Abstract
The microbiota–gut–brain axis is currently being explored in many types of rodent models, including models of behavioral, neurodegenerative and metabolic disorders. Our laboratory is interested in determining the mechanisms and consequences of activation of vagal afferent neurons that lead to activation of parasympathetic reflexes and changes in feeding behavior in the context of obesity. Obesity is associated with microbial dysbiosis, decreased intestinal barrier function, gut inflammation, metabolic endotoxemia, chronic low-grade systemic inflammation and desensitization of vagal afferent nerves. This review will present the evidence that altered gut microbiota together with decreased gut barrier function allows the passage of bacterial components or metabolites in obese individuals, leading to the disruption of vagal afferent signaling and consequently resulting in an increase in body weight. We first review the most recent descriptions of gut microbial dysbiosis due to a high fat diet and describe changes in the gut barrier and the evidence of increased intestinal permeability in obesity. We then will review the evidence to show how manipulating the gut microbiota via pre and probiotics can restore gut barrier function and prevent weight gain. Lastly, we present possible mechanisms by which the microbe–gut–brain axis may have a role in obesity. The studies mentioned in this review have provided new targets to treat and prevent obesity and have highlighted how the microbiota–gut–brain axis is involved.
The gastrointestinal (GI) tract has to balance two seemingly opposing functions. The digestive products of ingested food, including essential macronutrients and micronutrients, ions and water have to be absorbed across the intestinal epithelium. This vast epithelial surface represents a huge surface area that separates the outside from the inside environment and the GI tract has to maintain this barrier, whilst also allowing absorption to occur. This dual function, of absorption and protection, necessitates numerous control mechanisms, including endocrine, paracrine, neural and immune pathways. In order to fulfill these two roles, the gut is endowed with numerous sensory mechanisms to detect luminal contents and initiate changes in physiology and behavior.
Recent attention has focused on the bacteria that reside in the gut and their symbiotic relationship with the host. It is well known that the gut microbiota provide the host with a number of benefits including development of an effective immune system, provide essential vitamins for the host and have a role in the digestion of macronutrients, such as complex carbohydrates, that the host enzymes cannot digest. There is a complex interrelationship between diet, microbes and the host, all of which are dependent on each other in a dynamic way.1 Evidence has emerged to show that the gut microbiota can also influence behavior, such as food intake and anxiety.2
Our laboratory is interested in determining the mechanisms and consequences of activation of vagal afferent neurons, that lead to activation of parasympathetic reflexes and changes in feeding behavior.3 This pathway is important in mediating satiety signals from the gut to the brain to regulate food intake. However, there is evidence that in obesity and in animal models of obesity, there is impairment of signaling in the gut-brain pathway.4, 5, 6 Rats fed a high fat (HF) diet for several weeks have a higher energy intake than low fat fed lean controls.7 This hyperphagia is accompanied by desensitization of vagal afferent neurons to gut satiety hormones such as cholecystokinin (CCK). The mechanism by which these neurons become desensitized to intestinal satiety signals, leading to increased food intake, is unknown. We hypothesize that changes in vagal afferent neurons may be driven by alterations in the gut microbiota and the intestinal epithelium. Obesity is associated with microbial dysbiosis, decreased intestinal barrier function, gut inflammation, metabolic endotoxemia, and chronic low-grade systemic inflammation.8, 9 This review will present the evidence that altered gut microbiota together with decreases in gut barrier function allows the absorption of bacterial components or metabolites in obese individuals, leading to the disruption of vagal afferent signaling and consequently resulting in hyperphagia and increase in body weight.
Altered gut microbiota in obesity
The microbiota is known to digest otherwise indigestible carbohydrates, synthesize essential vitamins, educate the immune system, and protect against pathogens.10 Germ-free C57/Blk6 mice have less body fat than those raised in conventional conditions11 and are resistant to the obesogenic effects of a HF diet.12 Transfer of the microbiota from either lean or obese mice to germ-free mice results in recapitulation of the donor phenotype.13 Obese humans and animal models of obesity have comparable microbial dysbiosis when analyzed at the phylum level.14, 15 However, the specific contributions to body weight regulation of the microbiota at both the genus or phylum level are unknown.
The diversity and abundance of total bacteria increase from the proximal to the distal regions of the gut, with 101 cells per gram in the stomach to over 1012 cells per gram in the colon.16 Each GI region contains a different signature population of microbes and this signature is also influenced by diet.17 The two most abundant phyla in the gut, the Bacteroidetes and Firmicutes, are markedly different in the feces of obese subjects compared with lean controls and in both rodent genetic models of obesity or HF diet-induced obesity.14, 15 The Bacteroidetes/Firmicutes ratio has been shown to be influenced by the host antimicrobial response by removing α-defensin-5 (produced by Paneth cells in the gut wall)18 and obese humans express lower amounts of α-defensin-5.19 Proteobacteria and the lesser known Deferribacteres are two important gram-negative phyla that also increase in obesity.20, 21 Bacteria in the family Enterobacteriaceae, within the Proteobacteria phylum, are both commensal and pathogenic.22 These bacteria have a growth advantage in an inflamed gut due to their ability to use nitrogen as an energy source.23 A recent study investigating a specific species, Enterobacter clocae, isolated from an obese human, found that it alone can cause obesity when inoculated into a germ-free mouse.24 Gram-negative bacteria contain lipopolysaccharide (LPS) within their outer membrane. LPS causes a robust inflammatory response through Toll-like receptor 4 (TLR4), which is located on innate immune cells, intestinal enterocytes,25 enteroendocrine cells,26 and neurons.27 LPS is found at low concentrations in the blood of obese individuals,28, 29 termed metabolic endotoxemia, and it has been hypothesized to have a role in the onset of obesity and also in inflammatory comorbidities. In experimental models of obesity induced by HF feeding, metabolic endotoxemia has also been reported.30 Although high systemic levels of LPS associated with sepsis can induce anorexia, we hypothesized that the low, chronic levels of LPS seen in HF fed rodents might alter vagal afferent neuronal function and induce hyperphagia by inhibition of intestinal satiety signaling 31 which is further discussed below.
Strategies to prevent the growth of gram-negative bacteria have been suggested to help weight loss, yet have not met with great success. For example, antibiotic administration to obese-prone genetic knockout (KO) mice can prevent weight gain32 and reduced adipose tissue;33 however, in humans, antibiotic use has been shown to be associated with increased body mass index34 and increased childhood exposure to antibiotics is associated with obesity rates in later life.35 Other methods (discussed in more detail below) have a greater potential for decreasing the abundance of gram-negative bacteria; for example, short chain fatty acid (SCFA) producing bacteria have been shown to inhibit growth Enterobacteriaceae by lowering gut pH.36
We are interested in how changes in the gut microbiota contribute to the regulation of food intake, possibly by influencing the vagal afferent-gut-brain pathway. Two current hypotheses explaining the possible mechanisms of how the microbiota may influence body weight are:
microbes in obese individuals have an increased ability to extract energy from the diet which is then available to the host compared with the microbes in lean individuals, and
microbes in obese individuals interact with the host to promote chronic low-grade systemic inflammation driving alterations in food intake and the comorbidities seen in obesity.
Data to support the first hypothesis were obtained from studies performed in ob/ob genetically obese mice that had increased amounts of microbes from the Kingdom Archea and decreased energy in their stool.13 However, more recent studies have shown that obesity development does not necessitate microbiota changes that result in increased energy extraction,37 or an increase Archea abundance (personal observations, Figure 1). The latter hypothesis has gained support, yet the origin of inflammation is currently debated. There is evidence to suggest that the gut may be the origin of inflammation early in the response to HF diets.20, 38 We hypothesize that inflammation is observed after intestinal barrier function is disrupted and that alterations in the microbiota–gut–brain axis drive increased food intake leading to weight gain.8
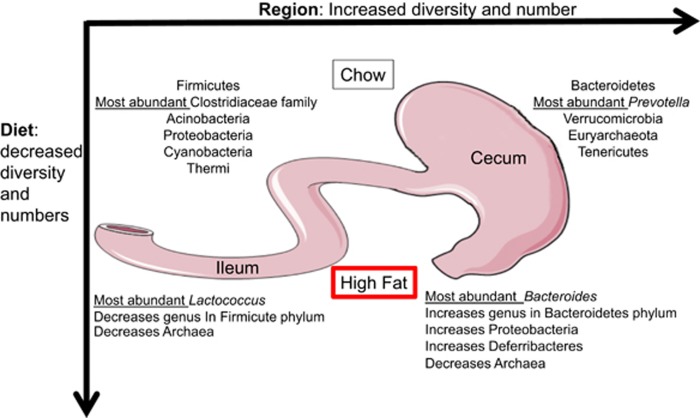
Figure 1.
Signature population of microbes at the phylum level showing significant differences between the small and large intestine. A HF diet reduces diversity, total numbers, and relative abundance of selected region specific microbes. Most abundant genus in each condition is listed in italics.
The altered microbiota in obesity has primarily been studied in rodent obese-prone genetic models, or in rodent models of HF diet-induced obesity, once body weight gain, adiposity, and metabolic disorders are well established.9 However, in order to understand the contribution of the GI tract and the gut microbiota to the initial alteration in food intake that lead to obesity, we described the early changes in the gut microbiota and the intestinal physiology in response to ingestion of a HF diet. We characterized the microbiota signature in the small and large intestine and found that a HF diet had region specific effects (Figure 1). Bacterial diversity decreased throughout the entire intestine; however, in the small intestine, significant changes due to a HF diet showed bacterial genus abundance decreasing, while in the large intestine, bacterial genus increased in abundance. HF diet resulted in the majority of changes in the small intestine to occur within the phylum Firmicutes, at the same time causing changes within the phylum Bacteroidetes in the large intestine.39
Microbe–gut and gut–microbe interactions: gut barrier function
The gut barrier is both an immunological, physiological and physical barrier that is highly regulated, and responsive to internal and external stimuli, such as hormones and nutrients. The immunological barrier consists of antimicrobial peptides released from Paneth cells in the intestinal wall, the immune cells within the lamina propria and Peyer’s patches, and dendritic cells that have projections in between epithelial cells into the gut lumen. The physical barrier consists of the epithelial cells connected via tight junctions, and a layer of mucus produced by goblet cells which keep the majority of luminal contents away from the epithelial cells.
Intestinal permeability is a target of interest in many GI diseases, including obesity and metabolic disease. There is evidence for an increase in intestinal permeability in obese rodents and humans.20, 40 The physical epithelial cell barrier has two routes of passage depending on molecule size. Small molecules (less than 3.6 Å) are able to cross the barrier via the paracellular pathway through pores within the tight junctions. This allows for increased passage of ions and monomers when the intestine is actively absorbing nutrients in the fed state. However, when tight junctions are impaired, larger molecules with radii of at least 7.5 Å are able to cross via the paracellular leak pathway.41 Larger molecules can cross the intestinal epithelium via the transcellular pathway, through either enterocyte endocytosis, immune cells, or goblet cells. The mechanisms of how bacterial components such as LPS, flagellin or peptidoglycan cross the intestinal epithelium during obesity is unknown.42 LPS translocation occurs via chylomicrons along with fat absorption43 and has been hypothesized to cross the leaky intestinal epithelium during obesity,30 however, LPS (28 Å)44 is larger than the paracellular leak pathway pores. Thus, there are three remaining possible reasons that HF fed rodents develop metabolic endotoxemia: LPS translocates due to increased luminal fat from the diet, dysbiosis leads to increased Gram-negative bacteria, or decreased barrier function leading to increased macromolecule transport via the transcellular pathway.
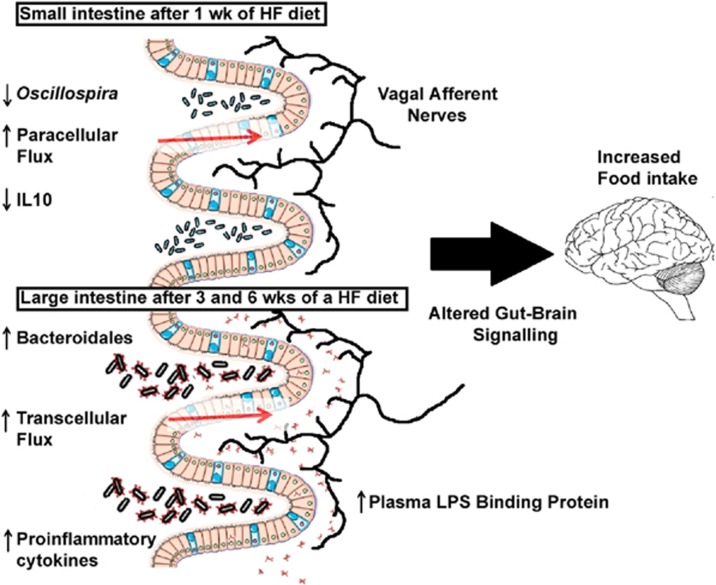
We have recently sought to understand how the paracellular and transcellular pathways are influenced by ingestion of a HF diet. In vivo measurement of intestinal permeability is limited to measuring the appearance of fluorescently tagged probes or lactulose/mannitol ratios in the blood.20, 40 However, this technique provides little information on the location of the deficit or its exact nature (paracellular or transcellular, or both). We utilized an ex vivo approach to understand how ingestion of a HF diet alters gut barrier function in different gut regions and how this correlates with changes in gut microbiota and development of the obese phenotype. We found that paracellular flux in the small intestine increases after one week of a HF diet, yet transcellular flux was unaltered. The response of the large intestine to the HF diet was different; there was no change in either paracellular permeability or transcellular flux after 1 week but there was a significant increase in transcellular flux after 3 and 6 weeks on a HF diet.39 It is of interest to note that this increase in transcellular flux in the large intestine correlated with adiposity and plasma levels of LPS binding protein (Figure 2).
Figure 2.
Schematic representation summarizing our results of a HF diet-induced obesity study. There are region and time dependent changes in microbial signature, gut barrier function, and gut cytokine expression. We hypothesis that decreased barrier function allows for the translocation of bacterial components in which alter vagal afferent nerve behavior and ultimately leads to increased food intake.
The cause and effect relationship between decreased barrier function and increased intestinal inflammation is unclear. Gut inflammation measured with myeloperoxidase, a neutrophil marker, is increased in rats after 12 weeks on a HF diet20 and proinflammatory cytokine (tumor necrosis factor-α) expression in the ileum has been shown to be positively correlated to adiposity, which is significantly increased compared with controls after 6 weeks in mice on a HF diet.38 We showed a positive correlation between proinflammatory cytokine IL-1β expression in the ileum with adiposity. Interestingly, we also showed that anti-inflammatory IL10 decreases in the ileum after one week on a HF diet, preceeding the increases in IL-1β expression. This timing is compelling given that it occurs concurrently with increased paracellular flux and decreased Oscillospira abundance (Figure 2). IL10 KO mice have spontaneous colitis45 and have increased intestinal permeability before the onset of microbiota-induced gut inflammation.46 In vitro studies have shown that adding IL10 to the media will maintain paracellular flux in the presence of inflammatory cytokines.47 IL10 expression in colonic immune cells is increased in rodents given Clostridum. This is particularly interesting because Oscillospira and Clostridum are in the same bacterial order. The association of increased paracellular flux with decreased Oscillospira is intriguing in light of a recent study showing that alcohol dependent subjects with high intestinal permeability had decreased Ruminococcaceae family,48 which includes the genus Oscillospira. These observations are the first step in defining and targeting microbe-host interactions in the altered gut physiology and gut-brain axis in the onset of obesity.
The microbiota and diet may interact with many receptors including Toll-like receptors (TLRs; detecting pathogen-associated molecular patterns) or pregnane X receptor (PXR; detecting xenobiotics) which may influence the gut barrier. HF diet will increase TLR4 (detects LPS) activation on enterocytes20 and TLR4 KO animals are resistant to the obesogenic effects of a HF diet.49 This is in contrast to TLR5 (detects flagellin) KO mice that become hyperphagic and obese on a chow diet.50 It has been debated that saturated fat can activate TLR4;51 however recent evidence has shown that saturated fat stimulates fetuin A secretion from the liver, which then binds to TLR4 and promotes its incorporation into lipid rafts, a relatively more ‘ordered’ area in the plasma membrane enriched with glycosphingolipids and cholesterol.52 TLR2 (detecting lipoteichoic acid) on intestinal dendritic cells has been shown to respond to capsular polysaccharide in outer membrane vesicles produced by Bacteroides fragilis, preventing experimental colitis53 and leaky gut.54 TLR2 has also been implicated to aid in preventing intestinal injury; Lactobacillus rhamnosus administration results in mesenchymal stem cells relocating to the intestinal crypt via TLR2, and increasing epithelial cell proliferation.55 PXR KO mice, unable to respond to ligands from microbial protein fermentation, have increased intestinal permeability; however permeability is restored if TLR4 is also deleted, showing how fermentation products from the microbiota directly can affect gut barrier function.56
Manipulation of the microbiota
Previous work from investigators focused on inflammatory GI diseases have used pre and probiotics to manipulate the gut microbiota and restore gut barrier function. In the last ten years, this approach has been used in studies of obesity and metabolic disease.57 Probiotic administration have also been a useful tool to study obesity in rodents: lactobacillus plantarum or paracasei with high fat feeding, reduced adipocyte size, body fat, and colonic IL10 was increased.58, 59, 60 Prebiotics, fibers that enhance the growth of benificial bacteria, may be a more advantageous way to manipulate the microbiota, eliminating the issue of probiotic survival upon administration. In a meta-analysis of human obesity, increased fiber intake in the obese population led to decreased inflammation, seen through measurement of high-sensitivity C-reactive protein (hsCRP).61 Inulin and oligofructose (a type of inulin with chain length less than 10 fructose molecules) are common prebiotics and have been shown to increase the abundance of beneficial bifidobacteria, prevent increased intestinal permeability, and decreased plasma LPS, food intake and weight gain in obese rodents.30, 62 Recent evidence has shown that oligofructose administration increases the number of goblet cells and mucus layer as well as mucus-associated bacteria.63 Gluco-oligosaccharide (GOS; dietary fiber) has also been shown to reduce cardiovascular disease marker PAI-1 when given with a HF diet.64
SCFA production by commensal bacteria has been hypothesized to be a possible mechanism by which both administration of prebiotics and probiotics might improve disease severity in obesity. Acetate, proprionate and butyrate are the three primary SCFAs produced by bacteria from fermentation in the gut lumen; these SCFAs can also be detected in the blood. Butyrate is of particular interest because it is used as an energy source for colonocytes and has been shown to be effective in the treatment of ulcerative colitis.45 Administration of SCFAs to germ-free mice increases colonic T regulatory cells, which are otherwise depleted in these mice.65 G-protein coupled receptors which detect SCFA (GPR41 and 43) are found on enterocytes, enteroendocrine and immune cells, and neurons. GPR41 (FFAR3) KO mice had reduced peptide YY expression, a gut-derived hormone involved in satiety and intestinal motility.66 Interestingly, mice with a GPR43 KO (FFAR2) fed a HF diet do not weigh significantly more than chow fed controls after 35 weeks; however during the first 20 weeks fed a HF diet, they grow at the same rate as wild-type mice on a HF diet.67 SCFAs are one possible mechanism by which microbes benefit the host; however because they have also been shown to be increased in a human obese population,68 we hypothesize that pre and probiotics may act via other mechanisms to produce beneficial effects. We have preliminary data from experiments in which HF diets were supplemented with either inulin or milk oligosaccharides (MO). MOs are the third largest component in milk,69 refer to more than 200 types of small glycans, and have been shown to enhance the growth of a beneficial microbe, Bifidobacterium longum ssp. Infantis.70 Addition of either MOs or inulin to a HF diet prevented the increase in intestinal permeability and also reduced body weight and adiposity gain.39 Thus, there is evidence for a beneficial effect of prebiotics and probiotics in changing the gut microbiota, improving intestinal barrier function, and reducing body weight. It is interesting to note that human studies using inulin and oligofructose have not achieved the weight loss success as seen in rodent models,71 even though inulin ingestion increases SCFAs.72
Gut–brain and microbe–brain signaling
Enteroendocrine cells (EECs) constitute less than one percent of the epithelial cells in the gut mucosa, yet produce over 30 different peptide hormones that are crucial to regulate gut function, released upon activation of their numerous luminal sensors. Nutrients will activate EECs causing the release of gut peptide hormones which act either in a humoral way on remote targets or act in a paracrine manner on vagal afferent nerves and epithelial cells.73 EECs can sense the microbiota and bile acids through TLRs26 and GPRs. In fact, bile acids have been shown to alter the microbiota and the receptor TGR5, found on EECs alters gut hormone GLP-1 secretion.74 Indeed, bile acids may serve as a new target for obesity.75 Another communication site by which EECs and microbiota interact could be through bitter taste receptors; bitter taste receptors expressed on EECs are differentially expressed after HF feeding.76 Altogether, the data are consistent with the proposal that microbe-host interactions are able to lead to altered hormone secretion. EECs are a possible treatment target for decreased barrier function and obesity via microbe-gut-brain interactions. Evidence to support this is that prebiotic oligofructose treatment is able to increase GLP-2 secretion, which will promote a healthy epithelium by increasing proliferation and mucosal repair.77
The microbiota–gut–brain axis was first explored in rodent models of anxiety. Probiotics (Lactobacillus) decreased corticosterone levels and stress behavior in mice and the beneficial effects of probiotics were attenuated in vagotomized animals.78 Desensitization of the vagal afferent nerves seen in obesity occurs after six weeks on a HF diet in rodents.79 However, we have shown that changes in barrier function happen before six weeks; there is a significant decrease in barrier function and a significant decrease in anti-inflammatory gene expression after only one week on a HF diet.39 Given that vagal afferent nerves express many types of receptors, including TLR4(ref. 27) and GPR40 (FFAR1),80 other studies have sought to find luminal components that can act on vagal afferent nerves. SCFA, propionate, activates vagal afferent nerves from the portal vein in vivo leading to activation of the brain stem, but did not alter food intake.81 Therefore, we tested whether chronic low doses of LPS could be a cause of nerve desensitization. We have recently shown that rodents given osmotic pumps of LPS in the intraperitoneal cavity leads to attenuated CCK-induced satiation and a dysregulation of anorexigenic and orexigenic expression on vagal afferent neurons which coincides with hyperphagia and obesity development.31 LPS exposure to nodose ganglia in vitro will activate SOCS-3, a marker for leptin resistance. Therefore, we hypothesize that decreased barrier function results in luminal components coming in contact with vagal afferent nerves which leads to desensitization to gut satiety hormones. Chen et al. expanded the microbiota–gut–brain axis field by administering Escherichia coli, engineered to express N-acylphosphatidylethanolamines (NAPEs; precursor to anorexigenic lipids), to mice resulting in decreased food intake and weight gain.82
Evidence to support our hypothesis that luminal components cross the barrier when intestinal permeability is high can result in negative outcomes has recently been published. For example, bacteroides fragilis was used to restore gut barrier function in a mouse model of autism spectrum disorder. After restoring the intestinal barrier, plasma levels of bacterial metabolite 4-ethylphenylsulfate were reduced, and behavioral parameters were improved.54
Conclusion
The microbiota–gut–brain axis is ideally poised to help us to understand the physiological changes seen in obesity. Many questions still remain concerning the complex microbe-host relationship such as the causal direction of microbiota dysbiosis, decreased gut barrier function, and gut inflammation; however, by using the approaches mentioned in this review, new targets to treat and prevent obesity will be discovered in the near future.
Acknowledgments
HER funded by NIH (DK41004). Publication of this article was sponsored by the Université Laval’s Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments, and prevention of obesity.
Footnotes
The authors declare no conflict of interest.
References
- Kashyap PC, Marcobal A, Ursell LK, Larauche M, Duboc H, Earle KA et al. Complex interactions among diet, gastrointestinal transit, and gut microbiota in humanized mice. Gastroenterology 2013; 144: 967–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, O'Mahony SM. The microbiome-gut-brain axis: from bowel to behavior. Neurogastroenterol Motil 2011; 23: 187–192. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, de La Serre CB, Raybould HE. Vagal afferent neurons in high fat diet-induced obesity; intestinal microflora, gut inflammation and cholecystokinin. Physiol Behav 2011; 105: 100–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino G, Barbier de la Serre C, Knotts TA, Oort PJ, Newman JW, Adams SH et al. Increased expression of receptors for orexigenic factors in nodose ganglion of diet-induced obese rats. Am J Physiol Endocrinol Metab 2009; 296: E898–E903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covasa M, Ritter RC. Rats maintained on high-fat diets exhibit reduced satiety in response to CCK and bombesin. Peptides 1998; 19: 1407–1415. [DOI] [PubMed] [Google Scholar]
- Brennan IM, Seimon RV, Luscombe-Marsh ND, Otto B, Horowitz M, Feinle-Bisset C. Effects of acute dietary restriction on gut motor, hormone and energy intake responses to duodenal fat in obese men. Int J Obes (Lond) 2011; 35: 448–456. [DOI] [PubMed] [Google Scholar]
- Paulino G, Darcel N, Tome D, Raybould H. Adaptation of lipid-induced satiation is not dependent on caloric density in rats. Physiol Behav 2008; 93: 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybould HE. Gut microbiota, epithelial function and derangements in obesity. J Physiol 2012; 590: 441–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H, Kaser A. Gut microbiome, obesity, and metabolic dysfunction. J Clin Invest 2011; 121: 2126–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neish AS, Naumann M. Microbial-induced immunomodulation by targeting the NF-κB system. Trends Microbiol 2011; 19: 596–605. [DOI] [PubMed] [Google Scholar]
- Backhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA 2004; 101: 15718–15723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backhed F, Manchester JK, Semenkovich CF, Gordon JI. Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc Natl Acad Sci USA 2007; 104: 979–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006; 444: 1027–1031. [DOI] [PubMed] [Google Scholar]
- Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 2005; 102: 11070–11075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: human gut microbes associated with obesity. Nature 2006; 444: 1022–1023. [DOI] [PubMed] [Google Scholar]
- Sekirov I, Russell SL, Antunes LC, Finlay BB. Gut microbiota in health and disease. Physiol Rev 2010; 90: 859–904. [DOI] [PubMed] [Google Scholar]
- Brown EM, Sadarangani M, Finlay BB. The role of the immune system in governing host-microbe interactions in the intestine. Nat Immunol 2013; 14: 660–667. [DOI] [PubMed] [Google Scholar]
- Salzman NH, Hung K, Haribhai D, Chu H, Karlsson-Sjoberg J, Amir E et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol 2010; 11: 76–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodin CM, Verdam FJ, Grootjans J, Rensen SS, Verheyen FK, Dejong CH et al. Reduced Paneth cell antimicrobial protein levels correlate with activation of the unfolded protein response in the gut of obese individuals. J Pathol 2011; 225: 276–284. [DOI] [PubMed] [Google Scholar]
- de La Serre CB, Ellis CL, J L, Al H, Jc R, HE R. Propensity to high-fat diet-induced obesity in rats is associated with changes in the gut microbiota and gut inflammation. Am J Physiol Gastrointest Liver Physiol 2010; 299: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, Stombaugh J et al. Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 2012; 20: 738–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinert M, Hentschel U, Hacker J. Symbiosis and pathogenesis: evolution of the microbe-host interaction. Naturwissenschaften 2000; 87: 1–11. [DOI] [PubMed] [Google Scholar]
- Winter SE, Winter MG, Xavier MN, Thiennimitr P, Poon V, Keestra AM et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 2013; 339: 708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei N, Zhao L. An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J 2013; 7: 880–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu MT. Toll-like receptor signalling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol 2010; 10: 131–144. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Dave SH, Tilstra JS, Chang DT, Harpaz N, Xiong H et al. Enteroendocrine cells express functional Toll-like receptors. Am J Physiol Gastrointest Liver Physiol 2007; 292: G1770–G1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Diet-induced obesity leads to the development of leptin resistance in vagal afferent neurons. Am J Physiol Endocrinol Metab 2011; 301: E187–E195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkesworth S, Moore SE, Fulford AJ, Barclay GR, Darboe AA, Mark H et al. Evidence for metabolic endotoxemia in obese and diabetic Gambian women. Nutr Diabetes 2013; 3: e83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 2007; 56: 1761–1772. [DOI] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57: 1470–1481. [DOI] [PubMed] [Google Scholar]
- de La Serre CB, de Lartigue G, Raybould HE. Chronic exposure to Low dose bacterial lipopolysaccharide inhibits leptin signaling in vagal afferent neurons. Physiol Behav 2014; 139C: 188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T et al. Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 2012; 482: 179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wernstedt Asterholm I, Tao C, Morley TS, Wang QA, Delgado-Lopez F, Wang ZV et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab 2014; 20: 103–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois F, Roper J, Joseph N, Pei Z, Chhada A, Shak JR et al. The effect of H. pylori eradication on meal-associated changes in plasma ghrelin and leptin. BMC Gastroenterol 2011; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trasande L, Blustein J, Liu M, Corwin E, Cox LM, Blaser MJ. Infant antibiotic exposures and early-life body mass. Int J Obes (Lond) 2013; 37: 16–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veiga P, Gallini CA, Beal C, Michaud M, Delaney ML, DuBois A et al. Bifidobacterium animalis subsp. lactis fermented milk product reduces inflammation by altering a niche for colitogenic microbes. Proc Natl Acad Sci USA 2010; 107: 18132–18137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koren O, Goodrich JK, Cullender TC, Spor A, Laitinen K, Backhed HK et al. Host remodeling of the gut microbiome and metabolic changes during pregnancy. Cell 2012; 150: 470–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding S, Chi MM, Scull BP, Rigby R, Schwerbrock NM, Magness S et al. High-fat diet: bacteria interactions promote intestinal inflammation which precedes and correlates with obesity and insulin resistance in mouse. PLoS ONE 2010; 5: e12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton MK, Boudry G, Lemay DG, Raybould HE. Changes in intestinal barrier function and gut microbiota in high-fat diet fed rats are dynamic and region-dependent. Am J Physiol Gastrointest Liver Physiol 2015; 308: G840–G851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira TF, Souza NC, Chiarello PG, Franceschini SC, Bressan J, Ferreira CL et al. Intestinal permeability parameters in obese patients are correlated with metabolic syndrome risk factors. Clin Nutr 2012; 31: 735–740. [DOI] [PubMed] [Google Scholar]
- Turner JR, Buschmann MM, Sailer A, Calvo IR, Shen L. The role of molecular remodeling in differential regulation of tight junction permeability. Semin Cell Dev Biol 2014; 36: 204–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Y, Moya-Perez A. Microbiota, inflammation and obesity. Adv Exp Med Biol 2014; 817: 291–317. [DOI] [PubMed] [Google Scholar]
- Ghoshal S, Witta J, Zhong J, de Villiers W, Eckhardt E. Chylomicrons promote intestinal absorption of lipopolysaccharides. J Lipid Res 2009; 50: 90–97. [DOI] [PubMed] [Google Scholar]
- Qiao S, Luo Q, Zhao Y, Zhang XC, Huang Y. Structural basis for lipopolysaccharide insertion in the bacterial outer membrane. Nature 2014; 511: 108–111. [DOI] [PubMed] [Google Scholar]
- Honda K, Littman DR. The microbiome in infectious disease and inflammation. Ann Rev Immunol 2012; 30: 759–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madsen KL, Malfair D, Gray D, Doyle JS, Jewell LD, Fedorak RN. Interleukin-10 gene-deficient mice develop a primary intestinal permeability defect in response to enteric microflora. Inflamm Bowel Dis 1999; 5: 262–270. [DOI] [PubMed] [Google Scholar]
- Madsen KL, Lewis SA, Tavernini MM, Hibbard J, Fedorak RN. Interleukin 10 prevents cytokine-induced disruption of T84 monolayer barrier integrity and limits chloride secretion. Gastroenterology 1997; 113: 151–159. [DOI] [PubMed] [Google Scholar]
- Leclercq S, Matamoros S, Cani PD, Neyrinck AM, Jamar F, Starkel P et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci USA 2014; 111: E4485–E4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierre N, Deldicque L, Barbe C, Naslain D, Cani PD, Francaux M. Toll-like receptor 4 knockout mice are protected against endoplasmic reticulum stress induced by a high-fat diet. PLoS ONE 2013; 8: e65061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay-Kumar M, Aitken JD, Carvalho FA, Cullender TC, Mwangi S, Srinivasan S et al. Metabolic Syndrome and Altered Gut Microbiota in Mice Lacking Toll-Like Receptor 5. Science 2010; 328: 228–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chait A, Kim F. Saturated fatty acids and inflammation: who pays the toll? Arterioscler Thromb Vasc Biol 2010; 30: 692–693. [DOI] [PubMed] [Google Scholar]
- Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S et al. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med 2012; 18: 1279–1285. [DOI] [PubMed] [Google Scholar]
- Shen Y, Giardino Torchia ML, Lawson GW, Karp CL, Ashwell JD, Mazmanian SK. Outer membrane vesicles of a human commensal mediate immune regulation and disease protection. Cell Host Microbe 2012; 12: 509–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013; 155: 1451–1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciorba MA, Riehl TE, Rao MS, Moon C, Ee X, Nava GM et al. Lactobacillus probiotic protects intestinal epithelium from radiation injury in a TLR-2/cyclo-oxygenase-2-dependent manner. Gut 2012; 61: 829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014; 41: 296–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz Y, Rastmanesh R, Agostoni C. Understanding the role of gut microbes and probiotics in obesity: how far are we? Pharmacol Res 2013; 69: 144–155. [DOI] [PubMed] [Google Scholar]
- Takemura N, Okubo T, Sonoyama K. Lactobacillus plantarum strain No. 14 reduces adipocyte size in mice fed high-fat diet. Exp Biol Med (Maywood) 2010; 235: 849–856. [DOI] [PubMed] [Google Scholar]
- Aronsson L, Huang Y, Parini P, Korach-Andre M, Hakansson J, Gustafsson JA et al. Decreased fat storage by Lactobacillus paracasei is associated with increased levels of angiopoietin-like 4 protein (ANGPTL4). PLoS ONE 2010; 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachon S, Lee B, Marco ML. Diet alters probiotic Lactobacillus persistence and function in the intestine. Environ Microbiol 2014; 16: 2915–2926. [DOI] [PubMed] [Google Scholar]
- Kantor ED, Lampe JW, Kratz M, White E. Lifestyle factors and inflammation: associations by body mass index. PLoS ONE 2013; 8: e67833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr 2007; 98: 32–37. [DOI] [PubMed] [Google Scholar]
- Everard A, Belzer C, Geurts L, Ouwerkerk JP, Druart C, Bindels LB et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci USA 2013; 110: 9066–9071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serino M, Luche E, Gres S, Baylac A, Berge M, Cenac C et al. Metabolic adaptation to a high-fat diet is associated with a change in the gut microbiota. Gut 2012; 61: 543–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly YM et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science 2013; 341: 569–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 2008; 105: 16767–16772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell M, Admyre T, Goransson M, Marley AE, Smith DM, Oscarsson J et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am J Physiol Endocrinol Metab 2011; 300: E211–E220. [DOI] [PubMed] [Google Scholar]
- Fernandes J, Su W, Rahat-Rozenbloom S, Wolever TM, Comelli EM. Adiposity, gut microbiota and faecal short chain fatty acids are linked in adult humans. Nutr Diabetes 2014; 4: e121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivkovic AM, German JB, Lebrilla CB, Mills DA. Human milk glycobiome and its impact on the infant gastrointestinal microbiota. Proc Natl Acad Sci USA 2011; 108, (Suppl 1) 4653–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Crécy-Lagard V, Kim J-H, An HJ, Garrido D, German JB, Lebrilla CB et al. Proteomic Analysis of Bifidobacterium longum subsp. infantis Reveals the Metabolic Insight on Consumption of Prebiotics and Host Glycans. PLoS ONE 2013; 8: e57535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewulf EM, Cani PD, Claus SP, Fuentes S, Puylaert PG, Neyrinck AM et al. Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 2013; 62: 1112–1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab 2010; 35: 9–16. [DOI] [PubMed] [Google Scholar]
- Vincent KM, Sharp JW, Raybould HE. Intestinal glucose-induced calcium-calmodulin kinase signaling in the gut-brain axis in awake rats. Neurogastroenterol Motil 2011; 23: e282–e293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov 2008; 7: 678–693. [DOI] [PubMed] [Google Scholar]
- Ma H, Patti ME. Bile acids, obesity, and the metabolic syndrome. Best Pract Res Clin Gastroenterol 2014; 28: 573–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vegezzi G, Anselmi L, Huynh J, Barocelli E, Rozengurt E, Raybould H et al. Diet-induced regulation of bitter taste receptor subtypes in the mouse gastrointestinal tract. PLoS ONE 2014; 9: e107732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Possemiers S, Van de Wiele T, Guiot Y, Everard A, Rottier O et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009; 58: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG et al. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA 2011; 108: 16050–16055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lartigue G, Barbier de la Serre C, Espero E, Lee J, Raybould HE. Leptin resistance in vagal afferent neurons inhibits cholecystokinin signaling and satiation in diet induced obese rats. PLoS ONE 2012; 7: e32967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darling RA, Zhao H, Kinch D, Li AJ, Simasko SM, Ritter S. Mercaptoacetate and fatty acids exert direct and antagonistic effects on nodose neurons via GPR40 fatty acid receptors. Am J Physiol Regul Integr Comp Physiol 2014; 307: R35–R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Vadder F, Kovatcheva-Datchary P, Goncalves D, Vinera J, Zitoun C, Duchampt A et al. Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 2014; 156: 84–96. [DOI] [PubMed] [Google Scholar]
- Chen Z, Guo L, Zhang Y, Walzem RL, Pendergast JS, Printz RL et al. Incorporation of therapeutically modified bacteria into gut microbiota inhibits obesity. J Clin Invest 2014; 124: 3391–3406. [DOI] [PMC free article] [PubMed] [Google Scholar]