alaska Native | 2 (5.0) | 0 | 0 | 1 (2.5) |
| Native Hawaiian/Pacific Islander | 1 (2.5) | 0 | 0 | 0 |
| Other | 1 (2.5)a | 2 (4.9)b | 1 (2.6)c | 5 (12.5)d |
| Weight [kg], mean (SD) | 71.4 (13.2) | 69.5 (12.1) | 70.6 (11.1) | 76.8 (13.0) |
| Body mass index [kg/m2], mean (SD) | 24.5 (3.0) | 24.0 (3.2) | 24.2 (2.8) | 25.3 (2.8) |
SD, standard deviation.
One subject identified as White/Asian.
One subject each identified as White/American Indian/Alaska Native; biracial White/African American.
One subject identified as White/African American.
One subject each identified as White/African American/American Indian; White/African American/Asian (Korean); White/Asian (Filipino); White/Asian; Black/Native Hawaiian/Pacific Islander.
The ECG analysis set comprised 148 subjects (placebo, n = 39; isavuconazole 200 mg, n = 37; isavuconazole 600 mg, n = 32; moxifloxacin, n = 40). The 24‐h time‐matched, baseline‐adjusted drug–placebo difference in the Fridericia‐corrected QT interval (ddQTcF) values for moxifloxacin and both doses of isavuconazole are shown in Figure 2. At 2 h postdose, the median tmax (time to mean maximal plasma concentration (mean Cmax)) for moxifloxacin, the mean time‐matched difference (90% confidence interval (CI)) between moxifloxacin and placebo, baseline adjusted, was 11.03 (7.14 to 14.92) ms, and the lower limit of the 90% CI exceeded 5 ms, confirming assay sensitivity. The upper limit of the 90% CI for the time‐matched difference between isavuconazole and placebo, baseline adjusted, did not exceed 10 ms at any time in either dose group, indicating that isavuconazole did not prolong the QTcF (Supplementary Table 2). None of the subjects in either isavuconazole dose group had a QTcF >450 ms based on the mean of the replicates at each timepoint and no subject had a >30 ms increase in QTcF (Table 2). In contrast, two subjects in the 200‐mg dose group and five subjects in the 600‐mg dose group had QTcF <360 ms for at least one timepoint. A decrease from baseline in QTcF of >30 ms for at least one timepoint was observed in 13 subjects in the 600‐mg isavuconazole dose group and seven subjects in the 200‐mg isavuconazole dose group.
Figure 2.
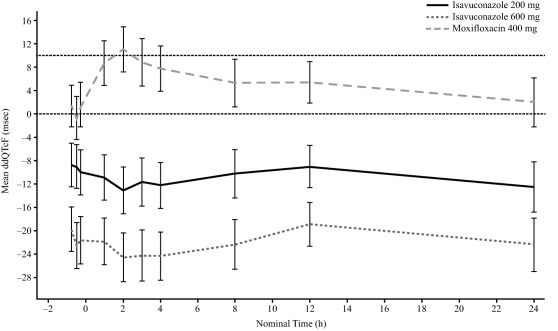
Mean change compared with placebo in baseline‐adjusted* QTcF over time on Day 13 in the phase I clinical study. Error bars represent 90% CI. QTcF, Fridericia‐corrected QT interval; ddQTcF, time‐matched, drug–placebo difference in QTcF interval, baseline‐adjusted. Zero on x‐axis represents time of Day 13 dose; horizontal dotted line represents threshold for significant prolongation of QTcF interval. *Baseline was defined as time‐matched measurement on Day −1.
Table 2.
Analysis of outliers for QTcF in the phase I clinical study
| Parameter | Placebo (n = 39)a n (%) | Isavuconazole | Moxifloxacin (n = 40)a n (%) | |
|---|---|---|---|---|
| 200 mg (n = 37)a n (%) | 600 mg (n = 32)a n (%) | |||
| Extreme valuesb | ||||
| <360 msec | 1 (2.6) | 2 (5.4) | 5 (15.6) | 1 (2.5) |
| <330 msec | 0 | 0 | 0 | 0 |
| >450 msec | 0 | 0 | 0 | 5 (12.5) |
| >480 msec | 0 | 0 | 0 | 0 |
| Change from baselinec | ||||
| >30 msec increase | 3 (7.7) | 0 | 0 | 4 (10.0) |
| >60 msec increase | 0 | 0 | 0 | 0 |
| >30 msec decrease | 1 (2.6) | 7 (18.9) | 13 (40.6) | 1 (2.5) |
| >60 msec decrease | 0 | 0 | 0 | 0 |
QTcF, Fridericia‐corrected QT interval.
Number of subjects with a nonmissing value.
Using the average of the replicates at each timepoint.
Baseline defined as average of replicates at each time point from Day 1; postbaseline defined as average of replicates from Day 13; percentages calculated as the total number of subjects within the change from baseline category divided by the total number of subjects with a nonmissing value, at each timepoint.
The pharmacokinetic (PK) analysis set comprised 109 subjects (isavuconazole 200 mg, n = 37; isavuconazole 600 mg, n = 32; moxifloxacin, n = 40). The mean plasma concentration‐time profiles of 200‐mg and 600‐mg isavuconazole groups following the Day 13 doses are shown in Figure 3 a. The mean plasma moxifloxacin concentration–time profile is shown in Figure 3 b. The PK parameters for study drugs are shown in Supplementary Table 3.
Figure 3.
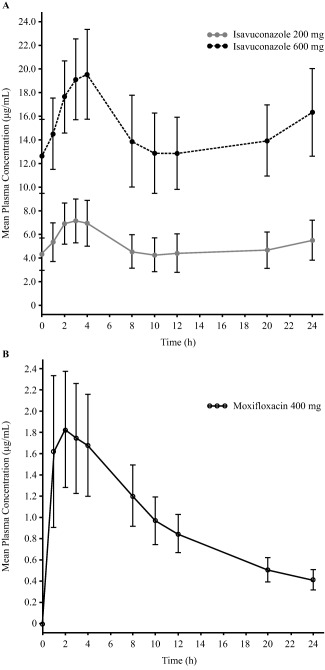
Mean ( ± standard deviation) plasma concentrations of isavuconazole (a) and moxifloxacin (b) following the Day 13 dose in the phase I clinical study.
Figure 4.
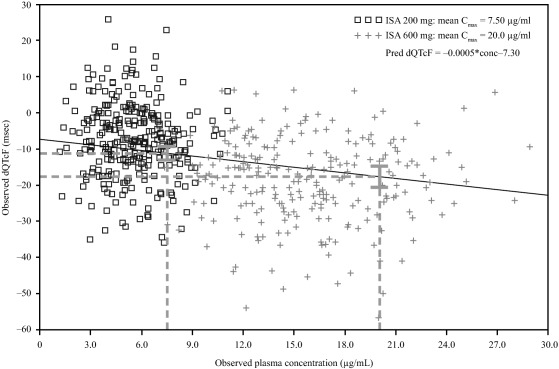
Scatterplot of individual dQTcF vs. isavuconazole plasma concentration in the phase I clinical study. C max, maximum concentration; dQTcF, time‐matched, baseline‐adjusted QTcF interval; QTcF, Fridericia‐corrected QT interval. Postdose timepoints are shown at 200 mg (□) and at 600 mg isavuconazole (+). Dashed lines represent predicted dQTcF at the Cmax for 200‐mg and 600‐mg doses, shown with 90% CI.
The relationship between isavuconazole plasma concentrations and dQTcF showed a negative relationship described by the equation:
The model was fitted using individual concentration and baseline‐adjusted QTc (dQTc) pairs. Based on this relationship, predicted mean dQTcF values (90% CI) at the mean Cmax for the 200‐mg and 600‐mg doses were −11.19 (–13.01, −9.37) ms and −17.68 (−20.54, −14.82) ms, respectively. Goodness of fit was based on the dQTc model as described previously.20, 21 The residual plots against dQTcF and time are presented in Supplementary Figure 1.
The safety analysis set comprised 160 subjects (placebo, n = 40; isavuconazole 200 mg, n = 41; isavuconazole 600 mg, n = 39; moxifloxacin, n = 40). Overall, more TEAEs were reported for the isavuconazole 600‐mg group compared with the placebo, isavuconazole 200 mg, and moxifloxacin groups (Supplementary Table 4). There were no serious TEAEs, severe TEAEs, or deaths. Many of the more frequently reported TEAEs (e.g., headache, dizziness, and paresthesia) were observed in approximately equal proportions of the placebo group and the isavuconazole 200‐mg group. However, more subjects in the isavuconazole 600‐mg group than in the isavuconazole 200‐mg and placebo groups experienced headache, hot flush, nausea, dizziness, paresthesia, anxiety, dry mouth, disturbance in attention, dysgeusia, palpitations, and oral hypoesthesia. TEAEs leading to discontinuation occurred in seven subjects in the isavuconazole 600‐mg group. TEAEs in the system organ class “Cardiac disorders” were only reported in the placebo and isavuconazole 600‐mg groups, and included palpitations (placebo, n = 1 (2.5%); isavuconazole 600 mg, n = 4 (10.3%)) and tachycardia (isavuconazole 600 mg, n = 2 (5.1%)). Concurrent ECGs performed in five of those six patients were either normal (n = 3) or abnormal but not clinically significant (n = 2). A summary of ECG abnormalities is provided in Supplementary Table 5. One subject who experienced a Cardiac disorder TEAE in the isavuconazole 600‐mg group discontinued because of palpitations. No important differences were found for changes from baseline in clinical laboratory values, and no clinically relevant changes from baseline were found for any vital signs. At 3 h postdose, an increase of 2 beats per minute (bpm) in the heart rate relative to placebo was seen for the 200‐mg dose and an increase of the same magnitude was seen for moxifloxacin. At 3 h postdose, a 6 bpm increase in heart rate was seen for the 600‐mg dose. The change in ventricular heart rate is summarized in Supplementary Table 6 and Supplementary Figure 2. Compared with placebo, PR was shortened by 6 ms at the 200‐mg dose and by 12 ms at the 600‐mg dose of isavuconazole. A very small and nonsignificant shortening of QRS was seen. The statistical assessments for PR and QRS intervals are provided in Supplementary Table 7. Plots of PR and QRS over the dosing interval and a statistical analysis at the PK tmax for the two doses of isavuconazole are provided in Supplementary Figure 3.
A scatterplot of QT vs. RR for all the Day −1 ECGs, i.e., ECGs taken on the day before first dosing of the study drugs, shows the expected increasing between QT and RR interval, i.e., decreasing heart rate (Supplementary Figure 4A). The corresponding scatterplot for QTcF vs. RR lacks this relationship, demonstrating that the Fridericia correction had appropriately corrected for heart rate (Supplementary Figure 4B).
Phase III SECURE Trial
In the SECURE trial, increases of >30 ms and >60 ms in QTcF postbaseline and at the end of treatment were numerically more frequent in the voriconazole group compared with the isavuconazole group (Supplementary Table 8). Similarly, greater numbers of voriconazole‐treated patients than isavuconazole‐treated patients had extreme values of QTcF >450 and >500 ms at any time postbaseline as well as >450 and >480 ms at the end of treatment. Conversely, decreases in QTcF of >30 ms and >60 ms, as well as extreme values of <300 and <360 ms were numerically more frequent in the isavuconazole group.
Overall, cardiac TEAEs by system organ class in the SECURE trial were numerically more frequent in the voriconazole arm compared with the isavuconazole arm (Supplementary Table 9). Tachycardia, QT interval prolongation, and cardiac arrest were all numerically more frequent in the voriconazole arm, whereas supraventricular tachycardia was numerically more frequent in the isavuconazole arm. All other cardiac‐related TEAEs occurred in similar proportions in each treatment arm.
Numerically fewer patients in the isavuconazole arm compared with the voriconazole arm had TEAEs related to Torsades de Pointes. (Table 3). Syncope and loss of consciousness were reported in a higher proportion of isavuconazole‐ than voriconazole‐treated patients. Among the seven patients in the isavuconazole group who experienced syncope, four were considered mild and unrelated to study drug, one was mild and considered possibly related to study drug (patient was receiving rituximab and diphenhydramine), one was considered moderate and possibly related to study drug (patient was on last dose of chemotherapy), and one was considered severe and possibly related to study drug (patient received concurrent morphine). Among the three patients in the isavuconazole group who experienced loss of consciousness, one was considered moderate and possibly related to study drug (patient received midazolam and morphine prior to event) and two were considered severe and unrelated to study drug. QT prolongation and cardiac arrest were reported in a lower proportion of isavuconazole than voriconazole‐treated patients. A total of five patients in both the isavuconazole and voriconazole treatment groups (3.8% and 3.5%, respectively) had a shift from normal to significantly abnormal ECG assessments between baseline and end of treatment. Another 3/99 patients in the isavuconazole group (3.0%) and 5/88 patients in the voriconazole group (5.7%) had abnormal ECG findings at baseline that became clinically significantly abnormal at the end of treatment.
Table 3.
Treatment‐emergent adverse events for Torsades de Pointes standardized MedDRA queries
| MedDRA v. 12.1, n (%) preferred term | Isavuconazole (n = 257) | Voriconazole (n = 259) |
|---|---|---|
| Patients with ≥ 1 Torsades de Pointes TEAE | 15 (5.8) | 19 (7.3) |
| Syncope | 7 (2.7) | 2 (0.8) |
| Loss of consciousness | 3 (1.2) | 0 |
| Cardio‐respiratory arrest | 2 (0.8) | 2 (0.8) |
| Electrocardiogram QT prolonged | 2 (0.8) | 8 (3.1) |
| Cardiac arrest | 1 (0.4) | 6 (2.3) |
| Sudden cardiac death | 0 | 1 (0.4) |
| Ventricular tachycardia | 0 | 2 (0.8) |
MedDRA, medical dictionary for regulatory activities; TEAE, treatment‐emergent adverse event.
DISCUSSION
The association of certain triazole antifungal agents with prolongation of the cardiac QT interval3, 4, 5, 6, 7, 8, 9, 10 underscores the need to test cardiac effects of new agents in this class. Depolarizing currents mediated by hCav1.2 L‐type Ca2+ channels were the most sensitive to inhibition by isavuconazole in vitro. The IC50 values for hCav1.2 (6.567 μM), as well as for hNav1.5 Na+ channels (0.36 μM [tonic] and 14.87 μM (phasic)), hERG, hKv4.3/KChiP2.2 (35.24 μM), and hKVLQT/hminK (24.02 μM) K+ channels were all markedly higher than the nonprotein‐bound Cmax observed in vivo at the therapeutic dose of 200 mg. Nevertheless, the dose‐related shortening of the QT interval observed clinically in this study is consistent with the possibility of some inhibition of L‐type Ca2+ channel currents by isavuconazole.
The effect on the late Na+ current was not directly evaluated; however, isavuconazole, at high in vitro concentrations (20 μM), inhibited radioligand binding to the Na+ channel site 2 by 78% (unpublished observations). While an effect on the late Na+ current cannot be excluded, the high concentrations needed to affect Na+ channel site 2 as well as the absence of an effect on the PR interval argues against clinically discernible effects of this current on the human ECG.
Irrespective of the underlying mechanism, this effect of isavuconazole on the QT interval did not appear to be associated with untoward cardiac effects at the therapeutic dose because cardiac‐related TEAEs that were observed in healthy subjects in the phase I trial (palpitations and tachycardia) occurred in those who received the supratherapeutic dose but not the therapeutic dose of isavuconazole. Although palpitations and tachycardia were observed at the therapeutic dose in small proportions of patients in the SECURE trial (less than 5% for each), an assessment of cardiac‐related TEAEs and Torsades de Pointes‐specific TEAEs from the SECURE trial also did not reveal signals indicative of an increased risk of cardiac events with isavuconazole treatment due to QT shortening.
Although concerns about the potential cardiac risk associated with drug‐induced shortening of the QT interval have been expressed,11 neither the actual risk nor the mechanism of QT shortening by isavuconazole are clear. Congenital short QT syndrome (SQTS) is associated with mutations in ion channels, including K+, Ca2+, and Na+ channels,22 and like congenital LQTS, marked QT interval shortening is associated with arrhythmogenic potential and an elevated risk for sudden cardiac death.22 However, some population‐based studies have found no evidence of increased cardiac risk in rare individuals with congenital short QTc interval.23, 24, 25
The arrhythmogenic potential of a given drug may be related to its net effect on several ion channels. Unlike drug‐induced QT interval prolongation, it is not clear whether there is an increased cardiac risk associated with drug‐induced QT shortening. For example, some antiepileptic drugs have been found to shorten the QT interval,26, 27, 28 but the associated cardiac risk is obscured both by the already elevated risk of sudden death in epileptic patients and by the potential for misdiagnosis of tachyarrhythmia as seizure activity.11 Finally, there is no consensus for a threshold beyond which QT shortening confers cardiac risk. Therefore, the absence of cardiac safety signals in these studies may indicate that the extent of shortening was not sufficient to elevate cardiac risk to detectable levels.
Notwithstanding the lack of any obvious cardiac risk associated with isavuconazole in studies performed to date, it is important that appropriate precautions be taken. Isavuconazole is contraindicated for patients with familial SQTS12 and due consideration should also be taken before prescribing isavuconazole with medication known to shorten the QT interval.
This analysis has some limitations. The phase I study enrolled only healthy subjects and risk factors for cardiac abnormalities were among the exclusion criteria in the phase III study.15 Therefore, it is not clear that the lack of cardiac safety signals in the clinical studies can be generalized to a real‐world population of patients who would be candidates for treatment with isavuconazole.
Taken together, these data demonstrate that isavuconazole shortens the cardiac QT interval in a dose‐related manner, with no evidence of an associated cardiac risk. Nevertheless, the findings indicate a need to be aware of the potential for isavuconazole to shorten the QT interval, and to consider the potential effects in patients with congenital SQTS or the use of concomitant medications with a similar effect.
METHODS
In vitro studies
The in vitro effects of isavuconazole on a panel of human cardiac Ca2+, K+, and Na+ channels were assessed by recording transmembrane currents in cultured cells stably transfected with genes encoding each channel. The details of cell culture, transfection, and test procedures for each ion channel are provided in Supplementary Material 1 and Supplementary Table 1.
Phase I clinical study
A phase I, single‐center, randomized, double‐blind, placebo‐ and active‐controlled, parallel‐group study evaluated the effect of repeat doses of isavuconazole on cardiac repolarization in healthy adult subjects (ClinicalTrials.gov identifier, NCT01565720). This study was conducted in accordance with the ethical principles consistent with the Declaration of Helsinki, Good Clinical Practice, International Conference on Harmonization guidelines, and all applicable laws and regulations. All subjects provided informed consent.
The study enrolled healthy males and females, aged 18–55 years, ≥45 kg, with a body mass index of 18–30 kg/m2 inclusive, sitting systolic blood pressure between 90 and 140 mm Hg inclusive, and diastolic blood pressure between 50 and 90 mm Hg inclusive. Exclusion criteria included previous history of clinically significant gastrointestinal, neurological, renal, hepatic, pulmonary, metabolic, cardiovascular, psychiatric, endocrine, or hematological disorders/diseases; cardiac conduction abnormalities based on ECG at screening (Fridericia's corrected QT interval (QTcF) >430 ms [male], >450 ms (female), or <360 ms (male or female)); history of unexplained syncope, cardiac arrest, cardiac arrhythmia, Torsades de Pointes, structural heart disease or family history of LQTS or SQTS; low levels of K+, Ca2+, or Mg2+; history of alcohol or drug abuse; use of tobacco products within 3 months prior to screening; and known or suspected hypersensitivity to any of the study drugs.
Subjects were randomized to 1 of 4 treatment groups (all treatments except moxifloxacin were double‐blinded), as follows:
Oral isavuconazole 200 mg (in capsules containing isavuconazonium sulfate 186 mg, each equivalent to isavuconazole 100 mg; hereafter, only the isavuconazole equivalent is used) 3 times daily (t.i.d.) on Days 1 and 2, then 200 mg once daily (q.d.) on Days 3–13.
Oral isavuconazole (200 mg) t.i.d. on Days 1 and 2, then 600 mg q.d. on Days 3–13.
Oral placebo t.i.d. on Days 1 and 2, then oral placebo q.d. on Days 3–13.
Oral placebo t.i.d. on Days 1 and 2, then oral placebo q.d. on Days 3–12, followed by oral moxifloxacin 400 mg (an established hERG channel inhibitor commonly used as a positive control for QT prolongation29) on Day 13.
The primary objective of the study was to assess the effect of near steady‐state plasma concentrations of isavuconazole (assessed following Day 13 dose) on cardiac repolarization. Continuous 12‐lead ECGs were recorded for ∼24 h on Day −2 (to familiarize the subjects with the study procedure; not analyzed), Day −1 (baseline), and on Day 13 starting ∼1 h before the planned dose. The ECG parameters collected and analyzed included ventricular heart rate, QT interval, PR interval, QRS duration, RR interval, and QTcF (QT/RR1/3).
Secondary objectives included evaluation of PK as well as safety and tolerability of isavuconazole in healthy adult subjects. Isavuconazole and moxifloxacin PK parameters (Cmax,tmax, 24‐h area under the plasma concentration–time curve (AUC24), trough plasma concentration (Ctrough; isavuconazole only)) were assessed. Blood samples for determining plasma isavuconazole concentrations were collected on Days 11 and 12 within 15 min prior to dosing, and on Day 13 at predose and 1, 2, 3, 4, 8, 10, 12, 20, and 24 h postdose. Blood samples for determination of plasma moxifloxacin concentrations were collected on Day 13 predose and at 1, 2, 3, 4, 8, 10, 12, 20, and 24 h postdose.
Safety and tolerability were assessed by TEAEs, vital signs, and laboratory findings.
Phase III SECURE study
Details of the SECURE study are provided elsewhere.15 Briefly, this was a randomized, double‐blind, noninferiority study comparing the safety and efficacy of isavuconazole (200 mg t.i.d. for 2 days, then 200 mg q.d. oral or i.v.) and voriconazole (6 mg/kg i.v. b.i.d., on Day 1, then 4 mg/kg i.v. b.i.d., then 4 mg/kg oral or i.v.) for treatment of patients with invasive fungal disease caused by Aspergillus spp. or other filamentous fungi. Men and women (aged ≥18 years) with possible or probable invasive mold disease (European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group Consensus Group criteria)30 received treatment for up to 84 days. The safety population included randomized patients who received at least one dose of study medication. Details of efficacy and safety have been presented previously.15 This analysis evaluated categorical changes from baseline for extreme values of QTcF (increases or decreases of >30 ms or >60 ms postbaseline and end of treatment), categorical values of <300, <330, <360, >450, >480, and >500 ms postbaseline and end of treatment and TEAEs related to cardiac function.
Statistical analyses
For the in vitro studies, descriptive statistics were performed using Excel software (Microsoft, Redmond, WA).
For the phase I study, a sample size of 160 subjects (40 per treatment arm, targeting 38 of whom completed in each arm) was estimated to provide 80% power to reject the null hypothesis that the difference in QTcF between isavuconazole and placebo, baseline adjusted, was >10 ms, assessed using the upper limit of a one‐sided 95% CI. These calculations assumed a maximum treatment effect of 2 ms and a standard deviation of the difference of 14 ms. Study populations included the safety analysis set (all randomized subjects who received at least one dose of study medication), the ECG analysis set (subjects who completed a least one time‐matched ECG extraction on Days −1 and 13) and the PK analysis set (all subjects in the safety analysis set whose PK data were adequate for calculation of at least one PK parameter). For ECG analyses, triplicate extractions from each of Days −1 and 13 were averaged at each timepoint. To evaluate the effect of isavuconazole on the QTcF interval at each timepoint, the change from baseline to Day 13 was assessed using analysis of covariance with treatment as a fixed effect and baseline QTcF as a covariate. A conclusion of no significant prolongation of the QTcF interval was reached if the upper bound of the two‐sided 90% CI (equivalent to the 95% CI of a one‐sided CI) was <10 ms at all timepoints. Assay sensitivity was assessed within the same model at the nominal time closest to the median tmax for moxifloxacin on Day 13, and was confirmed if the lower bound of the two‐sided 90% CI for the difference in least square means between moxifloxacin and placebo treatments, baseline adjusted, exceeded 5 ms, as per regulatory guidance.31 Summaries of the percentage of subjects with absolute QTcF interval <360 ms, <330 ms, <300 ms, >450 ms, >480 ms, and >500 ms, as well as increases/decreases from baseline in QTcF >30 ms and >60 ms, were conducted to evaluate outliers.
The relationship between isavuconazole plasma concentrations and dQTcF was explored using a random coefficients linear mixed effects model. Based on the relationship, the predicted dQTcF and its corresponding 90% CI were computed at the mean Cmax for each dose. A scatterplot of the dQTcF vs. isavuconazole plasma concentrations was produced, including the regression line and equation.
Baseline characteristics, PK data, and TEAEs in the phase I study as well as the cardiac‐related TEAEs in the phase III SECURE study (i.e., system organ classes of “Cardiac disorders” or “Electrocardiogram investigations”) were assessed using descriptive statistics.
CONFLICT OF INTEREST
Isavuconazonium sulfate was codeveloped by Astellas Pharma Global Development, Inc. and Basilea Pharmaceutica International Ltd. The studies were funded by Astellas Pharma Global Development, Inc. JK, AD, CL, SM, BP, MJS, RT, TW, and TY are employees of Astellas. PRK has been a paid consultant for Astellas. MY was an employee of PAREXEL International, who were contracted to perform studies related to this article. Editorial support for the article was provided by Ed Parr, PhD, CMPP, a medical writer at Envision Scientific Solutions, funded by Astellas Pharma Global Development, Inc. M Yen was employed by PAREXEL at the time of the study.
AUTHOR CONTRIBUTIONS
J.K., A.D., D.K., C.L., S.M., B.P., M.J.S., R.T., T.W., T.Y., M.Y., and P.R.K. wrote the artice; D.K., A.D., J.K., R.T., M.J.S., and T.W. designed the research; D.K. and M.Y. performed the research; D.K., J.K., T.W., and A.D. analyzed the data.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGMENTS
The authors thank the investigators and staff who conducted the clinical trials, and the subjects who volunteered for these studies. The authors thank Dr James Kramer and the staff at ChanTest Corporation, Cleveland, OH, for performing the in vitro ion channel experiments, the staff at ERT, Philadelphia, PA, for performing ECG analyses, and Dr Shahzad Akhtar of Astellas Research Institute of America LLC, Skokie, IL, for bioanalytical support.
References
- 1. Kannankeril, P. , Roden, D.M. & Darbar, D. Drug‐induced long QT syndrome. Pharmacol. Rev. 62, 760–781 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Owens, R.C., Jr. & Nolin, T.D. Antimicrobial‐associated QT interval prolongation: pointes of interest. Clin. Infect. Dis. 43, 1603–1611 (2006). [DOI] [PubMed] [Google Scholar]
- 3. Alkan, Y. , Haefeli, W.E. , Burhenne, J. , Stein, J. , Yaniv, I. & Shalit, I. Voriconazole‐induced QT interval prolongation and ventricular tachycardia: a non‐concentration‐dependent adverse effect. Clin. Infect. Dis. 39, e49–52 (2004). [DOI] [PubMed] [Google Scholar]
- 4. Dorsey, S.T. & Biblo, L.A. Prolonged QT interval and torsades de pointes caused by the combination of fluconazole and amitriptyline. Am. J. Emerg. Med. 18, 227–229 (2000). [DOI] [PubMed] [Google Scholar]
- 5. Dumaine, R. , Roy, M.L. & Brown, A.M. Blockade of HERG and Kv1.5 by ketoconazole. J. Pharmacol. Exp. Ther. 286, 727–735 (1998). [PubMed] [Google Scholar]
- 6. Aypar, E. et al. Voriconazole‐induced QT interval prolongation and torsades de pointes. Pediatr. Int. 53, 761–763 (2011). [DOI] [PubMed] [Google Scholar]
- 7. Brown, J.D. , Lim, L.L. & Koning, S. Voriconazole associated torsades de pointes in two adult patients with haematological malignancies. Med. Mycol. Case Rep. 4, 23–25 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elbey, M.A. , Cil, H. , Onturk, E. & Islamoglu, Y. OTc prolongation and torsade de pointes ventricular tachycardia in a small dose voriconazole therapy. Eur. Rev. Med. Pharmacol. Sci. 16, 100–102 (2012). [PubMed] [Google Scholar]
- 9. Philips, J.A. , Marty, F.M. , Stone, R.M. , Koplan, B.A. , Katz, J.T. & Baden, L.R. Torsades de pointes associated with voriconazole use. Transpl. Infect. Dis. 9, 33–36 (2007). [DOI] [PubMed] [Google Scholar]
- 10. Zeuli, J.D. , Wilson, J.W. & Estes, L.L. Effect of combined fluoroquinolone and azole use on QT prolongation in hematology patients. Antimicrob. Agents Chemother. 57, 1121–1127 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shah, R.R. Drug‐induced QT interval shortening: potential harbinger of proarrhythmia and regulatory perspectives. Br J. Pharmacol. 159, 58–69 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Astellas Pharma US & Inc . CRESEMBA® (isavuconazonium sulfate) prescribing information. <http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207500Orig1s000lbl.pdf> (2015). Accessed March 10, 2015.
- 13. European Medicines Agency . Cresemba (isavuconazole). <http://www.ema.europa.eu/ema/index.jsp?curl=pages/medicines/human/medicines/002734/human_med_001907.jsp&mid=WC0b01ac058001d124> (2015). Accessed November 11, 2015.
- 14. Falci, D.R. & Pasqualotto, A.C. Profile of isavuconazole and its potential in the treatment of severe invasive fungal infections. Infect. Drug Resist. 6, 163–174 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Maertens, J.A. et al. Isavuconazole vs. voriconazole for the primary treatment of invasive mould disease caused by Aspergillus and other filamentous fungi (SECURE): A pahse 3, randomised‐controlled, non‐inferiority trial. Lancet 387, 760–769 (2016). [DOI] [PubMed] [Google Scholar]
- 16. Marty, F.M. et al. Isavuconazole treatment for mucormycosis: a single‐arm open‐label trial and case‐control analysis. Lancet Infect. Dis. 16, 828–867 (2016). [DOI] [PubMed] [Google Scholar]
- 17. Perfect, J. et al. Outcomes, safety, and tolerability of isavuconazole for the treatment of invasive fungal disease (phase 3 VITAL trial). In International Society for Human and Animal Mycology – 2015 Congress.
- 18. Townsend, R . et al. Pharmacokinetic evaluation of CYP3A4‐mediated drug‐drug interactions of isavuconazole with rifampin, ketoconazole, midazolam, and ethinyl estradiol/norethindrone in healthy adults. Clin. Pharmacol. Drug Dev. (2016) [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Groll, A.H. et al. Pharmacokinetic assessment of drug‐drug interactions of isavuconazole with the immunosuppressants cyclosporine, mycophenolic acid, prednisolone, sirolimus, and tacrolimus in healthy adults. Clin. Pharmacol. Drug Dev. (2016); e‐pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Darpo, B . et al. Results from the IQ‐CSRC prospective study support replacement of the thorough QT study by QT assessment in the early clinical phase. Clin. Pharmacol. Ther. 97, 326–335 (2015). [DOI] [PubMed] [Google Scholar]
- 21. Garnett, C. , Needleman, K. , Liu, J. , Brundage, R. & Wang, Y. Operational characteristics of linear concentration‐QT models for assessing QTc interval in the thorough QT and phase 1 clinical studies. Clin. Pharmacol. Ther. 100, 170–178 (2016). [DOI] [PubMed] [Google Scholar]
- 22. Pérez Riera, A.R. et al. Congenital short QT syndrome: landmarks of the newest arrhythmogenic cardiac channelopathy. Cardiol. J. 20, 464–471 (2013). [DOI] [PubMed] [Google Scholar]
- 23. Anttonen, O. , Junttila, M.J. , Rissanen, H. , Reunanen, A. , Viitasalo, M. & Huikuri, H.V. Prevalence and prognostic significance of short QT interval in a middle‐aged Finnish population. Circulation 116, 714–720 (2007). [DOI] [PubMed] [Google Scholar]
- 24. Funada, A. et al. Assessment of QT intervals and prevalence of short QT syndrome in Japan. Clin. Cardiol. 31, 270–274 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gallagher, M.M. et al. Distribution and prognostic significance of QT intervals in the lowest half centile in 12,012 apparently healthy persons. Am. J. Cardiol. 98, 933–935 (2006). [DOI] [PubMed] [Google Scholar]
- 26. DeSilvey, D.L. & Moss, A.J. Primidone in the treatment of the long QT syndrome: QT shortening and ventricular arrhythmia suppression. Ann. Intern. Med. 93, 53–54 (1980). [DOI] [PubMed] [Google Scholar]
- 27. Dixon, R . et al. Lamotrigine does not prolong QTc in a thorough QT/QTc study in healthy subjects. Br. J. Clin. Pharmacol. 66, 396–404 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schimpf, R . et al. Drug‐induced QT‐interval shortening following antiepileptic treatment with oral rufinamide. Heart Rhythm 9, 776–781 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alexandrou, A.J. et al. Mechanism of hERG K+ channel blockade by the fluoroquinolone antibiotic moxifloxacin. Br. J. Pharmacol. 147, 905–916 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. De Pauw, B. et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46, 1813–1821 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. US Food and Drug Administration . E14 clinical evaluation of QT/QTc interval prolongation and proarrhythmic potential for non‐antiarrhythmic drugs — Questions and Answers. Guidance for Industry 2012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Supporting Information


