Abstract
In addition to an action on metabolism, anabolic/androgenic steroids also increase sex drive and mental acuity. If abused, such steroids can cause irritability, impulsive aggression, and signs of major depression [Pearson, H. (2004) Nature 431, 500-501], but the mechanisms that produce these symptoms are unknown. The present study investigates behavioral and neurochemical alterations occurring in association with protracted (3-week) administration of testosterone propionate (TP) to socially isolated (SI) and group-housed male and female mice. Male but not female SI mice exhibit aggression that correlates with the down-regulation of brain neurosteroid biosynthesis. However, in female mice, long-term TP administration induces aggression associated with a decrease of brain allopregnanolone (Allo) content and a decrease (≈40%) of 5α-reductase type I mRNA expression. In spayed mice treated with TP, restitution experiments with progesterone and estrogen normalize brain Allo content and prevent aggression. Submicromolar doses of S-norfluoxetine (S-NFLX) that are insufficient to inhibit serotonin reuptake selectively increase brain Allo content and abolish TP-induced aggression. Our results support the view that TP-induced aggressive behavior is the result of a TP-mediated neurosteroid biosynthesis down-regulation that can be reversed by the S-NFLX-induced increase of brain Allo content.
Keywords: 5α-reductase, anabolic steroids, neurosteroids, social isolation, castration
The athletic performance-enhancing anabolic/androgenic steroids (AAS) are becoming a major public health concern worldwide (1, 2). Contrary to expectations, AAS abuse is not a problem restricted to elite athletes, but, because of their ability to enhance mental acuity, these agents are becoming part of the general recreational drug armamentarium. In fact, not only non-athlete adolescents of both sexes but also elderly subjects abuse them (1-5). AAS abuse may increase the risk of cardiovascular and hepatic disorders and prostate cancer, may upset menstrual cycles (3, 6), and may elicit a wide range of psychiatric symptoms (e.g., episodic mania, impulsive aggression, and major depression) in both males and females (reviewed in refs. 7 and 8). If administered in large doses to female rodents, AAS promote the onset of male behavior by remodeling synaptic links in specific CNS circuits (reviewed in ref. 9). Episodes of irritability and aggressive behavior, commonly referred to as steroid rage (colloquially called “roid rage”), are among the side effects frequently expressed by AAS users (10, 11). These episodes can escalate to extreme violence, including murder attempts (7). AAS abuse may lead to dependence, and drug discontinuation can cause severe withdrawal symptoms that may lead to suicide attempts (12, 13).
Despite the social impact caused by the high incidence of AAS abuse, the neuronal mechanisms underlying this drug-induced syndrome are not understood.
The symptoms of such behavioral disorders strongly suggest a modification of neuronal events mediated by GABAergic transmission (8, 13-17). Although μM concentrations of testosterone can directly exert a positive allosteric modulation of the action of GABA at different recombinant GABAA receptors (16), it has also been shown that protracted daily exposure to large doses of testosterone results in the development of GABAergic transmission down-regulation (8, 16). Hence, GABAergic transmission may be an important mechanism underlying the action of AAS.
Several studies in mice unrelated to AAS have unequivocally shown that a GABAergic transmission impairment plays a pivotal role in the expression of aggressive behavior (18-20). For example, the down-regulation of GABAA receptor signal transduction (18-23) in socially isolated (SI) aggressive male mice is associated with a down-regulation of 3α-hydroxysteroid-5α-pregnan-20-one (allopregnanolone, Allo) biosynthesis (19-21, 24). Allo acts as a potent (nM concentrations) positive allosteric modulator of signal transduction at several GABAA receptor subtypes (25-27).
Unlike SI male mice, SI female mice fail to develop aggression or to express Allo down-regulation (19). Hence, these data strongly implicate a lack of positive GABAA receptor function modulation by 3α,5α-pregnane steroids and invite speculation on the pivotal mechanism underlying mouse aggressive behavior.
The experiments to be reported were designed to answer the broad question: Is testosterone propionate (TP)-elicited aggressive behavior mediated by brain Allo content down-regulation?
To answer this question, the following brain functions were studied: (i) Allo levels; (ii) the expression of the mRNA encoding the rate-limiting step enzyme of pregnane steroid biosynthesis, i.e., 5α-reductase type I (5α-RI) (28); and (iii) aggressive behavior in normal or spayed female mice treated or untreated for 3 weeks with a daily TP dose of 0.5 mg/kg s.c.
Methyltestosterone, oxandrolone, stanozolol, nandrolone, and a number of congeners of testosterone are among the most commonly abused AAS (7). However, the most extensively abused AAS is testosterone (2). Therefore, the present investigation is focused on the behavioral and neurochemical correlates of long-term TP administration.
Materials and Methods
Animals and Drug Treatment. Adult male and female Swiss-Webster mice (Harlan Breeders, Indianapolis), 22-25 g of body weight, maintained under a 12-h dark/light cycle, and food and water ad libitum, were used for all experiments. Animals were group housed (GH) (five to six per 24 × 17 × 12-cm cage) or SI in a cage of the same size for a period of 3 weeks, during which they were subjected to daily s.c. injections of TP, estradiol, progesterone, or vehicle. Treatment was discontinued 24 h preceding our behavioral and biochemical studies (19). Neurosteroids were measured in the olfactory bulbs (OB) because of the importance of this brain region in aggressive behavior (29), and because this area expresses the highest levels of neurosteroids in the rodent brain (23, 28).
Ovariectomy (dorsal route), orchiectomy, or olfactory bulbectomy were performed under nembutal anesthesia. Vehicle or tested drugs were prepared in sesame oil and given s.c., as 0.1 ml per 10 g of body weight. S-norfluoxetine (S-NFLX) was administered to SI mice i.p., 30 min before behavioral test.
Because estrous cycle stage was deemed an important variable in most of the behavioral and biochemical tests, vaginal smears were conducted in mice at the time of testing.
S-NFLX was a generous gift from Eli Lilly. Testosterone, estradiol, and progesterone were from Sigma. TP was from Steraloids (Newport, RI).
Resident-Intruder Test. Aggressive behavior in SI mice. To test the aggressive behavior of SI mice, an intruder mouse of the same gender as the SI resident male (male/male resident-intruder) (19, 20) or male bulbectomized intruder for SI resident female (female/male resident-intruder) (30) was placed in the resident home cage, and resident-intruder interactions were videotaped for 10 min. Olfactory bulbectomized males were used as intruders because they elicit aggressive behavior in SI female mice that received 3 weeks of TP treatment (30). The aggressive behavior of resident SI mice was characterized by an initial pattern of exploratory activity around the intruder, which was followed by rearing and tail rattle and, a few seconds later, by wrestling and/or a violent biting attack. The total duration of these attacks and/or wrestling during the 10-min observation period was evaluated as described (19).
Social interaction test. Because GH female mice fail to develop an overt territorial-dependent aggression, to test whether TP induces social interaction changes in GH female mice, female mice were placed individually in a novel cage for 60 min before introducing a bulbectomized male intruder. In TP-treated female mice, the resident initiates social interactions to a bulbectomized intruder that were characterized by exploratory activity, genitalia sniffing, and licking the male intruder, followed by occasional rough grooming and bites. These forms of resident/intruder interactions were expressed as duration of “aggressive grooming” during 10 min of the resident exposure to the intruder.
Measurement of Locomotor Activity. A computerized AccuScan 12 Animal Activity Monitoring System (Columbus Instruments, Columbus, OH) assisted by versamax software (AccuScan Instruments, Columbus, OH) was used to quantitatively monitor locomotor activity in mice, as described (31). Each activity cage consisted of a Perspex box (20 × 20 × 20 cm) surrounded by horizontal and vertical infrared sensor beams. The interruptions of the horizontal sensors during 15 min were taken as a measure of horizontal activity, whereas those of vertical sensors measured rearing activity. Between 1:00 and 3:00 p.m., activity was recorded from GH and SI mice for 15 min 24 h after drug discontinuation.
Measurement of Brain Neurosteroid Content. Extraction, derivatization, and GC-MS analyses of neurosteroids were performed with minor modifications as described (19). (i) Olfactory bulbs were homogenized in 10 vol of distilled water containing 2-5 fmol/ml [3H]progesterone, [3H]Allo, and [3H]testosterone (New England Nuclear) to monitor the HPLC retention profile and 1 pmol of deuterium-labeled progesterone (progesterone-1,2,6,7-D4), deuterium-labeled Allo (Allo-17,21,21,21-D4) and deuterium-labeled testosterone (17β-hydroxyandrost-4-en-3-one-16,16,17-D3) (Sigma) were used as internal standards. The supernatants were extracted with ethyl acetate and after lyophilization were purified with HPLC, as described (23). (ii) The HPLC fractions containing progesterone, Allo, or testosterone were derivatized with heptaf luorobutyric acid anhydride (HFBA) and subjected to GC-mass fragmentography analysis.
Mass fragmentography analysis of derivatized progesterone and Allo was performed in the standard electron impact (EI) mode. Testosterone was analyzed in the negative ion chemical ionization mode (NICI) by using methane as the reaction gas.
The detection limit for progesterone, Allo, and testosterone was ≈10 fmol; the standard curve was linear between 5 and 105 fmol. For progesterone, Allo, or testosterone quantification, the m/z ion-monitoring mode was 510 for HFBA-progesterone, 518 for HFBA-d-progsterone, 496 for HFBA-Allo, 500 for HFBA-D-Allo, 660 for HFBA-testosterone, and 663 for HFBA-d-testosterone.
Quantitative RT-PCR Analyses of 5α-RI mRNAs. The 5α-RI mRNAs were quantified with competitive RT-PCR as described by Dong et al., (28). Primers for 5α-RI mRNA quantification were as follows: reverse 308-331 (5′-ACCATGACTCATTGCTCCCTGCTT-3′) and forward 1-24 (5′-CATCATCAGTGGTACCTCGAGAAG-3′). Templates for 5α-RI internal standards contained a restriction endonucleases site XbaI, which on digestion generated fragments of 135 and 182 bp (28). Each primer pair annealed to a single RNA template on multiple blast comparisons and yielded a single band of the correct molecular size after amplification of the RNA isolated from the mouse brain.
Statistical Analyses. Data are given as means ± SEMs unless otherwise indicated. Comparisons between the control group and each of the treatment groups were performed by one-way ANOVA followed by Dunnett's test.
Results
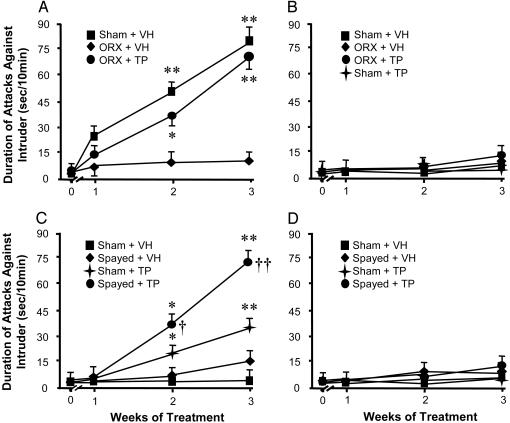
Testosterone-Induced Aggression in Orchiectomized (ORX) Mice. In most species, orchiectomy inhibits aggression (32, 33). Fig. 1A shows that when sham-operated adult (2.5-month-old) male mice are SI, there is a time-related increase of aggression intensity toward a same-sex intruder. After 3 weeks of social isolation (SI), the OB Allo content and the OB and frontal cortex 5α-RI mRNA expression are reduced by ≈50% (Table 1). In contrast, SI ORX mice not only exhibit a lower level of aggressive behavior (Fig. 1 A), but also a normal OB Allo level (Table 1) and a 10-fold reduction in OB testosterone levels (Table 1). To underscore the important interaction between testosterone brain levels and the expression of aggressive behavior, ORX mice were administered TP (0.5 mg/kg) once daily for 3 weeks of SI. Resident-intruder tests performed in these mice 24 h after the last TP dose showed an aggressive behavior level comparable with that of sham-operated SI mice (Table 1). This TP dose normalizes the OB testosterone level and reduces the OB Allo content to a level similar to that expressed by vehicle-treated sham-operated mice after an identical isolation period (Table 1).
Fig. 1.
Time course of aggressiveness development in male (A and B) and female (C and D) mice caged in SI (A and C) or GH (B and D). Aggressive behavior of a resident mouse against a same-sex (A and B) or a male bulbectomized (C and D) intruder was measured as the duration of attacks (sec.) in a 10-min exposure. TP (0.5 mg/kg s.c.) or vehicle were administered once daily. The resident-intruder test was performed 24 h after the last dose of TP. Each value is the mean ± SEM of six to eight animals. *, P < 0.05; **, P < 0.01 when the duration of attacks at a given time period is compared with period 0-week; †, P < 0.05; ††, P < 0.05 when the duration of attacks is compared with sham-operated plus TP group (one-way ANOVA followed by Dunnett's test).
Table 1. TP effects on the relationship between duration of attacks against an intruder, 5α-RI mRNA expression, progesterone (P), Allo, and testosterone (T) levels in the brain of SI and GH male and female mice.
| Mice | Aggression, attack duration sec/10 min | 5α-RI mRNA, attomol/μg total RNA | P, pmol/g | Allo, pmol/g | T, pmol/g |
|---|---|---|---|---|---|
| Male | |||||
| GH sham | None | 365 ± 21 | 19 ± 5.9 | 16 ± 3.6 | 31 ± 4.8 |
| SI sham | 68 ± 11 | 177 ± 15* | 18 ± 6.4 | 7.1 ± 1.2* | 35 ± 4.5 |
| SI + ORX | 9.4 ± 7.2† | ND | ND | 20 ± 2.8 | 3.4 ± 1.5* |
| SI + ORX + TP | 61 ± 13 | ND | ND | 5.2 ± 0.9* | 46 ± 9.3 |
| Female | |||||
| GH sham | None | 415 ± 41 | 65 ± 15 | 14 ± 2.3 | 12 ± 4.1 |
| SI sham | None | 437 ± 35 | 58 ± 13 | 16 ± 3.1 | 10 ± 5.0 |
| SI + sham + TP | 33 ± 7.3‡ | ND | 69 ± 20 | 8.2 ± 1.6‡ | 39 ± 5.2‡ |
| SI + spayed | 16 ± 6.1 | 378 ± 19 | ND | 14 ± 2.3 | 7.4 ± 1.7 |
| SI + spayed + TP | 75 ± 8.3§ | 280 ± 21§ | ND | 6.9 ± 1.3§ | 44 ± 18§ |
Allo, progesterone, and testosterone were determined in OB and 5α-RI in frontal cortex of SI and GH mice decapitated immediately after behavioral tests. TP (0.5 mg/kg s.c.) was administered daily for 3 weeks. Each value is the mean ± SEM of six to eight animals; ND = not determined. *, P < 0.01 compared with GH male mice; †, P < 0.01 compared with SI male mice; ‡, P < 0.05, compared with SI female mice; §, P < 0.01 compared with respective control group (one-way ANOVA followed by Dunnett's test).
Aggressive Behavior and Brain Neurosteroid Content in TP-Treated Female Mice. Behavioral correlates of TP administration. Unlike male mice, females fail to develop a territorially defined aggressiveness (see Materials and Methods) even after 3 weeks of SI (Fig. 1C). When female mice are SI for a period of 3 weeks and administered TP daily (0.5 mg/kg s.c.), they develop a time-related increase of aggression measured 24 h after the last TP injection (Fig. 1C). This dose of TP increases the female OB testosterone content to values similar to that of male mice (Table 1).
SI induces only modest levels of aggression in spayed mice (Fig. 1C). However, when SI spayed mice are administered TP daily for 3 weeks, they evince a time-dependent high level of aggression (Fig. 1C) against a bulbectomized male intruder. The intensity of aggression is comparable with that expressed by adult SI male mice toward a same-sex intruder. In this case, the OB testosterone content of SI spayed and TP-treated mice is slightly increased over the physiological levels found in adult male mice (Table 1).
Interestingly enough, when a TP-treated SI female mouse is exposed to a naive female intruder, it attempts to interact sexually with the intruder and also exhibits other courtship behavioral components that have been shown to be exclusively triggered in male resident mice by the introduction of a female intruder.
Unlike SI mice but similar to GH male mice, GH female mice with or without ovaries or with or without TP treatment and caged individually 60 min before the social interaction test fail to express a territorial-dependent aggressiveness (i.e., attacks or biting; see Fig. 1D). However, these mice show an increased level of interaction and aggressive grooming toward a bulbectomized male intruder when compared with vehicle-treated female mice (Table 2).
Table 2. Protracted TP treatment increases aggressive grooming and decreases Allo OB content in GH female mice.
| Mice | Aggressive grooming, duration sec/10 min | Allo, pmol/g |
|---|---|---|
| GH + Sham + VH | 96 ± 14 | 14.5 ± 2.1 |
| GH + Sham + TP | 191 ± 35* | 8.8 ± 1.4* |
| GH + Spayed + VH | 117 ± 16 | 13.4 ± 1.2 |
| GH + spayed + TP | 209 ± 31† | 7.9 ± 1.1† |
Resident-intruder test was performed 24 h after the last injection of 3 weeks of TP treatment (0.5 mg/kg s.c. once daily). Each value is the mean ± SEM of six to eight animals. *, P < 0.01 compared with GH plus VH group; †, P < 0.01 compared with GH plus Spayed plus VH group (one-way ANOVA followed by Dunnett's test).
Locomotor activity was not affected by any of the above reported treatments (data not shown). The TP-treated female mouse body weights increased during the course of 3-week treatment to an extent similar to groups receiving vehicle (data not shown).
In either SI or GH mice, the vaginal smears were used to assess the estrous phase. All mice SI for 3 weeks were found to be in metaestrus. Only one SI female mouse of five treated with vehicle was in estrus. GH female mice treated with vehicle were also in the metaestrus phase except for two mice: one was in diestrus and the other in estrus. All remaining GH females were in metaestrus. TP-treated females were all in metaestrus.
Neurosteroid correlates of TP-induced aggressive behavior. Table 1 reports the OB Allo content after 3 weeks of TP treatment during SI vis-à-vis the frontal cortex (FC) 5α-RI mRNA expression and aggression levels (measured by attack and biting duration) in sham-operated, ORX, and spayed mice. Sham-operated male mice SI for 3 weeks and receiving vehicle exhibit aggressive behavior and express a 57% decrease of OB Allo content (Table 1), a 40% decrease of OB 5α-RI mRNA expression, and a 48% decrease of FC 5α-RI mRNA expression (Table 1). In contrast, cycling sham-operated or spayed females that have been SI for the same time period express only modest aggression. They also fail to show a decrease of brain Allo content or a decrease of 5α-RI mRNA expression (Table 1). However, TP treatment of SI sham-operated or spayed females over a 3-week period induces a >50% decrease of brain Allo content (Table 1), vigorous aggressiveness (Table 1 and Fig. 1C), and a 50% decrease of 5α-RI mRNA expression in OB (705 ± 62 to 436 ± 65 attomol/μg total RNA, n = 4). Table 1 shows that 5α-RI mRNA expression in FC was likewise decreased by TP treatment. In contrast to the decrease of Allo, the OB progesterone content fails to decrease in TP-treated female mice (Table 1).
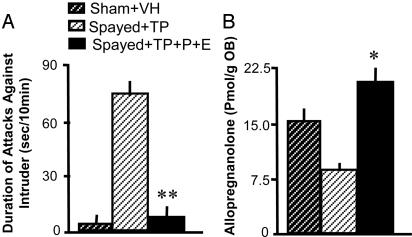
Fig. 2A shows that progesterone and estradiol coadministration to TP-treated spayed SI mice greatly reduces the expression of aggression. This effect is accompanied by an up-regulation of OB Allo content (see Fig. 2B).
Fig. 2.
Duration of attacks toward a male bulbectomized intruder (A) and OB Allo content (B) in 3-week SI sham-operated or spayed mice. TP (0.5 mg/kg s.c., once daily) and hormone-restitution with progesterone (1 mg/kg s.c., once daily) and estradiol (0.05 mg/kg s.c., once daily) were administered for 3 weeks during SI. Each value is the mean ± SEM of six animals. *, P < 0.05; **, P < 0.01 when compared with spayed plus TP group (one-way ANOVA followed by Dunnett's test).
Table 2 shows that TP treatment also decreases Allo brain content in GH female mice with or without ovaries. These mice express a higher level of interaction and aggressive grooming than vehicle-treated controls.
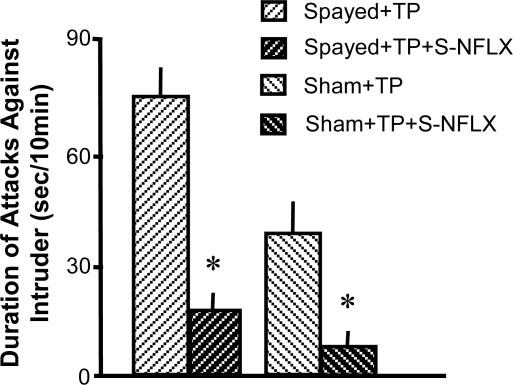
S-NFLX Antagonizes the Expression of Aggression in Testosterone-Treated Female Mice. Submicromolar doses of S-NFLX reduce aggressiveness in SI male mice. This effect was correlated with an up-regulation of brain Allo (19). Fig. 3 shows that aggressive female mice that have received long-term TP treatment respond to a single low dose of S-NFLX (1.8 μmol/kg) with a decrease of aggressiveness, presumably mediated by up-regulating brain Allo content.
Fig. 3.
S-NFLX reduces aggression in spayed or sham-operated SI mice. Each value is the mean ± SEM of four mice. S-NFLX (1.8 μmol/kg, i.p.) was given 30 min before resident-intruder tests. *, P < 0.01 when S-NFLX-treated groups are compared with respective control groups (one-way ANOVA followed by Dunnett's test).
Discussion
Testosterone Treatment and Aggression in Mice. The results obtained in this study establish that a 3-week administration of TP (0.5 mg/kg s.c., daily) consistently favors aggressive behavior induction in adult ORX, spayed, or cycling female mice (Fig. 1 and Tables 1 and 2).
The presence of aggressive behavior elicited by TP can be detected by (i) an increased level of social contact associated with aggressive grooming in GH mice (Table 2) and (ii) an increased number of biting and attack episodes against an intruder male mouse in SI mice (Fig. 1).
When the increased aggressiveness induced by protracted TP treatment in SI mice is compared with the relatively low level of aggression noted in GH mice, one may suggest that TP does not induce aggression per se but sensitizes mice responsiveness to environmental stimuli. Thus, our results confirm previous reports (34-37) indicating that TP and other AAS fail to induce aggressive behavior per se but they can lower the threshold of irritability induced by provocative environmental stimuli (36, 37).
Because insufficient research has been conducted on the AAS mode of action and aggression in female mice, our studies of TP action on female mice may be of relevance in developing new hypotheses on the molecular mechanisms underpinning the behavioral consequences of the growing use of AAS in adolescent girls (1-5).
In our experiments, aggressive behavior was measured 24 h after the last TP injection, and, at this time, the amount of brain testosterone in spayed mice was slightly increased compared with that found in the brain of adult untreated male mice. This finding indicates that the dose of TP used in the study of female mice is not “supraphysiological” (8) because it induces brain levels that are within the range detected in normal male mice.
Modulation of GABAA Receptors by Testosterone. Alterations of GABAergic transmission in the mammalian forebrain including the OB probably play a pivotal role in lowering the threshold for aggressive behavior (19, 30, 38-40). Furthermore, several lines of evidence recently reviewed by Clark and Henderson (8) suggest that down-regulation of the GABAergic signal transduction is prominent in the regulation of aggressive behavior by AAS (8).
For example, it has been reported that protracted exposure to high (7.5 mg/kg per day) doses of 17α-methyltestosterone significantly decreases the expression of GABAA receptor subunits (α1, α2, α5, γ1, and γ2) mRNAs preferentially in peripubertal females (41). In contrast, moderate (0.75 mg/kg per day) doses of 17α-methyltestosterone fail to induce significant changes of GABAA receptor subunit expression in adult mice of either sex (41).
We have shown (Table 1) that, after the TP schedule (0.5 mg/kg per day) used in our experiments, brain testosterone levels in SI ORX or spayed mice reach values slightly higher than those found in brain tissue of intact male mice. These concentrations are in the nM range, i.e., three orders of magnitude lower than the concentrations causing a direct positive allosteric modulatory action at GABAA receptors (8). It is also unlikely that these brain testosterone concentrations are sufficient to induce significant changes of GABAA receptor subunit expression in adult male or female mice (41).
Down-Regulation of Neurosteroid Biosynthesis. Because accumulating evidence suggests that a decreased signal transduction of GABAA receptors to GABA or GABAmimetic drugs in SI mice may be related to a down-regulation of Allo biosynthesis (19-23), we were prompted to study whether TP action on aggressive behavior is associated with a down-regulation of Allo brain content.
In this study, we provide evidence that TP reduces the brain Allo content by >50% in female and male mice that are either GH or SI, and sham-operated or spayed.
The rationale for measuring neurosteroids in the OB is supported by the crucial role that this brain region plays in aggressive behavior (29), and also by the finding that this brain structure expresses the highest levels of neurosteroids and presumably the highest rate of steroid biosynthesis (23, 28). Moreover, previous studies could correlate the biosynthesis rates of Allo in OB with the intensity of aggressive behavior (19).
To test the hypothesis that a decrease of Allo biosynthesis may modulate the threshold and duration of the aggressive behavior accompanying TP treatment, we injected mice with progesterone plus estradiol in doses that increase OB Allo levels (Fig. 2). We found that coadministration of progesterone and estradiol reduces aggression induced by SI and protracted treatment with TP.
S-NFLX is a pregnane steroid-enhancing drug that in submicromolar doses increases brain Allo content and attenuates aggressive behavior in SI male mice (19) in a manner that is independent of 5HT reuptake (22). S-NFLX administered in submicromolar concentrations to SI normal, or spayed and TP-treated mice 30 min before an aggression test, reduces aggression induced by protracted TP treatment (Fig. 3).
Electrophysiological recordings from cortical pyramidal neurons in brain slices obtained from mice with low levels of Allo treated with a 5α-RI inhibitor showed a GABAA receptor signal transduction down-regulation (25). Moreover, miniature inhibitory postsynaptic current recordings from such neurons exhibited faster decay kinetics than their wild-type counterparts. These electrophysiological changes are reversed by the application of low concentrations of Allo (25). Thus, one may hypothesize that a possible mechanism by which TP modulates aggression is by means of a reduction of the GABAergic signal transduction amplification elicited by Allo.
Regulation of Neurosteroid Biosynthesis. Brain Allo content but not that of its precursor progesterone is decreased after protracted administration of TP to female mice. Thus, a possible mechanism by which TP induces a brain Allo content decrease is a down-regulation of the expression of specific enzymes, i.e., 5α-RI or 3α-hydroxysteroid-oxidoreductase (3α-HSOR), which converts progesterone into 5α-dihydroprogesterone and Allo, respectively. In this study, we show that 5α-RI mRNA expression is decreased in brain tissue of aggressive male and female mice although it fails to decrease in brain tissue of mice that do not exhibit aggressive behavior. Studies by Torres and Ortega (42, 43) and by Torres et al. (44) have recently reported that protracted TP administration to rats results in a negative modulation of the expression of the gene encoding 5α-RI in brain, but not in peripheral tissues such as liver and prostate, where TP up-regulates the expression of 5α-RI mRNA. Because 5α-RI is the rate-limiting step enzyme in pregnane steroid metabolism (22, 28), these results suggest that the down-regulation of brain 5α-RI expression may be responsible for the decrease of brain Allo biosynthesis after TP administration. We cannot exclude, however, that, in addition to 5α-RI, 3α-HSOR also is down-regulated during TP treatment.
An alternative mechanism by which TP may cause a decrease of Allo brain content could be a competition between testosterone and progesterone in the catalytic action of 5α-RI (45). Thus, additional detailed time-course and dose-response studies are needed to precisely define the mechanisms by which testosterone induces brain Allo content decrease.
Conclusions
The neuronal mechanism underlying TP-induced aggression in female mice very likely is similar to that regulating aggression in untreated SI males. This mechanism involves the GABAergic system, which is positively and allosterically modulated by endogenous Allo (21-25, 46, 47). Thus, protracted exposure of rodents to TP may decrease GABAergic tone by reducing the bioavailability of pregnane steroids (by means of an inhibitory action on 5α-RI expression) that have a prominent endogenous regulatory role in the affinity of GABAA receptors for GABA. This action may mediate most of the behavioral abnormalities that are associated with AAS use and abuse.
Collectively, these findings suggest an important modulatory role for the endogenous pregnane steroids on GABAergic inhibitory synaptic transmission.
It is noteworthy that the pregnane-enhancing drug S-NFLX in submicromolar doses increases brain Allo content (19, 20, 22) and attenuates aggressive behavior in TP-treated mice. Likely, this observation may have important implications for designing future treatments for androgen abuse.
Acknowledgments
We thank Drs. Maria Luisa Barbaccia (Department of Neuroscience, School of Medicine, University of Rome “Tor Vergata,” Rome), Vassilios Papadopoulos (Departments of Cell Biology and Pharmacology and of Neuroscience, Georgetown University Medical Center, Washington, DC), and David H. Farb (Department of Pharmacology, School of Medicine, Boston University, Boston) for constructive criticism and suggestions in the preparation of the manuscript, and Ulana Liskevych for excellent technical assistance. This study was supported by National Institute of Mental Health Grants MH 56890 (to A.G.) and MH 071667-01A1 (to E.C.) and by a Campus Research Board Award (to G.P.).
Abbreviations: S-NFLX, S-norfluoxetine; SI, social isolation; SI, socially isolated; GH, group housed; ORX, orchiectomized; Allo, allopregnanolone; TP, testosterone propionate; AAS, anabolic/androgenic steroids; 5α-RI, 5α-reductase type I; OB, olfactory bulbs; HFBA, heptafluorobutyric acid anhydride.
References
- 1.National Institute on Drug Abuse (2000) NIDA Research Report: Steroid Abuse and Addiction (National Clearinghouse on Alcohol and Drug Information, Rockville, MD), NIH Publication No. 00-3721 (http://165.112.78.61/ResearchReports/Steroids/Anabolicsteroids.html).
- 2.Pearson, H. (2004) Nature 431, 500-501. [DOI] [PubMed] [Google Scholar]
- 3.Lukas, S. E. (1996) Annu. Rev. Pharmacol. Toxicol. 36, 333-357. [DOI] [PubMed] [Google Scholar]
- 4.Faigenbaum, A. D., Zaichkowshy, L. D., Gardner, D. E. & Micheli, L. J. (1998) Pediatrics 101, 1-6. [DOI] [PubMed] [Google Scholar]
- 5.Irving, L. M., Wall, M., Neumark-Sztainer, D. & Story, M. (2002) J. Adolesc. Health 30, 243-252. [DOI] [PubMed] [Google Scholar]
- 6.Urhausen, A., Albers, T. & Kindermann, W. (2004) Heart 90, 496-501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pope, H. G., Jr., & Brower, K. J. (2000) in Comprehensive Textbook of Psychiatry, eds. Sadock, B. J. & Sadock, B. A. (Lippincott William & Wilkins, Philadelphia), 7th Ed., pp. 1085-1095.
- 8.Clark, A. S. & Henderson, L. P. (2003) Neurosci. Biobehav. Rev. 27, 413-436. [DOI] [PubMed] [Google Scholar]
- 9.Morris, J. A., Jordan, C. L. & Breedlove, S. M. (2004) Nat. Neurosci. 7, 1034-1039. [DOI] [PubMed] [Google Scholar]
- 10.Corrigan, B. (1996) Med. J. Aust. 165, 222-226. [DOI] [PubMed] [Google Scholar]
- 11.Hartgens, F. & Kuipers, H. (2004) Sport Med. 34, 513-554. [DOI] [PubMed] [Google Scholar]
- 12.Brower, K. J., Blow, F. C., Beresford, T. P. & Fuelling, C. (1989) J. Clin. Psychiatry. 50, 31-33. [PubMed] [Google Scholar]
- 13.Thiblin, I., Lindquist, O. & Rajs, J. (2000) J. Forensic Sci. 45, 16-23. [PubMed] [Google Scholar]
- 14.Bitran, D., Kellogg, C. K. & Hilvers, R. J. (1993) Horm. Behav. 27, 568-583. [DOI] [PubMed] [Google Scholar]
- 15.Wilson, M. A. (1996) Crit. Rev. Neurobiol. 10, 1-37. [DOI] [PubMed] [Google Scholar]
- 16.Clark, A. S., Jones, B. L., Yang, P. & Henderson, L. P. (2004) in Neurosteroid Effects in the Central Nervous System: The Role of the GABAA Receptor, ed. Smith, S. S. (CRC, Boca Raton, FL), pp. 119-141.
- 17.Jorge-Rivera, J. C., McIntyre, K. L. & Henderson, L. P. (2000) J. Neurophysiol. 83, 3299-3309. [DOI] [PubMed] [Google Scholar]
- 18.Miczek, K. A., Fish, E. W. & De Bold, J. F. (2003) Horm. Behav. 44, 242-257. [DOI] [PubMed] [Google Scholar]
- 19.Pinna, G., Dong, E., Matsumoto, K., Costa, E. & Guidotti, A. (2003) Proc. Natl. Acad. Sci. USA 100, 2035-2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guidotti, A., Dong, E., Matsumoto, K., Pinna, G., Rasmusson, A. M. & Costa, E. (2001) Brain Res. Rev. 37, 110-115. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto, K., Uzunova, V., Pinna, G., Taki, K., Uzunov, D. P., Watanabe, H., Mienvielle, J. M., Guidotti, A. & Costa, E. (1999) Neuropharmacology 38, 955-963. [DOI] [PubMed] [Google Scholar]
- 22.Pinna, G., Costa, E. & Guidotti, A. (2004) Proc. Natl. Acad. Sci. USA 101, 6222-6225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pinna, G., Uzunova, V., Matsumoto, K., Puia, G., Mienville, J.-M., Costa, E. & Guidotti, A. (2000) Neuropharmacology 39, 440-448. [DOI] [PubMed] [Google Scholar]
- 24.Serra, M., Pisu, M. G., Littera, M., Papi, G., Sanna, E., Tuveri, F., Usala, L., Purdy, R. H. & Biggio, G. (2000) J. Neurochem. 75, 732-740. [DOI] [PubMed] [Google Scholar]
- 25.Puia, G., Mienville, J.-M., Matsumoto, K., Takahata, H., Watanabe, H., Costa, E. & Guidotti, A. (2002) Neuropharmacology 44, 49-55. [DOI] [PubMed] [Google Scholar]
- 26.Puia, G., Santi, M. R., Vicini, S., Pritchett, D. B., Purdy, R. H., Paul, S. M., Seeburg, P. H. & Costa, E. (1990) Neuron 4, 759-765. [DOI] [PubMed] [Google Scholar]
- 27.Lambert, J. J., Belelli, D., Peden, D. R., Vardy, A. W. & Peters, J. A. (2003) Prog. Neurobiol. 71, 67-80. [DOI] [PubMed] [Google Scholar]
- 28.Dong, E., Matsumoto, K., Uzunova, V., Sugaya, I., Costa, E. & Guidotti, A. (2001) Proc. Natl. Acad. Sci. USA 98, 2849-2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siegel, A., Roeling, T. A., Gregg, T. R. & Kruk, M. R. (1999) Neurosci. Biobehav. Rev. 23, 359-389. [DOI] [PubMed] [Google Scholar]
- 30.Denenberg, V. H., Gaulin-Kremer, E., Gandelman, R. & Zarrow, M. X. (1973) Anim. Behav. 21, 590-598. [DOI] [PubMed] [Google Scholar]
- 31.Pinna, G., Galici, R., Schneider, H. H., Stephens, D. N. & Turski, L. (1997) Proc. Natl. Acad. Sci. USA 94, 2719-2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knol, B. W. & Egberink-Alink, S. T. (1989) Vet. Q. 11, 102-107. [DOI] [PubMed] [Google Scholar]
- 33.Schneider, J. S., Stone, M. K., Wynne-Edwards K. E., Horton, T. H., Lydon, J., O'Malley, B. & Levine, J. E. (2003) Proc. Natl. Acad. Sci. USA 100, 2951-2956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Breuer, M. E., McGinnis, M. Y., Lumia, A. R. & Possidente B. P. (2001) Horm. Behav. 40, 409-418. [DOI] [PubMed] [Google Scholar]
- 35.McGinnis, M. Y., Lumia, A. R., Breuer, M. E. & Possidente, B. (2002) Horm. Behav. 41, 101-110. [DOI] [PubMed] [Google Scholar]
- 36.Bronson, F. H. (1996) Pharmacol. Biochem. Behav. 53, 329-334. [DOI] [PubMed] [Google Scholar]
- 37.Bronson, F. H., Nguyen, K. Q. & De La Rosa, J. (1996) Physiol. Behav. 59, 49-55. [DOI] [PubMed] [Google Scholar]
- 38.Mandel, P., Haug, M., Puglisi-Allegra, S., Kempf, E. & Mack, G. (1981) in The Biology of Aggression, eds. Brian, P. & Benton, D. (Sijthoff & Noordhoff, Alphen aan der Rijn, The Netherlands), pp. 169-173.
- 39.Puglisi-Allegra, S. & Mandel, P. (1980) Psychopharmacology (Berlin) 70, 287-290. [DOI] [PubMed] [Google Scholar]
- 40.Molina, V., Ciesielski, L., Gobaille, S. & Mandel, P. (1986) Pharmacol. Biochem. Behav. 24, 657-664. [DOI] [PubMed] [Google Scholar]
- 41.McIntyre, K. L., Porter, D. M. & Henderson, L. P. (2002) Neuropharmacology 43, 634-645. [DOI] [PubMed] [Google Scholar]
- 42.Torres, J. M. & Ortega, E. (2003) FASEB J. 17, 1428-1433. [DOI] [PubMed] [Google Scholar]
- 43.Torres, J. M. & Ortega, E. (2003) Biochem. Biophys. Res. Commun. 308, 469-473. [DOI] [PubMed] [Google Scholar]
- 44.Torres, J. M., Ruiz, E. & Ortega, E. (2003) Prostate 56, 74-79. [DOI] [PubMed] [Google Scholar]
- 45.Stürenburg, H. J., Fries, U. & Kunze, K. (1997) Neuropsychobiology 35, 143-146. [DOI] [PubMed] [Google Scholar]
- 46.Barbaccia, M. L., Serra, M., Purdy, R. H. & Biggio, G. (2001) Int. Rev. Neurobiol. 46, 243-272. [DOI] [PubMed] [Google Scholar]
- 47.Park-Chung, M., Malayev, A., Purdy, R. H., Gibbs, T. T. & Farb, D. H. (1999) Brain Res. 830, 72-87. [DOI] [PubMed] [Google Scholar]