Abstract
Objective
To identify risk factors independently predictive of pressure injury (also known as pressure ulcer) development among critical-care patients
Design
We undertook a systematic review of primary research based on standardized criteria set forth by the Institute of Medicine.
Data Sources
We searched the following databases: CINAHL (EBSCOhost), the Cochrane Library (Wilson), Dissertations & Theses Global (ProQuest), PubMed (National Library of Medicine), and Scopus. There was no language restriction.
Method
A research librarian coordinated the search strategy. Articles that potentially met inclusion criteria were screened by two investigators. Among the articles that met selection criteria, one investigator extracted data and a second investigator reviewed the data for accuracy. Based on a literature search, we developed a tool for assessing study quality using a combination of currently available tools and expert input. We used the method developed by Coleman and colleagues in 2014 to generate evidence tables and a summary narrative synthesis by domain and subdomain.
Results
Of 1753 abstracts reviewed, 158 were identified as potentially eligible and 18 fulfilled eligibility criteria. Five studies were classified as high quality, two were moderate quality, nine were low quality, and two were of very low quality. Age, mobility/activity, perfusion, and vasopressor infusion emerged as important risk factors for pressure injury development, whereas results for risk categories that are theoretically important, including nutrition, and skin/pressure injury status, were mixed. Methodological limitations across studies limited the generalizability of the results, and future research is needed, particularly to evaluate risk conferred by altered nutrition and skin/pressure injury status, and to further elucidate the effects of perfusion-related variables.
Conclusions
Results underscore the importance of avoiding overinterpretation of a single study, and the importance of taking study quality into consideration when reviewing risk factors. Maximal pressure injury prevention efforts are particularly important among critical-care patients who are older, have altered mobility, experience poor perfusion, or who are receiving a vasopressor infusion.
Keywords: Critical Care, Pressure Injury, Risk factor, Skin
Introduction
Hospital-acquired pressure injuries (formerly called pressure ulcers) are localized areas of damage to the skin, underlying tissue, or both, as a result of pressure. Hospital-aquired pressure injuries occur in 3% to 34% of hospitalized patients worldwide and result in longer hospital stays, increased morbidity, and increased human suffering.1–4
Due to negative outcomes associated with pressure injuries, standards of practice include a recommendation to conduct pressure injury risk assessment and comprehensive skin assessment upon admission and at any time there is a significant change in a patient’s condition.5 Accurate risk assessment along with comprehensive skin assessment enables prompt recognition and treatment of pressure injuries that occur among high-risk patients, which is important because early (Category 1) pressure injuries are highly treatable6; however, discernment of which individuals are at highest risk for pressure injuries in the intensive care unit (ICU) is problematic because the risk-assessment scales currently used for critical-care patients tend to identify almost all patients as “high risk.”7
Critical-care patients represent a highly specialized patient population, and risk for pressure injuries in this population is likely to be different than risk in other populations, particularly as it relates to perfusion and general skin status due to severity of illness and treatments, including vasopressor infusion, that are unique to critical-care patients.8 The purpose of the current review is to identify factors that are independently associated with increased risk for pressure injuries among critical-care patients specifically. An independent risk factor retains its statistical association with the outcome variable when other risk factors are included in the model; note that independence is a statistical concept and does not imply causality.9,10
We evaluated identified independent risk factors in relation to clinical relevance and in relation to recent pressure injury conceptual and theoretical frameworks.5,11 We also evaluated risk factors in relation to study quality, as a recent pressure injury study conducted in a general population determined that most of the included studies were of low or very low quality.9
Methods
Research Protocol
We undertook a systematic review of primary research. Our approach was based on the standardized criteria set forth by the Institute of Medicine12 for comparative effectiveness reviews and modified to appraise risk-factor/observational studies.9
Eligibility Criteria
We adapted inclusion criteria based on the method employed by Coleman and colleagues,9 to include (a) primary research; (b) adult sample; (c) ICU setting; (d) prospective cohort, retrospective record review, or controlled trial; and (e) identification of independent risk factors for pressure injury (multivariate analysis). Exclusion criteria included the following: (a) limited to pediatric patient population (age <18 years), (b) >25% of the study population were excluded from analysis due to loss to follow up or missing records, (c) prevalence or cross-sectional study, (d) limited to evaluation of a pressure injury risk-assessment scale, and (e) limited to spinal cord injury (SCI) patients (due to the specialized physiology involved in spinal cord injuries and the associated risk for pressure injury among individuals with SCI.13 There was no language restriction.
Search Strategy
We searched the medical subject headings pressure injury and intensive care units in addition to field-restricted keywords for the following databases: CINAHL (EBSCOhost), the Cochrane Library (Wilson), Dissertations & Theses Global (ProQuest), and PubMed (National Library of Medicine). We downloaded our final results on December 17, 2016. A complete description of the search is outlined in Appendix A.
Data Extraction
Two investigators (XX and XX) identified potentially eligible studies. Among those deemed potentially eligible, XX noted whether each study met inclusion criteria for this review (or stated the reason the study did not meet criteria) and XX checked XX’s categorizations. Disagreements were addressed by a third researcher, XX, and agreement was determined by consensus. In addition, one investigator (XX) extracted data pertaining to study design, population, setting, analysis, and results, and a second investigator (XX) reviewed the data for accuracy.
Quality Appraisal
In an effort to identify a quality-assessment tool for the current review, we conducted a literature search. We determined that no currently available checklists or scales fit closely with the objectives of the current review while offering adequate inter-rater reliability.
We used the available tools to guide development of our tool for assessing quality among pressure injury risk-factor studies. First, the authors of a systematic review of quality-assessment tools for observational studies concluded that available checklists and scales did not differentiate well between poor study reporting and a truly flawed study.14 The authors recommended that instead of assigning a summative score based primarily on reporting, quality assessment of observational risk-factor studies should be conducted by defining flaws in different domains—an approach that results in more transparent conclusions when compared with global scoring based on a checklist or summative evaluation tool. Similarly, authors of a systematic review of quality-appraisal tools for observational epidemiological studies recommended against summative scores and instead advised an approach based on evaluation of bias in particular quality domains.15
The quality-appraisal tool developed for the current review (see Appendix B) includes the domains identified in Sanderson and colleagues’15 review of quality appraisal among observational studies: methods for selecting participants, methods for measuring exposure and outcome variables, design-specific sources of bias, methods to control confounding, statistical methods (excluding control of confounding), and conflict of interest. Major and moderate flaws are noted in each domain in which presence of a major flaw is a significant indicator that the flaw has substantially compromised our confidence in the study conclusions.
Although the quality-appraisal method employed in this study was focused on sources of bias in different domains, we determined that an evaluative descriptor was necessary to facilitate study classification according to the degree of actual or potential bias. Using the rubric provided in Appendix B, we employed the following evaluation based on specific sources of bias:
High-quality studies had 0 potential sources of bias with major implications for study quality and <1 potential sources of bias with moderate implications for study quality;
Moderate-quality studies had 1 potential source of bias with major implications for study quality and <1 potential sources of bias with moderate implications for study quality; or 0 potential sources of bias with major implications for study quality and 2–3 potential sources of bias with moderate implications for study quality;
Low-quality studies had 1 potential source of bias with major implications for study quality and 2–4 potential sources of bias with moderate implications for study quality, or 0 potential sources of bias with major implications for study quality and 4–7 potential sources of bias with moderate implications for study quality; and
Very-low-quality studies had 2 or more potential sources of bias with major implications for study quality, or >8 potential sources of bias with moderate implications for study quality.
Indeterminate sources of bias were items that may or may not have introduced bias; indeterminate items were noted but did not count toward the evaluative descriptor category. We sought expert input during tool development, and the final tool reflects consensus among two experts in pressure injury research and one expert in observational research.
Data Synthesis
Meta-analysis was not feasible for this review because of a high degree of clinical heterogeneity related to population, predictor variable operationalization, preventive interventions, and different thresholds for the pressure injury outcome variable (new Category 1 and greater pressure injury vs. new Category 2 and greater) according to the international National Pressure Ulcer Advisory Panel/European Pressure Ulcer Advisory Panel (NPUAP/EPUAP) classification system.5 The purpose of the review was to identify risk factors rather than to quantify the effect size of the relationship between a given factor and pressure injury development; therefore, we conducted a narrative synthesis. We utilized the narrative synthesis method previously employed by Coleman and colleagues.9 We recorded all potential risk factors entered into multivariate analysis and identified the factors that emerged as independent factors for pressure injury risk. For studies using stepwise regression, we included factors that were not statistically significant upon bivariate analysis if those factors were identified as independent risk factors for pressure injuries in the final model.9 Finally, we categorized recorded risk factors and potential risk factors into domains and subdomains.
Domains were structured according to Coleman and colleagues’11 interpretation of the NPUAP/EPUAP conceptual framework (see Figure 1). Domain 1 encompasses mechanical boundary conditions to include sources of pressure and also friction and shear, which are conceptualized as mechanical boundary conditions rather than as patient characteristics.11 Domain 2 comprises those factors that influence the susceptibility and tolerance of the individual. Some factors have an effect on mechanical boundary conditions and on the susceptibly and tolerance of the individual, and therefore some overlap exists between the two major domains; for example, diabetes affects mechanical load through sensory deficits and affects individual tolerance and susceptibility through altered perfusion. We developed subdomains in relation to Coleman and colleagues’11 theoretical schema of a proposed causal pathway for pressure ulcer development (see Figure 2), which built upon the NPAUP/EPUAP/Pan Pacific Pressure Injury Alliance (PPPIA) conceptual framework5 and identified immobility, skin and pressure injury status, and poor perfusion as direct causal factors in pressure injury development.11
Figure 1.
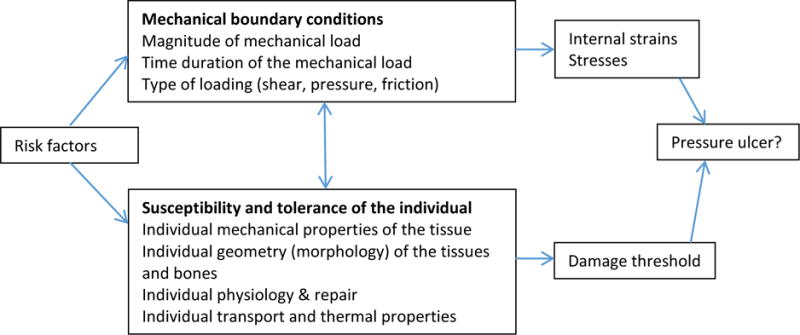
Enhancement of NPUAP/EPUAP (2009) factors that influence susceptibility for pressure ulcer development (Coleman et al., 2014, p. 2229, used with permission).
Figure 2.
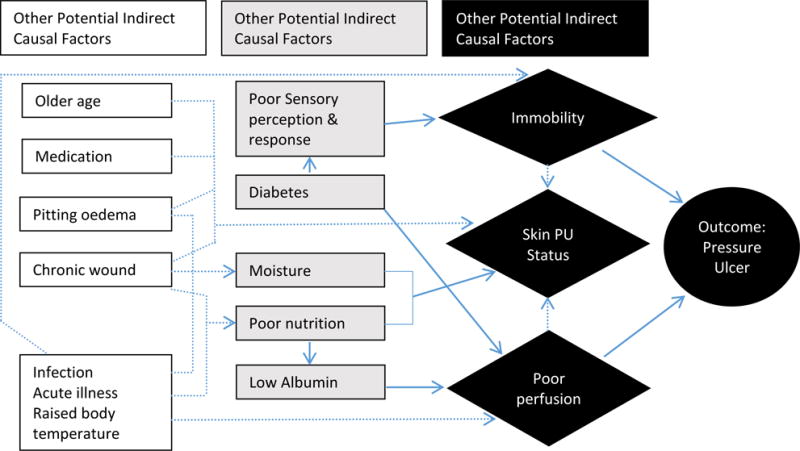
Theoretical schema of proposed causal pathway for pressure ulcer development. The solid arrows show the causal relationship between the key indirect causal factors and the outcome. Interrupted arrows show the causal relationship between other potential indirect causal factors and key indirect causal factors and between direct causal factors. Interrupted arrows also demonstrate interrelationships between direct causal factors and indirect causal factors (Coleman et al, 2014, p. 2229, used with permission).11
Results
Study Characteristics
Of 1753 abstracts reviewed, 158 were identified as potentially eligible and 18 fulfilled eligibility criteria (see Figure 3). The retained studies included 13 prospective cohort and five retrospective record reviews. A summary of the included studies is presented in Table 1.
Figure 3.
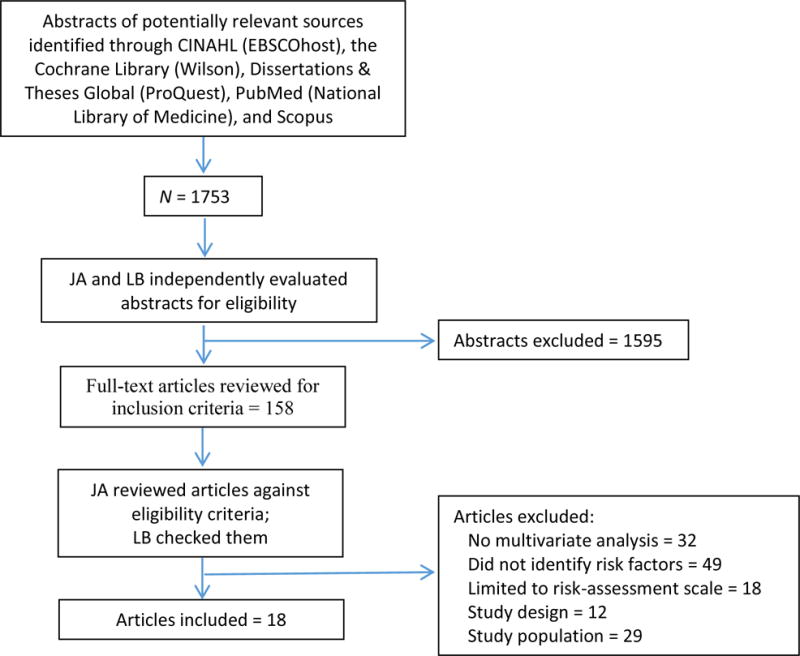
Decision Process
Table 1.
Summary of Studies
| Study Authors | Sample and Country | Inclusion Criteria | Design and Analysis | No. in Final Model (PI%), No. of PI and Category | Results: No. of Risk Factors (No. in Model), Model Risk-Factor Names: Odds Ration (95% Confidence Interval) | Study Quality | |
|---|---|---|---|---|---|---|---|
| Compton et al.28 | 713 general ICU patients in Germany | ≥72-hour stay No pressure injury upon admission |
Retrospective record review Logistic regression |
698 (17%), 121 Categories 2–4 |
32 (6) Male gender: 1.8 (NR) Moist skin: 2.4 (NR) Edematous skin: 2.2 (NR) Centralized circulation: 2.4 (NR) Mottled skin: 2.0 (NR) Reddened skin: 2.3 (NR) |
MQS | |
| Cox11 | 347 medical–surgical ICU patients in the United States | ≥24-hour stay No pressure injuey upon admission Age ≥18 years |
Retrospective record review Logistic regression |
Model 1: 347 (18.7%), 65 ≥Category 1 Model 2: 327 (13.7%), 45 ≥Category 2 |
Model 1: 15 (4) Mobility: 0.439 (0.21–0.95) Age: 1.033 (1.003–1.064) Length of ICU stay: 1.008 (1.005–1.011) Cardiovascular disease: 2.952 (1.3–6.4) Model 2: 15 (4) Friction/shear: 5.715 (1.423–22.95) Length of ICU stay: 1.008 (1.004–1.012) Norepinephrine: 1.017 (1.001–1.033) Cardiovascular disease: 3.380 (1.223–9.347) |
HQS | |
| Cox & Roche17 | 306 medical, surgical, and cardiothoracic ICU patients in the United States | ≥24-hour stay No pressure injury upon admission Age ≥18 Received a vasopressor during ICU stay |
Retrospective record review Logistic regression |
306 (13%), 41 ≥Category 1 |
11 (5) Cardiac arrest: 3.894 (0.998–15.118) Mechanical ventilation ≥72 hours: 23.604 (0.998–15.118) Hours of MAP less than 60 mm HG while on vasopressors: 1.096 (1.020–1.178) Vasopressin: 4.816 (1.666–13.925) Cardiac diagnosis at admission: 0.035 (0.002–0.764) |
HQS | |
| Cremasco et al.1 | 160 modical–surgical ICU patients in three ICUs in | ≥24-hour stay No pressure injury upon admission |
Prospective cohort Logistic regression |
160 (34.4%), 55, Category not reported | NR (4) Male gender: 5.4 (1.42–22.09) Length of ICU stay: 1.120 (1.943–1.202) SAPSI score: 1.058 (1.004–1.114) |
LQS | |
| Brazil | NAS score: 0.916 (0.855–0.980) | ||||||
| Eachempati et al.31 | Phase 2: 412 surgical ICU patients in the United States | Length of stay>7 days | Prospective cohort Logistic regression |
55 (60%), 33 ≥Category 2 | 7 (5) Emergent admission: 36 (0.2290–0.7694) Age: –0.0131) Days in bed: 1.05 (−0.0013–0.0156) CURS day 8: 1.45 (−0.0048–−0.0833) Days without any nutrition: 0.51 (−0.1095– −0.0334) |
VLQS | |
| Fife et al.29 | 186 neurologic ICU patients in the United States | No pressure injury upon admission No diagnosis of brain death on life support pending organ donation |
Prospective cohort Logistic regression |
186 (12%), 23 ≥Category 2 | NR (2) Braden score: NR (NR) Low body mass index (BMI): NR (NR) |
MQS | |
| Frankel et al.2 | 820 surgical ICU patients in the United States | Not reported | Retrospective record review Logistic regression |
820 (3%), 25 ≥Category 2 | 9 (4) Diabetes: 2.7 (1.1–6.4) Age: 2.9 (1.2–7.1) Creatinine: 3.7 (1.2–9.2) Spinal cord injury: 16.8 (1.5–182) |
MQS | |
| Kaitani et al.23 | 98 ICU and high-care-unit patients in Japan | Age≥20 years No pressure injury upon admisison ≥24-hour stay Unable to make major and frequent position changes independently |
Prospective cohort Logistic regression |
98 (11.2%), 11 Categories 1–4 | 6 (2) Scheduled admission: 0.04 (0–0.47) Frequency of turning: 0.45 (0.21–0.97) |
LQS | |
| Manzano et al.21 | 299 patients in nine ICUs in Spain | Mechanical ventilation Age≥18 years Nonpregnant |
Prospective cohort Logistic regression |
299 (15.7%), 47 ≥Category 2 | 16 (5) Day 1 respiratory SOFA: 1.56 (1.026–2.360) Day 4 cardiovascular SOFA: 1.33 (1.066–1.664) Age: 1.042 (1.013–1.072) Winter: 4.6 (1.99–10.59) Length of mechanical ventilation: 1.042 (1.005–1.080) |
HQS | |
| Nijs et al.22 | 520 surgical ICU patients in Belgium | Age≥16 years ≥24-hour expected stay Absence of burns |
Prospective cohort Logistic regression |
463 (28.9%), 134 Categories 2–4 | 19 (9) Dopamine <5 mcg/kg/min: 6.1 (1.9–19.5) Vascular disease: 4.5 (2.0–10.2) Dialysis: 3.8 (1.0–13.9) “Adequate prevention”: 6.0 (1.9–18.6) Frequency of turning six or more times daily or alternating mattress: 30.2 (12.2–74.8) “Turning”: 6.7 (2.7–16.4) Sedative use: 0.3 (0.1–0.7) Body temperature≤38.5: 0.2 (0.2–0.9) Sitting in chair: 0.1 (0.0–0.3) |
HQS | |
| O’Brien et al.19 | 2695 surgical and burn ICU patients in the United States | Age≥18 years ≥48-hour ICU stay Underwent a surgical procedure No pressure injury upon admission |
Retrospective record review | 2695 (10.7%), 288 ≥Category 2 | 12 (7) Existing airway: 5.28 (3.63–7.67) Low BMI: 2.7 (1.45–5.04) Noncardiac surgery: 1.84 (1.31–2.59) History of heart failure: 1.78 (1.27–2.49) History of renal failure: 1.75 (1.27–2.39) ASA class 4 or 5: 1.63 (1.19–2.29) Age: 1.02 (1.01–1.03) |
HQS | |
| Sayar et al.24 | 140 medical–surgical ICU patients in Turkey | At risk or at high risk on Waterlow pressure ulcer risk scale | Prospective cohort Logistic regression |
140 (14.3%), 20 ≥Category 1 | 5 (2) Length of stay: 1.2 (1.1–1.3) Activity level: 0.3 (.02–0.7) |
MQS | |
| Slowikowski & Funk4 | 369 surgical ICU petients in the United States | Age≥16 years | Prospective cohort Logistic regression |
369 (23.9%), 88, Category not reported | 8 (3) Braden Scale score: 1.3 (1.15–1.47) Diabetes: 1.93 (1.11–3.35) Age≥70 years: 2.14 (1.27–3.62) |
HQS | |
| Suriadi et al.18 | 253 general ICU patients in Indonesia | Age≥18 years Bedfast No pressure injury upon admission ≥24-hour stay and anticipated stay≥72 hours |
Prospective cohort Logistic regression |
253 (28.4%), 72 ≥Category 1 | NR (3) Interface pressure: 2.2 (1.6–2.9) Body temperature: 2.0 (1.7–2.5) Cigarette smoking: 1.6 (1.1–2.5) |
HQS | |
| Tayyib et al.25 | 84 general ICU patients in Saudi Arabia | Age≥18 years | Prospective cohort | 84 (39.3%), 33 Categories 1–4 | Model 1 Categories 1–4: 7 (3) Age: 1.254 (1.054–1.492) Longer ICU stay: 1.23 (1.014–3.309) Infrequent repositioning: 250.04 (230–11,954.16) Model 2 Categories 2–4: 3 (2) Longer ICU stay: 1.831 (1.054–1.492) Infrequent repositioning: 2.96 (1.23–7.153) |
MQS | |
| Theaker et al.26 | 286 general ICU patients in the United Kingdom | >24-hour stay No pressure injury upon admission Three or more pressure injury risk factors |
Prospective cohort Logistic regression |
286 (26.9%), 77 Categories 2–4 | 18 (5) Norepinephrine infusion: 8.11 (3.64–18) APACHE II≥13: 2.4 (1.4–7.92) Fecal incontinence: 3.27 (1.32–8.3) Anemia: 2.81 (1.24–6.34) Length of stay≥three days: 2.76 (1.06–7.05) |
LQS | |
| Ulker Efteli &Yapucu Gunes27 | 70 general ICU patients in Turkey | Age≥18 years Expected ICU stay≥7 days No pressure injury upon admission Braden Scale score<12 |
Prospective cohort Logistic regression |
70 (33%), 23 ≥Category 1 | 6 (2) Female gender: 0.15 (0.03–0.71) Lower serum albumin level: 11.6 (1.92–70.4) |
MQS | |
| Yepes et al.30 | 150 ICU patients in Bolivia | Intubated On mechanical ventilation Received vasopressor |
Prospective cohort Logistic regression |
150 (26.7%), 40 ≥Category 2 | 3 (3) Presence of infection: 4.39 (6.92–18.25) Length of stay in the ICU: 1.13 (1.06–1.22) APACHE II: 1.06 (1.0–1.12) |
LQS | |
NR = not reported
PI = pressure injury
ICU = intensive care unit
NR = not reported
MQS = moderate-quality study
HQS = high-quality study
MAP = mean arterial pressure
LQS = low-quality study
SAPSI = Simplified Acute Physiology Score
NAS = nursing activities score
VLQS = very-low-quality study
CURS = Corneil ulcer risk score
SOFA = sequential organ failure assessment
ASA = American Society of Anesthesiologists
APACHE =acute physiology and chronic health evaluation
Quality Appraisal
Two researchers conducted the quality appraisal and reached “substantial” agreement independently, as evidenced by Kappa = 0.72.16 After inter-rater reliability was calculated, the researchers reviewed any discrepancies and came to agreement. When possible, we contacted study authors for clarification purposes.
Quality appraisal results are identified in Table 2. The included studies had between zero and two major sources of bias, and between one and six moderate sources of bias; overall, five studies were classified as high quality,4,17–20 two were of moderate quality,21,22 nine were of low quality,2,23–30 and two were of very low quality1,31 (Table 2). The methodological limitations we found were similar to other reviews of pressure injury risk-factor studies in the sense that most of the included studies (61%) were of either low quality or very low quality.7,9 Eleven (64%) of the 17 included studies did not have adequate numbers of pressure injury events for analysis, a limitation that is reflected in some studies in the wide confidence intervals associated with reported odds ratios.
Table 2.
Study Quality: Potential Bias
| Study | Methods for Selecting Participants | Statistical Methods and Control of Confounding | Methods for Measuring Exposure | Methods for Measuring Outcome Variable | Conflict of Interest | Notes and Quality Appraisal |
|---|---|---|---|---|---|---|
| Compton et al.28 | – | Major: Inadequate number of events for analysis Moderate: Unclear statistical reporting |
– | Moderate: Nurses who were not specially trained identified pressure injuries |
– | LQS Strength: Used an independent cohort to validate model |
| Cox20 | – | Note on events for analysis: The author included a power analysis indicating there were enough events. | – | Moderate: Nurses who were not specially trained identified pressure injuries | – | HQS |
| Cox & Roche17 | – | – | – | – | – | HQS |
| Cremasco et al.1 |
– | Major: Inadequate number of events for analysis Moderate: Unclear statistical reporting Moderate: Non-independent factors included in the analysis without appropriate adjustment |
– | Major: No criteria for designation of wound as a pressure injury Moderate: Nurses who were not specially trained identified pressure injuries Moderate: Limited description of the outcome variable |
– | VLQS |
| Eachempati et al.31 | Moderate: Restricted sampling (included only patients with LOS>6 days) Moderate: Unclear inclusion/exclusion criteria |
Major: Clearly incorrect statistical methods Moderate: Inappropriate strategy for model building |
– | Major: No criteria for designation of wound as a pressure injury Moderate: Nurses who were not specially trained identified pressure injuries Moderate: Limited description of the outcome variable |
– | VLQS |
| Fife et al.29 | – | Major: Inadequate number of events for analysis Moderate: Unclear statistical reporting |
– | Moderate: Limited description of the outcome variable | – | LQS |
| Frankel et al.2 | Indeterminate: Individuals appear to have been excluded from the study but the inclusion/exclusion criteria are not defined | Major: Inadequate number of events for analysis | – | Moderate: Nurses who were not specially trained identified pressure injuries | – | LQS |
| Kaitani et al.23 | – | Major: Inadequate number of events for analysis Moderate: >15% lost to follow up or missing records/inadequate data collection Moderate: Inappropriate strategy for model building |
Moderate: Variable operation is unclear | – | – | LQS |
| Manzano et al.21 | – | Major: Inadequate number of events for analysis | Indeterminate: No reporting of missing data for predictor variables despite high likelihood of missing data | – | – | MQS |
| Nijs et al.22 | – | Major: Inadequate number of events for analysis Moderate: Problematic statistical methods with moderate implications for study findings |
Indeterminate: Potential temporal ambiguity (it is possible that the predictor variable occurred after the pressure ulcer event) | – | – | MQS |
| O’Brien et al.19 | – | – | – | Moderate: Nurses who were not specially trained identified pressure injuries | – | HQS |
| Sayar et al.24 | – | Moderate: Sampled from “high-risk” patients on a risk-assessment scale and then included attributes of the same scale as predictor variables | Moderate: Non-independent factors are included in the analysis without proper adjustment Moderate: Selective reporting of results Moderate: Unclear statistical reporting |
– | – | LQS |
| Slowikowski & Funk4 | – | – | – | Moderate: Limited description of the outcome variable | – | HQS |
| Suriadi et al.18 | Moderate: Unclear statistical reporting | – | – | HQS | ||
| Tayyib et al.25 | – | Major: Inadequate number of events for analysis Moderate: Nonindependent factors included in the analysis without appropriate adjustment |
– | Moderate: Nurses who were not specially trained identified pressure injuries | – | LQS |
| Theaker et al.26 | – | Major: Inadequate number of events for analysis Moderate: >15% lost to follow up or missing records Moderate: Nonindependent factors included in the analysis without appropriate adjustment |
– | Moderate: Nurses who were not specially trained identified pressure injuries Moderate: Limited description of the outcome variable | – | LQS |
| Ulker Efteli & Yapucu Gunes27 | Moderate: Restricted sampling (included only patients with LOS>6 days) | Major: Inadequate number of events for analysis | – | Moderate: Nurses who were not specially trained identified pressure injuries | – | LQS |
| Yepes et al.30 | Moderate: Restricted sampling (included only patients on mechanical ventilation and vasopressor support) | Moderate: Nonindependent factors included in the analysis without appropriate adjustment Moderate: Unclear statistical reporting |
– | Moderate: Nurses who were not specially trained identified pressure injuries | – | LQS |
Pressure Injury Outcome Variable
Two of the 18 studies included for review did not describe criteria used to designate a pressure injury.1,31 Two studies did not report specific pressure injury categories,1,4 six studies designated a pressure injury as a new injury ≥Category 1,17,23–27 eight studies included only new pressure injuries that were ≥Category 2,2,19,21,22,28–31 and two studies included separate models for pressure injuries ≥Category 1 and ≥Category 2 (Table 1).20,25
Risk-Factor Domains and Subdomains
The authors of 14 studies reported all of the risk factors entered into multivariate modeling as well as those that emerged as independently predictive of pressure injury,2,4,17,19–28,31 whereas authors of three studies reported only the variables that emerged as significant from multivariate modeling.1,18,29 A summary of risk factors entered into the multivariate model (when available) and those that emerged as independent risk factors are summarized by study (Table 1) and by risk-factor domain (see Table 3).9
Table 3.
Summary of Evidence for Risk Factor Domains and Subdomains
| Variable | Studies With Variable Significant in Multivariate Model Study Quality (Study Authors) Variable: Odds Ratio (95% Confidence Interval) | Studies With Variable Not Significant in Multivariate Model Study Quality (Study Authors) Variable |
|---|---|---|
| Domain 1: Mechanical Boundary Conditions | ||
| Body size | – | MQS (Manzano et al.21) Body weight LQS (Compton et al.28) Body weight and height |
| Friction and shear | HQS (Cox20) Friction/shear: 5.715 (1.423–22.95) | – |
| Emergent vs. scheduled admission | LQS (Kaitani et al.23) Scheduled admission: 0.04 (0–0.47) VLQS (Eachempati et al.31) Emergent admission: 36 (0.2290–0.7694) |
HQS (O’Brien et al.19) Emergent admission MQS (Manzano et al.21) Type of admission (medical vs. surgical) LQS (Tayyib et al.25) Emergent admission LQS (Kaitani et al.23) Admission type |
| Domain 1 Subdomain: Immobility | ||
| Mental/neurologic status | – | MQS (Nijs et al.22) GCS: opens eyes MQS (Nijs et al.22) GCS: movement, localizes pain MQS (Nijs et al.22) GCS: movement, follows commands LQS (Compton et al.28) Minimum GCS LQS (Compton et al.28) Maximum GCS LQS (Sayar et al.24) Consciousness LQS (Sayar et al.24) Cooperation LQS (Theaker et al.26) Pain |
| Mobility/activity | HQS (Cox20) Mobility: 0.439 (0.21–0.95) LQS (Sayar et al.24) Activity level: 0.3 (0.2–0.7) |
– |
| Sensory perception | – | HQS (Cox20) Sensory perception |
| Surgical factors | HQS (O’Brien et al.19) Noncardiac surgery: 1.84 (1.31–2.59) | LQS (Tayyib et al.25) Operation time |
| Turning/repositioning and surface | HQS (Suriadi et al.18) Interface pressure: 2.2 (1.6–2.9) MQS (Nijs et al.22) “Adequate prevention”: 6.0 (1.9–18.6) MQS (Nijs et al.22) Frequency of turning six or more times daily or alternating mattress: 30.2 (12.2–74.8) MQS (Nijs et al.22) “Turning”: 6.7 (2.7–16.4) MQS (Nijs et al.22) Sitting in chair: 0.1 (0.0–0.3) LQS (Tayyib et al.25) Infrequent repositioning: 2.96 (1.23–7.153) LQS (Kaitani et al.23) Frequency of turning: 0.45 (0.21–.0.97) |
HQS (Slowikowski & Funk4) Not repositioned LQS (Theaker et al.26) Too unstable to turn |
| Domain 2: Susceptibility and Tolerance of the Individual | ||
| Age | HQS (Cox20) Age: 1.033 (1.003–1.064) HQS (O’Brien et al.19) Age: 1.02 (1.01–1.03) HQS (Slowikowski & Funk4) Age≥70 years: 2.14 (1.27–3.62) MQS (Frankel et al.2) Age: 2.9 (1.2–7.1) LQS (Tayyib et al.25) Age: 1.254 (1.054–1.492) VLQS (Eachempati et al.31) Age: 1.08 (0.0026–0.0131) |
MQS (Manzano et al.21) Age |
| Body temperature | HQS (Suriadi et al.18) Body temperature: 2.0 (1.7–2.5) MQS (Nijs et al.22) Body temperature≥38.5: 0.2 (0.2–0.9) |
LQS (Compton et al.28) Maximum body temperature |
| Diagnosis (excepting diagnosis related to oxygenation and perfusion, included below under Subdomain: Poor Perfusion) | HQS (O’Brien et al.19) History of renal failure: 1.75 (1.27–2.39) LQS (Frankel et al.2) Spinal cord injury: 16.8 (1.5–182) LQS (Yepes et al.30) Presence of infection: 4.39 (6.92–18.25) |
HQS (O’Brien et al.19) History of liver disease MQS (Manzano et al.21) Multiple organ failure MQS (Nijs et al.22) Gastrointestinal diagnosis LQS (Tayyib et al.25) History of kidney disease |
| Laboratory values (excepting values related to oxygenation and perfusion, included below under Subdomain: Poor Perfusion) | LQS (Frankel et al.2) Creatinine: 3.7 (1.2–9.2) LQS (Theaker et al.26) Anemia: 2.81 (1.24–6.34) |
HQS (Cox & Roche17) Severe anemia LQS (Compton et al.26) Maximum serum potassium LQS (Compton et al.28) Maximum creatinine LQS (Compton et al.28) Maximum blood glucose LQS (Compton et al.28) Maximum c-reactive protein LQS (Compton et al.28) Minimum thromboplastin time LQS (Compton et al.28) Maximum serum bilirubin LQS (Ulker Efteli & Yapucu Gunes27) Hemoglobin LQS (Ulker Efteli & Yapucu Gunes27) Blood glucose LQS (Sayar et al.24) C-reactive protein LQS (Theaker et al.26) Coagulopathy |
| Length of stay | HQS (Cox20) Length of ICU stay: 1.008 (1.005–1.011) LQS (Sayar et al.24) Length of stay: 1.2 (1.1–1.3) LQS (Tayyib et al.25) Longer ICU stay: 1.831 (1.014–3.309) LQS (Yepes et al.30) Length of stay: 1.13 (1.06–1.22) LQS (Theaker et al.26) Length of stay > 3 days: 2.76 (1.08–7.05) VLQS (Cremasco et al.1) Length of ICU stay: 1.120 (1.943–1.202) VLQS (Eachempati et al.31) Days in bed: 1.05 (−0.0013–0.0156) |
HQS (Cox & Roche17) Hospital length of stay HQS (Cox & Roche17) Length of stay before ICU admission HQS (Cox & Roche17) ICU length of stay MQS (Manzano et al.21) ICU length of stay MQS (Manzano et al.21) Pre-ICU hospital stay LQS (Compton et al.28) Duration of ICU stay |
| Medication (excepting vasopressors) and treatments | MQS (Nijs et al.22) Sedative use: 0.3 (0.1–0.7) MQS (Nijs et al.22) Dialysis: 3.8 (1.0–3.9) |
HQS (O’Brien et al.19) Current corticosteroid use HQS (Slowikowski & Funk4) Orthotics HQS (Slowikowski & Funk4) Hemodialysis MQS (Nijs et al.22) Physical fixation MQS (Nijs et al.22) Major analgesics MQS (Nijs et al.22) “Floating heels” LQS (Compton et al.28) Sedation LQS (Compton et al.28) Insulin therapy LQS (Theaker et al.26) Current corticosteroid use |
| Nutrition and laboratory values related to nutrition status |
LQS (Ulker Efteli & Yapucu Gunes27) Lower serum albumin level: 11.6 (1.92–70.4) VLQS (Eachempati et al.31) Days without any nutrition 0.51 (−0.1095–−0.0334) |
HQS (Cox20) Nutrition LQS (Compton et al.28) Parenteral nutrition LQS (Kaitani et al.23) Nutrition LQS (Theaker et al.26) Serum albumin LQS (Theaker et al.26) Reduced nutritional intake |
| Severity of illness/health status | HQS (Cox & Roche17) Cardiac arrest: 3.894 (0.998–15.118) HQS (O’Brien et al.19) ASA class 4 or 5: 1.63 (1.19–2.23) MQS (Manzano et al.21) Day 1 respiratory SOFA: 1.56 (1.026–2.360) MQS (Manzano et al.21) Day 4 cardiovascular SOFA: 1.33 (1.066–1.664) LQS (Yepes et al.30) APACHE II: 1.06 (1.0–1.12) LQS (Theaker et al.26) APACHE II> 13: 2.4 (1.4–7.92) VLQS (Cremasco et al.1) SAPSII score: 1.058 (1.004–1.114) |
HQS (Cox20) APACHE HQS (Cox & Roche17) APACHE II HQS (Cox & Roche17) Died in ICU MQS (Manzano et al.21) Hospital mortality MQS (Nijs et al.22) APACHE II LQS (Ulker Efteli & Yapucu Gunes27) APACHE II LQS (Compton et al.28) ICU mortality LQS (Compton et al.28) TISS LQS (Kaitani et al.23) APACHE II LQS (Theaker et al.26) Peripheral vascular disease VLQS (Eachempati et al.31) MODS VLQS (Eachempati et al.31) APACHE III |
| Domain 2 Subdomain: Poor Perfusion Including Factors That Affect Oxygenation and Perfusion Status/Delivery of Oxygen to the Tissues | ||
| Blood pressure | HQS (Cox & Roche17) Hours of MAP less than 60 mm HG while on vasopressors: 1.096 (1.020–1.178) |
HQS (Cox20) Mean arterial pressure HQS (Cox20) Systolic blood pressure HQS (Cox20) Diastolic blood pressure |
| Diagnosis related to oxygenation and/or perfusion (also included in global diagnosis, above) | HQS (Cox20) Cardiovascular disease: 2.952 (1.3–6.4) HQS (Cox & Roche17) Cardiac diagnosis at admission: 0.035 (0.002–0.764) HQS (O’Brien et al.19) History of heart failure: 1.78 (1.27–2.49) HQS (Slowikowski & Funk4) Diabetes: 1.93 (1.11–3.35) HQS (Suriadi et al.18) Cigarette smoking: 1.6 (1.1–2.5) MQS (Nijs et al.22) Vascular disease: 4.5 (2.0–10.2) LQS (Frankel et al.2) Diabetes: 2.7 (1.1–6.4) |
HQS (O’Brien et al.19) History of diabetes MQS (Manzano et al.21) Septic shock MQS (Manzano et al.21) Acute respiratory distress syndrome LQS (Frankel et al.2) Vascular disease LQS (Compton et al.28) Sepsis LQS (Tayyib et al.25) History of cardiovascular disease LQS (Theaker et al.26) Diabetes LQS (Theaker et al.26) History of smoking |
| Heart rate and monitoring | LQS (Compton et al.28) Maximum heart rate LQS (Compton et al.28) Invasive monitoring |
|
| Oxygenation/ventilation | HQS (Cox & Roche17) mechanical ventilation longer than 72 hours: 23.604 (6.427–86.668) HQS (O’Brien et al.19) existing airway: 5.28 (3.63–7.67) MQS (Manzano et al.21) length of mechanical ventilation: 1.042 (1.005–1.080) |
HQS (Slowikowski & Funk4) Ventilator support MQS (Manzano et al.21) Pa02/Fi02 ratio on Day 1 MQS (Nijs et al.22) Mechanical ventilation LQS (Compton et al.28) Minimum PaCO2 LQS (Compton et al.28) Minimum arterial pH LQS (Compton et al.28) Mechanical ventilation LQS (Compton et al.28) Cyanosis LQS (Tayyib et al.25) Mechanical ventilation |
| Vasopressor | HQS (Cox20) Norepinephrine: 1.017 (1.001–1.033) HQS (Cox & Roche17) Vasopressin infusion: 4.816 (1.666–13.925) MQS (Nijs et al.22) Dopamine<5 mcg/kg/min: 6.1 (1.9–19.5) LQS (Theaker et al.26) Norepinephrine infusion: 8.11 (3.64–18) |
LQS (Compton et al.28) Vasopressor therapy LQS (Frankel et al.2) Vasopressor therapy LQS (Theaker et al.26) Dopamine LQS (Theaker et al.26) Epinephrine LQS (Theaker et al.26) Norepinephrine |
| Domain 2 Subdomain: Skin/Pressure Injury Status Including Factors That Affect Skin and Pressure Injury Status |
||
| Moisture | LQS (Compton et al.28) Moist skin: 2.4 (NR) | LQS (Theaker et al.26) Moisture |
| Skin/external skin factors/PI status | LQS (Compton et al.28) Edematous skin: 2.2 (NR) LQS (Compton et al.28) Centralized circulation: 2.4 (NR) LQS (Compton et al.28) Mottled skin: 2.0 (NR) LQS (Compton et al.28) Reddened skin: 2.3, (NR) LQS (Theaker et al.26) Fecal incontinence: 3.27 (1.32–8.3) |
HQS (Cox & Roche17) Peripheral necrosis in patients receiving vasopressors HQS (Slowikowski & Funk4) Edema MQS (Nijs et al.22) Pitting edema LQS (Compton et al.28) Livid skin LQS (Compton et al.28) Hyperemic skin LQS (Kaitani et al.23) Edema LQS (Theaker et al.26) Edema |
| Other Factors Not Included In Domains 1 or 2 | ||
| Gender | LQS (Ulker Efteli & Yapucu Gunes27) Female gender:0.15 (0.03–0.71) LQS (Compton et al.28) Male gender: 1.8 (NR) VLQS (Cremasco et al.1) Male gender: 5.6 (1.42–22.09) |
LQS (Kaitani et al.23) gender G |
| Risk-assessment scales | HQS (Slowikowski & Funk4) Braden Scale score: 1.3 (1.15–1.47) LQS (Fife et al.29) Braden Scale score: NR (NR) VLQS (Eachempati et al.31) CURS Day 8: 1.45 (−0.0048–−0.0833) |
HQS (Cox20) Braden Scale total HQS (Cox & Roche17) Braden Scale at hospital admission HQS (Cox & Roche17) Braden Scale at ICU admission LQS (Compton et al.28) Waterlow score LQS (Tayyib et al.25) Braden Scale score |
| Other factors | MQS (Manzano et al.21) Winter admission: 4.6 (1.99–10.59) VLQS (Cremasco et al.1) NAS score: 0.916 (0.855–0.980) |
– |
Adapted from Coleman et al.9
HQS = high-quality study
MQS = moderate-quality study
LQS = low-quality study
VLQS = very-low-quality study
GCS = Glaslow Coma Score
APACHE = Acute Physiology and Chronic Health Evaluation
TISS = Trauma Injury Severity Score
MODS = multiple organ dysfunction syndrome
PA02/FI02 = ratio of arterial oxygen partial pressure to fractional inspired oxygen PaCO2 = carbon dioxide partial pressure
MAP = mean arterial pressure
CURS = Corneil ulcer risk score
NAS = nursing activities score
PI = pressure injury
Domain 1: Mechanical Boundary Conditions
Mechanical boundary conditions are aspects that influence the magnitude of the mechanical load, the time duration, and also the type of loading (pressure, friction, shear; Figure 1).5 We extended this category to include body size because of the potential for increased mechanical load due to bony prominence among underweight individuals. We also included emergent admission because emergency department gurneys have a suboptimal surface,32 and surgical time as time in surgery confers immobility.
Body Size
One moderate-quality study21 and one low-quality study28 included body size in the multivariate analysis, but neither weight nor height emerged as significant upon multivariate analysis (Table 3). No study included change in weight, however, which might have been useful for assessing fluid shifts. Additionally, no study included a height/weight composite such as body mass index, which would have indicated underweight or excessive adipose tissue.
Friction and Shear
Recent developments in pressure injury research indicate that friction-induced skin injuries are not true pressure injuries, whereas shearing forces cause a decrease in regional blood flow and therefore are important in pressure injury risk.33,34 Authors of only one study20 entered a shear-related variable into multivariate modeling; the study, which was of high quality, found that friction/shear (as defined by the Braden Scale)35 was independently predictive of pressure injury development (Table 3).
Emergent Versus Scheduled Admission
We included emergent admission in Domain 1 because time in the emergency department is associated with time spent on suboptimal surfaces such as gurneys.32 Five study authors entered admission type into their statistical model.19,21,23,25,31 In two of those studies (33%),23,31 emergent admission was found to be independently predictive for pressure injury development; however, the two studies were of low- and very-low quality.
Domain 1 Subdomain: Immobility
Within Domain 1, Coleman and colleagues’11 schema depicts immobility as a direct causal factor (Figure 2). Therefore, factors associated with this subdomain are presented below.
Mental/Neurologic Status
Researchers in four studies,22,24,26,28 including one moderate-quality study22 and three low-quality studies,24,26,28 entered variables related to neurologic status into multivariate analysis. No variables related to mental status emerged in multivariate analysis (Table 3).
Mobility/Activity
One high-quality study20 and one low-quality study24 each identified mobility and activity level, respectively, as independently predictive of pressure injuries (Table 3).
Sensory Perception
Sensory perception was entered into the statistical model of one high-quality study but did not emerge as an independent risk factor.20
Surgical Factors
Information pertaining to surgical factors was limited. One high-quality study19 found that undergoing noncardiac surgery was an independent risk factor for pressure injury, whereas one low-quality study25 entered operative time into the multivariate model, but it did not emerge as an independent risk factor (Table 3).
Turning/Repositioning and Surface
Overall, authors of six studies entered one or more turning- and/or repositioning-related variables into the statistical model4,18,22,23,25,26; one study entered four variables related to positioning22 (Table 3). Results were conflicting. In their moderate-quality study, Nijs and colleagues22 found that more frequent turning was an independent risk factor for pressure injury development, whereas two low-quality studies23,25 each found that less frequent repositioning was independently predictive of pressure injury risk (Table 3). Nijs and colleagues speculated that perhaps high-risk patients experienced enhanced nursing vigilance in turning and repositioning.22
Domain 2: Susceptibility and Tolerance of the Individual
Domain 2 includes factors that influence the susceptibility and tolerance of the individual (Figure 1). Subdomains within Domain 2 are skin/pressure injury status, which includes existing and previous pressure injuries and general skin status, and poor perfusion, which encompasses conditions that alter oxygen delivery to the tissues.11
Body Temperature
Three studies,18,22,28 including one of high quality, one of moderate quality, and one of low quality, included body temperature in multivariate analysis, with conflicting results. The high-quality study found that fever was an independent risk factor for pressure injury development18; the moderate-quality study found that fever was a protective factor22, and in the low-quality study,28 fever did not emerge as significant in multivariate analysis (Table 2).
Diagnosis Not Directly Related to Oxygenation and Perfusion
Renal failure and high creatinine were each determined to be independent risk factors for pressure injury development in one high-quality study19 and one low-quality study,2 respectively. Researchers in one high-quality4 and one moderate-quality study22 entered dialysis into multivariate modeling. In the moderate-quality study, dialysis was independently predictive of pressure injury development, whereas dialysis did not emerge as an independent risk factor in the high-quality study. Serum creatinine was independently predictive of pressure injury development in one low-quality study2 (Table 3).
Laboratory Values
Researchers in six studies,2,17,24,26–28 including one high-quality study, entered laboratory values into multivariate analysis (apart from albumin, which is discussed under “Nutrition,” and blood-gas values, which are included in the oxygenation results; see Table 2). Only two laboratory values were statistically significant upon multivariate analysis: creatinine was an independent risk factor in one low-quality study,2 and anemia emerged in one low-quality study.26
Length of Stay
Length of stay (LOS) independently predicted risk for pressure injury development in seven1,20,24–26,30,31 of the 11 studies that included LOS in multivariate analysis (Table 2).1,17,20,21,24–26,28,30,31,36 Only one study,21 however, differentiated LOS prior to pressure injury development, which is important, because development of a pressure injury increases the length of a hospital stay.37
Medications
Among five studies that included medications other than vasopressors,4,19,22,26,28 one moderate-quality study22 found that sedative use was an independent risk factor for pressure injury development (Table 3).
Nutrition
In the current review, only one low-quality study determined that a nutrition-related variable (serum albumin) was independently predictive of pressure injury risk.27 Four other studies evaluated nutrition-related variables,20,23,26,28 but nutrition did not emerge as predictive in multivariate modeling (Table 3). Of note, one very-low-quality but frequently cited study indicated that days without nutrition was an independent risk factor for pressure injury development31; in that study, however, the data presented in tables and the associated odds ratio indicate the opposite: that days without nutrition was a protective factor. That paradoxical finding was actually replicated in the bivariate analysis conducted by Slowikowski and Funk,4 but the authors did not enter nutrition in the multivariate analysis because they thought it might have been a spurious finding.
Severity of Illness/Health Status
Eight studies included the Acute Physiology and Chronic Health Evaluation (APACHE) score as a marker of severity of illness in their multivariate model,17,20,22,23,26,27,30,31 and two low-quality studies26,30 identified the APACHE score as predictive of pressure injury risk (Table 2). The APACHE score is calculated using measurements that occur within 24 hours after admission, and the score is not repeated; therefore, the APACHE may not be a sensitive indicator of severity of illness throughout a several-day hospital course.38 Furthermore, experts contend that the APACHE should be used primarily to provide performance comparisons between ICUs rather than to provide an assessment of an individual patient’s illness severity.38
Among other markers of illness severity, an American Society of Anesthesiologists (ASA) Class-4 or Class-5 score was an independent risk factor for pressure injuries in one high-quality study,19 and sequential organ failure assessments on Days 1 and 4 were also independent risk factors for pressure injuries in a moderate-quality study21 (Table 3). Hospital and/or ICU mortality were considered in one high-quality study17 and two moderate-quality studies,21,28 but mortality did not emerge as statistically significant in the multivariate model.
Domain 2 Subdomain: Poor Perfusion
The subdomain of poor perfusion includes factors that alter oxygen delivery to tissues. Poor perfusion is included in Coleman and colleagues’ conceptual schema as a direct causal factor in pressure injury development.11
Blood Pressure
Two high-quality studies included blood pressure,17,20 and blood pressure was an independent risk factor in one of the studies.17 Cox defined blood pressure as the total number of hours in the first 48 hours that the patient had a mean arterial pressure <60 mm Hg, and/or systolic blood pressure <90 mm Hg, and/or diastolic blood pressure <60 mm Hg; however, in that study, the mean length of stay was five days, and therefore blood pressure readings were not recorded for more than half of a typical patient’s ICU stay.20 In a another study, Cox and Roche determined that the total number of hours a patient experienced a mean arterial blood pressure of <60mmHg while on vasopressors was independently predictive of pressure injury development.17
Diagnosis Related to Oxygenation and/or Perfusion
Researchers in 10 studies (including four high-quality studies4,17,19,20) entered diagnoses related to potentially altered perfusion (including diabetes, cardiovascular disease, and peripheral vascular disease) into multivariate modeling2,4,17,18,19,20,21,22,25,28; the diagnoses emerged as independent risk factors in six,2,4,17–19,22 including all four high-quality studies,4,17,19,20 one moderate-quality study,22 and one low-quality study2 (Table 2). Researchers in two studies included sepsis, another condition resulting in altered tissue perfusion, in their multivariate modeling, but sepsis did not emerge as a significant risk factor.21,28 In addition, researchers in two studies entered cigarette smoking into multivariate modeling18,26; smoking was an independent risk factor for pressure injury development in the high-quality study by Suriadi et al.18
Heart Rate and Monitoring
One low-quality study recorded heart rate and invasive monitoring and determined that neither variable was independently predictive of pressure injury development; however, the authors recorded variables only for the first 24 hours of a patient’s ICU stay, despite inclusion criteria that required an ICU length of stay >72 hours.28
Oxygenation and Ventilation
Authors of seven studies entered oxygenation and ventilation-related variables into multivariate modeling4,17,19,21,22,25,28; among those, one high-quality17 and one moderate-quality21 study identified length of mechanical ventilation as independently predictive of pressure injury risk. Other oxygenation and ventilation-related variables did not emerge as independently predictive (Table 3); however, variable operationalization limits the generalizability of the findings: only two studies included blood-gas results, and both studies limited their data collection to the first 24 hours.21,28 Furthermore, mechanical ventilation may be more indicative of severity of illness than oxygenation status because a patient could be stable from a respiratory standpoint but still require mechanical ventilation support due to other disease processes.
Vasopressors
Vasopressor infusion is commonly administered to critical-care patients to improve perfusion in shock states, with resulting peripheral vasoconstriction, which may confer risk for pressure injury.20 Authors of six studies entered a vasopressor variable into multivariate analysis2,17,20,22,26,28 and in four of those studies, including both of the high-quality studies,17,20 vasopressor infusion emerged as independently predictive of pressure injury development17,20,22,26 (Table 3). In their high-quality study, Cox and Roche found that patients receiving vasopressin were at increased risk for pressure injury development.17 Variable operationalization contributed to difficulty comparing across studies. Cox20 and Cox and Roche17 recorded hours of administration of specific vasopressor agents and hour/dose, respectively, whereas Nijs and colleagues22 recorded dose but not duration of vasopressor infusion and Theaker et al.26 dichotomized norepinephrine infusion as “yes/no.”
Domain 2 Subdomain: Skin/Pressure Injury Status
The subdomain of skin and pressure injury status includes existing and previous pressure injuries and general skin status. Skin/pressure injury status is included in Coleman and colleagues’11 conceptual schema as a direct causal factor in pressure injury development (Figure 2).
Moisture
Moisture is included in skin/pressure injury status due to its close relationship with skin condition.39 Two studies evaluated moisture,26,28 and it emerged as an independent risk factor for pressure injury in one moderate-quality study28 (Table 3).
External Skin Factors
Researchers in six studies entered variables related to skin status into multivariate modeling.4,17,22,23,26,28 The variables included external conditions (incontinence), assessment of the skin’s appearance, and edema (Table 2). Edema emerged from multivariate modeling in one low-quality study,28 but was not independently predictive of pressure injury risk in one high-quality study,4 one moderate-quality study,22 and two low-quality studies.23,26 Peripheral necrosis due to vasopressor use was not an independent predictor of pressure injury in one study.17 A single study recorded detailed examination of the skin’s condition28; that low-quality study found that centralized circulation, mottled skin, and reddened skin were independent predictors of pressure injury development, whereas livid skin and hyperemic skin did not emerge from the multivariate analysis (Table 2).
Other Factors Not Included in Domains 1 and 2
Gender
Four studies included gender in the multivariate model,1,23,27,28 and in three of the four,1,27,28 male gender was independently predictive of pressure injury risk.
Risk-Assessment Scales
Overall, seven studies included a risk-assessment-scale total score in their multivariate analysis,4,17,20,25,28,29,31 and in three studies (43%)4,29,31 the total score emerged as an independent risk factor (Table 3). The total score for the Braden Scale35 emerged in one high-quality study4 and one low-quality study,29 and did not emerge in two high-quality studies17,20 and one low-quality study.25
Other Factors
A high-quality study found winter season was a risk factor for pressure injury development.21 One low-quality study noted that increased nursing workload was a slightly protective factor.1
Discussion
Our findings reveal inconsistent results among studies, as well as marked variability in study quality, indicating that researchers should avoid overinterpretation of results from any single study. Each study was subjected to quality assessment, which will allow clinicians and researchers to take quality into consideration when evaluating results.
In the current review of pressure injury risk factors among critical-care patients, age, mobility/activity, perfusion, and vasopressor infusion frequently emerged as important factors in pressure injury development, particularly among high-quality studies. Findings for age and mobility/activity are consistent with the results from a systematic review conducted by Coleman and colleagues in an acute, rehabilitative, long-term-care population.11 The finding that mobility and poor perfusion are important subdomains is in keeping with current theoretical knowledge, given that mobility and poor perfusion are both direct causal factors in Coleman and colleagues’ conceptual model; however, results for skin and pressure injury status, which is also conceptualized as a direct causal factor, were mixed.11
Results for the perfusion subdomain were mixed; however, the bulk of evidence from high-quality studies favored perfusion as an important independent risk factor, whereas negative findings from lower quality studies may have reflected methodologic limitations. Perfusion is a dynamic process, particularly among critical-care patients, who are at risk for hemodynamic instability. Only one study incorporated perfusion-related measures throughout the patient’s entire ICU stay17; other studies that included perfusion-related variables utilized cut points that presented dynamic hemodynamic processes as dichotomous variables, an approach that fails to quantify the magnitude of hypotension. Similarly, only one study recorded the duration of hypotension.17
Vasopressor agents are an important element influencing perfusion among ICU patients, but are difficult to study due to variability in effects on peripheral circulation related to dose delivered and receptors targeted. Among studies in the current review, only one study included the dose of the vasopressor for the entire duration of administration, and the same study was the only one to capture the potentially synergistic effects of more than one vasopressor agent.17 Despite methodological limitations, however, results from the current review indicate that vasopressor agents are important in pressure injury development. Among two high-quality and one moderate-quality studies that examined various vasopressor-related variables, all found that vasopressors were independent predictors.17,20,22
Cox and Roche17 examined a population receiving vasopressor therapy and found increased risk among individuals receiving vasopressin, which is important because vasopressin is typically considered a second-line drug and is commonly administered along with norepinephrine for vasodilatory shock.40 This is particularly interesting in light of a prevalence study conducted by Bly and colleagues36 that determined that infusion of more than one vasopressor conferred risk for pressure ulcers.• Additional research is needed to elucidate the effects of individual vasopressor agents, the potentially synergistic effects of multiple agents (particularly concomitant use of norepinephrine and vasopressin), and the underlying effects of the shock state that the vasopressor agents treat.
Coleman and colleagues conceptual model indicates that skin and pressure injury status are direct causal factors in pressure injury development.11 The conclusion that skin status is important is also supported by current clinical practice guidelines and by the broader pressure injury literature.5 Unfortunately, however, information pertaining to skin and pressure injury status in the current review was extremely limited; only one study addressed skin status (excepting edema) throughout the hospitalization (vs. only on admission).17 Additionally, the authors of 10 (56%) of the 18 studies in the current review excluded patients who were admitted to the ICU with a pre-existing pressure injury, which is unfortunate, because individuals with proven skin compromise are therefore not represented in more than half of the included studies.1,17–20,23,26–29
Although nutrition is theoretically a factor in pressure injury development, results from the current review failed to demonstrate a connection between nutrition status and pressure injury development among critical-care patients. Eachempati and colleagues’ study concluded that more days without nutrition conferred risk for pressure injuries; however, careful analysis of their study shows the opposite.31 In Table 4 on page 1681, the 33 patients with a pressure injury experienced a mean of 1.9 days without nutrition, whereas the 22 patients without a pressure injury experienced a mean of 4.3 days without nutrition. Furthermore, the reported odds ratio of 0.51 indicates a protective effect.31 In their high-quality study, Slowikowski and Funk4 also found that patients receiving no nutrition had a lower incidence of pressure injury, but they chose not to enter nutrition in multivariate analysis because they were concerned that it was a spurious finding, citing Eachempati and colleagues’31 erroneous conclusion that days without nutrition conferred risk. In the future, researchers should utilize more sensitive nutrition indictors. Guidance on appropriate measurement of nutrition status among critical-care patients is available from the American Society for Parenteral and Enteral Nutrition in coordination with the Society of Critical Care Medicine.41
In addition to skin/pressure injury status and nutrition, more information is needed about the relationship between surgery and the risk for pressure injury development. A high-quality retrospective record review of 3225 surgical patients (not limited to critical care) found that multiple surgeries and total surgical time were independent risk factors for pressure injury development.42 Only two studies in the current review included surgical factors in multivariate analysis.19,25
Our study was limited to critical-care patients within the ICU setting. Therefore, it is possible that we failed to include research that featured critically ill patients in other settings, or subgroup analysis of studies that featured various levels of acuity among hospitalized patients. Finally, our search strategy included databases that are primarily in the English language—CINAHL (EBSCOhost), the Cochrane Library (Wilson), Dissertations & Theses Global (ProQuest), PubMed (National Library of Medicine), and Scopus—which may have failed to identify some articles in languages other than English.
Conclusion
Results from this review of pressure injury risk factors among critical-care patients underscore the importance of avoiding overinterpretation of a single study, and the importance of taking study quality into consideration when reviewing risk factors. Age, mobility/activity, perfusion, and vasopressor infusion emerged as important risk factors for pressure injury development, whereas results for risk categories that are theoretically important, including skin and pressure injury status and nutrition, were mixed.5 Methodological limitations across studies limit generalizability of results, and future research is needed, particularly to elucidate risk conferred by illness severity, nutrition, and skin and pressure injury status. Clinicians may consider extending maximal preventive interventions to critical-care patients who are older, experience altered mobility/activity, have altered perfusion, or receive vasopressor infusions. Future research examining the effects of poor nutrition, and especially skin and pressure injury status, is needed. In addition, research is still needed to elucidate the effects of specific perfusion related variables, including high doses of vasopressors, combinations of vasopressors, and duration of decreased oxygen delivery to tissues (hypotension and/or decreased blood oxygen content).
What is already known about this topic?
Critical care patients are exposed to unique potential risk factors for pressure injury (PI) development, such as vasopressor infusion and the effects of severe illness.
Although studies have examined PI risk among critical care patients, there is little consensus about which factors influence PI risk in the critical care population.
What this paper adds
Age, mobility/activity, poor perfusion, and vasopressor infusion are risk factors for pressure-injury development among critical care patients.
Future research is needed to evaluate risk conferred by malnutrition, and skin/pressure injury status.
Future research is also needed to further elucidate risk conferred by specific perfusion related variables including high doses of vasopressors, combinations of vasopressors, and duration of decreased oxygen delivery to tissues (hypotension and/or decreased blood oxygen content).
Acknowledgments
This publication was supported by the National Institute of Nursing Research of the National Institutes of Health under Award Number T32NR01345 and F31NR014608. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Appendix A: Database Search Strategies
| Search Lexicon | |
|---|---|
| MH | Restricts the search to MeSH headings assigned to the article |
| TI | Keyword search for terms in the article title |
| tiab | Keyword search for terms in the title or abstract |
| + | Medical subject heading exploded to include all narrower subject terms |
| “ ” | Exact phrase search |
| * | Wildcard - can replace any letter or, at the end of the word, multiple letters |
| su | ProQuest subject headings |
| Search Statements Employed | ||
|---|---|---|
| Database | Search Statement | Number of Results |
| Medline (EBSCO) | ((MH “Pressure Ulcer”) OR (TI “pressure ulcer*”)) AND ((MH “intensive care”) OR (MH “intensive care units”) OR (TI intensive care unit*) OR (TI “critical care”)) | 243 |
| Medline (EBSCO) | ((MH “Intensive Care Units+”) OR (MH “Critical Care+”)) AND (MH “Pressure Ulcer+”) | 334 |
| PubMed | (pssure injur*[TI] OR pressure ulcer*[TI] OR pressure sore*[TI] OR bed sore*[TI] OR bedsore*[TI] OR decubital ulcer*[TI] OR decubitus ulcer*[TI] OR ulcus decubitus[TI] OR “Pressure Ulcer”[Mesh]) AND (“Critical Care”[Mesh] OR “Intensive Care Units”[Mesh] OR “Burn Units”[Mesh] OR “Coronary Care Units”[Mesh] OR “Intensive Care Units, Pediatric”[Mesh] OR “Intensive Care Units, Neonatal”[Mesh] OR “Recovery Room”[Mesh] OR “Respiratory Care Units”[Mesh] OR “Critical Illness”[Mesh] OR “Critical Care Nursing”[Mesh] OR “Critical Care Outcomes”[Mesh] OR critical care[TI] OR Critically Ill[TI] OR critical ill*[TI] OR intensive care[TI] OR cardiovascular unit*[TI] OR coronary care[TI] OR Cardiac Care[TI] OR neurocritical care[TI] OR neurointensive care[TI] OR step-down unit*[TI] OR step down unit*[TI] OR burn unit*[TI] OR high dependency unit*[TI] OR neurosurgical unit*[TI] OR surgical intensive care[TI] OR Recovery Room*[TI] OR recovery unit*[TI] OR observation unit*[TI] OR observational unit*[TI] OR Respiratory Care[TI] OR ICU[tiab] OR ICUs[tiab] OR NICU[tiab] OR NICUs[tiab] OR CCU[tiab] OR CCUs[tiab] OR SICU[tiab] OR SICUs[tiab]) | 441 |
| CINAHL (EBSCO) | ((MH “Intensive Care, Neonatal+”) OR (MH “Intensive Care Units+”) OR (MH “Critical Care+”) OR (TI “intensive care”) OR (TI “critical care”)) AND ((MH “Pressure Ulcer+”) OR (TI “Pressure Ulcer”) OR (TI “Pressure ulcers”)) | 506 |
| Cochrane | pressure ulcer* AND (“intensive care” unit* OR “intensive care” OR “critical care”) in Title, abstract, kw | 113 |
| Scopus | pressure ulcer* AND (“intensive care” unit* OR “intensive care” OR “critical care”) in Title, abstract, kw | 926 |
| Dissertations and Theses | su(pressure ulcer*) AND su((intensive care OR critical care)) | 9 |
| Dissertations and Theses | diskw(pressure ulcer*) AND diskw((intensive care OR critical care)) | 8 |
Note. NLM subject headings: https://www.nlm.nih.gov/mesh/. With regard to database selection: Though the material indexed in Medline is also included in NLM PubMed, the search algorithms can vary between interface providers, as can post-limit features and other options, and thus can yield slightly different results sets.
Appendix B Quality Appraisal of Observational Studies of Pressure Ulcer Risk in Critical Care
| Domain | Major flaws | Moderate Flaws | Indeterminate Flaws |
|---|---|---|---|
| Methods for selecting participants | (More than 25% of sample lost to follow up and missing records were exclusion criteria for the current review.) | ≥15% of the population lost to follow up or missing records Restricted sampling, resulting in limited generalizability The study sampled from high-risk patients on a risk-assessment scale and then included the factors in the scale as potential predictor variables; or, very restricted sampling frame that resulted in limited generalizability |
Inclusion/exclusion criteria are unclear |
| Statistical methods and control of confounding | Clearly incorrect statistical methods Inadequate number of events (pressure ulcers) for analysis: <10 pressure ulcers per variable included in the multivariate analysis10,43 | Nonindependent factors are included in analysis without appropriate adjustment10 Time-dependent covariates (e.g., blood pressure) included without appropriate adjustment10 Selective reporting of results9 Inappropriate strategy for model building3 Unclear statistical reporting:
|
Unclear statistical reporting |
| Methods for measuring exposure | Temporal ambiguity: it is possible that the predictor variable occurred after the pressure ulcer event. | Variable operationalization is unclear or misleading. Incomplete data for predictor variables
|
No reporting of missing data for predictor variables despite high likelihood of missing data |
| Methods for measuring outcome variable | No criteria for wound designation as a pressure ulcer (e.g., NPUAP/EPUAP ≥category 1 or equivalent) | Nurses who were not wound nurses and not specially trained identified or categorized pressure ulcers. | Limited description of the outcome variable (e.g., no staging information) |
| Conflict of interest | Evidence of conflict of interest, with major implications for study results | Evidence of conflict of interest, with minor implications for study results | Evidence of conflict of interest, with unclear implications for study results |
References:
Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud. 2013;50(7):974–1003. doi:10.1016/j.ijnurstu.2012.11.019.
Harrell FE. Regression modeling strategies. New York, NY: Springer; 2001.
Peduzzi PJ, Concato AR, Feinstein X, Holford TR. Importance of events per independent variable in proportional hazards regression analysis. II. Accuracy and precision of regression estimates. J Clin Epidemiol. 1996;48(12):1503–1510.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The study by Bly et al.36 was a prevalence study, and therefore did not meet inclusion criteria for the current review.
References
- 1.Cremasco MF, Wenzel F, Zanei SSV, Whitaker IY. Pressure ulcers in the intensive care unit: the relationship between nursing workload, illness severity and pressure ulcer risk. J Clin Nurs. 2013;22(15/16):2183–2191. doi: 10.1111/j.1365-2702.2012.04216.x. [DOI] [PubMed] [Google Scholar]
- 2.Frankel H, Sperry J, Kaplan L. Risk factors for pressure ulcer development in a best practice surgical intensive care unit. Am Surg. 2007;73(12):1215–1217. [PubMed] [Google Scholar]
- 3.Graves N, Birrell F, Whitby M. Effect of pressure ulcers on length of hospital stay. Infect Cont Hosp Epidemiol. 2005;26(3):293–297. doi: 10.1086/502542. [DOI] [PubMed] [Google Scholar]
- 4.Slowikowski GC, Funk M. Factors associated with pressure ulcers in patients in a surgical intensive care unit. J Wound Ostomy Continence Nurs. 2010;37(6):619–626. doi: 10.1097/WON.0b013e3181f90a34. [DOI] [PubMed] [Google Scholar]
- 5.Haesler E, editor. National Pressure Ulcer Advisory Panel, European Pressure Ulcer Advisory Panel, Pan Pacific Pressure Injury Alliance. Prevention and treatment of pressure ulcers: clinical practice guideline. Osborne Park, Western Australia: Cambrighe Media; 2014. [Google Scholar]
- 6.Halfens RJ, Bours GJ, Van Ast W. Relevance of the diagnosis “stage 1 pressure ulcer”: an empirical study of the clinical course of stage 1 ulcers in acute care and long-term care hospital populations. J Clin Nurs. 2001;10(6):748–757. doi: 10.1046/j.1365-2702.2001.00544.x. [DOI] [PubMed] [Google Scholar]
- 7.Keller BP, Wille J, van Ramshorst B, van der Werken C. Pressure ulcers in intensive care patients: a review of risks and prevention. Intensive Care Med. 2002;28(10):1379–1388. doi: 10.1007/s00134-002-1487-z. [DOI] [PubMed] [Google Scholar]
- 8.Cox J. Pressure ulcer development and vasopressor agents in adult critical care patients: a literature review. Ostomy/Wound Mgmt. 2013;59(4):50–54. 56–60. [PubMed] [Google Scholar]
- 9.Coleman S, Gorecki C, Nelson EA, et al. Patient risk factors for pressure ulcer development: systematic review. Int J Nurs Stud. 2013;50(7):974–1003. doi: 10.1016/j.ijnurstu.2012.11.019. [DOI] [PubMed] [Google Scholar]
- 10.Harrell FE. Regression modeling strategies. New York, NY: Springer; 2001. [Google Scholar]
- 11.Coleman S, Nixon J, Keen J, et al. A new pressure ulcer conceptual framework. J Adv Nurs. 2014;70(10):2222–2234. doi: 10.1111/jan.12405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eden J, Levit L, Berg A, Morton S, editors. Finding what works in health care: standards for systematic reviews. National Academies Press; Washington, DC: 2011. [PubMed] [Google Scholar]
- 13.Rappl LM. Physiological changes in tissues denervated by spinal cord injury tissues and possible effects on wound healing. Int Wound J. 2008;5(3):35–444. doi: 10.1111/j.1742-481X.2007.00360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shamliyan T, Kane RL, Dickinson S. A systematic review of tools used to assess the quality of observational studies that examine incidence or prevalence and risk factors for diseases. J Clin Epidemiol. 2010;63(10):1061–1070. doi: 10.1016/j.jclinepi.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Sanderson S, Tatt ID, Higgins JP. Tools for assessing quality and susceptibility to bias in observational studies in epidemiology: a systematic review and annotated bibliography. Int J Epidemiol. 2007;36(3):666–676. doi: 10.1093/ije/dym018. [DOI] [PubMed] [Google Scholar]
- 16.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Fam Med. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 17.Cox J, Roche S. Vasopressors and development of pressure ulcers in adult critical care patients. Am J Crit Care. 2015;24(6):501–510. doi: 10.4037/ajcc2015123. [DOI] [PubMed] [Google Scholar]
- 18.Suriadi F, Sanada H, Sugama J, Thigpen B, Subuh M. Development of a new risk assessment scale for predicting pressure ulcers in an intensive care unit. Nurs Crit Care. 2008;13(1):34–43. doi: 10.1111/j.1478-5153.2007.00250.x. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien DD, Shanks AM, Talsma A, Brenner PS, Ramachandran SK. Intraoperative risk factors associated with postoperative pressure ulcers in critically ill patients: a retrospective observational study. Crit Care Med. 2014;42(1):40–47. doi: 10.1097/CCM.0b013e318298a849. [DOI] [PubMed] [Google Scholar]
- 20.Cox J. Predictors of pressure ulcers in adult critical care patients. Am J Crit Care. 2011;20(5):364–375. doi: 10.4037/ajcc2011934. [DOI] [PubMed] [Google Scholar]
- 21.Manzano F, Navarro MJ, Roldán D, et al. Pressure ulcer incidence and risk factors in ventilated intensive care patients. J Crit Care. 2010;25(3):469–476. doi: 10.1016/j.jcrc.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Nijs N, Toppets A, Defloor T, Bernaerts K, Milisen K, Van Den Berghe G. Incidence and risk factors for pressure ulcers in the intensive care unit. J Clin Nurs. 2009;18(9):1258–1266. doi: 10.1111/j.1365-2702.2008.02554.x. [DOI] [PubMed] [Google Scholar]
- 23.Kaitani T, Tokunaga K, Matsui N, Sanada H. Risk factors related to the development of pressure ulcers in the critical care setting. J Clin Nurs. 2010;19(3–4):414–421. doi: 10.1111/j.1365-2702.2009.03047.x. [DOI] [PubMed] [Google Scholar]
- 24.Sayar S, Turgut S, Dogan H, et al. Incidence of pressure ulcers in intensive care unit patients at risk according to the Waterlow scale and factors influencing the development of pressure ulcers. J Clin Nurs. 2009;18(5):765–774. doi: 10.1111/j.1365-2702.2008.02598.x. [DOI] [PubMed] [Google Scholar]
- 25.Tayyib N, Coyer F, Lewis P. Saudi Arabian adult intensive care unit pressure ulcer incidence and risk factors: a prospective cohort study. Int Wound J. 2015 doi: 10.1111/iwj.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Theaker C, Mannan M, Ives N, Soni N. Risk factors for pressure sores in the critically ill. Anaesthesia. 2000;55(3):221–224. doi: 10.1046/j.1365-2044.2000.01216.x. [DOI] [PubMed] [Google Scholar]
- 27.Ulker Efteli E, Yapucu Gunes U. A prospective, descriptive study of risk factors related to pressure ulcer development among patients in intensive care units. Ostomy/Wound Mgmt. 2013;59(7):22–27. [PubMed] [Google Scholar]
- 28.Compton F, Hoffmann F, Hortig T, et al. Pressure ulcer predictors in ICU patients: nursing skin assessment versus objective parameters [corrected] [published erratum appears in J Wound Care. 2008;17(11):493] J Wound Care. 2008;17(10):417. doi: 10.12968/jowc.2008.17.10.31304. [DOI] [PubMed] [Google Scholar]
- 29.Fife C, Otto G, Capsuto EG, et al. Incidence of pressure ulcers in a neurologic intensive care unit. Crit Care Med. 2001;29(2):283–290. doi: 10.1097/00003246-200102000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Yepes D, Molina F, León W, Pérez E. Incidence and risk factors in critically ill patients. Medicina Intensiva. 2009;33(6):276–281. doi: 10.1016/S0210-5691(09)72195-3. [DOI] [PubMed] [Google Scholar]
- 31.Eachempati SR, Hydo LJ, Barie PS. Factors influencing the development of decubitus ulcers in critically ill surgical patients. Crit Care Med. 2001;29(9):1678–1682. doi: 10.1097/00003246-200109000-00004. [DOI] [PubMed] [Google Scholar]
- 32.Denby A, Rowlands A. Stop them at the door: should a pressure ulcer prevention protocol be implemented in the emergency department? J Wound Ostomy Continence Nurs. 2010;37(1):35–38. doi: 10.1097/WON.0b013e3181c68b4b. [DOI] [PubMed] [Google Scholar]
- 33.Brienza D, Antokal S, Herbe L, et al. Friction-induced skin injuries—are they pressure ulcers? an updated NPUAP white paper. J Wound Ostomy Continence Nurs. 2015;42(1):62–64. doi: 10.1097/WON.0000000000000102. [DOI] [PubMed] [Google Scholar]
- 34.Manorama A, Meyer R, Wiseman R, Bush TR. Quantifying the effects of external shear loads on arterial and venous blood flow: implications for pressure ulcer development. Clin Biomech (Bristol, Avon) 2013;28(5):574–578. doi: 10.1016/j.clinbiomech.2013.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Braden B, Bergstrom N. A conceptual schema for the study of the etiology of pressure sores. Rehab Nurs. 1987;12(1):8–12. doi: 10.1002/j.2048-7940.1987.tb00541.x. [DOI] [PubMed] [Google Scholar]
- 36.Bly D, Schallom M, Sona C, Klinkenberg D. A model of pressure, oxygenation, and perfusion risk factors for pressure ulcers in the intensive care unit. Am J Crit Care. 2016;25(2):156–164. doi: 10.4037/ajcc2016840. [DOI] [PubMed] [Google Scholar]
- 37.Allman RM, Goode PS, Burst N, Bartolucci AA, Thomas DR. Pressure ulcers, hospital complications, and disease severity: impact on hospital costs and length of stay. Adv Wound Care. 1999;12(1):22–30. [PubMed] [Google Scholar]
- 38.Breslow MJ, Badawi O. Severity scoring in the critically ill: part 1—interpretation and accuracy of outcome prediction scoring systems. Chest. 2012;141(1):245–252. doi: 10.1378/chest.11-0330. [DOI] [PubMed] [Google Scholar]
- 39.Beeckman D, Van Lancker A, Van Hecke A, Verhaeghe S. A systematic review and meta-analysis of incontinence-associated dermatitis, incontinence, and moisture as risk factors for pressure ulcer development. Res Nurs Health. 2014;37(3):204–218. doi: 10.1002/nur.21593. [DOI] [PubMed] [Google Scholar]
- 40.Gordon AC, Russell JA. Vasopressin guidelines in surviving sepsis campaign: 2012. Crit Care Med. 2013;41(12):e482–483. doi: 10.1097/CCM.0b013e3182916fd3. [DOI] [PubMed] [Google Scholar]
- 41.McClave SA, Taylor BE, Martindale RG, et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (ASPEN) JPEN J Parenter Enteral Nutr. 2016;40(2):159–211. doi: 10.1177/0148607115621863. [DOI] [PubMed] [Google Scholar]
- 42.Tschannen D, Bates O, Talsma A, Ying G. Patient-specific and surgical characteristics in the development of pressure ulcers. Am J Crit Care. 2012;21(2):116–125. doi: 10.4037/ajcc2012716. [DOI] [PubMed] [Google Scholar]


