Abstract
The largest challenge for laparoscopic surgeons is the eye–hand coordination within a three-dimensional (3D) scene observed on a 2D display. The 2D view on flat screen laparoscopy is cerebrally intensive. The loss of binocular vision on a 2D display causes visual misperceptions, mainly loss of depth perception and adds to the surgeon's fatigue. This compromises the safety of laparoscopy. The 3D high-definition view with great depth perception and tactile feedback makes laparoscopic surgery more acceptable, safe and cost-effective. It improves surgical precision and hand–eye coordination, conventional and all straight stick instruments can be used, capital expenditure is less and recurring cost and annual maintenance cost are less. In this article, we have discussed the physics of 3D laparoscopy, principles of depth perception, and the different kinds of 3D systems available for laparoscopy. We have also discussed our experience of using 3D laparoscopy in over 2000 surgeries in the last 4 years.
Keywords: Gynecological surgery, laparoscopic hysterectomy, laparoscopic myomectomy, minimally invasive, three-dimensional laparoscopy
INTRODUCTION
Laparoscopic surgery is currently the standard technique for an increasing number of gynaecological operations. It enables faster recovery with shorter hospitalisation, improved cosmesis, decreased blood loss and less post-operative pain.[1,2]
Traditional laparoscopic systems provide the operator with indirect monocular views of the operative field. This means that the operator is denied the binocular depth cues that provide a sense of stereopsis. The loss of binocular vision on a two-dimensional (2D) display causes visual misperceptions, mainly loss of depth perception and adds to the surgeons fatigue. The largest challenge for laparoscopic surgeons is the eye–hand coordination within a 3D scene observed on a 2D display. The 2D view on flat screen laparoscopy is cerebrally intensive.[3] Experienced endoscopic surgeons learn to use monocular depth cues such as light and shade, relative size of objects, object interposition, texture gradient, aerial perspective and motion parallax instead of stereovision. Using these cues, all laparoscopic operations can be accomplished; however, time and accuracy may be lost as these techniques do not completely compensate for stereoscopic depth perception.[4]
The 3D view provides depth perception and correct measurement of the dimensions of the anatomical spaces contributing to increasing the skills of the laparoscopic surgeon to turn tissues, dissect, design surgical strategies and perform intracorporeal sutures.[5] Studies have reported less strain on the surgeon using 3D rather than 2D vision.[6]
PRINCIPLES OF DEPTH PERCEPTION
Depth perception is the visual ability to judge the relative distance of objects and the spatial relationship of objects at different distances. As the 3D world projects onto a 2D retina, this projection on its own cannot provide depth information. The brain has to combine various monocular and binocular cues given by the eyes to recover the depth, distance and 3D shape of objects.
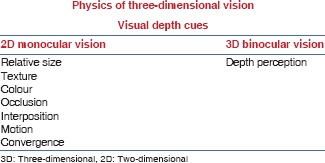
Stereopsis [Figure 1] is the most important cue for depth perception. It is the consequence of interpapillary separation between the two eyes, 6 cm, which causes each eye to have a slightly different view of the same scene. This is called ‘retinal disparity’. The brain is then able to combine the two views into a single 3D image – the process is called stereopsis. Stereoscopy (Greek, to look at a solid object) is the technique of creating or enhancing the illusion of depth in an image – by presenting two offset images separately to the left and right eye of the viewer. Both the 2D offset images are then combined in the brain to give the perception of depth. Stereoscopic vision improves accuracy in laparoscopic skills for novices, which was manifested in reduced numbers of repetitions and errors.[7]
Figure 1.
Stereoscopic vision: The perception of depth and three-dimensional structure is obtained on the basis of visual information derived from both eyes by processing the binocular disparities in the visual cortex
DOES THE TWO-DIMENSIONAL-THREE-DIMENSIONAL VISION HAVE AN INFLUENCE ON MOTOR PERFORMANCE IN MINIMALLY INVASIVE SURGERY?
The cognitive literature has shown that image in 2D and image in 3D do not contain the same information. Although 2D vision compensates somewhat for the lack of depth perception using a lot of monocular cues, image in 3D contains more information, especially to accurately and efficiently guide the action. Indeed, motion appears to rely on binocular depth cues particularly in reaching and grasping movements, main component of the surgical gesture.[4]
Our brain receives two pictures from our eyes that are not identical in angle, and the minute differences are used to process a 3D picture in specialised areas in the visual cortex. To mimic this process, several technologies to generate and display an artificial 3D image, or rather, a stereoscopic image, are used.[8]
THREE - DIMENSIONAL IMAGE GENERATION IN LAPAROSCOPY
The first surgical video system employed 3D monitors with standard resolution and low visual ergonomics and single-channelled laparoscope. Surgeons found the quality of the images was poor because of the heavy active shutter glasses. They also experienced side effects: Tiredness, headaches, ocular fatigue and nausea.
Recent technological advances have led to more flexible instruments, sophisticated high-resolution systems and light polarizing glasses that are lighter and more comfortable. The most recent instruments are quicker, more accurate and precise for surgical tasks and help shorten the learning curve.
Dual channel video
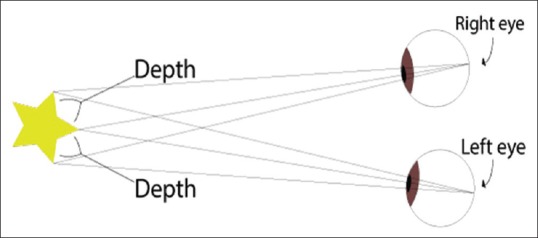
a dual-channel optical scope is connected to two video cameras and delivers two pictures that are displayed to the viewer on a stereoscopic display [Figure 2]. When the surgeon wears circular, polarised 3D glasses, the two images are merged by the brain into one, and this gives the perception of depth [Figure 3].
Figure 2.
Physics of a three-dimensional telescope: The telescope has two optical channels that carry two separate images which are fused together to produce a single binocular vision with depth perception
Figure 3.

Telescope showing two optical channels
Dual chip-on-the-tip
This closely related technology that uses dual channel video generated by two video chips that are mounted on the end of the scope. The two images created are digital, and bypass the disadvantages of the optic distortions created by the optic scope. However, the problems with separated images still exist, and the small distance between the chips enables to create a ‘weak’ 3D effect.
Deflectable tip laparoscope
With two lenses delivering high-definition (HD) 3D video, thanks to its flexibility (100 degrees tip rotation in four directions), it provides a critical clinical view while maintaining image orientation, greater depth of field and optimal depth perception.
Autostereoscopic displays
New developments in liquid crystal display (LCD) technology allowed the creation of stereoscopic images without using additional viewing devices such as the polarised glasses. The system is composed of an illumination screen divided into numerous lines that correspond to pixel columns in the LCD layer in the front.
BENEFITS OF THREE-DIMENSIONAL HIGH-DEFINITION LAPAROSCOPY
Depth perception
Tactile feedback retained
Accuracy
Safety
Surgical precision
Improves hand–eye coordination
Low capital expenditure
Low maintenance recurring cost
Conventional and new straight stick instruments can be used
Shorter learning curve.
OUR EXPERIENCE WITH THREE-DIMENSIONAL LAPAROSCOPY
Our centre is a dedicated high-volume gynaecological laparoscopy unit.
We started performing 3D laparoscopic surgeries since September 2011.
Till date, we have performed a total of 2050 3D laparoscopic surgeries which include hysterectomy, myomectomy, ovarian cyst excisions and others such as burch colposuspension, sacrocolpopexy and laparoscopy for ectopic pregnancy.
In 2013, we published our initial experience of 451 cases where we did a comparative analysis of 2D and 3D laparoscopy on the basis of the average time taken for surgery and morcellation for laparoscopic hysterectomy and myomectomy, the average blood loss and the learning curve for 3D laparoscopy.[3] The duration of surgery was found to be significantly reduced in 3D laparoscopy. The total blood loss during surgery with 2D and 3D was comparable, and the learning curve for 3D was found to be very short (4–5 cases).
The largest uterus removed was 4.87 kg.
All surgeries are performed under general anaesthesia using laryngeal mask airway with the patient in modified lithotomy position.[9]
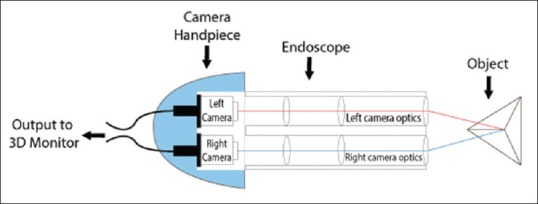
Veress needle is inserted at the Palmer's point, and the first blind 5-mm trocar–cannula is inserted in the left upper lumbar zone.[10] A 5-mm telescope (2D HD system) is introduced through this port; and the peritoneal cavity, uterus and adnexa are evaluated. A 10-mm port is inserted under vision at supraumbilical site or higher depending on the size of the pathology. The 3D HD telescope with a fore-oblique 30° view is then attached to the 3D HD camera and inserted through the 10-mm cannula. Two additional accessory 5-mm trocar–cannulas are inserted: One each in the left and right lateral upper quadrant. An optional robotic arm with a remote control is available to attach the camera for prolonged surgeries or for tremor-free camera stabilisation. The surgeon and the entire operation theatre team wear polarised glasses to appreciate the depth perception on a 3D medical grade 32-inch monitor [Figure 4].
Figure 4.
Operation theatre set up with surgeons wearing three-dimensional glasses: The central monitor is three-dimensional monitor and the right and left monitors show the right and left eye vision, respectively. Surgeons have to wear polarised glasses to view a three-dimensional picture on the monitor
Suggestions for practical use:[11]
To improve the visibility of the 3D image, the operating room can be darkened. For optimal orientation, the 3D camera should be kept in an upright position
When a bichannelled laparoscope is used with an angulated view, it might be possible to disassemble the camera and rotate the laparoscope by 180°.
COMPARING ROBOTIC-ASSISTED SURGERY AND THREE-DIMENSIONAL LAPAROSCOPY
The da Vinci Surgical System has some advantages compared to the conventional laparoscopic surgery such as the 3D vision, better ergonomics, higher degree of freedom of the robotic instruments and reduction of tremor interference.
The system employs a 3D vision system and endowrist technology. Endowrist technology facilitates instrument rotation through tiny incisions. The robotic instruments replicate the surgeon's hand, wrist and finger movement. This allows for extended range of motion and improved manipulation while conducting laparoscopic procedures.[12]
The evolving literature on robot-assisted surgery in gynaecology suggests that the surgical limitations of conventional laparoscopy can be overcome and that the skill level of the surgeon may be enhanced. At present, this seems to be the result of improved instrument, precision and dexterity and 3D imaging.[13]
After the first excitement over the innovative and sophisticated robotic technology has settled, critical assessment of the technique and costs are being considered.[14]
LEARNING CURVE
In robotic-assisted surgery, the learning curve for some gynaecologists was approximately 50 cases to develop consistent operative times and predictable outcomes. These learning curves were dramatically less than those reported for general surgeons and urologists who report learning curves for robotic-assisted laparoscopic prostatectomy to be 150–200 cases.[15,16,17]
Although some authors suggest that robotics can be a useful method for shortening the learning curve in physicians performing minimally invasive gynaecologic surgery, another study suggested that robotic surgery should not serve as a wholesale substitute for a skilled laparoscopic surgeon, especially in procedures where standard laparoscopy is routine.[18]
The learning curve for 3D laparoscopy is much shorter, approximately five cases, i.e., two or days to get used to the 3D vision on a 32-inch monitor using conventional instruments. The learning curve for 3D HD system is very short for all laparoscopic surgeons and this system will enhance the skills of good surgeons.[3]
3D significantly improves visualisation and ability to perform complex tasks. It is likely that 3D technology is very effective at shortening the learning curve to train surgeons in advanced laparoscopy.[19]
COSTS
Another important consideration is that the higher hospital costs associated with robotic surgery is specific to perioperative and post-operative costs (US$1,446 more than conventional laparoscopy)[20] and did not account for the acquisition costs. The robotic unit costs between US$1 million and US$2.3 million and is associated with annual maintenance costs of US$180,000 a year (Intuitive Surgical Investor presentation Q4-2009). In comparison, the total cost of the 3D HD System is much less while there is no recurring cost per patient.
OTHER LIMITATIONS OF ROBOTIC-ASSISTED SURGERY
Other disadvantages of robotic-assisted surgery are a lack of tactile feedback to the surgeon, inability to move the surgical table once the arms of the robot are fixed, and expenses related to the robot and its semi-disposable instruments.[21] surgeons benefit from using the ‘robot’ by sitting and from 3d view; in addition, the robot makes suturing faster to learn. It does not appear to make a significant difference in the outcome of the patient.[22] Women prefer both single site and traditional laparoscopic incisions over robotic procedures as aesthetics are an important consideration for many women. As clinical outcomes of both are similar, during the informed consent procedure, location and length of incisions should be included in the discussion of risks, benefits and alternatives.[23]
CONCLUSIONS
The major limitation in laparoscopy was its 2D image. This resulted in the lack of depth perception thus increasing the strain for the surgeon and compromise on the safety of laparoscopy. The 3D HD view with depth perception and tactile feedback will make laparoscopic surgery more acceptable, safe and cost-effective. It improves surgical precision and hand–eye coordination, conventional and all straight stick instruments can be used, capital expenditure is less and recurring cost and annual maintenance cost are less. In addition, it will enhance the skills of a good surgeon and shorten the learning curve of a novice surgeon and ultimately shortening the surgical time thereby reducing cost, exposure to anaesthetic agents and morbidity thus improving the quality of care dispensed to the patient.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Yuen PM, Yu KM, Yip SK, Lau WC, Rogers MS, Chang A. A randomized prospective study of laparoscopy and laparotomy in the management of benign ovarian masses. Am J Obstet Gynecol. 1997;177:109–14. doi: 10.1016/s0002-9378(97)70447-2. [DOI] [PubMed] [Google Scholar]
- 2.Lo L, Pun TC, Chan S. Tubal ectopic pregnancy: An evaluation of laparoscopic surgery versus laparotomy in 614 patients. Aust N Z J Obstet Gynaecol. 1999;39:185–7. doi: 10.1111/j.1479-828x.1999.tb03368.x. [DOI] [PubMed] [Google Scholar]
- 3.Sinha R, Sundaram M, Raje S, Rao G, Sinha M, Sinha R. 3D laparoscopy: Technique and initial experience in 451 cases. Gynecol Surg. 2013;10:123–8. [Google Scholar]
- 4.Blavier A, Gaudissart Q, Cadière GB, Nyssen AS. Impact of 2D and 3D vision on performance of novice subjects using da Vinci robotic system. Acta Chir Belg. 2006;106:662–4. doi: 10.1080/00015458.2006.11679976. [DOI] [PubMed] [Google Scholar]
- 5.Izquierdo L, Peri L, García-Cruz E, Musquera M, Ciudin A, Pérez M, et al. 3D advances in laparoscopic vision. Eur Urol Rev. 2012;7:137–9. [Google Scholar]
- 6.Currò G, La Malfa G, Lazzara S, Caizzone A, Fortugno A, Navarra G. Three-dimensional versus two-dimensional laparoscopic cholecystectomy: Is surgeon experience relevant? J Laparoendosc Adv Surg Tech A. 2015;25:566–70. doi: 10.1089/lap.2014.0641. [DOI] [PubMed] [Google Scholar]
- 7.Alaraimi B, El Bakbak W, Sarker S, Makkiyah S, Al-Marzouq A, Goriparthi R, et al. A randomized prospective study comparing acquisition of laparoscopic skills in three-dimensional (3D) vs. two-dimensional (2D) laparoscopy. World J Surg. 2014;38:2746–52. doi: 10.1007/s00268-014-2674-0. [DOI] [PubMed] [Google Scholar]
- 8.Zazzarini CC, Pansini A, Cerveri P, Zaltieri R, Lavizzari D. Design of a robotic endoscope for mini invasive surgery. ASME 2011 International Design Engineering Technical Conferences and Computers and Information in Engineering Conference. American Society of Mechanical Engineers. 2011:771–7. [Google Scholar]
- 9.Reich H, Roberts L. Laparoscopic hysterectomy in current gynecological practice. Rev Gynaecol Pract. 2003;3:32–40. [Google Scholar]
- 10.Hegde A, Sinha R. Comment on: Safe entry techniques during laparoscopy: Left upper quadrant entry using the ninth intercostal space-a review of 918 procedures. J Minim Invasive Gynecol. 2005;12:463–4. doi: 10.1016/j.jmig.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 11.Kunert W, Storz P, Kirschniak A. For 3D laparoscopy: A step toward advanced surgical navigation: How to get maximum benefit from 3D vision. Surg Endosc. 2013;27:696–9. doi: 10.1007/s00464-012-2468-0. [DOI] [PubMed] [Google Scholar]
- 12.Diaz-Arrastia C, Jurnalov C, Gomez G, Townsend C. Laparoscopic hysterectomy using a computer-enhanced surgical robot. Surg Endosc Other Intervent Tech. 2002;16:1271–3. doi: 10.1007/s00464-002-8523-5. [DOI] [PubMed] [Google Scholar]
- 13.Advincula AP, Wang K. Evolving role and current state of robotics in minimally invasive gynecologic surgery. J Minim Invasive Gynecol. 2009;16:291–301. doi: 10.1016/j.jmig.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Sarlos D, Kots L, Stevanovic N, Schaer G. Robotic hysterectomy versus conventional laparoscopic hysterectomy: Outcome and cost analyses of a matched case-control study. Eur J Obstet Gynecol Reprod Biol. 2010;150:92–6. doi: 10.1016/j.ejogrb.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 15.Patel HR, Ribal MJ, Arya M, Nauth-Misir R, Joseph JV. Is it worth revisiting laparoscopic three-dimensional visualization? A validated assessment. Urology. 2007;70:47–9. doi: 10.1016/j.urology.2007.03.014. [DOI] [PubMed] [Google Scholar]
- 16.Giulianotti PC, Coratti A, Angelini M, Sbrana F, Cecconi S, Balestracci T, et al. Robotics in general surgery: Personal experience in a large community hospital. Arch Surg. 2003;138:777–84. doi: 10.1001/archsurg.138.7.777. [DOI] [PubMed] [Google Scholar]
- 17.Talamini MA, Chapman S, Horgan S, Melvin WS. A prospective analysis of 211 robotic-assisted surgical procedures. Surg Endosc Other Intervent Tech. 2003;17:1521–4. doi: 10.1007/s00464-002-8853-3. [DOI] [PubMed] [Google Scholar]
- 18.Wexner SD, Bergamaschi R, Lacy A, Udo J, Brölmann H, Kennedy RH, et al. The current status of robotic pelvic surgery: Results of a multinational interdisciplinary consensus conference. Surg Endosc. 2009;23:438–43. doi: 10.1007/s00464-008-0202-8. [DOI] [PubMed] [Google Scholar]
- 19.Cologne KG, Zehetner J, Liwanag L, Cash C, Senagore AJ, Lipham JC. Three-dimensional Laparoscopy: Does Improved Visualization Decrease the Learning Curve Among Trainees in Advanced Procedures? Surgical Laparoscopy Endoscopy & Percutaneous Techniques. 2015;25:321–3. doi: 10.1097/SLE.0000000000000168. [DOI] [PubMed] [Google Scholar]
- 20.Pasic RP, Rizzo JA, Fang H, Ross S, Moore M, Gunnarsson C. Comparing robot-assisted with conventional laparoscopic hysterectomy: Impact on cost and clinical outcomes. J Minim Invasive Gynecol. 2010;17:730–8. doi: 10.1016/j.jmig.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 21.Nezhat C, Lavie O, Lemyre M, Unal E, Nezhat CH, Nezhat F. Robot-assisted laparoscopic surgery in gynecology: Scientific dream or reality? Fertil Steril. 2009;91:2620–2. doi: 10.1016/j.fertnstert.2008.03.070. [DOI] [PubMed] [Google Scholar]
- 22.Nezhat C, Lavie O, Hsu S, Watson J, Barnett O, Lemyre M. Robotic-assisted laparoscopic myomectomy compared with standard laparoscopic myomectomy – A retrospective matched control study. Fertil Steril. 2009;91:556–9. doi: 10.1016/j.fertnstert.2007.11.092. [DOI] [PubMed] [Google Scholar]
- 23.Bush AJ, Morris SN, Millham FH, Isaacson KB. Women's preferences for minimally invasive incisions. J Minim Invasive Gynecol. 2011;18:640–3. doi: 10.1016/j.jmig.2011.06.009. [DOI] [PubMed] [Google Scholar]


