Abstract
Objective
Mice with adipocyte-specific inactivation of LDL receptor related protein-1 (LRP1) are resistant to diet-induced obesity and hyperglycemia due to compensatory thermogenic response by muscle. However, the physiological function of LRP1 in mature adipocytes and its role in cardiovascular disease modulation is unknown. This study compared perivascular adipose tissues (PVAT) from wild type (adLrp1+/+) and adipocyte-specific LRP1 knockout (adLrp1−/−) mice in modulation of atherosclerosis progression.
Approach and Results
Analysis of adipose tissues from adLrp1+/+ and adLrp1−/− mice after Western diet feeding for 16 weeks revealed that, in comparison to adLrp1+/+ mice, the adipocytes in adLrp1−/− mice were smaller but their adipose tissues were more inflamed with increased monocyte-macrophage infiltration and inflammatory gene expression. The transplantation of PVAT from chow-fed adLrp1+/+ and adLrp1−/− mice into the area surrounding the carotid arteries of Ldlr−/− mice prior to feeding the Western diet revealed a contributory role of PVAT toward hypercholesterolemia-induced atherosclerosis. Importantly, recipients of adLrp1−/− PVAT displayed a 3-fold increase in atherosclerosis compared to adLrp1+/+ PVAT recipients. The increased atherosclerosis invoked by LRP1-deficient PVAT was associated with elevated monocyte-macrophage infiltration and inflammatory cytokine expression in the transplanted fat.
Conclusion
Perivascular adipose tissues provide outside-in signals through the adventitia to modulate atherosclerotic lesion progression in response to hypercholesterolemia. Moreover, adipocytes with LRP1 deficiency are dysfunctional and more inflamed. This latter observation adds the adipose tissue to the list of anatomic sites where LRP1 expression is important to protect against diet-induced atherosclerosis.
Keywords: Adipocytes, Lipoprotein receptors, Perivascular adipose tissues, Atherosclerosis
Subject Codes: Animal Models of Human Disease, Inflammation, Metabolism
The low density lipoprotein receptor related protein-1 (LRP1) is a type 1 transmembrane protein with both endocytic and cell signal transduction properties.1 The receptor is ubiquitously expressed in all tissues where it modulates physiological and pathophysiological processes in a cell type-specific manner.2 Animal studies suggested that the association between LRP1 polymorphisms and premature coronary artery disease observed in humans may be due to LRP1 dysfunction in several cell types. Specifically, LRP1 dysfunction in the liver increases the risk of atherosclerosis by potentiating diet-induced hyperlipidemia.3 In contrast, LRP1 dysfunction in smooth muscle cells potentiates growth factor signaling cascades to increase cell proliferation and disruption of the elastic layer,4–6 whereas dysfunction of LRP1 in macrophages promotes atherosclerosis by increasing inflammation7 and reducing their efferocytosis capabilities.8 Another cell type with high LRP1 expression levels is the adipocytes. Increasing evidence suggests that adipose tissues, particularly those in the perivascular area surrounding the vessel wall, also play a critical role in atherosclerosis pathogenesis.9, 10 The goal of this study is to evaluate the influence of LRP1 expressed in adipocytes in atherosclerosis development.
Materials and Methods
Materials and methods are available in the online-only Data Supplement.
Results
Consistent with results reported previously for chow-fed animals,11 adipocytes in adLrp1−/− mice were also smaller in size compared to adipocytes in adLrp1+/+ mice after 16 weeks of Western diet feeding. Interestingly, more crown-like structures indicative of macrophages surrounding dead adipocytes were observed in epididymal and periaortic adipose tissues of Western diet-fed adLrp1−/− mice compared to adLrp1+/+ mice (Fig. 1A–C, Supplemental Figure I). Plasma levels and adipose expression of the pro-inflammatory adipokine resistin were also higher in adLrp1−/− mice compared to adLrp1+/+ mice (Fig. 1D,E). Analysis of PVAT gene expression revealed significantly lower levels of leptin mRNA, which is consistent with the reduced adipocyte cell size and fat mass, in adLrp1−/− mice. Surprisingly, adiponectin mRNA levels were similar between adLrp1+/+ and adLrp1−/− mice (Fig. 1E). We interpret these data to indicate that LRP1-deficient adipocytes are not dysfunctional, capable of synthesizing adiponectin, but are pro-inflammatory with elevated resistin expression.
Figure 1.
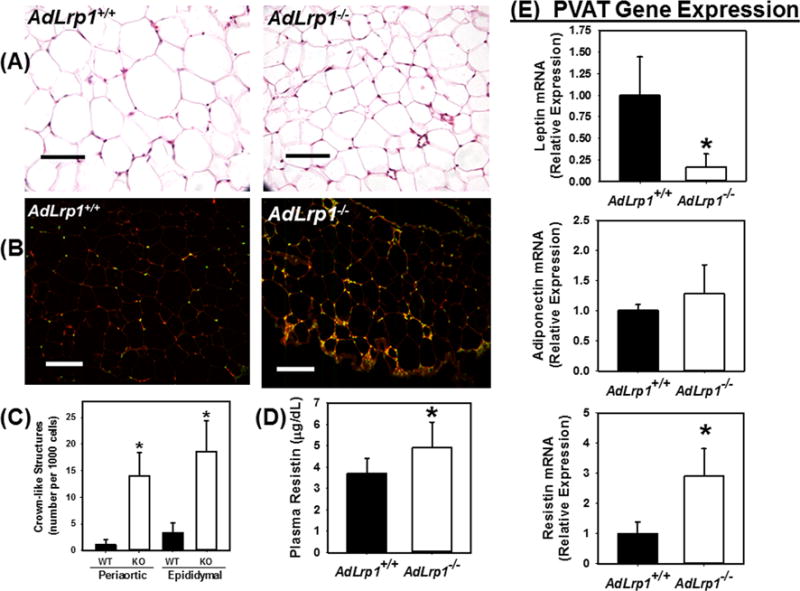
Adipocyte-specific LRP1 inactivation increases diet-induced inflammation in adipose tissues. AdLrp1+/+ and adLrp1−/− mice were fed Western diet for 16 weeks. Epididymal and periaortic adipose tissues were harvested for characterization. (A) Representative histological images of epididymal adipose tissues of adLrp1+/+ and adLrp1−/− mice. (B) Immunofluorescence detection of CD68+ cells with anti-CD68 (1:200 dilution) (red) and DAPI counterstain (green) in periaortic adipose tissues of adLrp1+/+ and adLrp1−/− mice. Scale bars = 100 μm. (C) Morphometric quantification of crown-like structures present in epididymal and periaortic adipose tissues of adLrp1+/+ (WT) and adLrp1−/− mice (KO). (D) Resistin levels in plasma of adLrp1+/+ and adLrp1−/− mice. (E) Expression levels of adipokines leptin, adiponectin, and resistin in PVAT of adLrp1+/+ and adLrp1−/− mice. All data represent mean ± SEM from N=6 mice in each group. * denotes difference from adLrp1+/+ (WT) mice at P < 0.05.
The influence of increased adipose tissue inflammation on atherosclerosis was examined by transplanting 2 mg of perivascular adipose tissues (PVAT) from adLrp1+/+ and adLrp1−/− mice to the left common arteries of 8-week old Ldlr−/− mice prior to feeding the recipient animals with the Western diet. Atherosclerosis analysis after 8 weeks of Western diet feeding revealed an ~3-fold increase in atherosclerosis in the carotid arteries of adLrp1−/− PVAT recipient mice compared to adLrp1+/+ recipients (Fig. 2A). The increased carotid atherosclerosis in adLrp1−/− recipients was associated with increased recruitment of CD68+ monocytes-macrophages (Fig. 2B, Supplemental Fig. II) and elevated expression of pro-inflammatory genes such as MCP-1/CCL2, IL6, and TNFα in the LRP1-deficient PVAT (Fig. 2C). Importantly, atherosclerosis was not observed in the contralateral carotid arteries without PVAT transplant in either groups (Supplemental Figure III).
Figure 2.
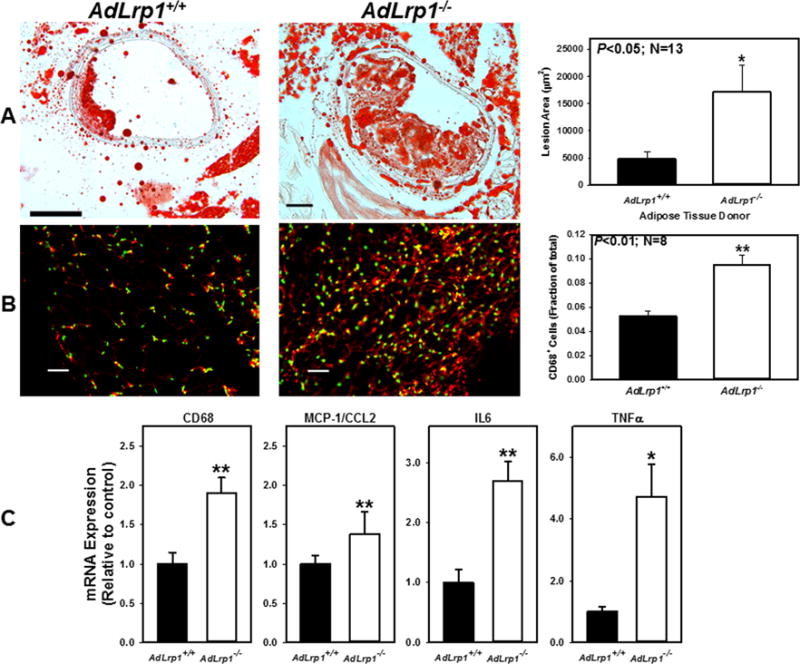
Transplant of adLrp1−/− PVAT accelerates atherosclerosis in carotid arteries of hyperlipidemic Ldlr−/− mice. PVAT from chow-fed adLrp1+/+ and adLrp1−/− mice were transplanted to surrounding areas of left carotid arteries in Ldlr−/− mice. Atherosclerosis in the carotid arteries were examined in 13 mice from each group after 8 weeks of Western diet feeding. (A) Representative images and morphometric quantification of atherosclerosis in the carotid arteries of mice after transplantation with adLrp1+/+ and adLrp1−/− PVAT. (B) Images and quantification of immunofluorescence staining of CD68 with anti-CD68 (1:200 dilution) (red) and DAPI (green) in the adLrp1+/+ and adLrp1−/− transplanted adipose tissues. Scale bars = 100 μm. (C) Expression of inflammatory genes in the transplanted PVAT relative to expression in adLrp1+/+ PVAT. * denotes difference from control at P < 0.05; ** denotes difference from control at P < 0.01.
Discussion
Atherosclerosis in Ldlr−/− and other commonly used mouse models is restricted to the aorta and the innominate arteries,12 which contrasts the humans where atherosclerosis may also occur in other vascular beds such as the coronary and carotid arteries. This difference may possibly be explained by differences in the architecture of the vasculatures between the two species. Whereas the coronary and carotid arteries in humans are surrounded by PVAT, these vessels are not surrounded by PVAT in mice.10 In the current study, we showed that transplanting PVAT to the adventitia surrounding the carotid arteries of Ldlr−/− mice resulted in atherosclerosis development in response to hypercholesterolemia similar to that observed in humans.
This study also showed that PVAT from adLrp1−/− mice invoked more atherosclerosis compared to PVAT from adLrp1+/+ mice. Intriguingly, we have shown previously that adLrp1−/− mice are resistant to high fat diet-induced obesity with improved glucose tolerance.11 The discrepancy between metabolic benefits and cardiovascular risk with adipocyte-specific LRP1 inactivation may be explained by the different context in which cardiometabolic effects of adipocyte LRP1 was assessed. The apparent metabolic benefit observed in adLrp1−/− mice is indirect and due primarily to compensatory increase of nutrient utilization by muscle cells to maintain body temperature.11 The current study showed that in animals with normal brown adipose tissue functions without the requirement of compensatory muscular thermogenesis, LRP1-deficient adipocytes are not dysfunctional, capable of synthesizing adiponectin, but are pro-inflammatory with elevated resistin expression. Previously, we showed that adipose tissues in adLrp1−/− mice expressed normal lipoprotein lipase activity,11 implying that fatty acid transport is normal in LRP1-deficient adipocytes. Hence, the impairment of lipid storage in adLrp1−/− adipocytes11 is likely due to reduced cholesterol uptake that is necessary for expansion and stabilization of lipid droplets.13, 14 As a consequence, excessive free fatty acids that cannot be stored as lipid droplets trigger lipotoxicity leading to monocyte-macrophage infiltration and inflammation. This mechanism is supported by the prevalence of crown-like structures indicative of dead adipocytes15 and CD68+ cells indicative of inflammation in adLrp1−/− adipose tissues. Thus, when PVAT from adLrp1−/− mice were transplanted to Ldlr−/− recipients, the heightened inflammation of adLrp1−/− adipose tissues provided outside-in signals through the adventitia to accelerate atherosclerotic lesion progression in the lumen. Taken together, this study adds the adipose tissue to the list of anatomic sites where LRP1 expression is important for atheroprotection.
Supplementary Material
Highlights.
Transplant of perivascular adipose tissue to carotid arteries promotes carotid atherosclerosis in mice.
Adipocytes with LRP1 deficiency are pro-inflammatory.
Adipocyte-specific LRP1 impairment promotes atherosclerosis.
Acknowledgments
None.
Source of Funding
This research was supported by a grant from the National Institutes of Health (RO1 DK045932).
Abbreviations
- PVAT
perivascular adipose tissue
- LRP1
LDL receptor related protein-1
Footnotes
Disclosure
None.
References
- 1.Herz J, Strickland DK. LRP: A multifunctional scavenger and signaling receptor. J Clin Invest. 2001;108:779–784. doi: 10.1172/JCI13992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lillis AP, Van Duyn LB, Murphy-Ullrich JE, Strickland DK. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol Rev. 2008;88:887–918. doi: 10.1152/physrev.00033.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding Y, Xian X, Holland WL, Tsai S, Herz J. Low density lipoprotein receptor related protein-1 protects against hepatic insulin resistance and hepatic steatosis. EBioMedicine. 2016;7:135–145. doi: 10.1016/j.ebiom.2016.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Basford JE, Moore ZWQ, Zhou L, Herz J, Hui DY. Smooth muscle LDL receptor-related protein-1 inactivation reduces vascular reactivity and promotes injury-induced neointima formation. Arterioscler Thromb Vasc Biol. 2009;29:1772–1778. doi: 10.1161/ATVBAHA.109.194357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boucher P, Gotthardt M, Li W-P, Anderson RGW, Herz J. LRP: Role in vascular wall integrity and protection from atherosclerosis. Science. 2003;300:329–332. doi: 10.1126/science.1082095. [DOI] [PubMed] [Google Scholar]
- 6.Boucher P, Li W-P, Matz RL, Takayama Y, Auwerx J, Anderson RGW, Herz J. LRP1 functions as an atheroprotective integrator of TGFb and PDGF signals in the vascular wall: Implications for marfan syndrome. PLoS ONE. 2007;2(5):e448. doi: 10.1371/journal.pone.0000448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.May P, Bock HH, Nofer J-R. Low density lipoprotein receptor-related protein 1 (LRP1) promotes anti-inflammatory phenotype in murine macrophages. Cell Tissue Res. 2013;354:887–889. doi: 10.1007/s00441-013-1699-2. [DOI] [PubMed] [Google Scholar]
- 8.Yancey PG, Ding Y, Fan D, Blakemore JL, Zhang Y, Ding L, Zhang J, Linton MF, Fazio S. Low density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6c(high) monocytosis. Circulation. 2011;124:454–464. doi: 10.1161/CIRCULATIONAHA.111.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omar A, Chatterjee TK, Tang Y, Hui DY, Weintraub NL. Proinflammatory phenotype of perivascular adipocytes. Arterioscler Thromb Vasc Biol. 2014;34:1631–1636. doi: 10.1161/ATVBAHA.114.303030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown NK, Zhou Z, Zhang J, Zeng R, Wu J, Eitzman DT, Chen YE, Chang L. Perivascular adipose tissue in vascular function and disease. A review of current research and animal models. Arterioscler Thromb Vasc Biol. 2014;34:1621–1630. doi: 10.1161/ATVBAHA.114.303029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hofmann SM, Zhou L, Perez-Tilve D, Greer T, Grant E, Wancata L, Thomas A, Pfluger PT, Basford JE, Gilham D, Herz J, Tschop MH, Hui DY. Adipocyte LDL receptor-related protein-1 expression modulates postprandial lipid transport and glucose homeostasis in mice. J Clin Invest. 2007;117:3271–3282. doi: 10.1172/JCI31929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.VanderLaan PA, Reardon CA, Getz GS. Site specificity of atherosclerosis. Site-selective responses to atherosclerotic modulators. Arterioscler Thromb Vasc Biol. 2004;24:12–22. doi: 10.1161/01.ATV.0000105054.43931.f0. [DOI] [PubMed] [Google Scholar]
- 13.Le Lay S, Ferre P, Dugail I. Adipocyte cholesterol balance in obesity. Biochem Soc Transac. 2004;32:103–106. doi: 10.1042/bst0320103. [DOI] [PubMed] [Google Scholar]
- 14.Le Lay S, Hajduch E, Lindsay MR, Le Liepvre X, Thiele C, Ferre P, Parton RG, Kurzchalia T, Simons K, Dugail I. Cholesterol-induced caveolin targeting to lipid droplets in adipocytes: A role for caveolar endocytosis. Traffic. 2006;7:549–561. doi: 10.1111/j.1600-0854.2006.00406.x. [DOI] [PubMed] [Google Scholar]
- 15.Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


