Abstract
Liver receptor homolog 1 (LRH-1) is an orphan nuclear receptor that synergizes with β-catenin/T cell factor 4 signaling to stimulate intestinal crypt cell renewal. We evaluated here the impact of haploinsufficiency of LRH-1 on intestinal tumorigenesis by using two independent mouse models of human colon tumorigenesis. Haploinsufficiency of LRH-1 blunts intestinal tumorigenesis in the ApcMin/+ mice, a genetic model of intestinal cancer. Likewise, Lrh-1+/– mice are protected against the formation of aberrant crypt foci in the colon of mice exposed to the carcinogen azoxymethane. LRH-1 gene expression is reduced in tumors that express elevated levels of the proinflammatory cytokine TNF-α. Reciprocally, decreased LRH-1 expression in Lrh-1+/– mice attenuates TNF-α expression. Compared with normal human colon, expression and subcellular localization of LRH-1 is significantly altered in neoplastic colon. In combination, these data suggest a role of LRH-1 in the initiation of intestinal tumorigenesis both by affecting cell cycle control as well as through its impact on inflammatory pathways.
Keywords: β-catenin, colon cancer, nuclear receptors
Liver receptor homolog 1 (LRH-1; also known as NR5A2) is an orphan nuclear receptor that belongs to the NR5A or the Ftz-F1 subfamily of nuclear receptors. Members of this subfamily all bind DNA as monomers, and the specificity of DNA recognition is to a large extent dictated by the Ftz-F1 box, a unique domain at the C terminus of the DNA-binding domain of NR5A members (1). Although no ligands for these receptors are yet described, all of the NR5A members display constitutive transcriptional activity. This constitutive activity has been attributed to the unique folding of the ligand-binding domain observed by crystal structure analysis (2). Like other members of the subfamily, LRH-1 is a critical factor in early development, as evidenced by the early lethality of LRH-1-null embryos (3, 4). High levels of LRH-1 are found in mouse pluripotent embryonic stem cells (5). During early mouse development, LRH-1 expression is detected in the mouse yolk sac endoderm, branchial arch, and neural crest (6). Later on during organogenesis, expression becomes confined to the developing intestine, liver, and pancreas. During this stage, LRH-1 is proposed to control and simultaneously to be under the control of a number of developmental transcription factors crucial in the specification of the enterohepatic phenotype (6–8). In adult mammals, LRH-1 expression is predominantly expressed in the liver (5), the exocrine pancreas (9) and the intestinal crypts (4), but high levels are also found in the ovary (10). In the enterohepatic tissues, LRH-1 participates in the control of complex regulatory pathways that govern cholesterol and bile acid homeostasis (for review, see ref. 11). In the ovary, its main function involves the regulation of cholesterol delivery and steroid production (12–14).
We recently provided evidence that LRH-1 facilitates cell proliferation in synergy with the β-catenin/T cell factor 4 signaling pathway (4). Whereas β-catenin coactivates LRH-1 on the cyclin E1 promoter, LRH-1 acts as a potent tissue-restricted coactivator of β-catenin on the cyclin D1 and c-Myc promoter. This unanticipated cross-talk between LRH-1 and the β-catenin/T cell factor 4 signaling pathway was shown to be of particular relevance for the renewal of intestinal crypt cells. The β-catenin protein is one of the key determinants in the pathogenesis of colon cancer (15). Levels of free β-catenin are usually tightly controlled in the cell through the interaction of a multiprotein complex that comprises conductin/axin, the adenomatous polyposis coli (APC), and the serine/threonine kinase GSK-3β, which phosphorylates β-catenin, thus inducing its degradation. Activation of Wnt signaling prevents this proteosomal destruction of β-catenin, which then associates with and coactivates the transcription factor T cell factor 4, ultimately leading to the induction of target genes stimulating cell-cycle progression. β-Catenin levels accumulate after mutations of the Apc gene, and heterozygous germline mutations of the human Apc gene predispose carriers to familial adenomatous polyposis, a syndrome characterized by the occurrence of large numbers of colon polyps at young age (16–19). In such familial adenomatous polyposis patients, the second APC allele is often inactivated subsequently in intestinal epithelial cells, leading to cell proliferation and ultimately colon polyps. Also, in up to 80% of the subjects with sporadic colon cancers, both Apc alleles are inactivated (15, 18), emphasizing the critical role of the APC complex in the maintenance of a normal balance between proliferation and differentiation in the intestinal crypts. The molecular link between β-catenin and LRH-1 signaling incited us to evaluate an eventual contribution of LRH-1 to intestinal tumorigenesis.
Experimental Procedures
Animal Experiments. Heterozygous Lrh-1 mice (4) were backcrossed for nine generations with C57BL/6J mice. Six-week-old male C57BL/6J-ApcMin/+ inbred mice were obtained from The Jackson Laboratory and were crossed with female LRH-1+/– mice to generate 10 pairs of male and 10 pairs of female Lrh-1+/+/ApcMin/+ F1 control mice and double heterozygous Lrh-1+/–/ApcMin/+ F1 littermates. Mice were group-housed and accustomed to a 12-h light/12-h dark cycle with free access to standard mouse chow (D03, SAFE, Villemoisson sur Orge, France) and tap water. At 18 weeks of age, mice were killed by CO2 asphyxiation. The gastrointestinal tract was removed, five sections from duodenum to the rectum were opened longitudinally, washed with PBS, spread out on filter paper, and fixed in 10% buffered formalin. Specimens were examined under a dissecting microscope, and location, volume, and number of tumors were determined. Tumor diameter (in mm) was measured by using a microscopic eyepiece grid. For the short-term chemically induced neoplasia model, 6-week-old male and female Lrh-1+/– mice and wild-type littermates were injected i.p. twice within a 1-week interval with 7.5 mg/kg azoxymethane (AOM; Sigma). Aberrant crypt foci in colon of mice were determined by methylene blue staining (0.2%) 5 weeks after the first AOM injection. Standardized segments of colon were taken for histological analysis. To obtain chemically induced colon tumors, mice were exposed to six i.p. injections of 10 mg/kg AOM during a period of 6 weeks. Mice were killed 23 weeks after the first AOM injection, and tumors were counted and harvested. For BrdUrd incorporation studies, mice were injected i.p. with 50 mg/kg BrdUrd 2 h before being killed. Inflammation was induced in 8-week-old C57BL/6 males, which were injected i.p. with 100 μg of LPS (L-4005, Sigma). Colons were harvested 6 and 24 h after LPS administration and immediately frozen for RNA analysis.
RNA Analysis. Total RNA from ileum and colon was purified by using the RNeasy Mini kit (Qiagen, Valencia, CA). Real-time quantitative PCR with gene-specific primers for LRH-1 and reference gene was performed as described in ref. 4. The relative expression level of TNF-α was determined with primer set 5′-GGG ACA GTG ACC TGG ACT GT-3′ and 5′-AGG CTG TGC ATT GCA CCT CA-3′.
Histological Analysis, Antibodies, and Statistics. Sections (5 μm) from paraffin-embedded mouse ileum and/or colon were stained with hematoxylin/eosin. For BrdUrd immunostaining, antigen-retrieved sections were incubated overnight with an anti-BrdUrd mouse monoclonal primary antibody (Roche Molecular Biochemicals), followed by incubation with a secondary Cy3-conjugated anti-mouse IgG antibody. Signals were revealed with a fluorescence microscope (Leica Microsystems, Rueil Malmaison, France). Human colon biopsies were collected during elective surgery after informed consent from patients and approval from the ethical review board at Katholieke Universiteit, Leuven. Immunohistochemistry was performed on 5-μm-thick sections from paraffin-embedded human colon. Incubation with the primary antibody (anti-hLRH-1, Perseus Proteomics, Tokyo, Japan) was performed at room temperature for 30 min, followed by treatment with peroxidase-conjugated anti-mouse EnVision+ reagent (DakoCytomation, Glostrup, Denmark). Except stated otherwise, results are expressed as mean ± SEM. Differences were considered statistically significant at P < 0.05.
Results
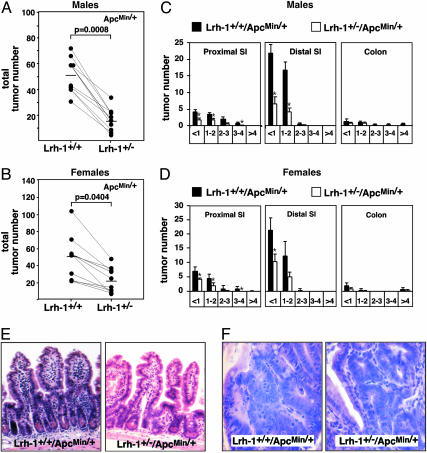
ApcMin/+ mice, heterozygous for a nonsense mutation in codon 850 of the Apc gene, develop multiple tumors throughout the intestinal tract and are a commonly used model for both familial adenomatous polyposis and sporadic colon cancer (20). ApcMin/Min mice, homozygous for the Min mutation, are not viable and heterozygous ApcMin/+ mice have a considerably reduced life span (21). To study the effect of LRH-1 on intestinal tumor development we crossed Lrh-1+/– mice with ApcMin/+ mice. Lrh-1+/+/ApcMin/+ single heterozygous mice developed a mean ± SEM of 52 ± 16 and 51 ± 11 intestinal tumors in male and female littermates respectively (Fig. 1 A and B). Strikingly, only 16 ± 3 and 23 ± 5 tumors were present in the intestine of male and female Lrh-1+/–/ApcMin/+ double heterozygous mice (Fig. 1 A and B), which resulted in a mean 3- and 2-fold decrease in tumor multiplicity in the intestine of male and female Lrh-1+/–/ApcMin/+ mice, respectively. Consistent with previous observations, the highest frequency of tumors was found in the distal part of the small intestine, whereas only very low numbers were observed in the colon (Fig. 1 C and D). In the small intestine, Lrh-1 haploinsufficiency did not cause a shift in tumor diameter and did not affect tumor distribution. Consistent with the decrease in tumor multiplicity, a proportional reduction could be observed in the number of small (<1 mm), intermediate (1–3 mm) and large (>3 mm) tumors in the proximal and distal part of the small intestine (Fig. 1 C and D). Multiplicity of small tumors in the colon of double heterozygous mutants did not differ at all from that of ApcMin/+ littermates. The absence of a clear effect of LRH-1 on colon tumors is likely related to the very low multiplicity of tumors in the colon of ApcMin/+ mice. Histological analysis of the nonneoplastic intestinal mucosa of both genotypes did not reveal any apparent differences (Fig. 1E). Also tumor morphology was similar in the Lrh-1+/+/ApcMin/+ and Lrh-1+/–/ApcMin/+ mice (Fig. 1F). In both cases, the tumors were mainly corresponding to well differentiated tubular adenomas.
Fig. 1.
Haploinsufficiency of LRH-1 reduces tumor multiplicity in ApcMin/+ mice. (A and B) Total tumor number in the gastrointestinal tract of 18-week-old male (A) and female (B) single C57BL/6J-Lrh-1+/+/ApcMin/+ and double C57BL/6J-Lrh-1+/–/ApcMin/+ heterozygous mutant littermates (n = 10 per genotype and per gender). Processing and analysis of the gastrointestinal tract is described in Experimental Procedures. Animals were studied on a pure C57BL/6J background and on a standard chow diet. Single and double heterozygous mutant littermates are represented by lines. The mean tumor number is indicated by a bar. Mann–Whitney U test was used to compare data from different genotypes. Differences were considered statistically significant at P < 0.05. (C and D) Tumor distribution by number and diameter in proximal small intestine (SI), distal small intestine, and colon of male (C) and female (D) single C57BL/6J-Lrh-1+/+/ApcMin/+ and double heterozygous C57BL/6J-Lrh-1+/–/ApcMin/+ mutant mice. x axis represents tumor diameter (in mm). *, Statistically significant at P < 0.05 (ANOVA). (E and F) Representative hematoxylin/eosin staining of normal (E) and adenomatous (F) ileum of single heterozygous C57BL/6J-Lrh-1+/+/ApcMin/+ and double heterozygous C57BL/6J-Lrh-1+/–/ApcMin/+ mice.
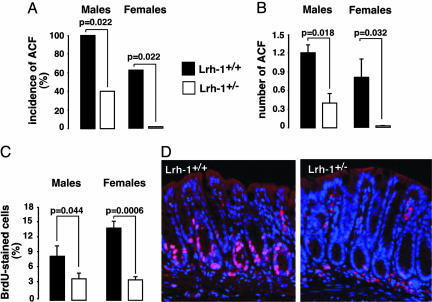
Despite the fact that ApcMin/+ mice represent a genetic mouse model to study colon carcinogenesis, these animals mainly develop tumors in their small intestine. Therefore, we also studied an independent model of colon carcinogenesis, i.e., the AOM-induced model. AOM is a chemical agent, which upon metabolic activation is converted into the carcinogen O6-methylguanine (22). AOM almost exclusively induces tumors in the colon of rodents although it is administered systematically. We performed a short-term treatment with AOM in both male and female mice to examine the early stages of colon neoplasia, which are characterized by the formation of aberrant crypt foci (ACF). The incidence of colonic ACF provoked by a short-treatment of AOM was significantly reduced in Lrh-1+/– littermates (Fig. 2A). Consistent with our data observed in the Min mouse model, the number of ACF formed after AOM treatment was significantly greater in Lrh-1+/+ compared with Lrh-1+/– mice (Fig. 2B). In addition, BrdUrd incorporation index, determined as a measure of DNA synthesis and cell proliferation, was higher in Lrh-1+/+ vs. Lrh-1+/– mice (Fig. 2 C and D). This increase in BrdUrd incorporation is in line with our previous analysis of intestinal cell proliferation in animals that were not exposed to AOM, and where several parameters of increased cell proliferation, such as BrdUrd incorporation, phosphohistone H3 and Ki67 staining, were enhanced in Lrh-1+/+ relative to Lrh-1+/– mice (4). Interestingly, because in our study the AOM-induced foci reflect the very early stages of tumorigenesis, these data indicate that LRH-1 may have a critical role in the initial phases of the neoplastic process. In regions where no neoplastic lesions were observed, the colonic epithelium of both genotypes looked grossly normal (data not shown). Also, ACF morphology did not show substantial differences among the two genotypes (data not shown).
Fig. 2.
Haploinsufficiency of LRH-1 reduces azoxymethane-induced neoplasia. (A) Incidence of mice with ACF in male and female Lrh-1+/+ and Lrh-1+/– mice after short-term treatment with AOM. Six-week-old animals (n = 5 per gender and per genotype) were injected i.p. twice for 2 weeks with 7.5 mg/kg AOM and killed 5 weeks after the first injection. Analysis of the colon is described in Experimental Procedures. Statistical significance (P < 0.05; χ2 test) is indicated. (B) Total number of ACF in male and female Lrh-1+/+ and Lrh-1+/– mice (n = 5 per genotype and gender). Comparisons were only performed between littermates. (C) BrdUrd labeling index expressed as the percentage of BrdUrd-positive cells in the colon of male and female BrdUrd-injected Lrh-1+/+ and Lrh-1+/– littermates. BrdUrd immunostaining was performed as described in Experimental Procedures. Percentage of BrdUrd-positive cells represents the number of BrdUrd-positive epithelial cells compared with the total number of epithelial cells in three random fields (×200). (D) BrdUrd immunofluorescence staining of colon sections from BrdUrd-injected Lrh-1+/+ and Lrh-1+/– mice that were challenged with AOM.
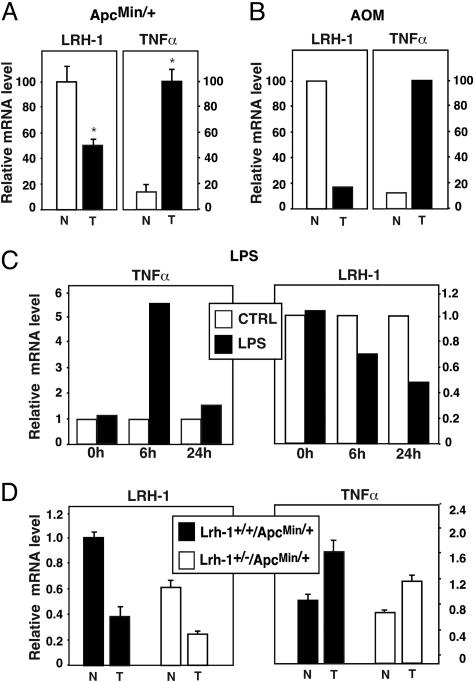
We next explored whether LRH-1 expression was changed in the intestine of the various animal models. Because our monoclonal LRH-1 antibody failed to recognize LRH-1 in mouse intestinal immunohistochemistry, we limited our analysis to mRNA expression studies. We first analyzed LRH-1 mRNA expression in normal intestine and in intestinal polyps of Lrh-1+/+/ApcMin/+ and Lrh-1+/–/ApcMin/+ mice. Contrary to our expectations, LRH-1 mRNA expression was reduced in the polyps relative to unaffected sections of the intestine (Fig. 3A). Also in tumors of long-term AOM-treated mice, LRH-1 mRNA levels were attenuated compared with nontumoral sections of the colon (Fig. 3B). Chronic intestinal inflammation is a high-risk factor for the development of intestinal cancer (23). Recently, support for a direct causal role of the NF-κB signaling pathway in epithelial tumorigenesis in the intestine has been provided (24). Several reports have suggested a link between LRH-1 and inflammation (3, 25). We therefore measured mRNA levels of the proinflammatory cytokine TNF-α in the same intestinal samples. Interestingly, all tumors showed a significant increase in TNF-α mRNA levels in the ApcMin/+ and AOM-induced tumors, which was inversely correlated with the decrease in LRH-1 mRNA (Fig. 3 A and B). To prove a direct causal link between inflammation and decreased LRH-1 expression, we injected wild-type C57BL/6J mice i.p. with LPS, an agent that induces circulating TNF-α levels (26), and measured LRH-1 mRNA levels subsequently in the intestine. LPS injection was associated with a significant decrease in intestinal LRH-1 mRNA expression (Fig. 3C). Although these data suggest that TNF-α can decrease LRH-1 expression, indications for a reciprocal relationship emerged when we analyzed LRH-1 and TNF-α expression in Lrh-1+/+/ApcMin/+ and Lrh-1+/–/ApcMin/+ mice. Compared with Lrh-1+/+/ApcMin/+ mice, Lrh-1+/–/ApcMin/+ double heterozygous mice showed consistently lower expression levels of LRH-1 mRNA, with a similar relative fold reduction in LRH-1 levels in the tumors (Fig. 3D). As shown in Fig. 3A, TNF-α was again higher in the tumors than in normal ileum of Lrh-1+/+/ApcMin/+ mice. Induction was, however, less pronounced and may be attributed to the gender or younger age of the mice analyzed in Fig. 3D. Interestingly, compared with ApcMin/+ mice harboring both alleles of LRH-1, ApcMin/+ mice with haploinsufficiency of LRH-1 showed a moderate decrease in TNF-α expression levels in both normal and tumoral intestine (Fig. 3D). These results indicate that a decrease of LRH-1 levels in mice on ApcMin/+ background also modulates inflammatory response, which in addition to decreased cell proliferation may also contribute to the protection against tumor development in these mice.
Fig. 3.
Proinflammatory factors decrease LRH-1 expression in normal and tumoral intestine. (A and B) LRH-1 and TNF-α expression are inversely correlated in the intestine. Relative mRNA levels of LRH-1 and TNF-α were analyzed in normal (N) versus tumoral (T) ileum of 22-week-old female C57BL/6J-Lrh-1+/+/ApcMin/+ mice (A) or in normal versus tumoral colon of C57BL/6J mice exposed to long-term AOM treatment (B) as described in Experimental Procedures.(C) Effect of LPS treatment on colon TNF-α and LRH-1 mRNA. Relative mRNA levels of TNF-α and LRH-1 were examined on colon biopsies from C57BL/6 mice killed before or 6 and 24 h after vehicle (CTRL) or LPS i.p. injection. (D) Effect of LRH-1 haploinsufficiency on the expression level of LRH-1 in normal and tumoral ileum of 18-week-old male ApcMin/+ mice.
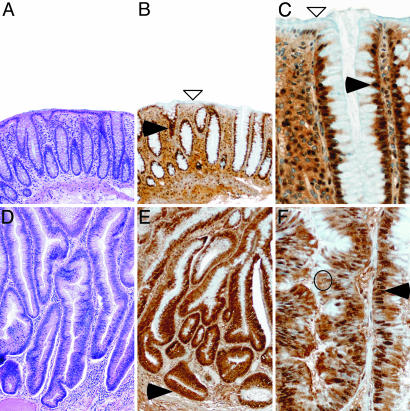
Whereas technical limitations of the available antibodies hampered immunohistochemical analysis on mouse intestinal tissues, LRH-1 could be readily detected on human tissues. To evaluate whether the results we obtained in our two mouse models could be extrapolated to man, we analyzed the expression of LRH-1 protein in human biopsies obtained from normal and neoplastic colon. Anti-LRH-1 staining showed nuclear expression in the cells lining the basal two-thirds of the mucosa of normal colon (Fig. 4 B and C). LRH-1 expression was stronger in the proliferative compartment, with a decrease in staining intensity as cells exit the cell cycle and migrate to the upper part of the crypts. Differentiated enterocytes at the surface of the intestine were almost negative for LRH-1. In the neoplastic lesions, characterized by high-degree dysplasia, subcellular levels of LRH-1 protein were changed, with significantly higher cytoplasmic levels (Fig. 4 E and F) compared with normal colon. In addition, LRH-1 staining was no longer limited to the cells lining the crypts but also present in the epithelial cells at the surface. These alterations in both expression and subcellular localization of LRH-1 further underscore the critical role of LRH-1 in intestinal tumorigenesis.
Fig. 4.
LRH-1 is differentially expressed in normal versus neoplastic colon. Shown is hematoxylin/eosin staining [A and D (magnification, ×80)] and LRH-1 immunostaining [B and E (magnification, ×80) and C and F (magnification, ×320)] of human normal colon mucosa (A–C) and neoplastic polyp (D-F). (A–C) In normal colon, LRH-1 is found predominantly in the nucleus of the crypt epithelial cells (filled arrowheads). LRH-1 immunostaining is detected in the epithelial cells lining the crypt compartment but absent in the surface epithelial cells (open arrowheads). (D–F) Neoplastic polyp characterized with high-grade dysplasia. A marked elongation and lack of differentiation and maturation of crypt and surface epithelial cells is observed. Nuclei are elongated and pseudostratified. Increased cytoplasmic (open circle) and nuclear (filled arrowheads) LRH-1 immunostaining is observed in all epithelial cells, including those lining the surface, which are normally nonproliferative and unstained.
Discussion
We show here that haploinsufficiency of LRH-1 reduces intestinal tumorigenesis in two independent models that are commonly used to study intestinal tumorigenesis, i.e., mice with a mutation in the Apc gene (or ApcMin/+ mice) and mice in which ACFs were induced by the administration of AOM. In view of the impact of gene-environment and gene–gene interactions on the pathogenesis of intestinal tumors, we took extreme caution to reduce variables that could confound the interpretation of our data. In the two studies, animals were studied on a pure C57BL/6J background and on the same diet, reducing contributions of confounders to the study outcome. Furthermore, in the ApcMin/+ study, tumor multiplicity was compared within animals from the same litter. The striking similarity in the reduction of tumor multiplicity in Lrh-1+/– animals in these two independent and well controlled models hence provides conclusive proof of the contribution of LRH-1 to intestinal tumor formation. Molecular analysis of human tumors has shown that the cell-cycle regulators cyclin D1, cyclin E1 and c-Myc, downstream targets of the LRH-1 and β-catenin-signaling pathways (4, 27–29) are frequently overexpressed in human gastrointestinal cancers (30–32) and have been shown to contribute to oncogenesis in animal models (33–35). Hence, our present in vivo data, which link LRH-1 with intestinal tumorigenesis, as well as our previous in vitro studies, which document the synergy between LRH-1 and β-catenin/T cell factor 4 signaling, warrant a thorough genetic and epigenetic exploration of the contribution of LRH-1/β-catenin signaling to the development of human intestinal cancer. Our data on gene expression in the intestine of Lrh-1+/+/ApcMin/+ and Lrh-1+/–/ApcMin/+ mice suggest furthermore the existence of a reciprocal relationship between TNF-α and LRH-1. In view of the established link between chronic intestinal inflammation and epithelial tumor development, a careful dissection of the relative contribution of changes in cell proliferation and inflammation to the pathogenesis of colon cancer will be required.
Acknowledgments
We thank C. Antal, G. Christoffel, and M. Teletin for technical assistance. This work was supported by grants from the Centre National de la Recherche Scientifique, the Institut National de la Santé et de la Recherche Médicale, the Hôpitaux Universitaires de Strasbourg, and the Association pour la Recherche contre le Cancer and by European Union Grant QLRT-2001-00930 and National Institutes of Health Grant 1-P01-DK59820-01. L.D. was supported by an Institut National de la Santé et de la Recherche Médicale fellowship.
Author contributions: K.S. and J.A. designed research; K.S., L.D., J.M., E.F., O.W., and C.H. performed research; J.M. and K.G. contributed new reagents/analytic tools; K.S., L.D., and K.G. analyzed data; and K.S. and J.A. wrote the paper.
Abbreviations: LRH-1, liver receptor homolog 1; ACF, aberrant crypt foci; APC, adenomatous polyposis coli; AOM, azoxymethane.
References
- 1.Ueda, H., Sun, G.-C., Murata, T. & Hirose, S. (1992) Mol. Cell. Biol. 12, 5667–5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sablin, E. P., Krylova, I. N., Fletterick, R. J. & Ingraham, H. A. (2003) Mol. Cell 11, 1575–1585. [DOI] [PubMed] [Google Scholar]
- 3.Paré, J. F., Malenfant, D., Courtemanche, C., Jacob-Wagner, M., Roy, S., Allard, D. & Belanger, L. (2004) J. Biol. Chem. 279, 21206–21216. [DOI] [PubMed] [Google Scholar]
- 4.Botrugno, O. A., Fayard, E., Annicotte, J. S., Haby, C., Brennan, T., Wendling, O., Tanaka, T., Kodama, T., Thomas, W., Auwerx, J. & Schoonjans, K. (2004) Mol. Cell 15, 499–509. [DOI] [PubMed] [Google Scholar]
- 5.Galarneau, L., Paré, J.-F., Allard, D., Hamel, D., Lévesque, L., Tugwood, J. D., Green, S. & Bélanger, L. (1996) Mol. Cell. Biol. 16, 3853–3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rausa, F. M., Galarneau, L., Bélanger, L. & Costa, R. H. (1999) Mech. Dev. 89, 185–188. [DOI] [PubMed] [Google Scholar]
- 7.Paré, J. F., Roy, S., Galarneau, L. & Belanger, L. (2001) J. Biol. Chem. 276, 13136–13144. [DOI] [PubMed] [Google Scholar]
- 8.Annicotte, J. S., Fayard, E., Swift, G. H., Selander, L., Edlund, H., Tanaka, T., Kodama, T., Schoonjans, K. & Auwerx, J. (2003) Mol. Cell. Biol. 23, 6713–6724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fayard, E., Schoonjans, K., Annicotte, J. S. & Auwerx, J. (2003) J. Biol. Chem. 278, 35725–35731. [DOI] [PubMed] [Google Scholar]
- 10.Boerboom, D., Pilon, N., Behdjani, R., Silversides, D. W. & Sirois, J. (2000) Endocrinology 141, 4647–4656. [DOI] [PubMed] [Google Scholar]
- 11.Fayard, E., Auwerx, J. & Schoonjans, K. (2004) Trends Cell Biol. 14, 250–260. [DOI] [PubMed] [Google Scholar]
- 12.Schoonjans, K., Annicotte, J. S., Huby, T., Botrugno, O. A., Fayard, E., Ueda, Y., Chapman, J. & Auwerx, J. (2002) EMBO Rep. 3, 1181–1187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hinshelwood, M. M., Repa, J. J., Shelton, J. M., Richardson, J. A., Mangelsdorf, D. J. & Mendelson, C. R. (2003) Mol. Cell. Endocrinol. 207, 39–45. [DOI] [PubMed] [Google Scholar]
- 14.Saxena, D., Safi, R., Little-Ihrig, L. & Zeleznik, A. J. (2004) Endocrinology 145, 3821–3829. [DOI] [PubMed] [Google Scholar]
- 15.Kinzler, K. W. & Vogelstein, B. (1996) Cell 87, 159–170. [DOI] [PubMed] [Google Scholar]
- 16.Nishisho, I., Nakamura, Y., Miyoshi, Y., Miki, Y., Ando, H., Horii, A., Koyama, K., Utsunomiya, J., Baba, S., Hedge, P., et al. (1991) Science 253, 665–669. [DOI] [PubMed] [Google Scholar]
- 17.Groden, J., Thliveris, A., Samowitz, W., Carlson, M., Gelbert, L., Albertsen, H., Joslyn, G., Stevens, J., Spirio, L. & Robertson, M. (1991) Cell 66, 589–600. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi, Y., Nagase, H., Ando, H., Horii, A., Ichii, S., Nakatsuru, S., Aoki, T., Miki, Y., Mori, T. & Nakamura, Y. (1992) Hum. Mol. Genet. 1, 229–233. [DOI] [PubMed] [Google Scholar]
- 19.Powell, S. M., Petersen, G. M., Krush, A. J., Booker, S., Jen, J., Giardiello, F. M., Hamilton, S. R., Vogelstein, B. & Kinzler, K. W. (1993) N. Engl. J. Med. 329, 1982–1987. [DOI] [PubMed] [Google Scholar]
- 20.Su, L. K., Kinzler, K. W., Vogelstein, B., Preisinger, A. C., Moser, A. R., Luongo, C., Gould, K. A. & Dove, W. F. (1992) Science 256, 668–670. [DOI] [PubMed] [Google Scholar]
- 21.Moser, A. R., Pitot, H. C. & Dove, W. F. (1990) Science 247, 322–324. [DOI] [PubMed] [Google Scholar]
- 22.Pegg, A. E. (1984) Cancer Invest. 2, 223–231. [DOI] [PubMed] [Google Scholar]
- 23.Itzkowitz, S. H. & Yio, X. (2004) Am. J. Physiol. Gastrointest. Liver Physiol. 287, G7–G17. [DOI] [PubMed] [Google Scholar]
- 24.Greten, F. R., Eckmann, L., Greten, T. F., Park, J. M., Li, Z. W., Egan, L. J., Kagnoff, M. F. & Karin, M. (2004) Cell 118, 285–296. [DOI] [PubMed] [Google Scholar]
- 25.Kim, M. S., Shigenaga, J., Moser, A., Feingold, K. & Grunfeld, C. (2003) J. Biol. Chem. 278, 8988–8995. [DOI] [PubMed] [Google Scholar]
- 26.Remick, D. G., Strieter, R. M., Lynch, J. P., III, Nguyen, D., Eskandari, M. & Kunkel, S. L. (1989) Lab. Invest. 60, 766–771. [PubMed] [Google Scholar]
- 27.He, T. C., Sparks, A. B., Rago, C., Hermeking, H., Zawel, L., da Costa, L. T., Morin, P. J., Vogelstein, B. & Kinzler, K. W. (1998) Science 281, 1509–1512. [DOI] [PubMed] [Google Scholar]
- 28.Shtutman, M., Zhurinsky, J., Simcha, I., Albanese, C., D'Amico, M., Pestell, R. & Ben-Ze'ev, A. (1999) Proc. Natl. Acad. Sci. USA 96, 5522–5527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tetsu, O. & McCormick, F. (1999) Nature 398, 422–426. [DOI] [PubMed] [Google Scholar]
- 30.Arber, N., Hibshoosh, H., Moss, S. F., Sutter, T., Zhang, Y., Begg, M., Wang, S., Weinstein, I. B. & Holt, P. R. (1996) Gastroenterology 110, 669–674. [DOI] [PubMed] [Google Scholar]
- 31.Donnellan, R. & Chetty, R. (1999) FASEB J. 13, 773–780. [DOI] [PubMed] [Google Scholar]
- 32.Sikora, K., Chan, S., Evan, G., Gabra, H., Markham, N., Stewart, J. & Watson, J. (1987) Cancer 59, 1289–1295. [DOI] [PubMed] [Google Scholar]
- 33.Wang, T. C., Cardiff, R. D., Zukerberg, L., Lees, E., Arnold, A. & Schmidt, E. V. (1994) Nature 369, 669–671. [DOI] [PubMed] [Google Scholar]
- 34.Yu, Q., Geng, Y. & Sicinski, P. (2001) Nature 411, 1017–1021. [DOI] [PubMed] [Google Scholar]
- 35.Bortner, D. M. & Rosenberg, M. P. (1997) Mol. Cell. Biol. 17, 453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]