Abstract
Developmental alcohol exposure causes a host of cognitive and neuroanatomical abnormalities, one of which is impaired executive functioning resulting from medial prefrontal cortex (mPFC) damage. This study determined whether third-trimester equivalent alcohol exposure reduced the number of mPFC GABAergic parvalbumin-positive (PV+) interneurons, hypothesized to play an important role in local inhibition of the mPFC. The impact on passive avoidance learning and the therapeutic role of aerobic exercise in adulthood was also explored. Male C57BL/6J mice received either saline or 5 g/kg ethanol (two doses, two hours apart) on PD5, 7, and 9. On PD35, animals received a running wheel or remained sedentary for 48 days before behavioral testing and perfusion on PD83. The number of PV+ interneurons was stereologically measured in three separate mPFC subregions: infralimbic, prelimbic and anterior cingulate cortices (ACC). Neonatal alcohol exposure decreased number of PV+ interneurons and volume of the ACC, but the other regions of the mPFC were spared. Alcohol impaired acquisition, but not retrieval of passive avoidance, and had no effect on motor performance on the rotarod. Exercise had no impact on PV+ cell number, mPFC volume, or acquisition of passive avoidance, but enhanced retrieval in both control and alcohol-exposed groups, and enhanced rotarod performance in the control mice. Results support the hypothesis that part of the behavioral deficits associated with developmental alcohol exposure are due to reduced PV+ interneurons in the ACC, but unfortunately exercise does not appear to be able to reverse any of these deficits.
Keywords: anterior cingulate cortex, executive function, inhibition, fear, running, fetal alcohol spectrum disorders
Developmental alcohol exposure in humans produces a wide range of deficits collectively referred to as Fetal Alcohol Spectrum Disorders (FASD). FASD occurs in approximately 2–5% of all live births and, despite growing awareness of this disease; the rate continues to increase (May et al., 2009). Patients with FASD exhibit neurobehavioral deficits, which include physical, cognitive, learning and behavioral disabilities. These effects are long lasting and have possible lifelong implications (Calhoun et al., 2006). Third trimester alcohol exposure is associated with deficits in executive functioning, planning, organized search, inhibition, working memory, and flexible thinking (Connor et al., 2000, Rasmussen, 2005). These behaviors are highly associated with brain regions that include, but are not limited to, the prefrontal cortex (Mattson et al., 2001, Kooistra et al., 2010, Mattson et al., 2011). Furthermore, maternal alcohol consumption reduces frontal lobe size and increases grey matter asymmetry between frontal lobes (Wass et al., 2001, Sowell et al., 2002, Rasmussen, 2005). Thus, FASD-related impairments in cognition and learning persist into adulthood and are accompanied by structural changes in the PFC.
The PFC is the last cortical structure to fully develop. Beginning with the generation of neurons in utero, development is not fully complete until late adolescence. Of note, during the third trimester, the PFC, along with other brain regions, goes through a transient period of mass neurodevelopment and rapid growth. This is referred to as the brain growth spurt. The equivalent of the brain growth spurt occurs over the first ten postnatal days in rodents (Dobbing and Sands, 1979). During this time period many essential features of the PFC are developing. In humans, it is during the third trimester that the formation of the six-layered pattern of the cortex occurs (Mrzljak et al., 1990). In rodents, the medial PFC (mPFC) laminar architecture is completed after birth. By postnatal day (PD) 6, thalamic fibers reach their layer of termination (Layer III) and it is not until PD 10 that all cortical layers can be recognized in the mPFC. The rodent mPFC consists of infralimbic (IL), prelimbic (PL) and anterior cingulate (ACC) subregions. Each subregion is made up of a different architectonic organization and laminar differentiation (Van Eden and Uylings, 1985) and these anatomical distinctions suggest functional differences. For example, each subregion has been associated with fear-associated learning; however, their roles appear slightly unique. The IL and PL subregions are more highly associated with either fear suppression or expression, respectively (for review see Giustino and Maren, 2015), while the ACC is associated with fear acquisition (Hamner et al., 1999, Bissiere et al., 2008, Etkin et al., 2011). Therefore, it is it is possible that alcohol exposure during the brain growth spurt, could differentially impact the mPFC subregions both anatomically and functionally.
Alcohol exposure during this period of rapid neurodevelopment leads to mPFC anatomical abnormalities. Not only does maternal alcohol consumption result in a smaller frontal lobe size in the offspring (Fakoya and Caxton-Martins, 2006), but also it changes the connectivity. For example, human literature demonstrates that maternal alcohol consumption alters mPFC pyramidal cell spine density and morphology (Ferrer and Galofre, 1987). Further rodent studies show third trimester equivalent alcohol exposure produces simplified dendritic organization and decreased spine density of Layer II/III mPFC pyramidal cells (Granato and Van Pelt, 2003, Whitcher and Klintsova, 2008, Hamilton et al., 2010). Ethanol is a known NMDA antagonist, and research shows that PD 7 treatment of a NMDA antagonist not only significantly reduced the number of Layer V pyramidal neurons, but it also halved the number of parvalbumin-positive (PV+) interneurons in the frontal cortex, when measured in adulthood (Coleman et al., 2009). Prenatal alcohol exposure also produced long-term anomalies in striatal PV+ interneurons, mainly represented by the shrinkage of their dendritic tree (De Giorgio et al., 2012). In the mPFC, PV+ interneurons primarily receive input from the mediodorsal nucleus of the thalamus (Rotaru et al., 2005) and project primarily on the proximal basilar dendrites of pyramidal neurons (Shibata, 1993, Rotaru et al., 2005). It is through this strong indirect inhibitory influence that the mediodorsal nucleus of the thalamus is able to gate pyramidal cell excitability to other inputs (Floresco and Grace, 2003, Kuroda et al., 2004, Rotaru et al., 2005). Therefore, alterations in PFC connectivity, perhaps specifically through altered GABAergic inhibition, may mitigate certain FASD-associated behavioral deficits.
Given the continuously increasing number of FASD patients who are undoubtedly suffering from behavioral deficits, it is essential to develop therapeutic interventions. Consistently, exercise participation has proven to improve brain health and function. In humans, participation in physical activity not only improves learning and memory (Colcombe et al., 2004) but is also argued to slow the progress of neurodegenerative diseases such as Alzheimer’s disease (Nelson & Tabet, 2015). Further, work with rodents has demonstrated increased levels of neurotrophic factors such as BDNF and VEGF in the cortex following voluntary exercise (Hopkins et al., 2011, Uysal et al., 2011, Aksu et al., 2012). These factors promote the differentiation, neurite extension, and survival of a variety of neuronal populations (Cotman and Berchtold, 2002). In particular, Marty and colleagues (1997) argue that because both the synthesis and secretion of BDNF by pyramidal neurons are regulated by neuronal activity, BDNF may mediate activity dependent interactions between interneurons and their target cells. In fact, voluntary exercise has been shown to enhance cellular plasticity in the PFC of corticosterone-treated (Ekstrand et al., 2008) and methamphetamine-treated (Mandyam et al., 2007) male adult rats. Further, voluntary exercise has been shown to rescue decreases in spine density and eliminate deficits in dendritic complexity in mPFC pyramidal cells of rats exposed to a third trimester equivalent alcohol exposure (Hamilton et al., 2015). Therefore, it is possible that by promoting neuroplasticity, exercise later in life could facilitate cell survival in the alcohol-exposed brain.
Although it is known that neonatal alcohol exposure leads to mPFC abnormalities such as altered pyramidal cell morphology (i.e. reduced spine density and simplified dendritic organization), to the best of our knowledge no previous study has specifically investigated the impact of neonatal alcohol exposure on the number of mPFC PV+ interneurons. Therefore, the goal of this study was first to establish the effects of neonatal alcohol exposure on the number of PV+ interneurons in distinct mPFC subregions. Second, given the unique contributions of each subregion to fear-associated learning, this study examined the impact of neonatal alcohol exposure on the passive avoidance task. Third, we sought to determine the extent to which wheel running could mitigate any observed deficits. To this regard, rotarod performance served as a positive control for the effects of alcohol and exercise, given that neonatal alcohol exposure has been known to influence motor performance (Goodlett et al., 1991, Klintsova et al., 2000) and exercise is known to mitigate cerebellar damage (Cotman and Berchtold, 2002, Hamilton et al., 2016). Given the role of PV+ neurons in local inhibition and the crucial role of the mPFC in fear-associated learning, we hypothesized that neonatal alcohol exposure may reduce PV+ interneurons in mPFC subregions and that these neuroanatomical abnormalities would be associated with impaired passive avoidance performance. Further, we hypothesized that wheel running would mitigate some of the alcohol-induced behavioral and neuroanatomical deficits, given its proven ability to improve brain health and function.
EXPERIMENTAL PROCEDURES
Subjects
The mice used in this study were the control (non-transgenic litter-mates) produced by crossing two hemizygous transgenic Nestin-TK mice (Yu et al., 2008). The transgenes were inserted in C57BL/6J mouse zygotes for an unrelated study. Genotyping confirmed the mice used in this study did not carry the transgene, hence they were plain C57BL/6J. A total of 37 male mice were used. Only males were used in this study, as we first wanted to establish that this alcohol model would impact the number of PV+ interneurons without the complication induced by the estrus cycle. It has been shown that female rats have a significantly higher number of PV+ interneurons in the prefrontal cortex than do male rats (Smiley et al., 2015, Wischhof et al., 2015). Therefore, it is possible that females would respond differently from males. All mice were bred in house at the Beckman Institute. Individual mice from each litter were assigned to different groups, to account for litter effects. Throughout the study, mice were housed in standard polycarbonate shoebox cages (29 cm × 19 cm × 13 cm; L × W × H) with corncob bedding (Teklad 7012; Harlan Teklad, Madison, WI, USA) and nesting material. Rooms were controlled for temperature (21°C ± 1°C) and photo-period (12:12 D:L; lights off at 9:00am CST and on at 9:00pm) with food and water provided ad libitum. The Beckman Institute Animal Facility is AAALAC approved. All procedures were approved by the University of Illinois Institutional Animal Care and Use Committee and adhered to NIH guidelines.
Neonatal Treatment
On PD 5, 7, and 9, whole litters were assigned to one of two treatment groups: saline or ethanol solution. Twenty ethanol treated pups were injected subcutaneously twice daily, two hours apart (9:00 AM and 11:00 AM) with 2.5 g/kg ethanol, amounting to a total of 5 mg/kg per day. The ethanol was delivered at a concentration of 20% (Decon Laboratories, Inc., King of Prussia, PA, USA) in sterile saline. This dose was determined based on the literature (Ikonomidou et al., 2000, Wozniak et al., 2004, Ieraci and Herrera, 2007, Lantz et al., 2012, Hamilton et al., 2016). Seventeen pups served as control animals by receiving saline injections. On PD 21, all animals were weaned into groups of four. Blood samples were not collected from animals, but previous work demonstrates that this concentration of alcohol is enough to produce a toxic level, above 200 mg/dl for four consecutive hours or more (Ikonomidou et al., 2000, Hamilton et al., 2016).
5′bromo-5′-deoxyuridine (BrdU) Injections
On PD 35–44, all mice received daily intraperitoneal injections of 50 mg/kg 5-Bromo-2′-Deoxyuridine in order to label newly dividing cells in the dentate gyrus of the hippocampus (data not included).
Exercise Intervention
Beginning on PD 35, all mice were placed in single housing where they either had access to or did not have access to running wheels. This method was chosen in order to obtain individual running levels. Therefore, final group numbers in each treatment condition were: saline sedentary: n= 8; saline runner; n = 9; alcohol sedentary, n = 10; alcohol runner: n = 10. Runners were placed in cages (36 cm × 20 cm × 14cm; L × W × H) with a 23 cm diameter wheel mounted to the cage top (Respironics, Bend, OR, USA) while sedentary animals remained in standard polycarbonate boxes without running wheels. Wheel rotations were monitored continuously in one-minute increments via magnetic switches interfaced to a computer running VitalView software (Respironics, Bend, OR, USA). Sedentary mice were not kept in cages with locked wheels, as mice will climb on top of locked wheels and this would introduce additional activity in the sedentary group (Koteja et al., 1999). Animals remained in their designated housing throughout the behavioral testing except when temporarily moved into behavioral testing apparatuses. Similar to previous studies from our lab, mice were given at least 30 days access to the exercise intervention prior to any behavioral testing. As such, animals began behavioral testing on PD 79. Animals remained on wheels until time of perfusion.
Rotarod
Children with FASD typically have poor motor coordination. As such, mice were tested on the rotarod task to establish whether a PD 5, 7, and 9 alcohol exposure would impair motor coordination. Further, we hoped to determine whether exercise could ameliorate any alcohol-induced deficit. The rotarod task was conducted on PD 79 and PD 80 to measure motor coordination. The apparatus was a horizontal rod positioned above a box (AccuRotor Rota Rod Tall Unit, 63cm fall height, 8 cm diameter rotating dowel; Accuscan, Columbus, OH). This rod rotated at a constantly accelerating rate of 60 rpm/minute. The rod was divided into four small, even compartments by disks. Mice were placed in their own compartments and motor coordination was assessed by latency for the animal to fall from the rod. The response variable that was statistically analyzed was average latency to fall across all 4 trials on each of the 2 days.
Passive avoidance
Passive avoidance testing occurred on PD 81- PD 82, one hour following lights off. On Day 1 (PD 81) animals were placed in a passive avoidance chamber (San Diego Instruments, San Diego, CA, USA) that consisted of two compartments, both with a metal grid flooring. The compartments, one of which was lit while the other was unlit, were separated by a guillotine door. At the beginning of each trial, animals were placed in the lit compartment and the guillotine door was raised. Mice have a natural aversion to light and were thus motivated to flee from the lit chamber through the open door and into the unlit compartment. When animals crossed fully into the unlit compartment, the guillotine door closed and a mild foot shock (0.5 mV) was delivered via the metal grid floor of the compartment. The door remained closed for a ten second post conditioned stimulus period, after which it opened and the house light was turned on in the compartment containing the mouse (previously unlit), while the other compartment went dark. This counterbalancing ensured that the animal was conditioned to the light/dark cues and not a particular side of the chamber. The animal repeated this task until it learned to remain in the lit compartment for 120 consecutive seconds. The time it took for the animal to reach criterion was determined for each subject (latency to reach criterion). On Day 2 (PD 82), animals were tested for task retention. They were placed into the lit compartment and the time it took for them to cross over to the unlit compartment was recorded (latency to cross).
Tissue Preparation and Immunohistochemistry
Perfusions
Perfusions occurred on PD 83. All mice were euthanized via transcardial injection of 4% paraformaldehyde in phosphate buffer solution (PBS; 0.287% sodium phosphate monobasic anhydrous, 1.102% sodium phosphate dibasic anhydrous, 0.9% sodium chloride in water). Brains were placed in 4% paraformaldehyde for two days and subsequently transferred to a 30% sucrose in PBS solution. Brains were sectioned into 40 μm coronal sections on a cryostat and then immediately stored in cryoprotectant (30% ethylene glycol, 25% glycerin, 45% PBS) at −20°C.
Immunohistochemistry
Using a 1-in-5 series the mPFC was stained for PV+ interneurons using the primary antibody: anti-PV in rat. Tissue sections were first rinsed in PBS, followed by a 30 minute immersion in 0.6% hydrogen peroxide. Sections were subsequently washed with PBS again and then exposed to a solution of 0.3% Triton-X and 3% goat serum in PBS for 30 minutes (PBS-X plus). Next, tissue sections were incubated with primary antibody anti PV in mouse (1:2000; AbD Serotec, Raleigh, NC, USA) in PBS-X plus for 24 hours at approximately 4 °C. After washing with PBS-X plus, sections were treated with goat anti-mouse secondary antibody (1:250) in PBS-X plus for 90 minutes at room temperature. Sections were then treated with the ABC system (Vector, Burlingame, CA, USA), washed in PBS-X, and stained using a diaminobenzidine kit. Tissue sections were mounted on microscope slides, left to dry overnight, dehydrated and stained using Methylene Azure and subsequently cover slipped.
Unbiased Stereology
Quantification of PV+ interneurons was performed in accordance with an unbiased stereology approach. A series of sections (every 5th section, 7–9 sections per animal) throughout the entire mPFC was used for each staining batch (Markham et al., 2007). All cell counts were made on coded slides by an investigator blind to the treatment conditions. Tracing of the mPFC was conducted for every tissue section by referencing the Allen Mouse Brain Atlas, for the location of the IL, PL and ACC subregions (Markham et al., 2007, Willing and Juraska, 2015). Each subregion was outlined on the digitized image on each section using StereoInvestigator software (Figure 1). Counts were made in an unbiased manner within a known volume of each mPFC subregion using the optical fractionator workflow (StereoInvestigator, MicroBrightField Inc., Williston, VT). The grid frame was set to 200 × 200 μm and the counting frame set to 200 × 200 μm. A guard zone of 2 μm and a dissector height of 14 μm were used. The frozen sections were originally cut at a nominal thickness of 40 μm. Immunostaining and mounting result in altered section thickness, which was measured at each counting site. Average section thickness was determined and used to estimate both the total volume of each mPFC subregion and also the total number of PV+ interneurons in each mPFC subregion. For each animal, the coefficient of error (CE) was used to determine the precision of PV+ cell number estimates in a set of sections (Gundersen et al., 1999, Slomianka and West, 2005). A CE of less than 0.10 was considered adequate. (Gundersen et al., 1999). Actual CEs ranged between 0.05 and 0.08.
Figure 1. mPFC subregion identification and staining.
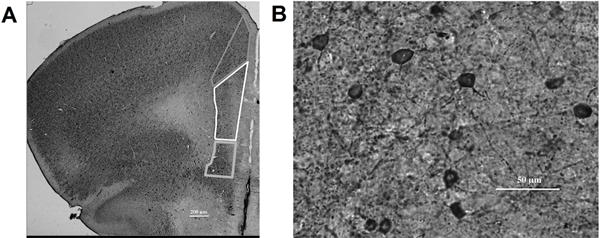
A) Methylene blue stained coronal mPFC section (2.5× magnification) with example tracings of the different subregions (top: anterior cingulate (ACC); middle: prelimbic (PL); bottom: infralimbic (IL)). B) mPFC PV/DAB labeled GABAergic interneurons (20× magnification).
Statistical Analysis
Data were analyzed using SPSS (version 22) statistical software. For all analyses, p < 0.05 was considered statistically significant. The following variables were analyzed using a repeated measures analysis with Day as the within measure and Postnatal Treatment (alcohol vs. saline) and Intervention (runner vs. sedentary) as the between factors: body weights (g), latency to fall from the rotarod (s). The following variables were analyzed using a two-way analysis of variance (ANOVA), with Postnatal Treatment (alcohol vs. saline) and Intervention (runner vs. sedentary) as the two factors: brain weights (g), total number of PV+ cells, average distance run on wheels, latency to reach criterion, latency to cross in passive avoidance (s). Post-hoc analyzes (LSD) were used to characterize treatment and interaction effects, when statistically significant (p < 0.05).
RESULTS
Body Weights
Body Weights for all animals are summarized in Table 1. All animals continued to gain weight throughout the experiment. Neonatal body weights (PD 5, 7, and 9) versus body weights from later in life (PD 21, 35, 44 and 83) were analyzed separately. To analyze neonatal body weights, a repeated measures ANOVA with Postnatal Treatment as an independent variable was used. Results show a main effect of Day (F(1, 360) = 3122.31, p < 0.001), with all animals gaining weight each day. A Postnatal Treatment × Day interaction (F(1, 360) = 131.14, p < 0.001) and a main effect of Postnatal Treatment (F(1, 360) = 82.75, p < 0.001) were also found. These results indicate that alcohol-exposed animals gained less weight between each weighing period when compared to their saline-exposed counterparts. This was evident on PD 5 (p < 0.05), PD 7 (p < 0.001) and PD 9 (p < 0.001). It should be noted that on PD 5, animals had not yet been injected with either ethanol or saline at the time of weighing, which indicates a preexisting weight difference between these randomly assigned groups.
Table 1.
Effect of postnatal alcohol exposure on body weight
| PD 5 | PD 7 | PD 9 | PD 21 | PD 35 | PD 45 | PD 83 | |
|---|---|---|---|---|---|---|---|
| Saline | 3.09 ± 0.04 | 4.13 ± 0.05 | 5.02 ± 0.06 | 10.24 ± 0.30 | 16.17± 0.52 | 17.28± 0.41 | 23.56± 0.43 |
| Alcohol | 2.97 ± 0.03* | 3.52 ± 0.03** | 4.24 ± 0.05** | 8.69 ± 0.27** | 14.90 ± 0.39* | 16.10 ± 0.29* | 22.76± 0.32 |
PD, postnatal day. The weights are reported as group means ± SEM.
p < 0.05;
p<0.001
Consistently, to analyze body weights from later in life (PD 21, 35, 44 and 83), a repeated measures ANOVA with Postnatal Treatment as an independent variable was performed. The results of which revealed not only a significant main effect of Day (F(1, 22) = 2753.23, p < 0.001) but also a main effect of Postnatal Treatment (F(1, 22) = 8.23, p < 0.01), suggesting a long-term detrimental impact of neonatal alcohol exposure on body weight. However, although alcohol-exposed animals exhibited lower body weights than their saline-exposed counterparts during the juvenile and adolescent time period (PD 21: p = 0.001; PD 35: p < 0.05; PD 45: p < 0.05), this effect was no longer significant when measured in adulthood (PD 83). Together these data indicate that neonatal alcohol exposure causes a transient reduction in body weight for male mice.
Brain Weights
Brain weights were recorded following perfusion in order to assess whether PD 5, 7, and 9 alcohol exposure had any long-term effects on brain weight. A univariate ANOVA revealed a main effect of Postnatal Treatment (F(1,35) = 7.75, p < 0.01). More specifically, these results demonstrated that mice exposed to neonatal alcohol exposure had significantly smaller brains when compared to their saline-exposed littermates (Figure 2a). Similarly, a decrease in the calculated brain/body weights ratios for both Treatment groups was also found (Saline: 0.0192g ± 0.0007g, Alcohol: 0.0175g ± 0.0004g). Further, an ANCOVA with PD 83 body mass as a covariate revealed that not only was body mass significantly correlated with brain weight (F(1,34) = 6.51, p < 0.05) but that the effect of Treatment was also significant (F(1,34) = 5,11, p < 0.05). Together, these data demonstrate that PD 5, 7 and 9 alcohol exposure has a significantly damaging impact on brain development that is still evident in adulthood and that this impact is not due to simply lower body weights (Figure 2b).
Figure 2. Persistent impact of PD 5, 7, and 9 alcohol exposure evident in brain weight as adults.
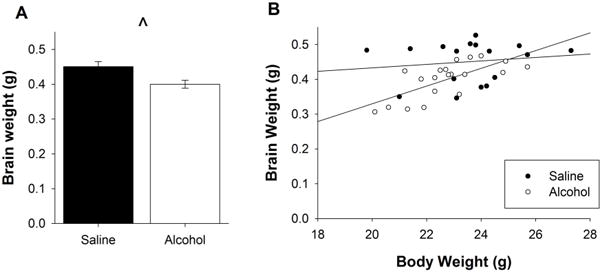
A. When measured in adulthood, the brains of mice exposed to a PD 5, 7, and 9 alcohol exposure are significantly smaller than those of their saline-exposed mice littermates. B. The negative impact of PD 5, 7, and 9 alcohol exposure on brain weight is still evident even when animals are matched for body size. All values represent mean ± SEM. *p < 0.05.
Wheel Running
Mice exposed to neonatal alcohol exposure run significantly less than their saline exposed counterparts (F(1, 17) = 6.222, p = 0.023; Figure 3a). This effect was persistent throughout the entirety of the intervention period (Figure 3b). This suggests neonatal alcohol exposure may suppress motivation for physical activity into adulthood, as suggested by the decreased running levels.
Figure 3. Wheel Running Levels.
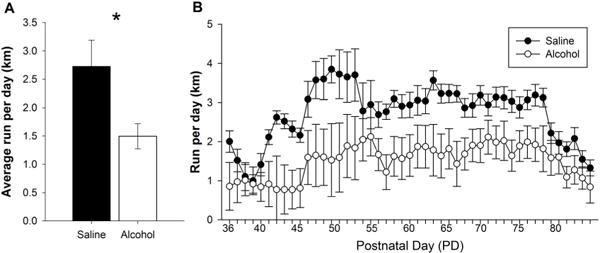
A. Data demonstrate the average running distance (km) per 24 hours of alcohol- and saline-exposed mice. Animals exposed to a PD5, 7, and 9 alcohol exposure ran significantly less than their saline-exposed littermates. B. Daily running distance (km) of alcohol-exposed and saline-exposed mice throughout the entirety of the intervention. All values represent mean ± SEM. *p < 0.05.
Rotarod
A repeated measures ANOVA revealed a main effect of Day (F(1, 33) = 22.828, p < 0.001), a main effect of Intervention (F(1, 33) = 8.102, p < 0.01) and a Day × Postnatal Treatment × Intervention interaction (F(1, 33) = 4.097, p = 0.05). Regardless of Postnatal Treatment, runners stayed significantly longer on the rod than their sedentary counterparts. However, it appears that although Saline Runners showed a significant enhancement from Day 1 to Day 2 (F(1, 10) = 11.080, p < 0.01), Alcohol Runners failed to exhibit this same benefit from previous running experience (Figure 4). These data suggest that although neonatal alcohol exposure does not negatively impact rotarod performance, it does limit the ability of exercise to enhance performance on this task.
Figure 4. Neonatal alcohol exposure has no impact on Rotarod performance, while previous exposure to exercise enhances performance.
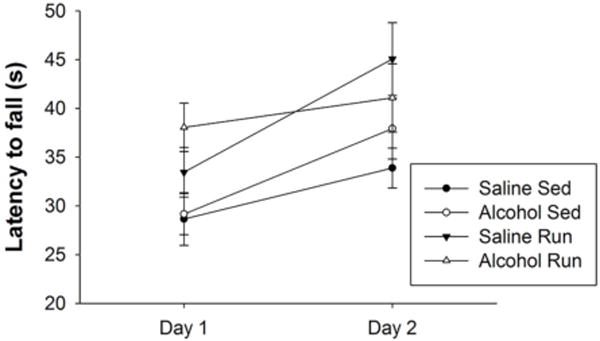
PD 5, 7, and 9 alcohol exposure fails to impact Rotarod performance in adulthood on both Days of Rotarod testing. Running wheel access improved Rotarod performance in both saline and alcohol-exposed mice on Day 1. On Day 2, Alcohol Runners failed to show behavioral improvements on the task, while Saline Runners showed a significant improvement across days. All values represent mean ± SEM. *p < 0.05.
Passive Avoidance
Day 1
A PD 5, 7, and 9 alcohol exposure resulted in long-term behavioral deficits on the passive avoidance task, regardless of whether or not mice had access to a running wheel. Alcohol exposed mice took a significantly longer time to reach criterion on the task than did their saline-exposed littermates. Specifically there was a main effect of Postnatal Treatment (F(1, 33) = 6.315, p < 0. 05; Figure 5a).
Figure 5. PD 5, 7, and 9 alcohol exposure impairs passive avoidance performance.
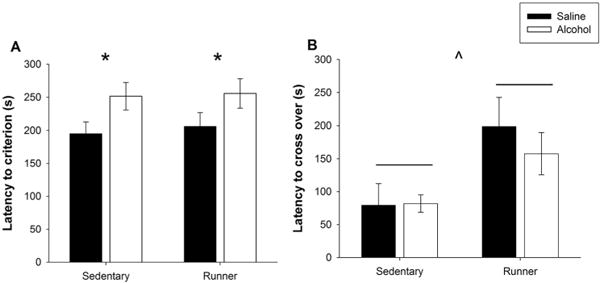
A. Latency to reach criterion on Day 1. When tested in adulthood, mice exposed to a PD 5, 7, and 9 alcohol exposure required a significantly longer time to reach criterion compared to their saline-exposed littermates, regardless of Intervention. B. Latency to cross over into the dark chamber on Day 2. Mice given access to a running wheel demonstrate significantly longer latencies to cross over than their sedentary littermates, regardless of Postnatal Treatment condition. All values represent mean ± SEM. *p < 0.05, ^p < 0.001.
Day 2
In contrast, Postnatal Treatment had no impact on Day 2 performance. Rather, runners showed a longer latency to cross over into the dark chamber when compared to their sedentary counterparts, indicating a better task retention (Figure 5b). Specifically, there was a significant main effect of Intervention (F(1, 31) = 8.433, p < 0.01). Therefore, it appears that a PD 5, 7, and 9 alcohol exposure is sufficient to impact acquisition but not retention of the passive avoidance task, while an exercise intervention is sufficient to enhance retention but not acquisition of the task.
Medial Prefrontal Cortex (mPFC) Volume
Neither a PD 5, 7, and 9 alcohol exposure, nor an exercise intervention impacted the overall volume of the mPFC. Specifically, no significant differences were found between the mPFC volumes of alcohol-exposed versus saline-exposed mice regardless of exercise intervention (Figure 6a). Interestingly, PD 5, 7, and 9 alcohol exposure did significantly reduce the volume of the ACC dorsal mPFC subregion (F(1, 20) = 7.081 p < 0.05; Figure 6b); while a lack of significant impact of PD 5, 7, and 9 alcohol exposure on the volume of the combined prelimbic and infralimbic (PL/IL) ventral mPFC subregion was found (Figure 6c). No significant impact of exercise intervention was present in any subregion. These results suggest that PD 5, 7, and 9 alcohol exposure has a persistent effect that specifically targets the ACC.
Figure 6. PD 5, 7, and 9 administration has long-term impact on not the overall volume, but rather distinct mPFC subregions.
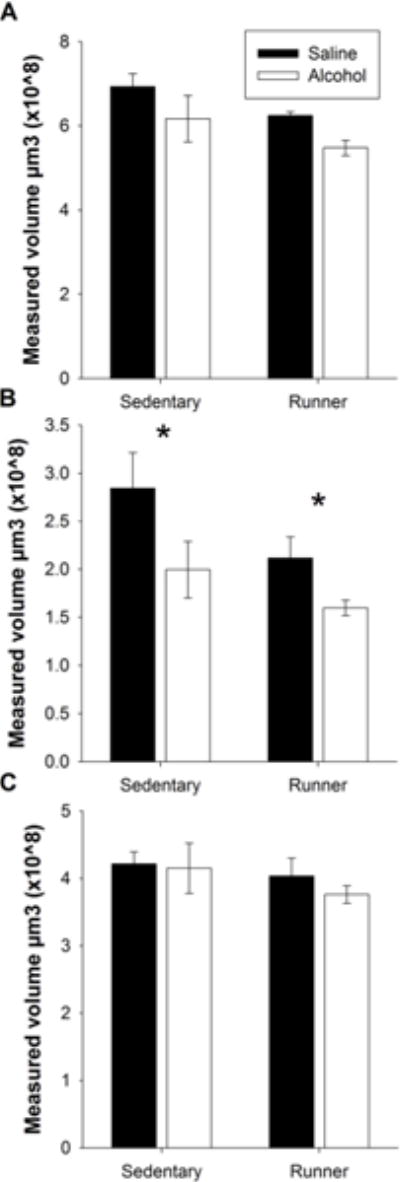
A. Neither PD 5, 7, and 9 alcohol exposure nor exercise impacts mPFC volume. B. PD 5, 7, and 9 alcohol exposure significantly reduces the volume of the ACC of the mPFC. Exercise has no impact on ACC volume. C. Neither PD 5, 7, and 9 alcohol exposure nor exercise impacts the volume of the PL/IL. All values represent mean ± SEM. *p < 0.05.
Parvalbumin-positive (PV+) Cell Counts
Despite having no impact on mPFC volume, PD 5, 7, and 9 alcohol exposure significantly reduced the number of mPFC PV+ GABAergic interneurons (F(1, 20) = 8.974 p < 0.01), while neither an impact of Intervention (F(1, 20) = 0.063 p = 0.80) nor a Postnatal Treatment × Intervention interaction (F(1, 20) = 2.716 p = 0.12) was found. When data were collapsed across the Intervention, a main effect of Postnatal Treatment was still evident, such that there was a significant reduction in the total number of mPFC PV+ GABAergic interneurons (F(1, 22) = 8.667 p < 0.01; Figure 7a). This effect was particularly prevalent in the ACC with brains of alcohol-exposed animals mice having significantly fewer PV+ cells than those of their saline counterparts (F(1, 22) = 7.755 p < 0.05; Figure 7b). No impact of PD 5, 7, and 9 alcohol exposure was seen in the PL/IL subregions (Figure 7c). Together, this suggests that PD 5, 7, and 9 alcohol exposure has a long-lasting impact, specifically on the ACC.
Figure 7. PD 5, 7, and 9 administration has long-term impact on the number of mPFC PV+ interneurons.
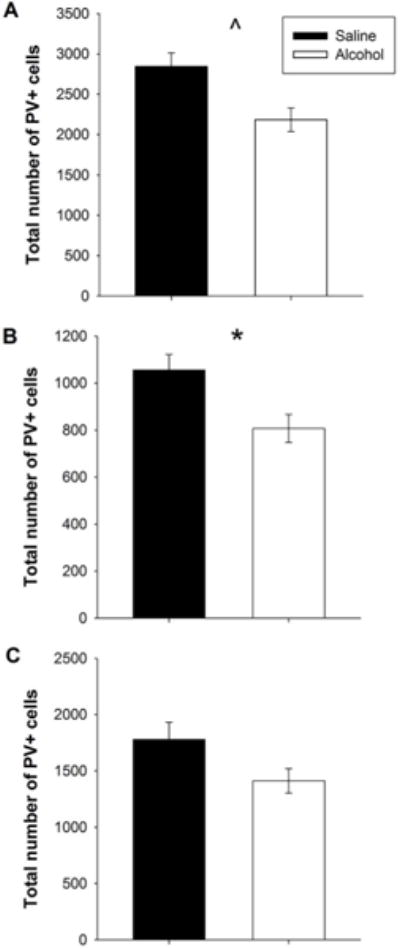
A. PD 5, 7, and 9 alcohol exposure significantly reduces the number of GABAergic PV+ interneurons in the mPFC. Exercise has no impact on number of PV+ interneurons. B. PD 5, 7, and 9 alcohol exposure significantly reduces the number of PV+ interneurons in the ACC. No effect of exercise. C. Neither PD 5, 7, and 9 alcohol exposure nor exercise impacts the number of PV+ GABAergic interneurons in the PL/IL. All values represent mean ± SEM. *p < 0.05, ^p < 0.001.
DISCUSSION
This study is the first to demonstrate that a third-trimester alcohol exposure reduces both the volume and the number of PV+ interneurons in the mPFC, an effect that is most profound in the ACC. PV+ interneurons provide a crucial inhibitory tone on mPFC pyramidal neurons, potentially gating their communication to other brain regions involved in higher cognitive processing. A loss of inhibitory tone, through a decreased number of PV+ interneurons, could result in an overactive mPFC. Thus, these novel findings could help explain alcohol-induced deficits in mPFC-associated behaviors such as inhibitory avoidance and fear-associated learning. Overall, these data are important because they illustrate the unique vulnerability of the PV+ cell population in the ACC to a third-trimester equivalent alcohol exposure and implicate the role of the ACC in FASD-associated behavioral impairments.
Although a few studies suggest neonatal alcohol exposure can either increase or have no impact on the number of PV+ interneurons (Mitchell et al., 2000, Skorput and Yeh, 2016), the current data add to a growing literature that shows a negative influence of neonatal alcohol exposure on PV+ interneurons (Moore et al., 1998, Coleman et al., 2009, Smiley et al., 2015). PD 7 ethanol treatment has been shown to result in over a 30% reduction in the number of PV+ interneurons in the cerebral cortex when measured in adulthood (Smiley et al., 2015) and rat pups born to dams fed an ethanol-containing liquid diet throughout gestation exhibit 45% fewer PV+ interneurons in the ACC in the absence of changes in structure volume on PD 60 (Moore et al., 1998). It is well acknowledged that PV+ interneurons have a strong influence over the output of mPFC pyramidal cells (Rotaru et al., 2005, Woo and Lu, 2006). Therefore, perhaps PD 5, 7, and 9 alcohol exposure impacts the role of PV+ interneurons, via population reduction, most notably in the ACC.
Alterations to the number of PV+ interneurons can dysregulate the information that is transmitted from the mediodorsal nucleus of the thalamus to mPFC pyramidal cells (Kuroda et al., 2004, Rotaru et al., 2005) and as such can affect mPFC networks. For example, research shows that by increasing the activity of PV+ fast spiking intervals, reward-related behavioral flexibility may be enhanced (Sparta et al., 2014). Moreover, Plaur null mice (decreased GABAergic interneurons in frontal and parietal regions) demonstrate impaired medial frontal cortical function in extinction of cued fear conditioning and the inability to form attentional sets (Bissonette et al., 2015). Therefore, fewer PV+ interneurons could result in less inhibition on the pyramidal cells and lead to an overexcited mPFC. In fact, methylazoxymethanol acetate-treated rats display a decreased density of PV+ cells throughout the mPFC and this is correlated with impaired behavioral expression of latent inhibition (Lodge et al., 2009). Therefore, by reducing numbers of PV+ interneurons in the ACC, PD 5, 7, and 9 alcohol exposure may likely result in altered ACC function.
The ACC is known to be involved in many behaviors, most notably inhibitory avoidance and fear-associated learning. Specifically, the ACC appears to be recruited during aversive conditions (Albrechet-Souza et al., 2009) and plays a key role in the acquisition of fear (Rotaru et al., 2005, Bissiere et al., 2008, Kooistra et al., 2010). Therefore, damage to the ACC would undoubtedly impact fear-conditioning performance. In fact, intra-ACC injections of muscimol have been shown to decrease freezing responses (Almada et al., 2015) while lesions to the ACC prior to training impair fear acquisition, while leaving fear expression intact (Bissiere et al., 2008). Further, rabbits that received ACC lesions exhibit delayed acquisition of discriminative avoidance learning (Gabriel et al., 1991). The passive avoidance task is a variety of fear conditioning that also requires the animal to demonstrate apt inhibitory avoidance performance. Therefore this is a behavioral task that most likely requires the proper functioning of the ACC. It is possible that alcohol-induced reductions in the number of PV+ interneurons in the ACC may be associated with the impaired passive avoidance performance seen in alcohol-exposed mice.
Behavioral deficits on the passive avoidance task could have been partially mediated by the loss and therefore function of PV+ cells in the anterior cingulate network. Our results demonstrate that a PD 5, 7, and 9 alcohol exposure impaired acquisition but not retrieval of passive avoidance. Acquisition of this task requires the animal to learn to ignore its innate response to move to the dark side of the box and instead stay in the light side. Therefore, this component of the task requires behavioral flexibility and proper inhibitory control, two highly PFC-associated behavioral traits, in order to be properly executed. And in fact, we saw that alcohol-exposed animals required a significantly longer time to learn this task than did their saline counterparts (Figure 5a). Bilateral electrolytic lesion of the mediodorsal nucleus in PD4 rat pups also produced delays in acquisition during the passive avoidance test when tested in young adulthood (Ouhaz et al., 2015). PV+ interneurons mediate mediodorsal nucleus-driven feedforward inhibition in the dorsal ACC (Delevich et al., 2015). In fact, Delevich and colleagues (2015) suggested that PV+ cells limit the time window during which pyramidal neurons are capable of integrating excitatory inputs. Therefore, PD 5, 7 and 9 alcohol exposure may impact passive avoidance acquisition by altering the mediodorsal-ACC circuit.
It is not completely clear why the ACC region was selectively sensitive to the PD 5, 7 and 9 alcohol exposure. It is possible that the mediodorsal-ACC circuit, which is still developing during this time window, is preferentially affected. It is also likely that the IL and PL are indeed sensitive to alcohol exposure; but that this is just not evident with the measures we chose. Still the ACC has shown a selective sensitivity to other neonatal insults. For example, in the Degus rodent, stress exposure during the first three postnatal weeks has been shown to produce a late developmental time window between puberty and adulthood in the density of interneurons in the ACC but not the PL or IL (Helmeke et al., 2008). Thus, more research is needed in understanding the selective sensitivity of the ACC to neonatal alcohol exposure.
Interestingly, PD 5, 7 and 9 alcohol exposure did not significantly impair rotarod performance. This was a little surprising given that motor deficits are well described in the alcohol literature (Goodlett et al., 1991, Klintsova et al., 2000). Still, alcohol exposed animals did exhibit a lack of benefit from the exercise intervention. This perhaps suggests that there were still some motor deficits present. Moreover, recently Fish and colleagues (2016) failed to see any impact of an acute gestational alcohol exposure on the rotarod performance of C57BL/6J males. Therefore, it is possible that the impact of neonatal alcohol exposure on motor performance is a little less reliable in mice than it is in rats.
Exercise did not prove to be an effective intervention for treating alcohol-induced mPFC deficits. It had no impact on the number of PV+ interneurons and it did not rescue behavioral deficits on Day 1 (acquisition) of the passive avoidance task. Previously, we have shown that exercise can both increase levels of adult hippocampal neurogenesis and enhance behavioral performance on Day 2 (retrieval). However, neither of these measures are impacted by PD 5, 7, and 9 alcohol exposure (Hamilton et al., 2016). Similarly, our current results show that although motor performance was not impacted by a PD 5, 7, and 9 alcohol exposure, alcohol-exposed mice did exhibit a limited benefit of the exercise intervention. This may be due to the fact that alcohol-exposed animals ran significantly less than their saline counterparts. Therefore, these results simply demonstrate the impact of exercise rather than judge its merit as an intervention. Exercise alone has been shown to increase the number of PV+ interneurons in the hippocampus. However, the hippocampus is a neurogenic region and it is likely a lot more susceptible to the benefits of exercise as there is a large literature that shows exercise increases levels of hippocampal plasticity (for review see Trivino-Paredes et al., 2016). Another commonly utilized intervention that sometimes includes aerobic exercise is environmental enrichment. Access to an enriched environment has been shown to increase the number of PV+ interneurons in the basolateral amygdala (Urakawa et al., 2013) and also rescue impaired freezing responses as well as developmental delays in PV+ interneurons in the PFC and hippocampus of animals exposed to a PD 6–8 NMDA antagonist (sevofluorance) exposure (Ji et al., 2016). It is possible that the running alone may not be sufficient to consistently rescue alcohol related deficits in the mPFC and that instead more complex interventions, including environmental enrichment components may be necessary.
Finally, there are a few important limitations that must be acknowledged within this study. The first is the use of single housing. This housing option was required in order to record individual running levels. We acknowledge that single housing has been found to induce stress responses in both rats (Chappell et al., 2013) and mice (Berry et al., 2012). Single housing has been linked to not only increased locomotor activity in several tests of exploration but also reduced anxiety-like behaviors in exploration (Haupt and Schaefers, 2010). However, a growing literature suggests that single housing may not be as stressful for mice as it is for rats. For example, a recent study by Lopez and Laber (2015) found that as long as mice were provided with nesting material during adolescence, they showed no difference in voluntary ethanol intake when compared to group-housed animals. In addition, studies have found no significant differences in stress marks of mice raised in single housing versus mice raised in group housing (Hunt and Hambly, 2006, Arndt et al., 2009). Rather, one study showed that group housing significantly increased corticosterone levels in male mice when compared to those housed singly (Kamakura et al., 2016). Regardless of the impact single housing may have had, all the groups were singly housed in our study, hence single housing was a controlled variable. Second, it is important to note that the authors chose to focus solely on PV+ interneurons. This was done because we were primarily interested in the crucial inhibitory role PV+ interneurons have on mPFC pyramidal neurons. However, it is very likely that neonatal alcohol exposure impacts other classes of interneurons. In fact, it was recently demonstrated that a PD7 alcohol exposure leads to long-term reductions in not only the number of PV+ (34%) but also the number of calretinin-positive (32%) neurons in the cerebral cortex when measured in adulthood (Smiley et al., 2015). Further, gestational alcohol exposure has been shown to cause alterations in the immunoreaction of calbindin-D28k, calretinin and PV in neurons of the cerebellar cortex of ten-day-old rat pups (Wierzba-Bobrowicz et al., 2011). Therefore, future research should explore this question of how PD 5, 7, and 9 alcohol exposure impacts other interneuron classes. This would provide a better understanding of how neonatal alcohol exposure impacts inhibitory connectivity in the mPFC. Lastly, we chose to focus on the impact of neonatal alcohol exposure on PV+ populations in specific mPFC subregions rather than the layer-specific distribution of PV+ cells. This was because we hypothesized there would be region-specific decreases in the number of PV+ interneurons and that they would coincide with functional deficits on the passive avoidance task. However, we must still acknowledge that interneurons generated from the caudal ganglionic eminence are still migrating to their layer II/III adult location during the first postnatal week (Miyoshi and Fishell, 2011) and that the PD5, 7 and 9 alcohol exposure likely impacted this migration pattern. On the other hand, it has been suggested that PV+ interneurons originate primarily within the medial ganglionic eminence (Xu et al., 2004) and therefore have finished migrating (Miyoshi and Fishell, 2011). Future research should examine the impact of a PD 5, 7, and 9 alcohol exposure on the layer-specific distribution of ont only PV+ interneurons but other interneuron classes as well. In doing so, it would provide us with a better understanding of how alcohol exposure impacts the migrational pattern of developing interneurons.
CONCLUSION
In summary, this study found for the first time that a PD 5, 7, and 9 alcohol exposure significantly reduces the number of PV+ interneurons in a specific subregion of the mPFC, the ACC. PV+ interneurons crucially regulate the activation of the mPFC, and the ACC plays an important role in behavioral inhibition and fear-associated learning. The current data suggest that impaired circuitry in the ACC may be associated with impaired performance on the Passive avoidance task, specifically during the acquisition stage. This possible association between brain region and functional deficit in the alcohol-exposed brain could be useful for developing more targeted interventions in order to mitigate alcohol-induced mPFC damage.
Research highlights.
PD5, 7 and 9 alcohol exposure impairs passive avoidance acquisition but not retrieval.
Impact of PD5, 7 and 9 alcohol exposure on the mPFC is most profound in the ACC.
PD5, 7 and 9 alcohol exposure has long-term impact on ACC volume but not overall mPFC volume.
PD5, 7 and 9 alcohol exposure significantly reduces the number of mPFC PV+ cells, most notably in the ACC.
Acknowledgments
The authors would like to extend their sincere gratitude to the Beckman Institute Animal Facility staff for excellent animal care. This work was supported by the National Institutes of Health (grant numbers: R01 MH083807 (JSR) and RO1 DA027487 (JSR) and the National Institute on Alcohol Abuse and Alcoholism (grant number: F32AA023444 (GFH). Additionally, support was provided by the Arnold O. and Mabel M. Beckman Foundation through the Beckman Institute Postdoctoral Fellowship Program at the University of Illinois at Urbana-Champaign (GFH).
GLOSSARY (as a separate list. the definitions of field-specific terms used in your article)
- ACC
anterior cingulate cortex
- FASD
fetal alcohol spectrum disorders
- IL
infralimbic cortex
- mPFC
medial prefrontal cortex
- PBS
phosphate buffering solution
- PD
postnatal day
- PL
prelimbic cortex
- PV+
parvalbumin-positive
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aksu I, Baykara B, Ozbal S, Cetin F, Sisman AR, Dayi A, Gencoglu C, Tas A, Buyuk E, Gonenc-Arda S, Uysal N. Maternal treadmill exercise during pregnancy decreases anxiety and increases prefrontal cortex VEGF and BDNF levels of rat pups in early and late periods of life. Neuroscience letters. 2012;516:221–225. doi: 10.1016/j.neulet.2012.03.091. [DOI] [PubMed] [Google Scholar]
- Albrechet-Souza L, Borelli KG, Carvalho MC, Brandao ML. The anterior cingulate cortex is a target structure for the anxiolytic-like effects of benzodiazepines assessed by repeated exposure to the elevated plus maze and Fos immunoreactivity. Neuroscience. 2009;164:387–397. doi: 10.1016/j.neuroscience.2009.08.038. [DOI] [PubMed] [Google Scholar]
- Almada RC, Coimbra NC, Brandao ML. Medial Prefrontal Cortex Serotonergic and Gabaergic Mechanisms Modulate the Expression of Contextual Fear: Intratelencephalic Pathways and Differential Involvement of Cortical Subregions. Neuroscience. 2015;284:988–997. doi: 10.1016/j.neuroscience.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Arndt SS, Laarakker MC, van Lith HA, van der Staay FJ, Gieling E, Salomons AR, van’t Klooster J, Ohl F. Individual housing of mice–impact on behaviour and stress responses. Physiology & behavior. 2009;97:385–393. doi: 10.1016/j.physbeh.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Berry A, Bellisario V, Capoccia S, Tirassa P, Calza A, Alleva E, Cirulli F. Social deprivation stress is a triggering factor for the emergence of anxiety- and depression-like behaviours and leads to reduced brain BDNF levels in C57BL/6J mice. Psychoneuroendocrinology. 2012;37:762–772. doi: 10.1016/j.psyneuen.2011.09.007. [DOI] [PubMed] [Google Scholar]
- Bissiere S, Plachta N, Hoyer D, McAllister KH, Olpe HR, Grace AA, Cryan JF. The rostral anterior cingulate cortex modulates the efficiency of amygdala-dependent fear learning. Biological psychiatry. 2008;63:821–831. doi: 10.1016/j.biopsych.2007.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissonette GB, Schoenbaum G, Roesch MR, Powell EM. Interneurons are necessary for coordinated activity during reversal learning in orbitofrontal cortex. Biological psychiatry. 2015;77:454–464. doi: 10.1016/j.biopsych.2014.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun F, Attilia ML, Spagnolo PA, Rotondo C, Mancinelli R, Ceccanti M. National Institute on Alcohol Abuse and Alcoholism and the study of fetal alcohol spectrum disorders. The International Consortium. Annali dell’Istituto superiore di sanita. 2006;42:4–7. [PubMed] [Google Scholar]
- Chappell AM, Carter E, McCool BA, Weiner JL. Adolescent rearing conditions influence the relationship between initial anxiety-like behavior and ethanol drinking in male Long Evans rats. Alcoholism, clinical and experimental research. 2013;37(Suppl 1):E394–403. doi: 10.1111/j.1530-0277.2012.01926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman LG, Jr, Jarskog LF, Moy SS, Crews FT. Deficits in adult prefrontal cortex neurons and behavior following early post-natal NMDA antagonist treatment. Pharmacology, biochemistry, and behavior. 2009;93:322–330. doi: 10.1016/j.pbb.2009.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor PD, Sampson PD, Bookstein FL, Barr HM, Streissguth AP. Direct and indirect effects of prenatal alcohol damage on executive function. Developmental neuropsychology. 2000;18:331–354. doi: 10.1207/S1532694204Connor. [DOI] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends in neurosciences. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- De Giorgio A, Comparini SE, Intra FS, Granato A. Long-term alterations of striatal parvalbumin interneurons in a rat model of early exposure to alcohol. Journal of neurodevelopmental disorders. 2012;4:18. doi: 10.1186/1866-1955-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delevich K, Tucciarone J, Huang ZJ, Li B. The mediodorsal thalamus drives feedforward inhibition in the anterior cingulate cortex via parvalbumin interneurons. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2015;35:5743–5753. doi: 10.1523/JNEUROSCI.4565-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbing J, Sands J. Comparative aspects of the brain growth spurt. Early human development. 1979;3:79–83. doi: 10.1016/0378-3782(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Ekstrand J, Hellsten J, Tingstrom A. Environmental enrichment, exercise and corticosterone affect endothelial cell proliferation in adult rat hippocampus and prefrontal cortex. Neuroscience letters. 2008;442:203–207. doi: 10.1016/j.neulet.2008.06.085. [DOI] [PubMed] [Google Scholar]
- Etkin A, Egner T, Kalisch R. Emotional processing in anterior cingulate and medial prefrontal cortex. Trends in cognitive sciences. 2011;15:85–93. doi: 10.1016/j.tics.2010.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakoya FA, Caxton-Martins EA. Neocortical neurodegeneration in young adult Wistar rats prenatally exposed to ethanol. Neurotoxicology and teratology. 2006;28:229–237. doi: 10.1016/j.ntt.2005.11.001. [DOI] [PubMed] [Google Scholar]
- Ferrer I, Galofre E. Dendritic spine anomalies in fetal alcohol syndrome. Neuropediatrics. 1987;18:161–163. doi: 10.1055/s-2008-1052472. [DOI] [PubMed] [Google Scholar]
- Fish EW, Holloway HT, Rumple A, Baker LK, Wieczorek LA, Moy SS, Paniagua B, Parnell SE. Acute alcohol exposure during neurulation: Behavioral and brain structural consequences in adolescent C57BL/6J mice. Behavioural brain research. 2016;311:70–80. doi: 10.1016/j.bbr.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Floresco SB, Grace AA. Gating of hippocampal-evoked activity in prefrontal cortical neurons by inputs from the mediodorsal thalamus and ventral tegmental area. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2003;23:3930–3943. doi: 10.1523/JNEUROSCI.23-09-03930.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel M, Kubota Y, Sparenborg S, Straube K, Vogt BA. Effects of cingulate cortical lesions on avoidance learning and training-induced unit activity in rabbits. Experimental brain research. 1991;86:585–600. doi: 10.1007/BF00230532. [DOI] [PubMed] [Google Scholar]
- Giustino TF, Maren S. The Role of the Medial Prefrontal Cortex in the Conditioning and Extinction of Fear. Frontiers in behavioral neuroscience. 2015;9:298. doi: 10.3389/fnbeh.2015.00298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlett CR, Thomas JD, West JR. Long-term deficits in cerebellar growth and rotarod performance of rats following “binge-like” alcohol exposure during the neonatal brain growth spurt. Neurotoxicology and teratology. 1991;13:69–74. doi: 10.1016/0892-0362(91)90029-v. [DOI] [PubMed] [Google Scholar]
- Granato A, Van Pelt J. Effects of early ethanol exposure on dendrite growth of cortical pyramidal neurons: inferences from a computational model. Brain research Developmental brain research. 2003;142:223–227. doi: 10.1016/s0165-3806(03)00094-4. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology–reconsidered. Journal of microscopy. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Hamilton GF, Bucko PJ, Miller DS, DeAngelis RS, Krebs CP, Rhodes JS. Behavioral deficits induced by third-trimester equivalent alcohol exposure in male C57BL/6J mice are not associated with reduced adult hippocampal neurogenesis but are still rescued with voluntary exercise. Behavioural brain research. 2016 doi: 10.1016/j.bbr.2016.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Criss KJ, Klintsova AY. Voluntary exercise partially reverses neonatal alcohol-induced deficits in mPFC layer II/III dendritic morphology of male adolescent rats. Synapse. 2015;69:405–415. doi: 10.1002/syn.21827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton GF, Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure decreases dendritic complexity while increasing the density of mature spines in mPFC Layer II/III pyramidal neurons. Synapse. 2010;64:127–135. doi: 10.1002/syn.20711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamner MB, Lorberbaum JP, George MS. Potential role of the anterior cingulate cortex in PTSD: review and hypothesis. Depression and anxiety. 1999;9:1–14. [PubMed] [Google Scholar]
- Haupt M, Schaefers AT. Effects of postweaning social and physical deprivation on locomotor activity patterns and explorative behavior in female CD-1 mice. Developmental psychobiology. 2010;52:383–393. doi: 10.1002/dev.20439. [DOI] [PubMed] [Google Scholar]
- Helmeke C, Ovtscharoff W, Jr, Poeggel G, Braun K. Imbalance of immunohistochemically characterized interneuron populations in the adolescent and adult rodent medial prefrontal cortex after repeated exposure to neonatal separation stress. Neuroscience. 2008;152:18–28. doi: 10.1016/j.neuroscience.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, Bucci DJ. Physical exercise during adolescence versus adulthood: differential effects on object recognition memory and brain-derived neurotrophic factor levels. Neuroscience. 2011;194:84–94. doi: 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt C, Hambly C. Faecal corticosterone concentrations indicate that separately housed male mice are not more stressed than group housed males. Physiology & behavior. 2006;87:519–526. doi: 10.1016/j.physbeh.2005.11.013. [DOI] [PubMed] [Google Scholar]
- Ieraci A, Herrera DG. Single alcohol exposure in early life damages hippocampal stem/progenitor cells and reduces adult neurogenesis. Neurobiology of disease. 2007;26:597–605. doi: 10.1016/j.nbd.2007.02.011. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Bittigau P, Ishimaru MJ, Wozniak DF, Koch C, Genz K, Price MT, Stefovska V, Horster F, Tenkova T, Dikranian K, Olney JW. Ethanol-induced apoptotic neurodegeneration and fetal alcohol syndrome. Science. 2000;287:1056–1060. doi: 10.1126/science.287.5455.1056. [DOI] [PubMed] [Google Scholar]
- Ji MH, Wang ZY, Sun XR, Tang H, Zhang H, Jia M, Qiu LL, Zhang GF, Peng YG, Yang JJ. Repeated Neonatal Sevoflurane Exposure-Induced Developmental Delays of Parvalbumin Interneurons and Cognitive Impairments Are Reversed by Environmental Enrichment. Molecular neurobiology. 2016 doi: 10.1007/s12035-016-9943-x. [DOI] [PubMed] [Google Scholar]
- Kamakura R, Kovalainen M, Leppaluoto J, Herzig KH, Makela KA. The effects of group and single housing and automated animal monitoring on urinary corticosterone levels in male C57BL/6 mice. Physiological reports. 2016;4 doi: 10.14814/phy2.12703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klintsova AY, Goodlett CR, Greenough WT. Therapeutic motor training ameliorates cerebellar effects of postnatal binge alcohol. Neurotoxicology and teratology. 2000;22:125–132. doi: 10.1016/s0892-0362(99)00052-5. [DOI] [PubMed] [Google Scholar]
- Kooistra L, Crawford S, Gibbard B, Ramage B, Kaplan BJ. Differentiating attention deficits in children with fetal alcohol spectrum disorder or attention-deficit-hyperactivity disorder. Developmental medicine and child neurology. 2010;52:205–211. doi: 10.1111/j.1469-8749.2009.03352.x. [DOI] [PubMed] [Google Scholar]
- Koteja P, Garland T, Jr, Sax JK, Swallow JG, Carter PA. Behaviour of house mice artificially selected for high levels of voluntary wheel running. Animal behaviour. 1999;58:1307–1318. doi: 10.1006/anbe.1999.1270. [DOI] [PubMed] [Google Scholar]
- Kuroda M, Yokofujita J, Oda S, Price JL. Synaptic relationships between axon terminals from the mediodorsal thalamic nucleus and gamma-aminobutyric acidergic cortical cells in the prelimbic cortex of the rat. The Journal of comparative neurology. 2004;477:220–234. doi: 10.1002/cne.20249. [DOI] [PubMed] [Google Scholar]
- Lantz CL, Wang W, Medina AE. Early alcohol exposure disrupts visual cortex plasticity in mice. International journal of developmental neuroscience: the official journal of the International Society for Developmental Neuroscience. 2012;30:351–357. doi: 10.1016/j.ijdevneu.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lodge DJ, Behrens MM, Grace AA. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2009;29:2344–2354. doi: 10.1523/JNEUROSCI.5419-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez MF, Laber K. Impact of social isolation and enriched environment during adolescence on voluntary ethanol intake and anxiety in C57BL/6J mice. Physiology & behavior. 2015;148:151–156. doi: 10.1016/j.physbeh.2014.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandyam CD, Wee S, Eisch AJ, Richardson HN, Koob GF. Methamphetamine self-administration and voluntary exercise have opposing effects on medial prefrontal cortex gliogenesis. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2007;27:11442–11450. doi: 10.1523/JNEUROSCI.2505-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Morris JR, Juraska JM. Neuron number decreases in the rat ventral, but not dorsal, medial prefrontal cortex between adolescence and adulthood. Neuroscience. 2007;144:961–968. doi: 10.1016/j.neuroscience.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Marty S, Berzaghi Mda P, Berninger B. Neurotrophins and activity-dependent plasticity of cortical interneurons. Trends in neurosciences. 1997;20:198–202. doi: 10.1016/s0166-2236(96)01026-0. [DOI] [PubMed] [Google Scholar]
- Mattson SN, Crocker N, Nguyen TT. Fetal alcohol spectrum disorders: neuropsychological and behavioral features. Neuropsychology review. 2011;21:81–101. doi: 10.1007/s11065-011-9167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson SN, Schoenfeld AM, Riley EP. Teratogenic effects of alcohol on brain and behavior. Alcohol research & health : the journal of the National Institute on Alcohol Abuse and Alcoholism. 2001;25:185–191. [PMC free article] [PubMed] [Google Scholar]
- May PA, Gossage JP, Kalberg WO, Robinson LK, Buckley D, Manning M, Hoyme HE. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Developmental disabilities research reviews. 2009;15:176–192. doi: 10.1002/ddrr.68. [DOI] [PubMed] [Google Scholar]
- Mitchell JJ, Paiva M, Heaton MB. Effect of neonatal ethanol exposure on parvalbumin-expressing GABAergic neurons of the rat medial septum and cingulate cortex. Alcohol. 2000;21:49–57. doi: 10.1016/s0741-8329(99)00101-9. [DOI] [PubMed] [Google Scholar]
- Miyoshi G, Fishell G. GABAergic interneuron lineages selectively sort into specific cortical layers during early postnatal development. Cereb Cortex. 2011;21:845–852. doi: 10.1093/cercor/bhq155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DB, Quintero MA, Ruygrok AC, Walker DW, Heaton MB. Prenatal ethanol exposure reduces parvalbumin-immunoreactive GABAergic neuronal number in the adult rat cingulate cortex. Neuroscience letters. 1998;249:25–28. doi: 10.1016/s0304-3940(98)00378-4. [DOI] [PubMed] [Google Scholar]
- Mrzljak L, Uylings HB, Van Eden CG, Judas M. Neuronal development in human prefrontal cortex in prenatal and postnatal stages. Progress in brain research. 1990;85:185–222. doi: 10.1016/s0079-6123(08)62681-3. [DOI] [PubMed] [Google Scholar]
- Ouhaz Z, Ba-M’hamed S, Mitchell AS, Elidrissi A, Bennis M. Behavioral and cognitive changes after early postnatal lesions of the rat mediodorsal thalamus. Behavioural brain research. 2015;292:219–232. doi: 10.1016/j.bbr.2015.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen C. Executive functioning and working memory in fetal alcohol spectrum disorder. Alcoholism, clinical and experimental research. 2005;29:1359–1367. doi: 10.1097/01.alc.0000175040.91007.d0. [DOI] [PubMed] [Google Scholar]
- Rotaru DC, Barrionuevo G, Sesack SR. Mediodorsal thalamic afferents to layer III of the rat prefrontal cortex: synaptic relationships to subclasses of interneurons. The Journal of comparative neurology. 2005;490:220–238. doi: 10.1002/cne.20661. [DOI] [PubMed] [Google Scholar]
- Shibata H. Efferent projections from the anterior thalamic nuclei to the cingulate cortex in the rat. The Journal of comparative neurology. 1993;330:533–542. doi: 10.1002/cne.903300409. [DOI] [PubMed] [Google Scholar]
- Skorput AG, Yeh HH. Chronic Gestational Exposure to Ethanol Leads to Enduring Aberrances in Cortical Form and Function in the Medial Prefrontal Cortex. Alcoholism, clinical and experimental research. 2016;40:1479–1488. doi: 10.1111/acer.13107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slomianka L, West MJ. Estimators of the precision of stereological estimates: an example based on the CA1 pyramidal cell layer of rats. Neuroscience. 2005;136:757–767. doi: 10.1016/j.neuroscience.2005.06.086. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Saito M, Bleiwas C, Masiello K, Ardekani B, Guilfoyle DN, Gerum S, Wilson DA, Vadasz C. Selective reduction of cerebral cortex GABA neurons in a late gestation model of fetal alcohol spectrum disorder. Alcohol. 2015;49:571–580. doi: 10.1016/j.alcohol.2015.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Mattson SN, Tessner KD, Jernigan TL, Riley EP, Toga AW. Regional brain shape abnormalities persist into adolescence after heavy prenatal alcohol exposure. Cereb Cortex. 2002;12:856–865. doi: 10.1093/cercor/12.8.856. [DOI] [PubMed] [Google Scholar]
- Sparta DR, Hovelso N, Mason AO, Kantak PA, Ung RL, Decot HK, Stuber GD. Activation of prefrontal cortical parvalbumin interneurons facilitates extinction of reward-seeking behavior. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2014;34:3699–3705. doi: 10.1523/JNEUROSCI.0235-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivino-Paredes J, Patten AR, Gil-Mohapel J, Christie BR. The effects of hormones and physical exercise on hippocampal structural plasticity. Frontiers in neuroendocrinology. 2016;41:23–43. doi: 10.1016/j.yfrne.2016.03.001. [DOI] [PubMed] [Google Scholar]
- Urakawa S, Takamoto K, Hori E, Sakai N, Ono T, Nishijo H. Rearing in enriched environment increases parvalbumin-positive small neurons in the amygdala and decreases anxiety-like behavior of male rats. BMC neuroscience. 2013;14:13. doi: 10.1186/1471-2202-14-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uysal N, Sisman AR, Dayi A, Aksu I, Cetin F, Gencoglu C, Tas A, Buyuk E. Maternal exercise decreases maternal deprivation induced anxiety of pups and correlates to increased prefrontal cortex BDNF and VEGF. Neuroscience letters. 2011;505:273–278. doi: 10.1016/j.neulet.2011.10.039. [DOI] [PubMed] [Google Scholar]
- Van Eden CG, Uylings HB. Cytoarchitectonic development of the prefrontal cortex in the rat. The Journal of comparative neurology. 1985;241:253–267. doi: 10.1002/cne.902410302. [DOI] [PubMed] [Google Scholar]
- Wass TS, Persutte WH, Hobbins JC. The impact of prenatal alcohol exposure on frontal cortex development in utero. American journal of obstetrics and gynecology. 2001;185:737–742. doi: 10.1067/mob.2001.117656. [DOI] [PubMed] [Google Scholar]
- Whitcher LT, Klintsova AY. Postnatal binge-like alcohol exposure reduces spine density without affecting dendritic morphology in rat mPFC. Synapse. 2008;62:566–573. doi: 10.1002/syn.20532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wierzba-Bobrowicz T, Lewandowska E, Stepien T, Szpak GM. Differential expression of calbindin D28k, calretinin and parvalbumin in the cerebellum of pups of ethanol-treated female rats. Folia neuropathologica. 2011;49:47–55. [PubMed] [Google Scholar]
- Willing J, Juraska JM. The timing of neuronal loss across adolescence in the medial prefrontal cortex of male and female rats. Neuroscience. 2015;301:268–275. doi: 10.1016/j.neuroscience.2015.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wischhof L, Irrsack E, Osorio C, Koch M. Prenatal LPS-exposure–a neurodevelopmental rat model of schizophrenia–differentially affects cognitive functions, myelination and parvalbumin expression in male and female offspring. Progress in neuropsychopharmacology & biological psychiatry. 2015;57:17–30. doi: 10.1016/j.pnpbp.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Woo NH, Lu B. Regulation of cortical interneurons by neurotrophins: from development to cognitive disorders. The Neuroscientist: a review journal bringing neurobiology, neurology and psychiatry. 2006;12:43–56. doi: 10.1177/1073858405284360. [DOI] [PubMed] [Google Scholar]
- Wozniak DF, Hartman RE, Boyle MP, Vogt SK, Brooks AR, Tenkova T, Young C, Olney JW, Muglia LJ. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiology of disease. 2004;17:403–414. doi: 10.1016/j.nbd.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu TS, Zhang G, Liebl DJ, Kernie SG. Traumatic brain injury-induced hippocampal neurogenesis requires activation of early nestin-expressing progenitors. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2008;28:12901–12912. doi: 10.1523/JNEUROSCI.4629-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]


