Abstract
Cortical DNA-methyltransferase 1 (DNMT1) is preferentially expressed in interneurons secreting GABA where it very likely contributes to promoter CpG island hypermethylation, thus causing a down-regulation of promoter functions. To consolidate and expand on previous findings that, in the cortex of schizophrenia (SZ) brains, glutamic acid decarboxylase 67 (GAD67) expression is down-regulated whereas that of DNMT1 is up-regulated, we studied both parameters in Brodmann's area (BA) 9 from the McLean 66 Cohort Collection (Harvard Brain Tissue Resource Center, Belmont, MA). In BA9 of SZ and bipolar disorder patients with psychosis, DNMT1 mRNA and protein expression preferentially increases in layer I, II, and IV interneurons, and this increase is paralleled by a decreased number of GAD67 mRNA-positive neurons. The increase in DNMT1 and the decrease in GAD67-expressing neurons were unrelated to postmortem interval, pH, RNA quality, or to the presence, dose, or duration of antipsychotic (APS) medication, with the exception of a subgroup of SZ patients treated with a combination of valproate and APS in which the expression of DNMT1 failed to change. The DNMT1 increase and the GAD67 decrease in BA9 interneurons are significant features of SZ and bipolar disorder with psychosis. Interestingly, the DNMT1 increase failed to occur when patients with psychosis received a combination of valproate and APS treatment but not APS monotherapy.
Keywords: bipolar disorder, glutamic acid decarboxylase, schizophrenia, valproate, antipsychotics
Although monozygotic twins carry identical genomic sequences, they can exhibit a number of differences. In fact, in the absence of nucleotide sequence mutations, DNA can be modified by methylation of the cytosine ring. Such structural DNA modifications can be termed “epigenetic,” to indicate a modification that occurred after the operation of conventional genetic mechanisms (1).
In neurons, epigenetic hypermethylation in the 5′ position of the cytosine ring expressed in promoter CpG islands of various genes including that of glutamic acid decarboxylase 67 (GAD67) and reelin is catalyzed by DNA-methyltransferases (2, 3). One of them, the DNA-methyltransferase 1 (DNMT1), is preferentially expressed in cortical GABA-secreting neurons of Brodmann's area (BA) 10, and BA17, and in the medium spiny neurons of the caudate nucleus (4).
GABAergic interneurons of schizophrenia (SZ) patient (SZP) brains (BA10) exhibit a decrease of reelin and GAD67 expression (4–6) and an increased expression of DNMT 1 (4).
In a recent study (7), it was found that the selective expression of the human reelin gene in GABAergic neurons is regulated by an epigenetic cytosine hypermethylation of promoter CpG islands. In fact, when DNMT1 function is inhibited by the addition of aza-2′-deoxycytidine or down-regulated by the induction of demethylation elicited by histone deacetylase inhibitors, the in vitro expression of reelin increases severalfold (7). Moreover, in the hippocampus and frontal cortex of mice receiving methionine (6.6 mmol/kg s.c.) for 15 days, the expression of reelin and GAD67 was down-regulated (8, 9).
Collectively, these data support the hypothesis that the decrease of reelin (4, 6, 10–12) and GAD67 (5, 13–15) expression in BA10 or BA9 of SZP reported by several groups using different patient cohorts may be related to an epigenetic hypermethylation of the CpG islands in the above-mentioned two gene promoters. Furthermore, this decrease may be mediated by DNMT1 overexpression in cortical GABAergic neurons of SZP (4). The only exception to the down-regulation of GAD67 expression seems to be found in a population of chronically hospitalized elderly and demented SZP (16).
In a study of five brain cohorts from the Stanley Foundation, each characterized by the presence or absence of a specific psychiatric disorder [(i) SZ; (ii) bipolar disorder (BP) with psychosis (BP+); (iii) BP without psychosis (BP-); (iv) major depression without psychosis; and (v) nonpsychiatric subjects (NPS)], reelin and GAD67 were down-regulated not only in BA9 of SZP but also in BA9 of BP+ patients (10). Interestingly, GAD67 and reelin expression was not decreased in the BA9 GABAergic neurons of patients with major depression without psychosis. Moreover, in situ hybridization studies of different cohorts of psychiatric patients have demonstrated that GAD67 mRNA-positive neurons are decreased in the middle layers of the prefrontal cortex of subjects with SZ or BP+ (5, 13, 15).
In the present study, we tested whether cortical GABAergic neurons from BA9 of SZ and BP+ patients express a selective increase of DNMT1 mRNA. This study was carried out in BA9 tissue slices obtained from the McLean 66 Cohort Collection (Harvard Brain Tissue Resource Center, Belmont, MA). This collection contains (i) SZP, (ii) BP+ patients, (iii) BP- patients, and (iv) NPS. We have addressed the following experimental questions:
Is the up-regulation of DNMT1 mRNA expression observed in BA10 interneurons of SZP brains (4) also present in the homologous interneurons of BA9?
Is the up-regulation of DNMT1 expression a specific feature in the brains of both SZ and BP+ patients in which reelin and GAD67 are also down-regulated?
Is the increase of DNMT1 mRNA expression associated with an increase of DNMT1 protein levels?
-
Is the extent of GAD67 down-regulation correlated with the level of DNMT1 overexpression in the same neurons?
A new trend in SZ treatment is the administration of a combination of antipsychotics (APS) and valproate (VPA) that enhances APS efficacy (17, 18). Because VPA seems to induce changes in chromatin remodeling mediated by (i) inhibition of histone deacetylases (8, 9, 19) or (ii) induction of DNA-demethylation (9), it could be surmised that this VPA-induced chromatin remodeling contributes to the enhancement of APS efficacy. Hence, this hypothesis tacitly assumes that APS efficacy depends on chromatin remodeling.
Because we noticed that VPA was often used as an adjunct to APS in the treatment of the SZ and BP+ patients in the McLean 66 Cohort, we could also ask:
Is the up-regulation of DNMT1 expression in cortical GABAergic neurons of SZ or BP+ patients down-regulated by a combination of VPA plus APS?
Methods
Subjects. The BA9 specimens obtained from the Harvard Brain Tissue Resource Center include: 19 SZ patients (3 schizoaffective and 16 with a SZ diagnosis), 19 BP patients (14 with psychosis and 5 without psychosis), and 26 NPS brains matched for postmortem interval (PMI), gender, and age (Table 1 and Table 3, which is published as supporting information on the PNAS web site). These psychiatric diagnoses were established by using DSM IV criteria by two senior psychiatrists and were based on clinical and family histories.
Table 1.
Demographic characteristics of brain samples* from the Harvard Brain Tissue Resource Center (The McLean 66)
| Patient cohort
|
|||||
|---|---|---|---|---|---|
| NPS (n = 27) | SZP (n = 20) | BP+ (n = 14) | BP- (n = 5) | P | |
| Male/female | 19/8 | 13/7 | 7/7 | 4/1 | 0.06 |
| Hemisphere (L/R) | 15/12 | 12/8 | 9/5 | 1/4 | 0.39 |
| Age, y | 58 ± 18 | 56 ± 18 | 64 ± 16 | 55 ± 23 | 0.46 |
| PMI, h | 21 ± 5.8 | 21 ± 5.5 | 21 ± 9.5 | 19 ± 11 | 0.92 |
| Fixation days × 103 | 1.5 ± 0.6 | 1.9 ± 0.4 | 1.7 ± 0.6 | 1.6 ± 1.0 | 0.32 |
| pH | 6.4 ± 0.3 | 6.4 ± 0.3 | 6.4 ± 0.2 | 6.6 ± 0.3 | 0.10 |
| 3′/5′ G3PDH ratio | 1.5 ± 0.4 | 1.6 ± 0.6 | 1.6 ± 0.8 | 1.4 ± 0.3 | 0.10 |
| 3′/5′ β-actin ratio | 2.3 ± 0.9 | 2.4 ± 0.8 | 2.7 ± 1.3 | 2.6 ± 1.1 | 0.76 |
| RNA 28S/18S ratio | 1.1 ± 0.3 | 1.1 ± 0.5 | 1.0 ± 0.3 | 1.1 ± 0.2 | 0.28 |
| % present call | 46 ± 4.0 | 46 ± 4.1 | 44 ± 7.1 | 46 ± 2.4 | 0.51 |
| Illness onset, y | - | 23 ± 9.9 | 38 ± 17 | 33 ± 7.6 | 0.66 |
| Illness duration, y | - | 34 ± 18 | 28 ± 18 | 24 ± 16 | 0.50 |
| % Suicide | 0 | 16 | 21 | 40 | 0.44 |
| % Abuse/dependence | 0 | 47 | 43 | 60 | 0.63 |
| % CNS medications | |||||
| Atypical APS | 0 | 32 | 43 | 40 | 0.95 |
| Typical APS | 0 | 37 | 29 | 0 | 0.95 |
| Typical plus atypical | 0 | 5 | 0 | 0 | 0.95 |
| VPA | 0 | 16 | 71 | 40 | 1.00 |
The values are mean ± SD. L, left; R, right; G3PDH, glyceraldehyde-3-phosphate dehydrogenase; y, years; h, hours.
Formaldehyde-fixed BA9
Tissue Collection and Evaluation of RNA Integrity. Blocks of BA9 (Table 3) were fixed in 4% formaldehyde at the time of autopsy. The brain was cut rostro-caudally in 0.5-cm-thick slices along the coronal axis, starting from the frontal pole. Because BA9 was included between sections 2 and 5 for our studies, we received identical sections of each brain. Each sample studied was transferred into 30% sucrose in 0.1 M PBS 72 h before histological preparation.
RNA quality was established by Harvard Brain Tissue Resource Center (http://www.brainbank.mclean.org/). The percentage of the total number of probe sets detected as present on the array (percent present call), the gene 3′/5′ [glyceraldehyde-3-phosphate dehydrogenase and β-actin] ratios, and chip scale factor obtained with Affymetrix's mas software were used together with Agilent rRNA 18S/28S ratios to include or exclude a case in the National Brain Databank.
In Situ Hybridization (DNMT1 and GAD67). To assess changes in DNMT1 and GAD67 mRNA expression in BA9 obtained from the psychiatric patients and NPS of the above described cohort, samples were individually matched by using the following order of criteria: (i) pH and mRNA quality, (ii) gender, (iii) age difference, (iv) PMI, and (v) hemisphere. One patient from each diagnostic group was routinely processed simultaneously. However, because the number of patients in the NPS and SZP groups was larger than in the BP group, in some cases two NPS and two SZP samples were processed with one BP patient sample.
To visualize the DNMT1 mRNA signal, 40-μm-thick free-floating sections were incubated with a mixture of two antisense oligonucleotide probes complementary to bases 1627–1650 and 4801–4824 of the human DNMT1 cDNA (accession no. NM_001379). To detect GAD67 mRNA, we hybridized the slices with antisense probes 1063–1086 and 2674–2697 of the human GAD67 cDNA (GenBank accession no. NM_000817). The specificity of such probes was described in detail in Veldic et al. (4). In situ hybridization was performed according to Rodriguez et al. (20) and Veldic et al. (4).
Immunohistochemistry [DNMT1 and Neuron-Specific Nuclear Protein (NeuN)]. To obtain NeuN immunolabeling, 40-μm floating sections were incubated for 3 days at 4°C with a mouse anti-NeuN mono-clonal antiserum (Chemicon) diluted 1:500 according to the procedure described by Rodriguez et al. (20)
DNMT1 protein-positive neurons were visualized in 40-μm floating sections incubated for 3 days with a rabbit anti-DNMT1 antiserum (New England Biolabs) diluted 1:2,500. In pilot experiments, it was established that incubation with anti-DNMT1 antibody at a 1:2,500 dilution was necessary and sufficient to label the highest number of neurons in every given slice.
DNMT1 antibody specificity was tested by Western blot analysis of human cerebellar extracts. After 10% SDS/PAGE and blotting, a major immunoreactivity band was detected. This band mobility was identical to that obtained by running 10 ng of recombinant DNMT1 protein on gel.
Unbiased Three-Dimensional Counts. The criteria for unbiased three-dimensional counts and the reproducibility of our measurements are described in detail in Supporting Text, which is published as supporting information on the PNAS web site.
Cell counts were performed with confocal microscopy at a magnification of ×40 to obtain serial optical sections from each specimen throughout the z axis. This method allows counts of diaminobenzidine-positive cells in a counting box (100 × 100 × 20 μm) entirely embedded in the tissue section by using a modification by Veldic et al. (4) of the three-dimensional cell-counting procedure described by Williams and Rakic (21).
The cells counted in each box were corrected for tissue shrinkage, which in our experimental conditions was between 30% and 35%. The thickness of each section was measured by confocal microscopy, focusing from the upper to the lower surface of the specimen on sections with a division spacing of 2 μm according to the specifications of the manufacturer (Leica, Wetzlar, Germany).
To count strongly stained cells and exclude weakly stained cells or nonspecifically labeled cells from the analysis, the threshold intensity of staining was established at 3× the background (measured by Leica Confocal Software).
Digital Photomicrography. Images were captured by axiovision 3.1 (Zeiss). The final composites were processed by using photoshop (Adobe Systems, San Jose, CA) and powerpoint (Microsoft).
Statistical Analyses. To test whether demographic and other background variables influenced the difference between the experimental and control groups, we applied a variance analysis test for those variables that were meaningful in normal controls and the three diagnostic groups. We evaluated the interaction of each variable with the different diagnosis effects, and we present the F values and the P values of these evaluations in the tables and figure legends.
Results
Density of DNMT1 mRNA-Positive Neurons Is Increased in BA9 of SZ and BP+ Patients. The overall counts of DNMT1 mRNA-positive neurons are higher in patients with SZ (Table 2) but not in the overall group of patients with BP compared with NPS. The cohort of patients with BP was subdivided into two subgroups: (i) those with psychosis (BP+) (n = 14) and (ii) those without psychosis (BP-) (n = 5) (Table 2). A two pair-wise comparison between BP+ patients vs. NPS and BP- patients vs. NPS reveals a significant increase in the density of DNMT1 mRNA-positive neurons in the BP+ group but not in the BP- group (Table 2).
Table 2. DNMT1 mRNA-positive neurons in cortical layers I-VI of BA9.
| Patients | n | DNMT1 mRNA-positive neurons, mm3 × 103 (mean ± SD) |
|---|---|---|
| NPS | 26 | 24 ± 3.9 |
| SZP | 19 | 28 ± 3.6* |
| BP+ | 14 | 27 ± 4.5** |
| BP- | 5 | 20 ± 4.5 |
Overall ANOVA: F3,63 = 8.0; P < 0.001. Multiple comparisons (Bonferroni t test): *, NPS vs. SZP (P = 0.004); *, SZP vs. BP- (P < 0.001); BP+ vs. BP- (P = 0.325) Multiple comparisons (Student-Newman-Keuls Method): *, NPS vs. SZP (P = 0.002); **, NPS vs. BP+ (P = 0.032); SZP vs. BP+ (P = 0.309); *, SZP vs. BP- (P < 0.001); **, BP+ vs. BP- (P = 0.006).
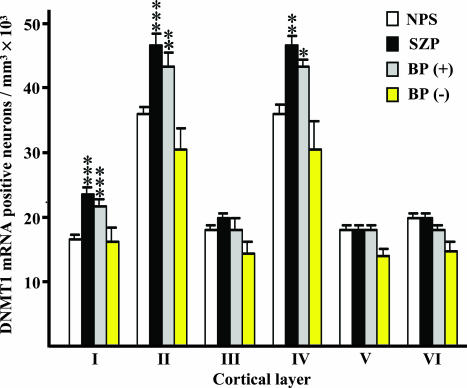
Furthermore, this effect seems to be layer specific. Thus, the number of DNMT1 mRNA-expressing neurons showed a significant 30–40% increase in layer I of SZ and BP+ patients compared with NPS (Fig. 1). The number of DNMT1 mRNA-positive neurons was also significantly increased in layers II and IV of SZ and BP+ patients, although this increase was more modest (Fig. 1). In all layers, the increased density of DNMT1 mRNA-positive neurons was slightly lower in BP+ patients than in SZP, but no significant differences were observed in the extent of DNMT1 mRNA increase between SZ and BP+ patients. No significant changes in the number of DNMT1 mRNA-positive neurons were detected in layers III, V, and VI.
Fig. 1.
DNMT1 mRNA-positive neuronal counts in various layers of BA9 from nonpsychiatric subjects and psychiatric patients. For NPS, n = 26; for SZP, n = 19; for BP+ patients, n = 14; for BP- patients, n = 5. The difference between SZP and BP patients with or without psychosis versus NPS was calculated by ANOVA, and P values were evaluated by Dunnett's two-sided test comparison. ***, P < 0.001; **, P < 0.01; *, P < 0.05. Error bars represent SEM.
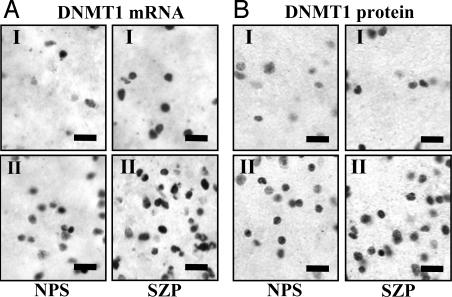
A typical example showing an increased numerical density of DNMT1 mRNA in layers I and II of SZP vs. NPS is shown in Fig. 2A. Note that, both in layers I and II, the in situ hybridization signal for DNMT1 is localized to the small round cells (Fig. 2). Although it is known that neurons in layer I are almost exclusively GABAergic (22), we have shown that, in layer II of BA10, the DNMT1 mRNA signal colocalizes with GAD65/67 and reelin immunoreactivity but not with glial fibrillary acidic protein (a marker of glial cells) or with vesicular glutamate transporter 2 (a marker of pyramidal neurons) immunoreactivity (4). Distribution and morphology of DNMT1 mRNA-positive neurons in BA9 is virtually identical to that in BA10. This similarity in distribution and morphology allows us to infer that DNMT1 mRNA overexpression also occurs in GABAergic interneurons in BA9.
Fig. 2.
Photomicrographs showing examples of DNMT1 mRNA and DNMT1 protein neuronal expression in layers I and II of BA9 from one NPS and one SZP. (A) DNMT1 mRNA in situ hybridization signal. (B) DNMT1 protein immunolabeling. Note an increase of neuronal DNMT1 mRNA and protein labeling in SZP. (Scale bars, 20 μm.)
In subjects with BP- (n = 5) the number of DNMT1 mRNA-positive neurons was not increased compared with NPS (Fig. 1).
The numerical density of NeuN-immunopositive neurons in the above-mentioned cortical layers of the various groups studied was similar. For example, in layer II, where the biggest increase in DNMT1 occurs, the number of NeuN-positive cells ± SD was virtually identical in SZP [(92 ± 15)/mm3 × 103], BP+ patients [(94 ± 16)/mm3 × 103], BP- patients [(90 ± 13)/mm3 × 103], and NPS [(88 ± 12)/mm3 × 103].
DNMT1-Like Immunoreactivity (LI) in BA9 Neurons. In the same group of patients in which we measured the numerical density of DNMT1 mRNA-positive neurons, we also measured the numerical density of DNMT1-LI-positive neurons.
In BA9 of a combined group of psychotic patients (SZP and BP+ patients, n = 33), the number of neurons expressing DNMT1-LI [(layer I, 18 ± 0.5; layer II, 41 ± 1.6; layer IV, 34 ± 1.2 SEM) × 103/mm3] was higher than in NPS (n = 26) [(layer I, 15 ± 0.6; layer II, 35 ± 1.8; layer IV, 30 ± 1.3 SEM) × 103/mm3]. P = 0.009 in layer I, P = 0.03 in layer II, and P = 0.028 in layer IV in psychotic patients compared with NPS.
Layers III and V of NPS and psychotic patients express similar DNMT1-LI counts. A typical example of the increased numerical density of DNMTI-LI-positive neurons in layers I and II of SZ patients vs. NPS is shown in Fig. 2B.
Density of GAD67 mRNA-Positive Neurons Is Decreased in SZ and BP+ Patients. The numerical density of GAD67 mRNA-positive neurons is decreased in layer I (P < 0.001) and II (P < 0.001) of SZP [(layer I, 16 ± 0.7; layer II, 36 ± 1.4 SEM) × 103/mm3] and BP+ patients [(layer I, 16 ± 0.8; layer II, 36 ± 1.3 SEM) × 103/mm3] compared with NPS [(layer I, 20 ± 0.7; layer II, 44 ± 1.0 SEM) × 103/mm3] (P < 0.001 of NPS vs. SZP and P = 0.002 of NPS vs. BP+ patients in layer I; P < 0.001 of NPS vs. SZP and P < 0.001 of NPS vs. BP+ patients in layer II).
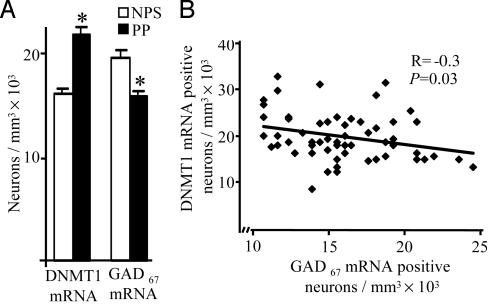
We also measured the numerical density of GAD67 mRNA-positive neurons vis-à-vis DNMT1 mRNA-positive neurons. In combined SZP and BP+ groups (Fig. 3), the density of GAD67 mRNA-positive neurons was decreased by ≈20% in layer I whereas, in the same patients, the numerical density of DNMT1 mRNA-positive neurons was increased by 37%. In layer II, the density of GAD67 mRNA-positive neurons in a combined SZP and BP+ group was decreased by ≈19% (F1,58 = 33.0; P < 0.001) whereas, in the same patients, the numerical density of DNMT1 mRNA-positive neurons was increased by 25% (F1,58 = 22.7; P < 0.001). Interestingly, in layer I, the number of GAD67 mRNA-positive neurons negatively correlates with the number of DNMT1 mRNA-positive neurons (see Fig. 3).
Fig. 3.
Negative correlation between GAD67 and DNMT1 mRNAs-positive neurons in layer I of BA9 from NPS (n = 26) and psychotic (SZ plus BP+) patients (PP) (n = 33). (A) The difference between NPS and PP for GAD67 and DNMT1 mRNAs-positive neurons was calculated by ANOVA followed by Bonferroni comparison. *, P < 0.001. Error bars represent SEM. (B) Pearson correlation between number of DNMT1 mRNA-positive neurons and number of GAD67 mRNA-positive neurons.
Demographic Variables. The average values of PMI and other demographic variables (gender, age of death, cause of death, brain pH, 3′/5′ glyceraldehyde-3-phosphate dehydrogenase mRNA ratio, 3′/5′ β-actin mRNA ratio, RNA 28S/18S ratio, the percent present call, the duration of storing the samples in formaldehyde, and difference in hemispheric laterality) were similar in all four groups (Table 1). Pearson correlation of all these variables with numerical densities of DNMT1 mRNA, DNMT1 protein, and GAD67 mRNA-positive neurons in layers I and II were all nonsignificant (Table 4, which is published as supporting information on the PNAS web site). Postmortem brains have a PMI of several hours; moreover, the PMI ranges from 5 h to more than a day (i.e., 41 h). Although in preliminary light microscopy studies with Nissl staining and with electron microscopy the brain structures of the Harvard Collections seem to be well preserved, a legitimate question is whether PMI duration influences the efficacy of our measures by a comparable extent. We have thus studied whether the levels of DNMT1 mRNA expression change in mouse brains fixed at different PMI. We failed to detect any significant changes in the number of DNMT1 mRNA-positive cells in the mouse frontal cortex for up to 24 h (4 h at room temperature and 20 h at 4°C).
There are reports indicating that agonal factors including coma, hypoxia, pyrexia, injuries, and neurotoxic substances influence brain pH and may change RNA integrity in postmortem brain tissue (23). Pearson's correlations of pH or mRNA quality compared with DNMT1 mRNA, DNMT1 protein, and GAD67 mRNA-positive neurons for NPS, SZ, or BP patients were all nonsignificant (Table 4).
As mentioned, several RNA integrity indicators based on Affymetrix GeneChip microarray data were provided by the Harvard Brain Tissue Resource Center. Although all samples seem to have a reasonably good mRNA quality judged by the percentage of the total number of probe sets detected as present on the array (percent present call), a few samples in each group have a relatively low RNA 28S/18S ratio and a relatively high 3′/5′ RNA ratio (Table 3).
When these samples were excluded, the density of DNMT1 mRNA-positive neurons was still found to be statistically significant, i.e., they were increased by 43%, 27%, and 19% in layers I, II, and IV, respectively, in SZP [layers I (P < 0.001), II (P < 0.001), and IV (P = 0.01)] and by 34%, 24%, and 22%, respectively, in BP+ patients [layers I (P = 0.003), II (P = 0.003), and IV (P = 0.01)].
Although due to PMI and pH alterations in postmortem brains RNAs may show various degree of degradation, only a reduction in the transcript length and not a reduction in the number of transcripts has been reported (24). This finding implies that an antisense probe directed toward the 3′ region can hybridize a transcript even if the 5′ end region has been degraded. Thus, the lack of a significant correlation between RNA quality or pH and expression level of DNMT1 mRNA using antisense probes directed toward the 3′ end region of the transcript should not be a surprise.
In our cohort, several patients had a history of either alcohol use or use of other substances of abuse. There is no statistically significant interaction between the drug of abuse and dependence and the DNMT1 mRNA increase in layers I, II, and IV [layer I (F = 1.835; P = 0.059); layer II (F = 1.349; P = 0.214); layer IV (F = 1.302; P = 0.241)]. Brain, blood, and urine were screened for alcohol and other substances of abuse. There were no statistically significant interactions between DNMT1 mRNA content and substances found in toxicology reports in any of the groups studied (F = 0.160; P = 0.853).
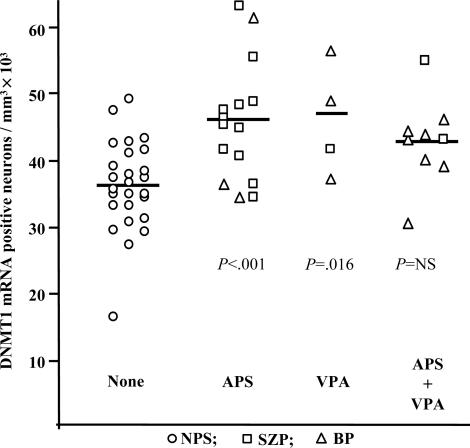
APS and VPA Medication. Most SZ or BP patients exhibiting severe psychotic symptoms had received APS medications (Tables 1 and 3). In a combined group of patients including both those with SZ and those with BP+, there was no significant or consistent correlation between intake of typical or atypical APS and expression levels of DNMT1 mRNA. In fact, in two patients never treated with APS drugs and in seven patients APS-free for at least 3 months before death, DNMT1 mRNA expression was virtually identical to the average levels in SZ or BP patients receiving APS treatment. Because some SZP or BP+ patients in our cohort received the mood stabilizer VPA in addition to APS, we evaluated the possible effects of this drug on the increase of DNMT1-positive neurons in a combined group of SZ and BP+ patients. As shown in Fig. 4, in layer II of the combined SZ and BP+ groups treated with only APS or only with VPA, the density of DNMT1 mRNA-positive neurons was significantly increased (F3,54 = 7.614, P < 0.001), whereas the relatively smaller increase of DNMT1 mRNA-positive neurons in the SZ and BP+ group treated with APS and VPA did not achieve statistical significance under the stringent Bonferroni criterion [NPS vs. VPA (P = 0.015); NPS vs. APS (P < 0.001); NPS vs. (APS plus VPA) (P = 0.067)].
Fig. 4.
DNMT1 mRNA-positive interneurons in layer II of BA9 from NPS (n = 26), SZP (n = 15), and BP patients (n = 12). APS, patients treated with antipsychotics; VPA, patients treated with valproate. *, P < 0.001 measured by ANOVA followed by Bonferroni multiple comparison.
Discussion
There is general consensus that a dysfunction of GABAergic neurons along with the down-regulation of the expression of several GABAergic neuronal proteins including reelin and GAD67 represent key features of SZ and BP pathophysiology (2, 5, 10, 14, 15, 25). Thus, SZ morbidity includes a GABAergic functional alteration that should be considered in establishing new directions for pharmacological interventions.
When we looked for a common mechanism that could explain the concurrent expression down-regulation of these and other specific genes [i.e., parvalbumin and GABA transporter (GAT1)] (26–28) in cortical GABAergic neurons of SZP, we observed that BA10 GABAergic neurons of SZP express an up-regulation of DNMT1 mRNA and protein that may cause hypermethylation of the gene promoters expressed in these interneurons. These findings are consistent with the hypothesis that the increase of DNMT1 expression in telencephalic GABAergic interneurons of SZP should be studied as a possible cause of a promoter hypermethylation of reelin and GAD67 genes and perhaps of other promoter genes expressed in these interneurons.
DNMT1 Expression and Psychiatric Pathology. In this study, we extended previous findings on the increase in DNMT1 mRNA from a small cohort of BA10 samples of patients with SZ to a larger cohort of BA9 samples of patients with SZ and in addition to a similar cohort of BP+ patients. We found that the expression of DNMT1 mRNA is equally up-regulated in BA9 of patients with SZ or BP+. In contrast, the expression of DNMT1 mRNA-positive neurons in a small group of BP- patients is virtually identical to that of NPS (Fig. 1). The increase of DNMT1 mRNA is paralleled by a similar increase in nuclear DNMT1 protein expression (Fig. 2). Hence, our data are consistent with the hypothesis that an increase in DNMT1 expression is very likely associated with the expression of psychotic symptomatology.
An increased number of DNMT1 mRNA-positive neurons in both SZ and BP+ patients seems to be generalized to all layers, but it is higher and it reaches significant levels only in layers I, II, and IV. It is interesting that in layer I, where the neurons are almost exclusively GABAergic (horizontal and bitufted cells), the increase in DNMT1 mRNA-positive neurons in SZ and BP+ patients was proportionately larger than in layers II and IV. However, as mentioned, in layers II (Fig. 2) and IV (not shown), the increase seems restricted to small ovoid, presumably GABAergic neurons and not to an increased expression of DNMT1 mRNA or protein in pyramidal neurons.
DNMT1 Overexpression and GAD67 Down-Regulation in Various Cortical Layers of SZ and BP+ Patients. We have reported that the numerical density of reelin mRNA-positive neurons in BA10 of SZ is decreased in layers I, II, and IV and that this decrease is associated with an increased expression of DNMT1 (4). Here, we show that, in layers I and II, an increase of DNMT1 is associated with a statistically significant decrease of GAD67 mRNA-positive neurons to similar extent in both SZ and BP+ patients. Statistical analysis of the ratio of DNMT1 and GAD67 in layers I and II consistently shows a highly significant increase of this ratio in SZ and BP+ patients. Thus, the selectivity of GABAergic neuron dysfunction in SZ and BP+ patients provides further insight into the similarities of the pathophysiological mechanisms underlying altered prefrontal cortex GABAergic neurotransmission in SZ and BP patients. Furthermore, our data suggest that the decrease of GAD67 mRNA and the increase of DNMT1 mRNA or protein expression may not be generalized but may occur in a selective subset of cortical GABAergic neurons. Because GABAergic interneurons are anatomically and functionally heterogeneous, the identification of the GABAergic interneurons that show an increased expression of DNMT1 may shed additional light on the location and functional consequences of neuron circuit disturbances and their symptomatic impact in SZ and BP symptomatology. This analysis may further support the hypothesis that an intervention with GABAergic drugs in SZ morbidity would be recommended.
The data on the numerical density of GAD67-positive neurons are in general agreement with data reported by others in SZ and BP patients using either the in situ hybridization technique or quantitative RT-PCR (5, 10, 14, 15, 27). Because the density of neurons evaluated with NeuN immunostaining is apparently not decreased (see also previous studies in refs. 4 and 10), it is suggested that the decreased number of GAD67-expressing interneurons cannot be attributed to neuronal loss but more likely relates to changes in the extent of GAD67 expression down-regulation.
It is noteworthy that the magnitude of GAD67 mRNA expression deficits measured in this cohort by the in situ hybridization histochemistry technique in BA9 layers I and II (20–25%) of SZP and BP+ patients is lower than that evaluated with quantitative RT-PCR measurements in BA9 tissue extracts obtained from the Stanley Foundation, which show a decrease of ≈40% (10).
This discrepancy is expected because histochemical evaluation of RNA expression is devoid of an internal standard correction and has a relatively low discrimination capacity. Hence, the histochemical in situ hybridization method, although appropriate for the study of cell location and selectivity, may not be the most appropriate method to conduct accurate measurements of the extent of DNMT1 or GAD67 mRNA changes.
DNA Methylation Controls Reelin and GAD67 Expression. Because cortical DNMT1 is primarily expressed in GABAergic neurons (13), we are inclined to consider that DNMT1 expression up-regulation is responsible for the epigenetically induced reelin and GAD67 promoter hypermethylation and the down-regulation of their expression in SZP and BP+ patients.
One should also consider the possibility that this DNMT1 increase could be treated with doxorubicin (adriamycin), which is a very potent inhibitor of DNMT1 activity (29). Experiments in animals are needed to support or negate this consideration. A pertinent question is whether epigenetic mechanisms are associated with the onset of psychopathology or are the result of the degree and type of symptomatology. Although this is an inference that cannot be studied in postmortem brains, in preliminary studies in heterozygous reeler mice, which express a 50% decrease of cortical reelin mRNA and protein content, we observed that DNMT1 mRNA is not changed compared with wild-type mice. However, in this case, the reelin deficit is primarily due to haploinsufficiency whereas, in SZ, this deficit is epigenetic, probably secondary to DNMT1 overexpression. This result suggests but does not prove that DNMT1 up-regulation is not the consequence of reelin or GAD67 down-regulation. Further experiments in animals (for example with doxorubicin) are needed to establish whether the increase of DNMT1 produces the decrease of GAD67 expression, or whether the decrease of GAD67 expression underlies the up-regulation of DNMT1 mRNA in cortical GABAergic neurons of SZP.
Reelin and GAD67 are not the only genes whose expression is down-regulated in GABAergic neurons of SZ and BP+ patients. For example, the expression of GABA transporter (GAT1) (30), parvalbumin (25) or N-methyl-d-aspartate receptor subunits (15) is also down-regulated in GABAergic neurons of SZ and BP+ patients. It remains to be studied whether the increase of DNMT1 in GABAergic neurons of SZ or BP+ patients is directly or indirectly responsible for the transcriptional down-regulation of these genes or other still unidentified genes operative in the regulation of reelin and GAD67 expression.
Does APS Medication Influence Changes in DNMT1 in SZ and BP+ Patients? Because patients with SZ and BP+ have similar psychotic symptoms, they often receive APS medication. Thus, it is important to test whether APS medication is a factor in the changes of DNMT1 expression. In a combined group of patients including both those with SZ and those with BP+, no correlation was found between levels of DNMT1 and the use of APS medications. Moreover, we have reported that protracted haloperidol and clozapine treatments in mice fail to change DNMT1 mRNA content in the cortex and cerebellum (4). Collectively, these data are consistent with the view that the increased expression of DNMT1 is not the consequence of neuroleptic treatment and that neuroleptic treatment does not prevent DNMT1 expression up-regulation. However, as reported in Fig. 4, the combination of VPA and APS may cause a reduction of DNMT1 overexpression.
Because SZP are often heavy cigarette smokers, we have considered whether the increase of DNMT1 expression is a consequence of exposure to high levels of nicotine. To test this hypothesis, in a preliminary study, mice (B6C3Fe strain) were treated for 8 days twice a day with 1.5 mg/kg nicotine bitartrate. Although this dose of nicotine elicits severe dacryorrhea, miosis, and whole body tremors that last for >2 h, no increase in DNMT1 mRNA-positive neurons was detected in the frontal cortex of these mice (M.V., unpublished data). These data suggest that nicotine per se is not the cause of DNMT1 overexpression. However, we cannot exclude that active compounds other than nicotine present in the cigarette smoke may contribute to the DNMT1 increase in psychosis.
Does VPA Medication Influence Changes in DNMT1 in SZ and BP+ Patients? Recently, there has been increased use of VPA in conjunction with APS in the treatment of SZ and BP patients (17, 18). Because several SZ and BP+ patients in our cohort treated with APS also received VPA (from 0.750 to 1.5 g/day), we studied whether VPA had any interactions with APS treatment in the expression of DNMT1. We found that SZ and BP+ patients treated with APS and VPA exhibited a lower increase in DNMT1 mRNA expression than patients treated with APS or VPA alone (Fig. 4). Hence, the hypothesis that VPA combined with APS may reduce the increase in DNMT1 mRNA expression remains a possibility that should be studied in a large number of patients treated with these drugs.
These results suggest that focusing on other and more efficacious histone deacetylase (HDAC) inhibitors may provide the required demonstration of whether what we attribute to the inhibition of HDAC is instead due to an induction of a DNA-demethylase, which has been reported to occur after VPA treatment (31). This would be a good lead to follow to explain the reduction of the increase in DNMT1 expression elicited by the combination of VPA and APS.
In conclusion, these studies contribute indirect evidence that the increase of DNMT1 in GABAergic neurons plays a role in the down-regulation of GAD67 and reelin expression. Particularly important is the fact that VPA contributes to a down-regulation of the DNMT1 increase, which may be considered a possible mechanism in the beneficial action of this drug and could be associated with the increased gene expression elicited by VPA. In fact, because this work suggests that down-regulation of the overexpression of DNMT1 is diminished by VPA treatment, perhaps the demethylation elicited by VPA is a mechanism important in reducing the increased expression of DNMT1 in GABAergic neurons.
Supplementary Material
Acknowledgments
We thank Dr. Bryan L. Roth (Department of Biochemistry, School of Medicine, Case Western Reserve University, Cleveland) and Dr. Daniel R. Weinberger (Genes, Cognition and Psychosis Program, National Institute of Mental Health, National Institutes of Health, Bethesda) for constructive criticism and suggestions in the preparation of the manuscript. This work was supported in part by National Institute of Mental Health Grants MH062188 (to A.G.) and MH062090 (to E.C.) and Merck & Co., Inc. Grant 03-4-699 (to E.C.).
Author contributions: M.V., A.G., and E.C. designed research; M.V. and E.M. performed research; M.V., A.G., E.M., J.M.D., and E.C. analyzed data; and M.V., A.G., and E.C. wrote the paper.
Abbreviations: GAD67, glutamic acid decarboxylase 67; DNMT1, DNA-methyltransferase 1; BA, Brodmann's area; SZ, schizophrenia; SZP, SZ patients; BP, bipolar disorder; BP+, BP with psychosis; BP-, BP without psychosis; NPS, nonpsychiatric subject; APS, antipsychotics; VPA, valproate; LI, like immunoreactivity; PMI, postmortem interval; NeuN, neuron-specific nuclear protein.
References
- 1.Petronis, A. (2004) Biol. Psychiatry 55, 965-970. [DOI] [PubMed] [Google Scholar]
- 2.Costa, E., Chen, Y., Davis, J. M., Dong, E., Noh, J., S., Tremolizzo, L., Veldic, M., Grayson, D. R. & Guidotti, A. (2002) Mol. Interv. 2, 47-57. [DOI] [PubMed] [Google Scholar]
- 3.Costa, E., Grayson, D. R., Mitchell, C. P., Tremolizzo, L., Veldic, M. & Guidotti, A. (2003) Crit. Rev. Neurobiol. 15, 121-142. [DOI] [PubMed] [Google Scholar]
- 4.Veldic, M., Caruncho, H. J., Liu, W. S., Davis, J. M., Satta, R., Grayson, D. R., Guidotti, A. & Costa, E. (2004) Proc. Natl. Acad. Sci. USA 101, 348-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akbarian, S., Kim, J. J., Potkin, S. G., Hagman, J. O., Tafazzoli, A., Bunney, W. E., Jr., & Jones, E. G. (1995) Arch. Gen. Psychiatry 52, 258-266. [DOI] [PubMed] [Google Scholar]
- 6.Impagnatiello, F., Guidotti, A. R., Pesold, C., Dwivedi, Y., Caruncho, H., Pisu, M., G., Uzunov, D. P., Smalheiser, N. R., Davis, J. M., Pandey, G., N., et al. (1998) Proc. Natl. Acad. Sci. USA 95, 15718-15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen, Y., Sharma, R. P., Costa, R. H., Costa, E. & Grayson, D. R. (2002) Nucleic Acids Res. 30, 2930-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tremolizzo, L., Carboni, G., Ruzicka, W. B., Mitchell, C. P., Sugaya, I., Tueting, P., Sharma, R., Grayson, D. R., Costa, E. & Guidotti, A. (2002) Proc. Natl. Acad. Sci. USA 99, 17095-17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tremolizzo, L., Doueiri, M., S., Dong, E., Grayson, D., R., Davis, J.M., Pinna, G., Tueting, P., Rodriguez-Menendez, V., Costa, E. & Guidotti, A. (2004) Biol. Psychiatry, in press. [DOI] [PubMed]
- 10.Guidotti, A., Auta, J., Davis, J. M., DiGiorgi Gerevini, V., Dwivedi, Y., Grayson, D. R., Impagnatiello, F., Pandey, G., Pesold, C., Sharma, R., et al. (2000) Arch. Gen. Psychiatry 57, 1061-1069. [DOI] [PubMed] [Google Scholar]
- 11.Fatemi, S. H., Earle, J. A. & McMenomy, T. (2000) Mol. Psychiatry 5, 654-663. [DOI] [PubMed] [Google Scholar]
- 12.Eastwood, S. L. & Harrison, P. J. (2003) Mol. Psychiatry 8, 821-831. [DOI] [PubMed] [Google Scholar]
- 13.Volk, D. W., Austin, M. C., Pierri, J. N., Sampson, A. R. & Lewis, D. A. (2000) Arch. Gen. Psychiatry 57, 237-245. [DOI] [PubMed] [Google Scholar]
- 14.Benes, F. M. & Berretta, S. (2001) Neuropsychopharmacology 25, 1-27. [DOI] [PubMed] [Google Scholar]
- 15.Woo, T. U., Walsh, J. P. & Benes, F. M. (2004) Arch. Gen. Psychiatry 61, 649-657. [DOI] [PubMed] [Google Scholar]
- 16.Hakak, Y., Walker, J. R., Li, C., Wong, W. H., Davis, K. L., Buxbaum, J. D., Hartounian, V. & Feinberg, A. A. (2001) Proc. Natl. Acad. Sci. USA 98, 4746-4751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wassef, A., Baker, J. & Kochan, L. D. (2003) J. Clin. Psychopharmacol. 23, 601-640. [DOI] [PubMed] [Google Scholar]
- 18.Casey, D. E., Daniel, D. G., Wassef, A. A., Tracy, K. A., Wozniak, P. & Sommerville, K. W. (2003) Neuropsychopharmacology 28, 182-192. [DOI] [PubMed] [Google Scholar]
- 19.Phiel, C. J., Zhang, F., Huang, E. Y., Guenther, M. G., Lazar, M. A. & Klein, P. S. (2001) J. Biol. Chem. 276, 36734-36741. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez, M. A., Caruncho H. J., Costa, E., Pesold, C., Liu, W. S. & Guidotti, A. (2002) J. Comp. Neurol. 451, 279-288. [DOI] [PubMed] [Google Scholar]
- 21.Williams, R. W. & Rakic, P. (1988) J. Comp. Neurol. 278, 344-352. [DOI] [PubMed] [Google Scholar]
- 22.DeFelipe, J. (1993) Cereb. Cortex 3, 273-289. [DOI] [PubMed] [Google Scholar]
- 23.Tomita, H., Vawter, M. P., Walsh, D. M., Evans, S. J., Choudary, P. V., Li, J., Overman, K. M., Atz, M. E., Myers, R. M., Jones E. G., et al. (2004) Biol. Psychiatry 55, 346-352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ryan, M. M., Huffaker, S. J., Webster, M. J., Wayland, M., Freeman, T. & Bahn, S. (2004) Biol. Psychiatry 55, 329-336. [DOI] [PubMed] [Google Scholar]
- 25.Lewis, D. A., Volk, D. W. & Hashimoto, T. (2004) Psychopharmacology 174, 143-150. [DOI] [PubMed] [Google Scholar]
- 26.Hashimoto, T., Volk, D. W., Eggan, S. M., Mirnics, K., Pierri, J. N., Sun, Z., Sampson, A. R. & Lewis, D. A. (2003) J. Neurosci. 23, 6315-6326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Volk, D., Austin, M., Pierri, J., Sampson, A. & Lewis, D. (2001) Am. J. Psychiatry 158, 256-265. [DOI] [PubMed] [Google Scholar]
- 28.Schleimer, S. B., Hinton, T., Dixon, G. & Johnston G. A. (2004) Neuropsychobiology 50, 226-230. [DOI] [PubMed] [Google Scholar]
- 29.Yokochi, T. & Robertson, K. D. (2004) Mol. Pharmacol. 66, 1415-1420. [DOI] [PubMed] [Google Scholar]
- 30.Woo, T. U., Whitehead, R. E., Melchitzky, D. S. & Lewis D. A. (1998) Proc. Natl. Acad. Sci. USA 95, 5341-5346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Detich, N., Bovenzi, V. & Szyf, M. (2003) J. Biol. Chem. 278, 27586-27592. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.