Abstract
The relatively stable body weight during adulthood is attributed to a homeostatic regulatory mechanism residing in the brain which uses feedback from the body to control energy intake and expenditure. This mechanism guarantees that if perturbed up or down by design, body weight will return to pre-perturbation levels, defined as the defended level or set point. The fact that weight re-gain is common after dieting suggests that obese subjects defend a higher level of body weight. Thus, the set point for body weight is flexible and likely determined by the complex interaction of genetic, epigenetic and environmental factors. Unlike dieting, bariatric surgery does a much better job in producing sustained suppression of food intake and body weight, and an intensive search for the underlying mechanisms has started. Although one explanation for this lasting effect of particularly Roux-en-Y gastric bypass surgery (RYGB) is simple physical restriction due to the invasive surgery, a more exciting explanation is that the surgery physiologically reprograms the body weight defense mechanism. In this non-systematic review, we present behavioral evidence from our own and other studies that defended body weight is lowered after RYGB and sleeve gastrectomy. After these surgeries, rodents return to their preferred lower body weight if over- or underfed for a period of time, and the ability to drastically increase food intake during the anabolic phase strongly argues against the physical restriction hypothesis. However, the underlying mechanisms remain obscure. Although the mechanism involves central leptin and melanocortin signaling pathways, other peripheral signals such as gut hormones and their neural effector pathways likely contribute. Future research using both targeted and non-targeted ‘omics’ techniques in both humans and rodents as well as modern, genetically targeted, neuronal manipulation techniques in rodents will be necessary.
Introduction
Gastric bypass and other bariatric surgeries are the most effective treatment option for obesity and its associated comorbidities such as diabetes, cardiovascular disease, sleep disturbances and certain cancers. Although serious complications can occur, most patients report significant improvements in general health and quality of life, and there is an intensive search for the mechanisms underlying these beneficial effects. A key observation is that the large weight loss after gastric bypass surgery does not seem to make patients hungrier and hypo-metabolic, responses typically seen after calorie restriction-induced weight loss. Instead, many gastric bypass patients seem both psychologically and physiologically ‘at ease’ with their reduced body weight. The surgery seems to have reprogrammed the defended body weight at a lower level. In this non-systematic review, we highlight recent observations in rodent models of bariatric surgery that support the notion of resetting the level of defended body weight and discuss potential underlying mechanisms.
Behavioral evidence for RYGB-induced change in defended body weight
Given the remarkable constancy of body weight over the adult lifespan, the concept of a homeostatically controlled body weight set point was introduced decades ago.1, 2 The basic concept is that a certain level of body weight (often referred to as the set point) is actively defended through a neural mechanism that uses feedback from the body to control energy intake and expenditure. Although the definition of set point has been debated over the years (for example, refs 3, 4, 5), it is now generally agreed that the set point is not ‘set in stone’, but rather is flexible and adaptable, taking into account essential biological and environmental circumstances such as pregnancy, season and long-term nutrient availability.6 In this review, the terms ‘set point’ and ‘level of defended body weight’ are used interchangeably without implying a defined underlying mechanism.
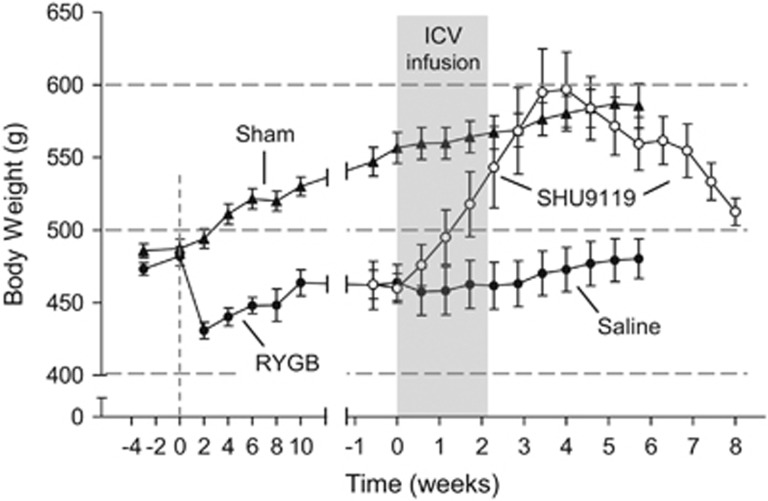
Because bariatric surgeries result in drastic reductions of food intake and weight loss, it is a commonly held view that they at least partially restrict total food intake in a non-physiological way. When these surgeries were pioneered decades ago, simple physical restriction of food intake and some malabsorption may have been intended, but resetting the defended body weight to a lower level clearly is a more physiological and thus preferred mechanism. Defense of a certain body weight can be ascertained by observing the metabolic and behavioral reactions to experimental perturbations usually accomplished by transient over- or underfeeding.7, 8 In our rat model of RYGB,9, 10 we have used chronic intracerebroventricular infusion of the melanocortin receptor antagonist SHU9119 to stimulate food intake.11 As shown earlier,12 SHU9119 powerfully stimulates food intake and over a 2-week infusion period results in significant weight gain and obesity in normal, chow fed as well as high-fat fed rats,11 reminiscent of rats and mice with MC4R deficiency.13, 14 Importantly, when the antagonist infusion was halted, rats gradually returned to their pre-infusion body weight, thus demonstrating the concept of defense of their lower body weight. We thus used this approach to test whether rats that had settled at a lower body weight about 3 months after RYGB surgery would show a similar behavior. Indeed, upon SHU9119 infusion, RYGB rats increased body weight to levels seen before surgery and slightly above that of sham-operated rats (Figure 1).11 When infusion was stopped, they returned to the same low body weight before infusion, clearly demonstrating that this low body weight is actively defended and not the result of an imposed physical restriction. We preferred this approach over forced intragastric feeding that results in the same voluntary feeding suppression7, 8 because it is more powerful and less invasive. Consistent with our findings, transient hyperphagia and weight gain was also observed in female rats with sleeve gastrectomy during pregnancy and lactation.15
Figure 1.
Behavioral demonstration of defense of reduced body weight level in rats with Roux-en-Y gastric (RYGB) bypass surgery. Rats that had reduced body weight after RYGB received infusion of saline or SHU9119, a potent melanocortin-4 receptor (MC4R) antagonist. MC4R blockade induced rapid weight gain to obese (sham-operated) body weight levels. After cessation of MC4R blockade body weight promptly returned to pre-infusion levels. Modified with permission from Mumphrey et al.,11 copyright John Wiley.
In our mouse RYGB model,16, 17 we used a different approach, in that we lowered pre-surgical body weight by calorie-restriction before RYGB surgery. After 12 weeks on high-fat diet, mice weighed about 35 g, 12 g of which was fat mass. After calorie restriction and before surgery, they weighed 26 g, with only 4 g of fat mass. Instead of losing weight, these mice gained weight and fat mass after surgery to plateau at about 30 g, with 8 g of fat mass (unpublished observations). These findings clearly demonstrate that RYGB surgery does not indiscriminately lower body weight level by physically limiting food intake. Rather, special circumstances are taken into account and lean body mass is defended. Furthermore, in a larger cohort of high-fat-fed mice, RYGB-induced weight as well as fat mass loss was positively correlated with pre-surgical body weight and fat mass. The fatter the animal was before surgery, the more fat mass was lost after surgery. In contrast, lean mass was completely conserved by the surgery.
In summary, our findings suggest that RYGB results in the establishment of a new level of defended body weight and adiposity. A similar conclusion was reached in a previous study with calorie-restriction in rats with sleeve gastrectomy.18
Meal size is restricted after gastric bypass surgery
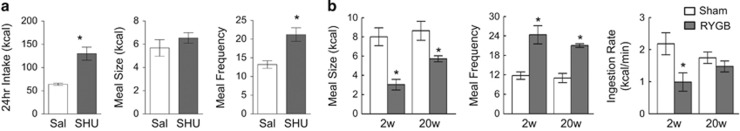
Meal pattern analysis in the above SHU9119-induced overfeeding study revealed different strategies to increase food intake in rats with RYGB vs sham surgery. Although sham rats increased meal size but not meal frequency, RYGB rats used the completely opposite strategy of increasing meal frequency but not meal size (Figure 2a). We had previously analyzed meal patterns in RYGB and sham-operated rats at an early and late time point after surgery with both liquid and solid food.10 Two weeks after surgery, liquid (Ensure) meal size of RYGB rats was less than half that of sham-operated animals, while meal frequency was increased (Figure 2b). The decrease in meal size was entirely accounted for by the rate of eating, with no change in meal duration. Twenty weeks after surgery the difference in meal size had somewhat moderated (35% lower), but meal frequency was still twice as high in RYGB rats. Thus, our studies in rats indicate that although meal size and eating rate are restricted after RYGB, animals compensate by increasing meal frequency, resulting in an only slight or no reduction in total food intake.
Figure 2.
RYGB restricts meal size but not total food intake. (a) Total daily food intake, meal size and meal frequency of RYGB rats during 14-day ICV infusion of SHU9119. (b) Meal size, meal frequency and ingestion rate of liquid formula (Ensure) of rats at 2 weeks (2w) and 20 weeks (20w) after RYGB or sham surgery. *P<0.05, RYGB vs Sham, *P<0.05, SHU9119 vs Saline. Modified with permission from Zheng et al.,10 copyright American Physiological Association.
In studies with bariatric surgery patients, it is standard procedure to provide a very low calorie diet for a week or two after gastric bypass surgery19, 20, 21 and caloric intake is often also restricted before surgery. In addition, patients typically receive heavy dietary counseling before and after surgery, making it difficult to analyze quantity and pattern of voluntarily ingested food, particularly during the early post-surgical period. Nevertheless careful studies in patients from 6 weeks to 2 years after RYGB22 revealed essentially similar effects on meal patterns as observed in our rats. RYGB patients show reduced meal size and a tendency for increased meal frequency, with decreased eating rate but maintained meal duration.22 Also in agreement with our rat study, the effect on meal size and eating rate was strongest early after surgery and moderated somewhat later.22
In summary, studies in both rodent models and patients with RYGB clearly demonstrate changes in eating patterns indicative of major changes in appetite control mechanisms. RYGB clearly does restrict meal size and eating rate, particularly early after surgery. However, RYGB animals can increase total food intake if properly stimulated, and thus the weight loss is not simply due to a mechanical restriction of meal size. Mechanistically, it is highly likely that these are behavioral adaptations to avoid discomfort, nausea and pain generated by eating as usual.22 It will be interesting to analyze the underlying adaptive neural mechanisms (aversive learning) for these dynamic interactions between the rearranged gut and the brain.
Potential underlying mechanisms for change in set point
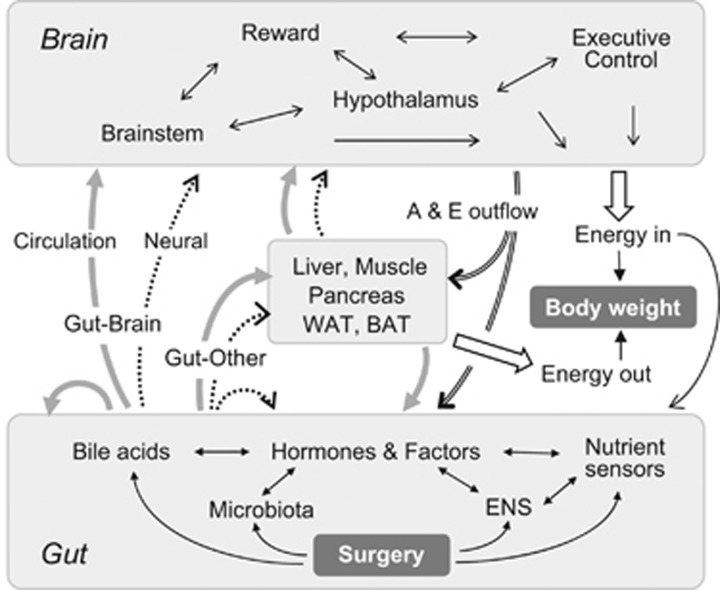
The multiple reciprocal signaling pathways between the gut and other organs and tissues that are potentially recruited in the effects of bariatric surgeries are depicted in Figure 3. Gut-to-brain communication through the circulation and neural connections is thought to be important, particularly for effects on eating and physical activity. However, communication with other organs that are important for energy and glucose homeostasis is also involved. In the following sections, mechanisms that have received the most attention will be discussed.
Figure 3.
Schematic diagram showing the flow of information between the gut and the brain that is potentially important for the dynamically emerging beneficial effects of bariatric surgeries on body weight and glucose homeostasis. The primary impact of surgery leads to changes in gut structure and function that result in changes of humoral (solid gray lines and closed arrows) and neural (broken black lines and open arrows) signaling within the gut itself and to the brain and other organs such as liver, muscle, pancreas, white (WAT) and brown (BAT) adipose tissue. The secondary impact of surgery on these ‘other’ organs changes their signaling to the brain and back to the gut via humoral and neural mechanisms. The brain integrates humoral and neural signals from gut and other organs and orchestrates adaptive behavior and metabolic control through changes in eating behavior and autonomic/endocrine outflow (A & E outflow). Abbreviation: ENS, enteric nervous system.
Role of leptin signaling
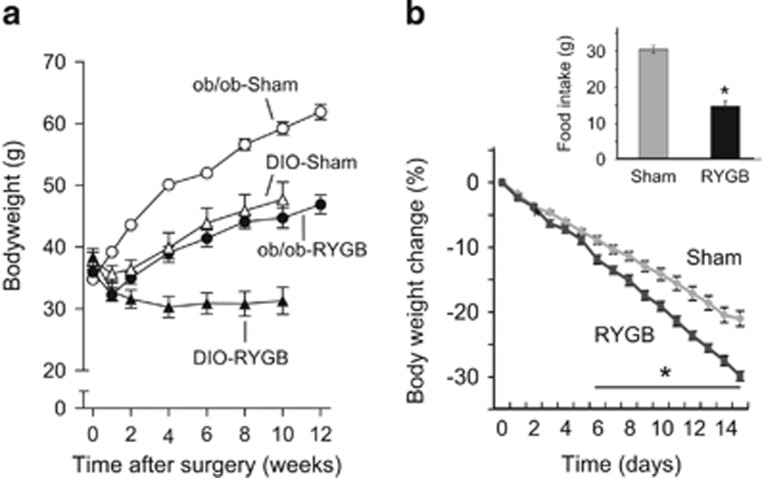
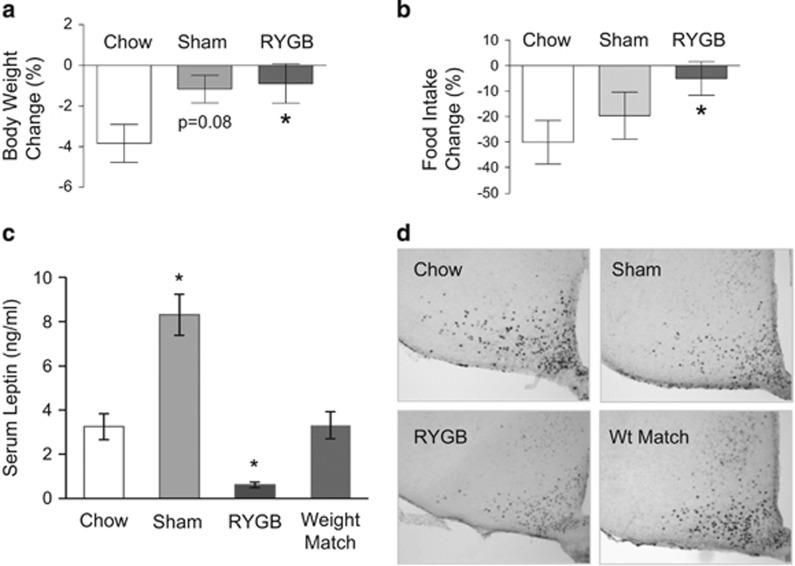
The discovery of leptin has provided an important missing link for the negative lipostatic feedback regulation of body weight, hypothesized long before by Kennedy.1 It has become clear that leptin action on the hypothalamus is a major mechanism for the homeostatic control of body weight/adiposity.23 In the absence of leptin signaling or after destruction of critical hypothalamic components of leptin signaling such as after VMH lesions, rodents and humans appear to regulate at a higher defended body weight.24, 25 It is thus possible that bariatric surgeries, particularly RYGB, change the defended body weight by restoring ‘normal’ leptin signaling and/or critical downstream signaling pathways in the hypothalamus and elsewhere. We have recently tested this hypothesis by carrying out RYGB surgery in leptin-deficient ob/ob mice.26 Leptin-deficient mice lost significantly less body weight and fat mass after RYGB, compared with wild-type mice, but more than sham-operated mice (Figure 4a). Furthermore, leptin-treatment of RYGB mice led to exaggerated percent body weight loss in ob/ob compared with wild-type mice (Figure 4b), suggesting that normal leptin signaling has at least a partially permissive role for the full beneficial effects of RYGB on body weight.26 However, it is not clear how RYGB-induced changes in leptin signaling lead to a lower defended body weight, as leptin levels rapidly decrease after the surgery, commensurate with the rapid weight loss. We therefore tested the possibility that RYGB reverses obesity-associated leptin resistance, making up for falling leptin levels, by measuring leptin-induced reduction of food intake and induction of phospho-STAT3 in the basomedial hypothalamus (Figure 5). Neither leptin-induced food intake suppression, nor leptin-induced pSTAT3 induction was augmented in RYGB mice compared with mice with sham surgery (Figure 5). Thus, increased leptin sensitivity is unlikely to be the mechanism for the beneficial effects of RYGB. This conclusion is corroborated by lack of increased leptin sensitivity after sleeve gastrectomy in rats18 and after RYGB in humans27 although intact leptin signaling is required for the full beneficial effects of RYGB in mice, the exact site and mechanism of leptin action remains to be investigated.
Figure 4.
Partial attenuation of body weight-lowering effects of RYGB in leptin-deficient ob/ob mice. (a) Effect of RYGB (filled symbols) and sham surgery (open symbols) on body weight in genetically (ob/ob, circles) and diet-induced (triangles) obese mice. (b) Leptin treatment (daily injections of 1 mg kg−1, i.p.) reduces body weight and food intake (inset) more in ob/ob mice with RYGB compared with sham surgery. *P<0.05, RYGB vs sham. Modified with permission from Hao et al.26
Figure 5.
RYGB does not restore two measures of leptin sensitivity in mice. (a, b) Effect of 4-day leptin treatment (daily injections of 1 mg kg−1, i.p.) on suppression of food intake and body weight in non-surgical mice fed chow (lean controls) and mice with RYGB or sham surgery fed high-fat (60% energy) diet. *P<0.05 vs chow. (c) Serum leptin levels of non-surgical mice fed chow and mice with RYGB or sham surgery and non-surgical mice weight-matched to RYGB, all fed high-fat diet. *P<0.05 vs chow. (d) Leptin-induced (1 mg kg−1, i.p.) phosho-STAT3 expression in basomedial hypothalamus in non-surgical mice fed chow and mice with RYGB or sham surgery and non-surgical mice weight-matched to RYGB, all fed high-fat diet.
Although absence of leptin triggers a strong anabolic response and safeguards dangerous weight loss, increased leptin, even at high pharmacological doses, seems unable to trigger a catabolic response to safeguard weight gain. The existence of another circulating factor that stimulates catabolic mechanisms during states of overnutrition has recently been hypothesized.28 Like leptin, this other factor may act on the hypothalamic circuit responsible for fine-tuning body weight homeostasis by differentially affecting energy intake and expenditure. Although this factor is distinct from leptin, it requires intact leptin signaling, as shown by the absence of food intake suppression following a period of intragastric overnutrition in leptin receptor-deficient Zucker rats.8 Although normal rats intragastrically ‘overfed’ for 10 days progressively suppress voluntary food intake and exhibit complete anorexia for a few days following cessation of infusion, Zucker rats immediately return to pre-infusion food intake upon cessation.8 It is thus possible that this factor has a role in the suppression of food intake and increase of energy expenditure after RYGB.
Fibroblast growth factor 21 (FGF21) could potentially fulfill the role of this unknown factor. FGF21 is primarily stimulated in adaptation to fasting,29 particularly protein-restriction.30 However, prolonged systemic infusion of FGF21 in the Siberian hamster, a model with seasonal body weight fluctuation, suppresses food intake and body weight and increases energy expenditure selectively during the high body weight phase (long days), but not during the low body weight phase (short days).31 Furthermore, a single injection of antibodies to the FGF receptor-1c splice variant with dual agonist/antagonist activity produced a long-lasting suppression of food intake and body weight in diet-induced obese mice.32 It will thus be interesting to test the potential role of FGF21 signaling in the beneficial effects of RYGB.
Role of melanocortin signaling
Melanocortin-4 receptor (MC4R) signaling is the most powerful catabolic downstream pathway of the basomedial hypothalamus, which is highly sensitive to signals of nutrient availability, including leptin. It is thus ideally positioned to mediate the catabolic effects of bariatric surgeries. The requirement of MC4R signaling to achieve reduced body weight with bariatric surgeries has been directly tested in MC4R-deficient rodents. One study concluded that MC4R signaling is not required for the full effects of sleeve gastrectomy in rats, because weight loss was identical in MC4R-deficient and wild-type rats.14 However, interpretation was complicated by the fact that in this model of sleeve gastrectomy there is only a transient reduction of body weight, returning to pre-surgical levels already after 20–30 days and surpassing it by 25% at 10 weeks. The conclusion was entirely based on the higher body weight levels reached in sham-operated rats and ignored the massive weight regain of both genotypes after surgery.14 Another study concluded that complete absence of MC4R signaling in homozygous, but not in heterozygous knockout mice, abrogated the effectiveness of RYGB.33 Interpretation of that study is made difficult due to high mortality, low number of animals and the unusually low weight gain in MC4R-deficient mice, which was less than observed in other studies using homozygous MC4R-deficient mice.34
Using chronic pharmacological blockade of brain MC4R signaling 3–4 months after RYGB or sham surgery in rats (see Discussion above and Figure 1), we concluded that brain MC4R signaling is not the critical mechanism by which RYGB lowers defended body weight level.11 However, interpretation of our findings was also not without problems. Although RYGB rats (that had plateaued at the lower body weight level) gained body weight during MC4R blockade, sham-operated rats showed an even stronger weight gain response. Thus, a final conclusion regarding requirement of MC4R signaling for the effects of bariatric surgeries in animal models is premature and additional studies will be necessary. In contrast to rodent models, studies in patients with heterozygous mutations at various loci are more consistent. With the exception of a few rare variants,35 all studies looking at patients with MC4R variants found that gastric bypass surgery was fully effective.14, 33, 35, 36 However, gastric banding in one rare patient with complete loss of MC4R functionality did not produce a lasting reduction in body weight.
In summary, the most parsimonious conclusion from both rodent and human studies is that complete absence of MC4R signaling diminishes, but does not completely abolish, effectiveness of bariatric surgeries. However, the partial absence of MC4R signaling, due to heterozygous mutations, has relatively little influence on surgical outcome, even if it results in obesity before surgery. Thus, increased catabolic signaling through MC4R expressing effector pathways may be partly responsible for defense of a lower body weight level after bariatric surgery. It is interesting in this respect that basomedial hypothalamic mRNA expression levels of MC4R, as well as POMC and AGRP, the major MC4R ligands, were not altered at 10 and 35 days after sleeve gastrectomy.18 Although this outcome suggests that the surgery-induced hypocaloric state and weight loss did not trigger the expected below set point counter-regulatory hypothalamic responses, there is no evidence that RYGB induces a permanent change in melanocortin signaling within the mediobasal hypothalamus. However, melanocortin signaling is not limited to the arcuate nucleus but extends to other hypothalamic and extra-hypothalamic sites. Specifically, MC4 receptors on preganglionic autonomic neurons are required for the full effects of RYGB on body weight reduction and increased energy expenditure.37
Role of extrahypothalamic mechanisms
Recent research has shown that feedback signals from the metabolic periphery such as leptin, GLP-1, ghrelin and insulin not only act on the hypothalamus and brainstem, but also on brain areas not classically associated with homeostatic regulation such as the mesolimbic dopamine system, sensory processing pathways and cortico-limbic structures (see Berthoud38 for recent review). These extra-hypothalamic areas should be considered as part of a larger neural system regulating body weight homeostasis, one that takes into consideration environmental factors and their interaction with cognitive and emotional processes. Defended body weight may thus be determined by an interaction between the classical hypothalamic and these extra-hypothalamic brain areas in an environment-dependent manner, and bariatric surgeries may act on any component of this larger neural system. For instance, RYGB has been linked to changes in taste reactivity,39, 40, 41, 42 food reward39, 43, 44 and cognitive functions,45 which could all contribute to the mechanism defending a new body weight. However, before such conclusions can be substantiated, the nature and exact site of action of the mediating signals needs to be demonstrated in future experiments.
Role of gut hormones
Early experimental studies showing that infusion of blood from a donor rat with intestinal bypass surgery suppressed food intake in recipient control rats suggested increased secretion of a humoral factor after surgery.46 Later studies in human subjects and rats after RYGB showed greatly increased meal-induced circulating levels of GLP-19, 47, 48, 49 and PYY9, 47, 50 as well as reduced levels of ghrelin,9, 50 and these gut hormones became prime candidate mechanisms for the beneficial effects of RYGB.51 However, subsequent studies in rodents directly testing roles for each of these gut hormones in the beneficial effects of RYGB and sleeve gastrectomy were largely negative. Neither GLP-1 receptor deficiency nor ghrelin deficiency appreciably changed the effects of RYGB17, 52 or sleeve gastrectomy,53, 54 and neither GLP-1 receptor nor PYY/Y2 receptor blockade in the brain attenuated RYGB-induced body weight suppression17 (but see Chandarana et al.55 for a different outcome). Although these studies do not lend much support for individual roles of these hormones, it is possible that they act synergistically. Thus, the effect of RYGB may be mediated by the combined induction of multiple gut hormones and factors, such that removal of any single factor has little or no consequence. It is also possible that compensatory mechanisms shift control from the deficient signaling pathway to other hormones. Future experiments should thus use loss-of-function strategies that are either inducible and/or involve more than one signaling pathway. For example, because GLP-2 has been demonstrated to act in addition to GLP-1 on appetite in the brain56 and the two hormones may substitute for each other, a double GLP-1R/GLP-2R knockout strategy may be necessary. This line of reasoning is supported by studies using octreotide, a non-specific inhibitor of all gut hormones.51 Inhibiting postprandial gut hormone responses with octreotide normalized RYGB-induced reduction of appetite and food intake at least in the short term.51
Besides the L-cell hormones GLP-1 and PYY, other gut hormones and secreted factors such as neurotensin,57 CCK,58 ApoA-IV,59 FGF 15/1960 and bile acids61, 62, 63, 64 have been shown to be increased after RYGB and may thus be involved in mediating some of the beneficial effects of bariatric surgeries. The beneficial effects of sleeve gastrectomy have been demonstrated to require FXR signaling, an important target of bile acids.65
In summary, there is considerable indirect evidence for important roles of hormones and other factors secreted from the surgically rearranged gut in reducing energy intake in helping to implement a new, lower defended body weight. However, direct evidence from specific loss-of-function studies directed at single mechanisms or factors is still missing, at least for RYGB.
Role of microbiota–host interactions
The gut microbiome changes after gastric bypass surgery in humans,66, 67, 68 rats69 and mice,70 and a recent transplant study in mice suggests that the changes in gut microbiome are responsible for at least some effects on body weight.70 The signaling pathways leading from the microbiota in the intestinal lumen to reduced body weight are not known (Figure 3). One possibility is that microbiota-induced effects on intestinal barrier function reverse obesity-associated endotoxemia and inflammation.71 Consistent with this idea is the observation of decreased paracellular permeability after gastric bypass in humans.72 Reduced systemic inflammatory signaling could thereby lead to reduced inflammation of hypothalamic areas involved in set point regulation.73 However, it is still not clearly understood which circulating inflammatory signals are involved and how they engage brain inflammatory processes. Another possibility is that the microbiota change bile acid profile and abundance which in turn signal through FXR and TGR5 receptors to peripheral organs involved in energy metabolism and to the brain. Finally, microbiota-derived factors such as neurotransmitters or neurotransmitter-like substances and other small circulating molecules may signal directly to the brain. This will require the full assessment of circulating metabolites of genomic and metagenomic origin by using global metabolomics approaches.
Role of neural communication pathways between the periphery and the brain
Besides the blood circulation, vagal afferents are in an ideal position to communicate information from the gut to the brain74 and we have recently examined the role of this communication pathway in the body weight lowering effects of RYGB in rats. In the first study, eliminating vagal communication through the common hepatic branch, which innervates the liver, hepatic portal vein and the upper duodenum75 had no effect on RYGB-induced reduction of food intake and body weight.76 In the second study, eliminating vagal communication through the celiac branches that innervate most of the small and large intestines, including the Roux- and common limbs, attenuated RYGB-induced body weight loss by about 20%, consistent with increased vagal afferent signaling from these limbs.77 A similar attenuation of RYGB-induced weight loss was observed with transection of the dorsal vagal para-esophageal bundle, which includes the celiac branches.78 A recent clinical study in a large cohort of patients undergoing RYGB with or without vagotomy found no effect of vagotomy on percent extra weight loss.79 However, because all these vagal lesions did not differentiate between afferents and efferents, the conclusions are limited and await more selective vagal manipulations such as targeted genetic deletions in mouse models of RYGB.37, 80
Conclusions
Studies using forced over- or under-feeding paradigms in rodents clearly demonstrate defense of a new body weight set point after RYGB and sleeve gastrectomy, but the underlying mechanisms have not yet been fully revealed. Specifically, it is not clear what signals are generated in the re-arranged gut, how they reach the brain, and where exactly in the brain they act to evade a state of hunger and hypo-metabolism that is typically encountered with dieting-induced weight loss. Deletion of single factors hypothesized to be the critical surgery-induced signals has not yielded the expected results. Total absence of leptin and MC4R signaling seem to attenuate but not abolish the beneficial weight loss effects of RYGB, but partial loss of MC4R signaling has no effect, even though it is obesogenic. Part of the complication in defining the critical brain circuitry is the recent realization that body weight homeostasis is not limited to parts of the hypothalamus, but includes other brain systems including sensory and corticolimbic systems, brainstem, and even autonomic outflow pathways.
Acknowledgments
Partially funded by National Institutes of Health Grants DK047348 (HRB); DK 085495 and DK068036 (JY); DK092587 (HM); and DK081563 (CM). Publication of this article was sponsored by the Université Laval’s Research Chair in Obesity in an effort to inform the public on the causes, consequences, treatments, and prevention of obesity.
Footnotes
H-RB has received lecture fees from Novo Nordisk, and grant support from the National Institutes of Health. CDM and HM have also received grant support from the National Institutes of Health. JY has received grant support from Suntory Foundation. The remaining authors declare no conflict of interest.
References
- Kennedy GC. The role of depot fat in the hypothalamic control of food intake in the rat. Proc R Soc Lond B Biol Sci 1953; 140: 578–596. [DOI] [PubMed] [Google Scholar]
- Keesey RE, Powley TL. Hypothalamic regulation of body weight. Am Sci 1975; 63: 558–565. [PubMed] [Google Scholar]
- Wirtshafter D, Davis JD. Set points, settling points, and the control of body weight. Physiol Behav 1977; 19: 75–78. [DOI] [PubMed] [Google Scholar]
- Harris RB. Role of set-point theory in regulation of body weight. FASEB J 1990; 4: 3310–3318. [DOI] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Berthoud HR. An expanded view of energy homeostasis: neural integration of metabolic, cognitive, and emotional drives to eat. Physiol Behav 2009; 97: 572–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan PJ, Ross AW, Mercer JG, Barrett P. Photoperiodic programming of body weight through the neuroendocrine hypothalamus. J Endocrinol 2003; 177: 27–34. [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Matson CA, Chavez M, Woods SC, Dallman MF, Schwartz MW. Behavioral, endocrine, and hypothalamic responses to involuntary overfeeding. Am J Physiol 1996; 271: R819–R823. [DOI] [PubMed] [Google Scholar]
- White CL, Purpera MN, Ballard K, Morrison CD. Decreased food intake following overfeeding involves leptin-dependent and leptin-independent mechanisms. Physiol Behav 2010; 100: 408–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Townsend RL, Sigalet DL, Berthoud HR. Meal-induced hormone responses in a rat model of Roux-en-Y gastric bypass surgery. Endocrinology 2010; 151: 1588–1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Shin AC, Lenard NR, Townsend RL, Patterson LM, Sigalet DL et al. Meal patterns, satiety, and food choice in a rat model of Roux-en-Y gastric bypass surgery. Am J Physiol Regul Integr Comp Physiol 2009; 297: R1273–R1282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumphrey MB, Hao Z, Townsend RL, Patterson LM, Morrison CD, Munzberg H et al. Reversible hyperphagia and obesity in rats with gastric bypass by central MC3/4R blockade. Obesity (Silver Spring) 2014; 22: 1847–1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrera JG, Jones KR, Herman JP, D'Alessio DA, Woods SC, Seeley RJ. Hyperphagia and increased fat accumulation in two models of chronic CNS glucagon-like peptide-1 loss of function. J Neurosci 2011; 31: 3904–3913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AA, Marks DL, Fan W, Kuhn CM, Bartolome M, Cone RD. Melanocortin-4 receptor is required for acute homeostatic responses to increased dietary fat. Nat Neurosci 2001; 4: 605–611. [DOI] [PubMed] [Google Scholar]
- Mul JD, Begg DP, Alsters SI, van Haaften G, Duran KJ, D'Alessio DA et al. Effect of vertical sleeve gastrectomy in melanocortin receptor 4-deficient rats. Am J Physiol Endocrinol Metab 2012; 303: E103–E110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grayson BE, Schneider KM, Woods SC, Seeley RJ. Improved rodent maternal metabolism but reduced intrauterine growth after vertical sleeve gastrectomy. Sci Transl Med 2013; 5: 199ra112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Zhao Z, Berthoud HR, Ye J. Development and verification of a mouse model for roux-en-Y gastric bypass surgery with a small gastric pouch. PLoS One 2013; 8: e52922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Hao Z, Mumphrey MB, Townsend RL, Patterson LM, Stylopoulos N et al. GLP-1 receptor signaling is not required for reduced body weight after RYGB in rodents. Am J Physiol Regul Integr Comp Physiol 2014; 306: R352–R362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefater MA, Perez-Tilve D, Chambers AP, Wilson-Perez HE, Sandoval DA, Berger J et al. Sleeve gastrectomy induces loss of weight and fat mass in obese rats, but does not affect leptin sensitivity. Gastroenterology 2010; 138: 2426–2436, 2436 e2421-2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackness C, Karmally W, Febres G, Conwell IM, Ahmed L, Bessler M et al. Very low calorie diet mimics the early beneficial effect of Roux-en-Y gastric bypass on insulin sensitivity and beta-cell function in type 2 diabetic patients. Diabetes 2013; 62: 3027–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lips MA, de Groot GH, van Klinken JB, Aarts E, Berends FJ, Janssen IM et al. Calorie restriction is a major determinant of the short-term metabolic effects of gastric bypass surgery in obese type 2 diabetic patients. Clin Endocrinol (Oxf) 2014; 80: 834–842. [DOI] [PubMed] [Google Scholar]
- Isbell JM, Tamboli RA, Hansen EN, Saliba J, Dunn JP, Phillips SE et al. The importance of caloric restriction in the early improvements in insulin sensitivity after Roux-en-Y gastric bypass surgery. Diabetes Care 2010; 33: 1438–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenius A, Larsson I, Bueter M, Melanson KJ, Bosaeus I, Forslund HB et al. Changes in eating behaviour and meal pattern following Roux-en-Y gastric bypass. Int J Obes (Lond) 2012; 36: 348–355. [DOI] [PubMed] [Google Scholar]
- Schwartz MW. Brain pathways controlling food intake and body weight. Exp Biol Med (Maywood) 2001; 226: 978–981. [DOI] [PubMed] [Google Scholar]
- Keesey RE, Hirvonen MD. Body weight set-points: determination and adjustment. J Nutr 1997; 127: 1875S–1883S. [DOI] [PubMed] [Google Scholar]
- Levin BE, Keesey RE. Defense of differing body weight set points in diet-induced obese and resistant rats. Am J Physiol 1998; 274: R412–R419. [DOI] [PubMed] [Google Scholar]
- Hao Z, Munzberg H, Rezai-Zadeh K, Keenan M, Coulon D, Lu H et al. Leptin deficient ob/ob mice and diet-induced obese mice responded differently to Roux-en-Y bypass surgery. Int J Obes (Lond) 2015; 39: 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Conroy R, Febres G, McMahon DJ, Conwell I, Karmally W et al. Randomized double-blind placebo-controlled study of leptin administration after gastric bypass. Obesity (Silver Spring) 2013; 21: 951–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravussin Y, Leibel RL, Ferrante AW Jr. A missing link in body weight homeostasis: the catabolic signal of the overfed state. Cell Metab 2014; 20: 565–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galman C, Lundasen T, Kharitonenkov A, Bina HA, Eriksson M, Hafstrom I et al. The circulating metabolic regulator FGF21 is induced by prolonged fasting and PPARalpha activation in man. Cell Metab 2008; 8: 169–174. [DOI] [PubMed] [Google Scholar]
- Laeger T, Henagan TM, Albarado DC, Redman LM, Bray GA, Noland RC et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest 2014; 124: 3913–3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Samms R, Warner A, Bolborea M, Barrett P, Fowler MJ et al. Increased responses to the actions of fibroblast growth factor 21 on energy balance and body weight in a seasonal model of adiposity. J Neuroendocrinol 2013; 25: 180–189. [DOI] [PubMed] [Google Scholar]
- Lelliott CJ, Ahnmark A, Admyre T, Ahlstedt I, Irving L, Keyes F et al. Monoclonal antibody targeting of fibroblast growth factor receptor 1c ameliorates obesity and glucose intolerance via central mechanisms. PLoS One 2014; 9: e112109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatoum IJ, Stylopoulos N, Vanhoose AM, Boyd KL, Yin DP, Ellacott KL et al. Melanocortin-4 receptor signaling is required for weight loss after gastric bypass surgery. J Clin Endocrinol Metab 2012; 97: E1023–E1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR et al. Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 1997; 88: 131–141. [DOI] [PubMed] [Google Scholar]
- Moore BS, Mirshahi UL, Yost EA, Stepanchick AN, Bedrin MD, Styer AM et al. Long-term weight-loss in gastric bypass patients carrying melanocortin 4 receptor variants. PLoS One 2014; 9: e93629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aslan IR, Campos GM, Calton MA, Evans DS, Merriman RB, Vaisse C. Weight loss after Roux-en-Y gastric bypass in obese patients heterozygous for MC4R mutations. Obes Surg 2011; 21: 930–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zechner JF, Mirshahi UL, Satapati S, Berglund ED, Rossi J, Scott MM et al. Weight-independent effects of roux-en-Y gastric bypass on glucose homeostasis via melanocortin-4 receptors in mice and humans. Gastroenterology 2013; 144: 580–590 e587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR. Metabolic and hedonic drives in the neural control of appetite: who is the boss? Curr Opin Neurobiol 2011; 21: 888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Pistell PJ, Berthoud HR. Roux-en-Y gastric bypass surgery changes food reward in rats. Int J Obes (Lond) 2011; 35: 642–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepino MY, Bradley D, Eagon JC, Sullivan S, Abumrad NA, Klein S. Changes in taste perception and eating behavior after bariatric surgery-induced weight loss in women. Obesity (Silver Spring) 2014; 22: E13–E20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burge JC, Schaumburg JZ, Choban PS, DiSilvestro RA, Flancbaum L. Changes in patients' taste acuity after Roux-en-Y gastric bypass for clinically severe obesity. J Am Diet Assoc 1995; 95: 666–670. [DOI] [PubMed] [Google Scholar]
- Hajnal A, Kovacs P, Ahmed TA, Meirelles K, Lynch CJ, Cooney RN. Gastric bypass surgery alters behavioral and neural taste functions for sweet taste in obese rats. Am J Physiol Gastrointest Liver Physiol 2010; 299: G967–G979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholtz S, Miras AD, Chhina N, Prechtl CG, Sleeth ML, Daud NM et al. Obese patients after gastric bypass surgery have lower brain-hedonic responses to food than after gastric banding. Gut 2014; 63: 891–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochner CN, Stice E, Hutchins E, Afifi L, Geliebter A, Hirsch J et al. Relation between changes in neural responsivity and reductions in desire to eat high-calorie foods following gastric bypass surgery. Neuroscience 2012; 209: 128–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman RL, Canterberry M, Borckardt JJ, Madan A, Byrne TK, George MS et al. Executive control circuitry differentiates degree of success in weight loss following gastric-bypass surgery. Obesity (Silver Spring) 2013; 21: 2189–2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson RL, Brent EL. Appetite suppressant activity in plasma of rats after intestinal bypass surgery. Am J Physiol 1982; 243: R60–R64. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Aylwin SJ, Batterham RL, Borg CM, Coyle F, Prasad V et al. Gut hormone profiles following bariatric surgery favor an anorectic state, facilitate weight loss, and improve metabolic parameters. Ann Surg 2006; 243: 108–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Bessler M, Inabnet W, Taveras C, Holst JJ. Exaggerated glucagon-like peptide-1 and blunted glucose-dependent insulinotropic peptide secretion are associated with Roux-en-Y gastric bypass but not adjustable gastric banding. Surg Obes Relat Dis 2007; 3: 597–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laferrere B, Heshka S, Wang K, Khan Y, McGinty J, Teixeira J et al. Incretin levels and effect are markedly enhanced 1 month after Roux-en-Y gastric bypass surgery in obese patients with type 2 diabetes. Diabetes Care 2007; 30: 1709–1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korner J, Bessler M, Cirilo LJ, Conwell IM, Daud A, Restuccia NL et al. Effects of Roux-en-Y gastric bypass surgery on fasting and postprandial concentrations of plasma ghrelin, peptide YY, and insulin. J Clin Endocrinol Metab 2005; 90: 359–365. [DOI] [PubMed] [Google Scholar]
- le Roux CW, Welbourn R, Werling M, Osborne A, Kokkinos A, Laurenius A et al. Gut hormones as mediators of appetite and weight loss after Roux-en-Y gastric bypass. Ann Surg 2007; 246: 780–785. [DOI] [PubMed] [Google Scholar]
- Mokadem M, Zechner JF, Margolskee RF, Drucker DJ, Aguirre V. Effects of Roux-en-Y gastric bypass on energy and glucose homeostasis are preserved in two mouse models of functional glucagon-like peptide-1 deficiency. Mol Metab 2014; 3: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers AP, Kirchner H, Wilson-Perez HE, Willency JA, Hale JE, Gaylinn BD et al. The effects of vertical sleeve gastrectomy in rodents are ghrelin independent. Gastroenterology 2013; 144: 50–52 e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson-Perez HE, Chambers AP, Ryan KK, Li B, Sandoval DA, Stoffers D et al. Vertical sleeve gastrectomy is effective in two genetic mouse models of glucagon-like peptide-1 receptor deficiency. Diabetes 2013; 62: 2380–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandarana K, Gelegen C, Karra E, Choudhury AI, Drew ME, Fauveau V et al. Diet and gastrointestinal bypass-induced weight loss: the roles of ghrelin and peptide YY. Diabetes 2011; 60: 810–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan X, Shi X, Li X, Chang B, Wang Y, Li D et al. GLP-2 receptor in POMC neurons suppresses feeding behavior and gastric motility. Am J Physiol Endocrinol Metab 2012; 303: E853–E864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen SH, Olesen SC, Dirksen C, Jorgensen NB, Bojsen-Moller KN, Kielgast U et al. Changes in gastrointestinal hormone responses, insulin sensitivity, and beta-cell function within 2 weeks after gastric bypass in non-diabetic subjects. Obes Surg 2012; 22: 1084–1096. [DOI] [PubMed] [Google Scholar]
- Holdstock C, Zethelius B, Sundbom M, Karlsson FA, Eden Engstrom B. Postprandial changes in gut regulatory peptides in gastric bypass patients. Int J Obes (Lond) 2008; 32: 1640–1646. [DOI] [PubMed] [Google Scholar]
- Culnan DM, Cooney RN, Stanley B, Lynch CJ. Apolipoprotein A-IV, a putative satiety/antiatherogenic factor, rises after gastric bypass. Obesity (Silver Spring) 2009; 17: 46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen PL, van Werven J, Aarts E, Berends F, Janssen I, Stoker J et al. Alterations of hormonally active fibroblast growth factors after Roux-en-Y gastric bypass surgery. Dig Dis 2011; 29: 48–51. [DOI] [PubMed] [Google Scholar]
- Ahmad NN, Pfalzer A, Kaplan LM. Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes (Lond) 2013; 37: 1553–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pournaras DJ, Glicksman C, Vincent RP, Kuganolipava S, Alaghband-Zadeh J, Mahon D et al. The role of bile after Roux-en-Y gastric bypass in promoting weight loss and improving glycaemic control. Endocrinology 2012; 153: 3613–3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohli R, Bradley D, Setchell KD, Eagon JC, Abumrad N, Klein S. Weight loss induced by Roux-en-Y gastric bypass but not laparoscopic adjustable gastric banding increases circulating bile acids. J Clin Endocrinol Metab 2013; 98: E708–E712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakatani H, Kasama K, Oshiro T, Watanabe M, Hirose H, Itoh H. Serum bile acid along with plasma incretins and serum high-molecular weight adiponectin levels are increased after bariatric surgery. Metabolism 2009; 58: 1400–1407. [DOI] [PubMed] [Google Scholar]
- Ryan KK, Tremaroli V, Clemmensen C, Kovatcheva-Datchary P, Myronovych A, Karns R et al. FXR is a molecular target for the effects of vertical sleeve gastrectomy. Nature 2014; 509: 183–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A 2009; 106: 2365–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong LC, Tap J, Aron-Wisnewsky J, Pelloux V, Basdevant A, Bouillot JL et al. Gut microbiota after gastric bypass in human obesity: increased richness and associations of bacterial genera with adipose tissue genes. Am J Clin Nutr 2013; 98: 16–24. [DOI] [PubMed] [Google Scholar]
- Graessler J, Qin Y, Zhong H, Zhang J, Licinio J, Wong ML et al. Metagenomic sequencing of the human gut microbiome before and after bariatric surgery in obese patients with type 2 diabetes: correlation with inflammatory and metabolic parameters. Pharmacogenomics J 2013; 13: 514–522. [DOI] [PubMed] [Google Scholar]
- Osto M, Abegg K, Bueter M, le Roux CW, Cani PD, Lutz TA. Roux-en-Y gastric bypass surgery in rats alters gut microbiota profile along the intestine. Physiol Behav 2013; 119: 92–96. [DOI] [PubMed] [Google Scholar]
- Liou AP, Paziuk M, Luevano JM Jr, Machineni S, Turnbaugh PJ, Kaplan LM. Conserved shifts in the gut microbiota due to gastric bypass reduce host weight and adiposity. Sci Transl Med 2013; 5: 178ra141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 2008; 57: 1470–1481. [DOI] [PubMed] [Google Scholar]
- Casselbrant A, Elias E, Fandriks L, Wallenius V. Expression of tight-junction proteins in human proximal small intestinal mucosa before and after Roux-en-Y gastric bypass surgery. Surg Obes Relat Dis 2015; 11: 45–53. [DOI] [PubMed] [Google Scholar]
- Thaler JP, Choi SJ, Schwartz MW, Wisse BE. Hypothalamic inflammation and energy homeostasis: resolving the paradox. Front Neuroendocrinol 2010; 31: 79–84. [DOI] [PubMed] [Google Scholar]
- Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil 2008; 20 (Suppl 1): 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthoud HR, Neuhuber WL. Functional and chemical anatomy of the afferent vagal system. Auton Neurosci 2000; 85: 1–17. [DOI] [PubMed] [Google Scholar]
- Shin AC, Zheng H, Berthoud HR. Vagal innervation of the hepatic portal vein and liver is not necessary for Roux-en-Y gastric bypass surgery-induced hypophagia, weight loss, and hypermetabolism. Ann Surg 2012; 255: 294–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Z, Townsend RL, Mumphrey MB, Patterson LM, Ye J, Berthoud HR. Vagal innervation of intestine contributes to weight loss After Roux-en-Y gastric bypass surgery in rats. Obes Surg 2014; 24: 2145–2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bueter M, Lowenstein C, Ashrafian H, Hillebrand J, Bloom SR, Olbers T et al. Vagal sparing surgical technique but not stoma size affects body weight loss in rodent model of gastric bypass. Obes Surg 2010; 20: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafor PN, Lien C, Bairdain S, Simonson DC, Halperin F, Vernon AH et al. Effect of vagotomy during Roux-en-Y gastric bypass surgery on weight loss outcomes. Obes Res Clin Pract 2015; 9: 274–280. [DOI] [PubMed] [Google Scholar]
- de Lartigue G, Ronveaux CC, Raybould HE. Deletion of leptin signaling in vagal afferent neurons results in hyperphagia and obesity. Mol Metab 2014; 3: 595–607. [DOI] [PMC free article] [PubMed] [Google Scholar]