Abstract
The gastrointestinal (GI) tract has a central role in nutritional homeostasis, as location for food ingestion, digestion and absorption, with the gut endocrine system responding to and regulating these events, as well as influencing appetite. One key GI hormone with the full spectrum of these activities is cholecystokinin (CCK), a peptide released from neuroendocrine I cells scattered through the proximal intestine in response to fat and protein, with effects to stimulate gall bladder contraction and pancreatic exocrine secretion, to regulate gastric emptying and intestinal transit, and to induce satiety. There has been interest in targeting the type 1 CCK receptor (CCK1R) for drug development to provide non-caloric satiation as an aid to dieting and weight loss; however, there have been concerns about CCK1R agonists related to side effects and potential trophic impact on the pancreas. A positive allosteric modulator (PAM) of CCK action at this receptor without intrinsic agonist activity could provide a safer and more effective approach to long-term administration. In addition, CCK1R stimulus–activity coupling has been shown to be negatively affected by excess membrane cholesterol, a condition described in the metabolic syndrome, thereby potentially interfering with an important servomechanism regulating appetite. A PAM targeting this receptor could also potentially correct the negative impact of cholesterol on CCK1R function. We will review the molecular basis for binding natural peptide agonist, binding and action of small molecules within the allosteric pocket, and the impact of cholesterol. Novel strategies for taking advantage of this receptor for the prevention and management of obesity will be reviewed.
Introduction
The prevalence of obesity has been progressively increasing throughout the United States and around the world, contributing to a parallel increase in the prevalence of type 2 diabetes mellitus and its associated comorbidities.1 These problems have been responsible for extraordinary morbidity, suffering, loss in productivity and premature mortality. In spite of major efforts to develop effective weight reduction diets, diet aids, medications, medical devices and surgical procedures, the trajectory for this continues in the wrong direction. Bariatric surgery has proven to be quite effective in patients with morbid obesity;2 however, this is quite expensive and not scalable, certainly not keeping up with the increasing prevalence of the most severe end of this clinical continuum. The activity of acute dieting and exercise has been similarly effective in inducing weight loss; however, it has not been durable, with an extremely high incidence of regaining weight to or even beyond the starting point. After a long hiatus in approved drugs, three new diet medications have recently been approved,3, 4, 5 but all carry concerns about safety, and there are requirements for registration and careful clinical follow-up, resulting in use for highly selected subjects who are not surgical candidates and in whom the risks associated with their level of obesity are greater than the risks associated with these agents.
There is clearly a need for new, safer and more effective medications that can effectively reduce appetite and the quantity of food ingested. As this is such a pervasive problem affecting so many people and often providing life-long challenges at weight control, there is a very high bar for regulatory approval, with such drugs needing to be extremely safe and highly effective. One place to look for clues to such agents is in normal physiology. The gastrointestinal (GI) tract is an important source of bioactive agents that have various roles in nutrient assimilation, coordinating the regulation of digestive processes, motility, absorption and even appetite control. GI hormones and neuronal transmitters have even been implicated in some of the successful outcomes of bariatric surgery.6
Cholecystokinin physiology
One GI hormone that was recognized as having a role in appetite control is cholecystokinin (CCK).7 This is a peptide hormone synthesized and secreted from neuroendocrine I cells scattered along the proximal two-thirds of the small intestine.8 It is released in response to luminal nutrients, with fat and protein most potent. Physiological effects of this hormone are to stimulate pancreatic exocrine secretion and gall bladder contraction that are so critical for digestion of these nutrients, regulation of gastric emptying and bowel transit to titrate the delivery of nutrients so as not to overwhelm the gut digestive and absorptive machinery, and even stimulation of satiety centers in the brain as a result of acting on receptors expressed on vagal afferent neurons.9, 10
Cholecystokinin and satiety
In a classical series of studies by Gibbs et al.11 in 1973, CCK was shown to reduce food intake when administered to rats. This observation was subsequently reproduced in various species, including humans.12 In addition, this effect was also observed for peptoid13 and non-peptidyl CCK-like agonists.14 The type 1 CCK receptor (CCK1R), a class A G protein-coupled receptor, became a very popular target for the pharmaceutical industry to focus on for the development of non-caloric satiety agents in the form of CCK-like agonists.10, 15, 16 Indeed, multiple companies were successful in developing small-molecule CCK agonists, with some of these agents entering into clinical trials.17, 18, 19, 20 Whereas those candidates that had been shown to be quite active in vitro in cell studies and in vivo in animal studies were also shown to reduce food intake in human clinical trials, these drugs were no better than acute dieting, the requirement that the Food and Drug Administration had in place for the approval of a diet drug. None of these agents advanced to being approved for clinical use. This decision was probably also contributed to by concerns about on-target side effects and potential toxicity for CCK1R-active agonists, particularly associated with the most potent and long lasting of these agents. Some of these drugs had a tendency to induce abdominal cramping, nausea and diarrhea, all explained by physiologic effects of this hormone. In addition, there has been a concern about potential induction of pancreatitis, with CCK (caerulein) hyperstimulation being a prominent investigative strategy to induce experimental pancreatitis in rodents,21 and the trophic effect of this hormone to stimulate pancreatic cell growth,22, 23 with concern about induction and/or progression of pancreatic cancer. These toxic effects have not been observed in clinical trials with these drugs. The pancreatitis is likely species-specific, as the rodent pancreatic acinar cells express large numbers of CCK1Rs, whereas the human pancreatic acinar cells express few, if any, of these receptors.24, 25, 26
Allosteric modulation of receptors
A different pharmacologic strategy that may overcome all of these concerns has come into popularity recently. This involves receptor-active drugs acting via allosteric modulation,27 rather than acting at the orthosteric site of action of the natural agonists of specific receptor targets. This approach has the advantage of increased selectivity, as allosteric modulators act at evolutionarily less well-conserved sites within the receptor.28 They also can possess a subset of biological actions of the natural agonist,28 and can even exhibit no intrinsic agonist activity at all.29 Their modulatory role can be positive, negative or even neutral as well.27, 28 In thinking about this broad spectrum of activity, it is intriguing to imagine the unique utility of a positive allosteric modulator (PAM) of the CCK1R with no intrinsic agonist activity. Such an agent would only act for a short and finite period of time after ingestion of a meal, when nutrients start to empty into the duodenum and are able to stimulate an increase in CCK levels. This hormone has a very short half-life in the circulation, measured in minutes.30 This is also the ideal time to stimulate a satiety response when it would be able to limit the size of the meal ingested. Because of the short duration of action of CCK when the physiological hormone needs to be active, accentuation of that response might increase appetite suppression, while not being overly stimulatory and certainly not acting over a longer duration of time that might be associated with trophic effects.31, 32, 33
Characterization of the orthosteric natural peptide ligand-binding site
As a first step toward the development of an allosteric modulator, it is important to understand how the orthosteric natural agonist binds and acts at its receptor. CCK, like most peptide hormones, approaches the CCK1R from outside the membrane. This peptide is hydrophilic and cannot cross the lipid bilayer without pore induction or use of detergents. The binding site for this hormone at the CCK1R, its physiological target for induction of satiety,8 is at the external surface of the lipid bilayer, with contributions by the amino-terminal tail and extracellular loop regions of this receptor.34 This has been established by receptor mutagenesis and chimeric receptor analysis,34, 35 as well as by the direct approach of intrinsic photoaffinity labeling of receptor residues in spatial approximation with residues within the natural peptide ligand.36 With the pharmacophoric region of CCK that is responsible for activity at the CCK1R representing the carboxyl-terminal heptapeptide amide, photoaffinity labeling of this receptor has been successful with probes incorporating a photolabile site of crosslinking at six of these seven positions within this portion of the peptide, as well as just beyond this region at the peptide amino terminus.37 All of these points of experimentally established spatial approximation with specific residues within the CCK1R have been accommodated into working models of the CCK-occupied CCK1R.36 This has even been further constrained with a series of fluorescent analogs of CCK and use of fluorescence resonance energy transfer with a series of fluorescently tagged CCK1R constructs to elucidate and apply 12 distance constraints to this model.38 Of note, the carboxyl-terminal end of CCK in this model resides adjacent to the amino-terminal tail of the receptor, whereas it dips into the bilayer within the helical bundle in a competing model that was based exclusively on indirect effects of mutagenesis.39 This becomes particularly important as the region in which this end of the peptide resides in the alternate model would interfere with the small-molecule-binding site that could be the site of action of allosteric modulators.39
Characterization of the small-molecule ligand-binding pocket
The small-molecule-binding site within the helical bundle has been directly mapped as well. This was achieved with photoaffinity labeling using 14C-labeled benzodiazepine agonist and antagonist ligands.40 It was also confirmed using fluorescence quenching of ligand binding41 and the impact of receptor mutagenesis on ligand binding.42, 43 The allosteric nature of this small-molecule-binding site was further confirmed using pharmacologic analyses, including the kinetics of ligand dissociation and the impact of orthosteric and possible allosteric ligands on the functions of each other.42, 43, 44
Radioiodinated small-molecule CCK1R ligands have been developed45, 46 that provide a means for direct displacement from this allosteric pocket to establish the molecular basis for the binding of small-molecule ligands. This was successfully applied to antagonist ligands to gain insights into the inactive conformation of this pocket. This effort included not only the docking of a single benzodiazepine antagonist ligand, but also a ligand-guided computational approach using many molecules that have been reported and that reside in public databases to gain more general understanding of this pocket.42, 43 This molecular model has substantial predictive power, with the model being able to distinguish ligands of this receptor from those of other receptors, and it was even able to very effectively distinguish ligands binding to the CCK1R from those binding to the very closely related type 2 CCK receptor (CCK2R).
A unique small-molecule ligand that is an agonist at the CCK1R and an antagonist at the CCK2R provided a very powerful tool to apply similar techniques to the active conformation of this same allosteric pocket.47 This, too, was used in a ligand-directed computational effort to generalize our understanding of the active conformation of this pocket.43 It also provided molecular insights into the determinants of biological activity, with evidence for a key interaction between a ‘trigger functionality’ within a benzodiazepine agonist and the receptor residues it likely interacts with.20 Once again, this model had great predictive power, being able to effectively distinguish CCK1R agonists from CCK1R antagonists.43 This has provided clear evidence of the differences in the shape of this pocket when in inactive and active conformations.
Development of a CCK1R PAM without intrinsic agonist activity
A number of strategies are possible to identify and/or develop a PAM without intrinsic agonist activity. It is possible that one or more of the existing small-molecule agonist ligands already has PAM activity. This needs to be explored. If so, the molecular basis of that ligand could be examined using analogous approaches to those described above, with the resulting model contrasted with the active and inactive conformations of this pocket, with this ultimately utilized in an in silico drug discovery ligand-screening effort. Similarly, if such an agonist with PAM activity were identified, the ‘agonist trigger’ could be modified in a medicinal chemical approach to modification of such a ligand in a drug development effort. Finally, a high-throughput screening effort would be possible. It is important to recognize that most, if not all, of the efforts to develop CCK1R-active drugs for appetite control that were utilized previously were designed to seek full agonists and would have almost certainly missed PAMs having no intrinsic agonist activity.
Lateral allosterism of membrane receptors
Just as drugs can act as guest allosteric modulators, there is also a phenomenon that has been called lateral allosterism in which interacting membrane proteins or lipids within the bilayer can also act as allosteric modulators of a membrane receptor, such as a G protein-coupled receptor.48 Indeed, the first indication that this could be relevant to the CCK1R came from a set of observations in patients with cholesterol gallstones.49 It was shown that gall bladders from such patients had abnormal contractile responses to CCK.49 In contrast, gall bladders from patients having pigment gallstones had normal responses to this hormone.49, 50 A major difference between these two clinical settings is the presence of supersaturated cholesterol in lithogenic bile.51 It was postulated that the cholesterol could get into the membrane and be responsible for the gall bladder muscle dysfunction.52 Indeed, direct quantitation of the level of cholesterol in the membrane of gall bladder smooth muscle cells in patients with cholesterol gallstones was found to be elevated, and extraction of the cholesterol from these cells resulted in normalization of the contractile response to CCK.52, 53 In addition, an animal model in which prairie dogs were fed high-cholesterol diets and then went on to develop cholesterol gallstones exhibited all the same features, with increased gall bladder membrane cholesterol, abnormal CCK responsiveness and normalization upon extraction of the cholesterol.54
Impact of membrane cholesterol on CCK1R structure and function
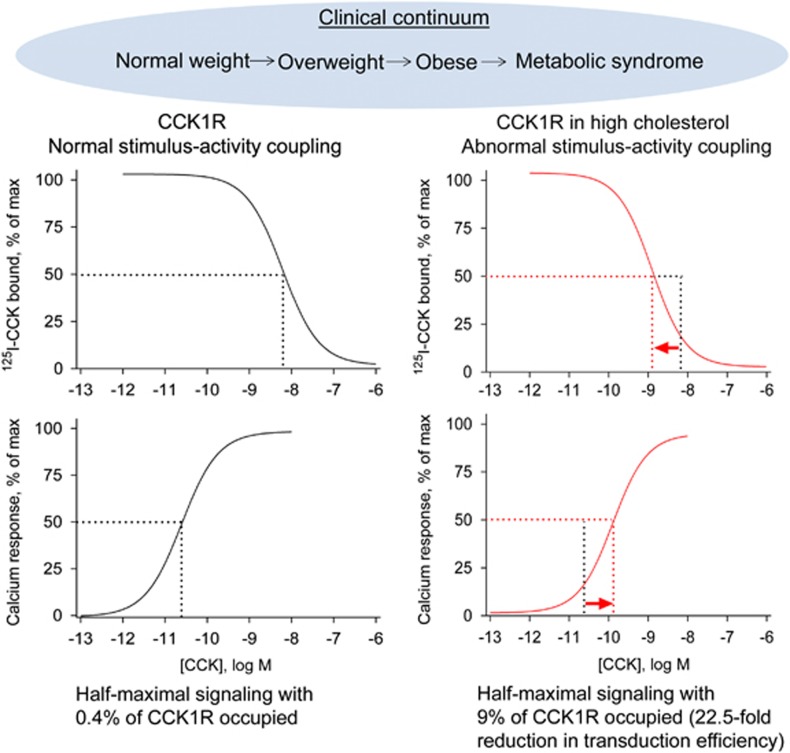
A series of studies was done in model cell systems in which the CCK1R could be expressed and the molecular mechanism of the impact of the cholesterol could be studied.55, 56 In these studies, the impact of the cholesterol was reproduced, with elevated cholesterol resulting in higher-affinity CCK binding and lower signaling responsiveness to CCK (Figure 1).56 This was shown to be specific to the CCK1R, with the CCK2R being resistant to the deleterious effects of cholesterol.56 It is helpful to think about the CCK1R as having three dominant domains, the extracellular domain where the natural peptide ligand binds, the intracellular domain where the heterotrimeric G protein interacts with the receptor, and the intramembranous core helical bundle domain in which a network of hydrogen bonds helps to stabilize conformations and to mediate conformational changes associated with agonist binding and activation (Figure 2). Clearly, the impact of the cholesterol is to cause a conformational change in this receptor that renders the latter domain dysfunctional. In that situation, CCK binds with higher than normal affinity, yet the G protein is not stabilized in a receptor-associated state where its activation can be initiated. This suggests that the major defect when expressed in a high-cholesterol membrane environment is in the transduction function of the core helical bundle of this receptor.
Figure 1.
CCK1R dysfunction in the obesity continuum. Shown is the CCK1R dysfunction in the clinical continuum that initially starts with susceptibility to gain weight, and eventually leads to obesity and metabolic syndrome. In the earlier stages, CCK1R exhibits normal binding and stimulus–activity coupling, whereas during the later stages there is a significant increase in membrane cholesterol, which results in an increased binding affinity, but reduced G protein coupling efficiency, with 22.5-fold more receptors required to achieve the same half-maximal response. Dotted lines indicate the IC50 and EC50 values at normal and high levels of membrane cholesterol, respectively.
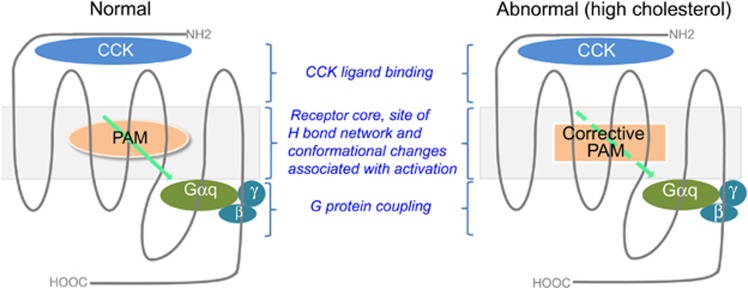
Figure 2.
Strategy for correcting abnormal stimulus–activity coupling at the CCK1R. When the CCK1R is present in a normal membrane microenvironment and functions normally, such as in normal weight people or those with a tendency to gain weight who are at the early stages of this clinical continuum, there could be clinical benefit from a PAM devoid of intrinsic activity that might augment CCK-stimulated satiety responses (left). In the later stages of this continuum when people become obese and may have metabolic syndrome, and in which CCK1R stimulus–activity coupling can be compromised because of the presence of high membrane cholesterol, a ‘corrective PAM’ might be beneficial, and perhaps even necessary, to recalibrate the function of the CCK1R (right).
A number of tools have been developed to help provide additional insights into this abnormal state of the CCK1R in a high-cholesterol environment. Methods to increase the cholesterol in the cell membrane using methyl-β-cyclodextrin–cholesterol complex or LDL were established and applied to CCK1R-bearing cells.56 Cells in which mutations in the lipid synthetic machinery were present and that resulted in elevated cholesterol were further engineered to express the CCK1R.57 In addition, a CCK1R mutant affecting a residue at the bottom of TM3, near the DRY ionic lock mechanism and involving a cholesterol-binding motif, was shown to have all of the structural and functional effects typical of the wild-type CCK1R in a high-cholesterol environment.55 This turned out to be a very powerful new tool. It could be used in analogous approaches to that used to characterize the allosteric pocket in agonist and antagonist modes, to try to characterize the abnormal pocket in the setting of elevated membrane cholesterol. Indeed, this was done and it showed that this pocket was in a different conformation than either the agonist or antagonist pockets in a normal membrane.55 This raised the extremely interesting possibility that unique drugs would have to be developed for this situation.
Membrane cholesterol composition in obesity
It is noteworthy that cellular and membrane cholesterol have been reported to be elevated in obesity and metabolic syndrome.58, 59 If this holds true, it is possible that certain patients who likely are quite advanced along the clinical continuum that starts with tendency toward mild weight gain and dieting, and moves toward obesity and metabolic syndrome have enough excess cholesterol in their cell membranes to interfere with the CCK satiety servomechanism (Figure 1). It is possible that these patients will need a ‘corrective PAM’ without intrinsic agonist activity, representing a second drug, to complement the PAMs without intrinsic agonist activity that act at the normal receptor in a normal membrane environment. It will ultimately be important to determine whether this situation of an abnormal CCK1R conformation really exists clinically, and if so, which patients would benefit from and/or require a second type of drug.
The location of the relevant CCK1R for the treatment of obesity is believed to be on vagal afferent neurons.10 Activation of this receptor has been shown in experimental models to result in stimulation of a variety of central nervous system nuclei, including the nucleus of the solitary tract that is involved in appetite control.60, 61, 62 Unfortunately, direct characterization of this receptor in intact human subjects is difficult or impossible. Such an analysis will require creative approaches to establish the sensitivity of this particular receptor to CCK and to determine whether the abnormal CCK stimulus–activity coupling observed on the gall bladder muscle cells in the setting of cholesterol gallstone disease and in vitro in model cells with elevated membrane cholesterol might also be relevant to the vagal afferent receptors.
The true test will ultimately require the development of drugs, such as are discussed above, with activity to act as a ‘corrective PAM’ without intrinsic agonist activity at the CCK1R, which can also gain access to the vagal afferent receptors. These can be tested in clinical trials to determine whether they are active at the physiologically relevant target and whether this treatment will be useful to facilitate weight reduction in patients far along the clinical continuum toward obesity and metabolic syndrome.
Conclusions
CCK is a physiologically important GI peptide hormone with multiple actions that support nutritional homeostasis, including the stimulation of satiety after a meal when nutrients that stimulate its release enter the proximal small intestine. The potent and prolonged activation of the CCK1R on vagal afferent neurons by full agonists of the CCK1R carries the risk of side effects and toxicity that can potentially be circumvented and avoided by drugs acting as PAMs without intrinsic agonist activity at this receptor. These agents would only be active during a finite and physiologically relevant point in time when CCK action could be augmented to limit the size of the meal being ingested. The case is developed that different types of PAMs without intrinsic agonist action might be necessary to serve the full clinical continuum in which early on, when people are struggling to maintain a normal weight, a simple such drug would suffice, while later in this course, when people might be obese with or without metabolic syndrome, their CCK1R conformation and stimulus–activity coupling might be so abnormal that a ‘corrective PAM’ might be necessary. It is possible that both of these activities might be satisfied by the same drug, but currently no agents with such actions have yet been recognized. It will be critical to develop and evaluate these types of drugs in an effort to address the particularly difficult and refractory public health problem of obesity, with its extensive disease associations and comorbidities.
Acknowledgments
This work was supported by a grant from the National Institutes of Health (DK032878) and by the Mayo Clinic. Publication of this article was sponsored by the Université Laval’s Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments and prevention of obesity.
Footnotes
The authors declare no conflict of interest.
References
- Forouhi NG, Wareham NJ. The EPIC-InterAct Study: a study of the interplay between genetic and lifestyle behavioual factors on the risk of type 2 diabetes in European populations. Curr Nutr Rep 2014; 3: 355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christou NV, Sampalis JS, Liberman M, Look D, Auger S, McLean AP et al. Surgery decreases long-term mortality, morbidity, and health care use in morbidly obese patients. Ann Surg 2004; 240: 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim GW, Lin JE, Blomain ES, Waldman SA. Antiobesity pharmacotherapy: new drugs and emerging targets. Clin Pharmacol Ther 2014; 95: 53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda-Clausen CF, Padwal RS, Sharma AM. New pharmacological approaches for obesity management. Nat Rev Endocrinol 2013; 9: 467–478. [DOI] [PubMed] [Google Scholar]
- Shyh G, Cheng-Lai A. New antiobesity agents: lorcaserin (Belviq) and phentermine/topiramate ER (Qsymia). Cardiol Rev 2014; 22: 43–50. [DOI] [PubMed] [Google Scholar]
- Pournaras DJ, Le Roux CW. The effect of bariatric surgery on gut hormones that alter appetite. Diabetes Metab 2009; 35: 508–512. [DOI] [PubMed] [Google Scholar]
- Little TJ, Horowitz M, Feinle-Bisset C. Role of cholecystokinin in appetite control and body weight regulation. Obes Rev 2005; 6: 297–306. [DOI] [PubMed] [Google Scholar]
- Chandra R, Liddle RA. Cholecystokinin. Curr Opin Endocrinol Diabetes Obes 2007; 14: 63–67. [DOI] [PubMed] [Google Scholar]
- Simasko SM, Wiens J, Karpiel A, Covasa M, Ritter RC. Cholecystokinin increases cytosolic calcium in a subpopulation of cultured vagal afferent neurons. Am J Physiol Regul Integr Comp Physiol 2002; 283: R1303–R1313. [DOI] [PubMed] [Google Scholar]
- Li Y, Owyang C. Endogenous cholecystokinin stimulates pancreatic enzyme secretion via vagal afferent pathway in rats. Gastroenterology 1994; 107: 525–531. [DOI] [PubMed] [Google Scholar]
- Gibbs J, Young RC, Smith GP. Cholecystokinin decreases food intake in rats. J Comp Physiol Psychol 1973; 84: 488–495. [DOI] [PubMed] [Google Scholar]
- Ballinger AB, Clark ML. L-phenylalanine releases cholecystokinin (CCK) and is associated with reduced food intake in humans: evidence for a physiological role of CCK in control of eating. Metabolism 1994; 43: 735–738. [DOI] [PubMed] [Google Scholar]
- Elliott RL, Kopecka H, Bennett MJ, Shue YK, Craig R, Lin CW et al. Tetrapeptide CCK agonists: structure-activity studies on modifications at the N-terminus. J Med Chem 1994; 37: 309–313. [DOI] [PubMed] [Google Scholar]
- Elliott RL, Cameron KO, Chin JE, Bartlett JA, Beretta EE, Chen Y et al. Discovery of N-benzyl-2-[(4S)-4-(1H-indol-3-ylmethyl)-5-oxo-1-phenyl-4,5-dihydro-6H-[1,2,4]triazolo[4,3-a][1,5]benzodiazepin-6-yl]-N-isopropylacetamide, an orally active, gut-selective CCK1 receptor agonist for the potential treatment of obesity. Bioorg Med Chem Lett 2010; 20: 6797–6801. [DOI] [PubMed] [Google Scholar]
- Chaudhri OB, Salem V, Murphy KG, Bloom SR. Gastrointestinal satiety signals. Annu Rev Physiol 2008; 70: 239–255. [DOI] [PubMed] [Google Scholar]
- Dockray GJ. Cholecystokinin and gut-brain signalling. Regul Pept 2009; 155: 6–10. [DOI] [PubMed] [Google Scholar]
- Berger R, Zhu C, Hansen AR, Harper B, Chen Z, Holt TG et al. 2-Substituted piperazine-derived imidazole carboxamides as potent and selective CCK1R agonists for the treatment of obesity. Bioorg Med Chem Lett 2008; 18: 4833–4837. [DOI] [PubMed] [Google Scholar]
- Sherrill RG, Berman JM, Birkemo L, Croom DK, Dezube M, Ervin GN et al. 1,4-Benzodiazepine peripheral cholecystokinin (CCK-A) receptor agonists. Bioorg Med Chem Lett 2001; 11: 1145–1148. [DOI] [PubMed] [Google Scholar]
- Zhu C, Hansen AR, Bateman T, Chen Z, Holt TG, Hubert JA et al. Discovery of imidazole carboxamides as potent and selective CCK1R agonists. Bioorg Med Chem Lett 2008; 18: 4393–4396. [DOI] [PubMed] [Google Scholar]
- Aquino CJ, Armour DR, Berman JM, Birkemo LS, Carr RA, Croom DK et al. Discovery of 1,5-benzodiazepines with peripheral cholecystokinin (CCK-A) receptor agonist activity. 1. Optimization of the agonist ‘trigger’. J Med Chem 1996; 39: 562–569. [DOI] [PubMed] [Google Scholar]
- Niederau C, Ferrell LD, Grendell JH. Caerulein-induced acute necrotizing pancreatitis in mice: protective effects of proglumide, benzotript, and secretin. Gastroenterology 1985; 88: 1192–1204. [DOI] [PubMed] [Google Scholar]
- Solomon TE, Petersen H, Elashoff J, Grossman MI. Interaction of caerulein and secretin on pancreatic size and composition in rat. Am J Physiol 1978; 235: E714–E719. [DOI] [PubMed] [Google Scholar]
- Mainz D, Webster PD. Pancreatic carcinoma. A review of etiologic considerations. Am J Dig Dis 1974; 19: 459–464. [DOI] [PubMed] [Google Scholar]
- Tang C, Biemond I, Lamers CB. Cholecystokinin receptors in human pancreas and gallbladder muscle: a comparative study. Gastroenterology 1996; 111: 1621–1626. [DOI] [PubMed] [Google Scholar]
- Ji B, Bi Y, Simeone D, Mortensen RM, Logsdon CD. Human pancreatic acinar cells do not respond to cholecystokinin. Pharmacol Toxicol 2002; 91: 327–332. [DOI] [PubMed] [Google Scholar]
- Murphy JA, Criddle DN, Sherwood M, Chvanov M, Mukherjee R, McLaughlin E et al. Direct activation of cytosolic Ca2+ signaling and enzyme secretion by cholecystokinin in human pancreatic acinar cells. Gastroenterology 2008; 135: 632–641. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat Rev Drug Discov 2009; 8: 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wootten D, Christopoulos A, Sexton PM. Emerging paradigms in GPCR allostery: implications for drug discovery. Nat Rev Drug Discov 2013; 12: 630–644. [DOI] [PubMed] [Google Scholar]
- Nemeth EF, Heaton WH, Miller M, Fox J, Balandrin MF, Van Wagenen BC et al. Pharmacodynamics of the type II calcimimetic compound cinacalcet HCl. J Pharmacol Exp Ther 2004; 308: 627–635. [DOI] [PubMed] [Google Scholar]
- Thompson JC, Fender HR, Ramus NI, Villar HV, Rayford PL. Cholecystokinin metabolism in man and dogs. Ann Surg 1975; 182: 496–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi H, Logsdon CD. Both low- and high-affinity CCK receptor states mediate trophic effects on rat pancreatic acinar cells. Am J Physiol 1993; 265: G1177–G1181. [DOI] [PubMed] [Google Scholar]
- Dawra R, Saluja A, Lerch MM, Saluja M, Logsdon C, Steer M. Stimulation of pancreatic growth by cholecystokinin is mediated by high affinity receptors on rat pancreatic acinar cells. Biochem Biophys Res Commun 1993; 193: 814–820. [DOI] [PubMed] [Google Scholar]
- Smith JP, Solomon TE. Cholecystokinin and pancreatic cancer: the chicken or the egg? Am J Physiol 2014; 306: G91–G101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LJ, Gao F. Structural basis of cholecystokinin receptor binding and regulation. Pharmacol Ther 2008; 119: 83–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller LJ, Lybrand TP. Molecular basis of agonist binding to the type A cholecystokinin receptor. Pharmacol Toxicol 2002; 91: 282–285. [DOI] [PubMed] [Google Scholar]
- Dong M, Lam PC, Pinon DI, Abagyan R, Miller LJ. Elucidation of the molecular basis of cholecystokinin peptide docking to its receptor using site-specific intrinsic photoaffinity labeling and molecular modeling. Biochemistry 2009; 48: 5303–5312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XQ, Dolu V, Hadac EM, Holicky EL, Pinon DI, Lybrand TP et al. Refinement of the structure of the ligand-occupied cholecystokinin receptor using a photolabile amino-terminal probe. J Biol Chem 2001; 276: 4236–4244. [DOI] [PubMed] [Google Scholar]
- Harikumar KG, Gao F, Pinon DI, Miller LJ. Use of multidimensional fluorescence resonance energy transfer to establish the orientation of cholecystokinin docked at the type A cholecystokinin receptor. Biochemistry 2008; 47: 9574–9581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer-Lahlou E, Tikhonova I, Escrieut C, Dufresne M, Seva C, Pradayrol L et al. Modeled structure of a G-protein-coupled receptor: the cholecystokinin-1 receptor. J Med Chem 2005; 48: 180–191. [DOI] [PubMed] [Google Scholar]
- Hadac EM, Dawson ES, Darrow JW, Sugg EE, Lybrand TP, Miller LJ. Novel benzodiazepine photoaffinity probe stereoselectively labels a site deep within the membrane-spanning domain of the cholecystokinin receptor. J Med Chem 2006; 49: 850–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Cawston EE, Miller LJ. Fluorescence polarization screening for allosteric small molecule ligands of the cholecystokinin receptor. Assay Drug Dev Technol 2011; 9: 394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cawston EE, Lam PC, Harikumar KG, Dong M, Ball AM, Augustine ML et al. Molecular basis for binding and subtype selectivity of 1,4-benzodiazepine antagonist ligands of the cholecystokinin receptor. J Biol Chem 2012; 287: 18618–18635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Cawston EE, Lam PC, Patil A, Orry A, Henke BR et al. Molecular basis for benzodiazepine agonist action at the type 1 cholecystokinin receptor. J Biol Chem 2013; 288: 21082–21095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Sexton PM, Christopoulos A, Miller LJ. Benzodiazepine ligands can act as allosteric modulators of the type 1 cholecystokinin receptor. Bioorg Med Chem Lett 2008; 18: 4401–4404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akgun E, Korner M, Gao F, Harikumar KG, Waser B, Reubi JC et al. Synthesis and in vitro characterization of radioiodinatable benzodiazepines selective for type 1 and type 2 cholecystokinin receptors. J Med Chem 2009; 52: 2138–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong M, Vattelana AM, Lam PCH, Orry A, Abagyan R, Christopoulos A et al. Development of a highly selective allosteric antagonist radioligand for the type 1 cholecystokinin receptor and elucidation of its molecular basis of binding. Mol Pharmacol 2015; 87: 130–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke BR, Aquino CJ, Birkemo LS, Croom DK, Dougherty Jr RW, Ervin GN et al. Optimization of 3-(1H-indazol-3-ylmethyl)-1,5-benzodiazepines as potent, orally active CCK-A agonists. J Med Chem 1997; 40: 2706–2725. [DOI] [PubMed] [Google Scholar]
- Desai AJ, Miller LJ. Sensitivity of cholecystokinin receptors to membrane cholesterol content. Front Endocrinol 2012; 3: 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar J, Lee KY, Thompson WR, Biancani P. Gallbladder contraction in patients with pigment and cholesterol stones. Gastroenterology 1989; 97: 1479–1484. [DOI] [PubMed] [Google Scholar]
- Xiao ZL, Amaral J, Biancani P, Behar J. Impaired cytoprotective function of muscle in human gallbladders with cholesterol stones. Am J Physiol 2005; 288: G525–G532. [DOI] [PubMed] [Google Scholar]
- McDougall RM, Walker K, Thurston OG. Prolonged secretion of lithogenic bile after cholecystectomy. Ann Surg 1975; 182: 150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao ZL, Chen Q, Amaral J, Biancani P, Jensen RT, Behar J. CCK receptor dysfunction in muscle membranes from human gallbladders with cholesterol stones. Am J Physiol 1999; 276: G1401–G1407. [DOI] [PubMed] [Google Scholar]
- Chen Q, Amaral J, Biancani P, Behar J. Excess membrane cholesterol alters human gallbladder muscle contractility and membrane fluidity. Gastroenterology 1999; 116: 678–685. [DOI] [PubMed] [Google Scholar]
- Yu P, Chen Q, Biancani P, Behar J. Membrane cholesterol alters gallbladder muscle contractility in prairie dogs. Am J Physiol 1996; 271: G56–G61. [DOI] [PubMed] [Google Scholar]
- Desai AJ, Harikumar KG, Miller LJ. A type 1 cholecystokinin receptor mutant that mimics the dysfunction observed for wild type receptor in a high cholesterol environment. J Biol Chem 2014; 289: 18314–18326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter RM, Harikumar KG, Wu SV, Miller LJ. Differential sensitivity of types 1 and 2 cholecystokinin receptors to membrane cholesterol. J Lipid Res 2012; 53: 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harikumar KG, Potter RM, Patil A, Echeveste V, Miller LJ. Membrane cholesterol affects stimulus-activity coupling in type 1, but not type 2, CCK receptors: use of cell lines with elevated cholesterol. Lipids 2013; 48: 231–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seres I, Foris G, Varga Z, Kosztaczky B, Kassai A, Balogh Z et al. The association between angiotensin II-induced free radical generation and membrane fluidity in neutrophils of patients with metabolic syndrome. J Membr Biol 2006; 214: 91–98. [DOI] [PubMed] [Google Scholar]
- Paragh G, Kovacs E, Seres I, Keresztes T, Balogh Z, Szabo J et al. Altered signal pathway in granulocytes from patients with hypercholesterolemia. J Lipid Res 1999; 40: 1728–1733. [PubMed] [Google Scholar]
- Chen DY, Deutsch JA, Gonzalez MF, Gu Y. The induction and suppression of c-fos expression in the rat brain by cholecystokinin and its antagonist L364, 718. Neurosci Lett 1993; 149: 91–94. [DOI] [PubMed] [Google Scholar]
- Monnikes H, Lauer G, Arnold R. Peripheral administration of cholecystokinin activates c-fos expression in the locus coeruleus/subcoeruleus nucleus, dorsal vagal complex and paraventricular nucleus via capsaicin-sensitive vagal afferents and CCK-A receptors in the rat. Brain Res 1997; 770: 277–288. [DOI] [PubMed] [Google Scholar]
- Day HE, McKnight AT, Poat JA, Hughes J. Evidence that cholecystokinin induces immediate early gene expression in the brainstem, hypothalamus and amygdala of the rat by a CCKA receptor mechanism. Neuropharmacology 1994; 33: 719–727. [DOI] [PubMed] [Google Scholar]