Plasmodesmata (PD), the intercellular channels that span plasma membranes and cell walls between adjacent plant cells, are unique structures that create cytoplasmic continuity, termed the symplast, between individual cells. Within a symplastic domain, a group of cells that are interconnected by PD are able to exchange a wide range of molecules, from ions and water to proteins and nucleic acids. Although the importance of macromolecular trafficking through PD for plant physiology is well established, the mechanism(s) by which this cell-to-cell transport process is regulated remains largely unclear. PD transport can be classified into two distinct types: passive translocation by simple diffusion [also termed nontargeted movement (1)] of molecules smaller than the PD size exclusion limit (SEL), and active transport by PD dilation [also termed targeted movement (1)] of molecules larger than their SEL. The passive type of PD transport is determined solely by the PD SEL and, thus, by the PD structure and development. Thus, marker molecules, such as fluorescently labeled dextrans or GFP, that passively move between plant cells can be used as molecular tools for mapping developmental changes in PD permeability; this issue of PNAS includes a report by Kim et al. (2) that elegantly uses GFP transport to characterize changes in cell-to-cell communication during embryogenesis and early seedling development in Arabidopsis.
The Younger They Are, the Larger Their PD
Kim et al. (2) used autofluorescent markers of two different sizes, i.e., single soluble GFP (sGFP, 27 kDa) and tandem-fused soluble GFP (2xsGFP, 54 kDa), to demonstrate passive intercellular trafficking of both macromolecules from the meristem region during early stages of Arabidopsis thaliana development. To achieve specific expression within the meristem, Kim et al. first produced transgenic plants with sGFP and 2xsGFP under the control of a yeast GAL4 UAS minimal promoter. Then they used another transgenic line, J2341, that carried a cell type-specific enhancer driving expression of the yeast transcriptional activator GAL4 in the meristem and endoplasmic reticulum (ER)-tethered GFP (erGFP) under the UAS GAL4 minimal promoter. Finally, the sGFP and 2xsGFP lines were crossed to J2341, producing the MSG1 and MSG2 lines, respectively, which expressed erGFP as well as sGFP or 2xsGFP in their meristems. In these plants, erGFP, which is unable to exit the expressing cell because of its association with the ER, conveniently identifies the meristematic cells in which the GFP expression has occurred, whereas sGFP and 2xsGFP act as transport substrates for mapping of PD permeability.
In early heart embryos, late heart embryos, and midtorpedo embryos of the MSG1 and MSG2 lines, the cell-autonomous erGFP remained largely confined to the meristem, whereas sGFP and 2xsGFP displayed a much broader pattern of sGFP fluorescence indicative of intercellular movement. Interestingly, cell-to-cell transport of sGFP and 2xsGFP revealed differences in intercellular communication between different stages of Arabidopsis embryonic development. Specifically, sGFP spread to all cells at all three embryonic stages tested, but 2xsGFP movement was reduced most drastically at the midtorpedo stage. That these differences indeed were caused by marker protein movement rather than ectopic expression was confirmed by in situ hybridization. Thus, in plant embryos, the SEL of PD of the shoot apical meristem (SAM) and its surrounding cells is at least 54 kDa, considerably exceeding the known PD SEL for mature tissues (e.g., ref. 3) (Fig. 1). That sGFP spreads throughout the entire embryo indicates that the plant embryo may be viewed as a single symplast connected with PD with relatively high permeability. Furthermore, PD permeability may be developmentally dynamic because it decreases as the embryo matures.
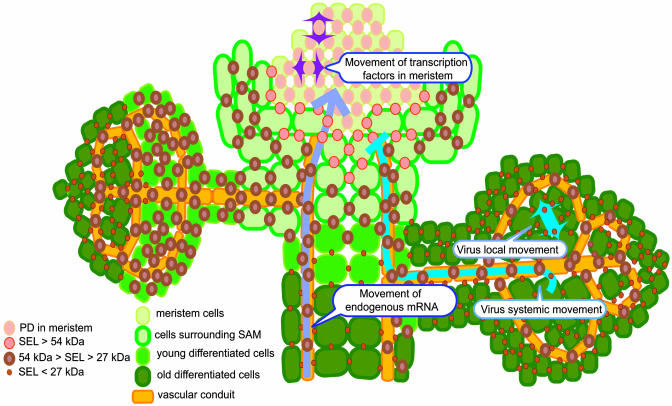
Fig. 1.
A schematic model for distribution of PD with different SELs in various plant tissues and their function in macromolecular movement. According to ref. 2, the PD SEL can be classified into at least three types: (i) >54 kDa in undifferentiated tissues around the meristem, (ii) <54 kDa and >27 kDa in young tissues, and (iii) <27 kDa in mature nonvascular tissues. SELs determined for nontargeted transport in different tissues, however, do not necessarily correlate with their ability to support targeted movement. For example, plant viruses move freely in fully developed leaves with small SELs but are unable to invade the meristematic tissues surrounded by cells with a larger PD SEL. On the other hand, endogenous mRNAs traffic through the vascular conduit and enter the meristem region. Many transcription factors are also known to move in the meristem. For further details, see text.
Cell-to-cell movement of sGFP and 2xsGFP in shoot apices was also analyzed in seedlings at 3, 8, and 14 days postgermination (dpg). In these plants, the cell-autonomous erGFP was expressed strongly in the SAM and in a small group of cells in petioles of rosette leaves at 3 and 8 dpg, whereas at 14 dpg, the expression occurred not only in SAM and basal petioles but also in the upper part of leaf petioles. In contrast, 1xsGFP was observed to move out from the expression region to different types of surrounding tissues, such as cotyledons, hypocotyls, petioles, and rosette leaves, at all tested periods. The pattern of sGFP movement changed as seedlings developed, from movement predominantly into cotyledons and hypocotyls (3 dpg) to movement into rosette leaves (8 and 14 dpg). 2xsGFP showed a similar movement pattern, albeit to a lesser extent and with preference for spread to the petioles of young rosette leaves. At 14 dpg, movement of sGFP in leaves was more restricted compared with that in leaf primodia; sGFP moved throughout the entire primordia, but it spread only into about half of the leaf blade in young leaves, again highlighting the enhanced PD SEL in younger, undifferentiated tissues. Importantly, sGFP, which spread throughout very young leaves, retained its ability to move freely through the central vascular tissues of petioles and leaf veins of mature leaves; moreover, these vascular tissues supported even movement of 2xsGFP, which did not move into primordia or young leaves from the SAM. Thus, the PD permeability of the vascular system remains high. This latter transport, even in differentiated (vascular) tissues, likely reflects the specific function of such tissues to act as conduits.
Careful investigation of the sGFP spread in the developing leaves uncovered another interesting feature of these tissues, directionality of symplastic movement. As primordia developed, PD in the regions distal to the SAM became less permeable, whereas PD in the areas proximal to the petiole and the SAM remained competent for sGFP traffic (2).
Overall, the study by Kim et al. provides three important insights into PD transport: (i) traffic of macromolecules between plant cells is most promiscuous in young, undifferentiated tissues, and it becomes much more restricted as tissues mature, (ii) cells destined to form the vascular system retain higher SELs even after differentiation, and (iii) macromolecular transport between cells of a developing leaf is polar.
Large PD Can Still Exercise Control
PD in young tissues are less restrictive for GFP exchange between cells (2) (Fig. 1), but does it mean that these PD are simply “leaky” and any macromolecules can traffic through them freely? Increasing evidence suggests that transport of many transcription factors that move from cell to cell within the meristem and function cell nonautonomously (e.g., refs. 4–6) is not uncontrolled; for example, a relatively small homeodomain protein DEFICIENS moves directionally, from L2 and L3 to L1 cell layers, but it does not move into L2 and L3 when expressed ectopically in L1 (4). Thus, although “free exchange of macromolecules” through PD may represent a default transport mode in the apical meristem (ref. 5 and references therein), these PD still exhibit a degree of selectivity, possibly by sorting out proteins that should not be transported.
Are plasmodesmata simply “leaky,” and can any macromolecules traffic through them freely?
Similarly to intercellular transport within the meristem, import of some macromolecules into the meristem is often controlled, potentially depending on the nature of the transported molecule rather than on its size. This controlled transport is reminiscent of the nuclear import of histone H1, which occurs actively even though H1 is small enough to diffuse through the nuclear pore (7). The best-known example of control over the macromolecular transport into the meristem is the case of many plant viruses, which, although able to dilate and move through PD both locally and systemically in most plant tissues, often are excluded from the meristem (reviewed in refs. 8 and 9). In contrast, some cellular transcripts, such as LeT6 mRNA (10), can gain entrance into the meristem (Fig. 1). Thus, the plant meristem may be under a quarantine for macromolecular import, and the inspection process may occur at PD at the tissue boundary.
Even though the PD SEL in the mature vascular tissues remains high (2), it may be insufficient for certain types of macromolecular transport because PD at the boundaries between different types of phloem cells often restrict movement of plant viruses. For example, a coat protein-deficient mutant of Tobacco mosaic virus accumulates in vascular parenchyma but not in companion cells of Nicotiana tabacum cv. Xanthinn, whereas bromoviruses are unable to cross the interface between bundle sheath and phloem cells in some hosts (reviewed in ref. 8), indicating that PD at these cellular boundaries within the vascular tissues can restrict viral movement. Furthermore, in a restrictive host, N. tabacum cv. V20, a “HAT” strain of Tobacco etch virus enters the vascular tissue but cannot unload from it (11), and nontoxic concentrations of heavy metal cadmium or overexpression of the tobacco cdiGRP protein block phloem unloading, but not entry into the phloem, of some tobamoviruses (12, 13), suggesting unidirectional transport of macromolecules through PD at the boundary between the vascular and nonvascular tissues. Taken together, our present knowledge about PD transport suggests that it is determined not only by the size of the transported molecule but also by its nature and the direction of movement.
Acknowledgments
The work in our laboratory is supported by grants from the National Institutes of Health, the National Science Foundation, the U.S. Department of Agriculture, the U.S.-Israel Binational Agricultural Research and Development Fund, and the U.S.-Israel Binational Science Foundation (to V.C.).
See companion article on page 2227.
References
- 1.Crawford, K. M. & Zambryski, P. C. (2001) Plant Physiol. 125, 1802–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kim, I., Cho, E., Crawford, K., Hempel, F. D. & Zambryski, P. C. (2005) Proc. Natl. Acad. Sci. USA 102, 2227–2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wolf, S., Deom, C. M., Beachy, R. N. & Lucas, W. J. (1989) Science 246, 377–379. [DOI] [PubMed] [Google Scholar]
- 4.Perbal, M. C., Haughn, G., Saedler, H. & Schwarz-Sommer, Z. (1996) Development (Cambridge, U.K.) 122, 3433–3441. [DOI] [PubMed] [Google Scholar]
- 5.Wu, X., Dinneny, J. R., Crawford, K. M., Rhee, Y., Citovsky, V., Zambryski, P. C. & Weigel, D. (2003) Development (Cambridge, U.K.) 130, 3735–3745. [DOI] [PubMed] [Google Scholar]
- 6.Hantke, S. S., Carpenter, R. & Coen, E. S. (1995) Development (Cambridge, U.K.) 121, 27–35. [DOI] [PubMed] [Google Scholar]
- 7.Breeuwer, M. & Goldfarb, D. G. (1990) Cell 60, 999–1008. [DOI] [PubMed] [Google Scholar]
- 8.Waigmann, E., Ueki, S., Trutnyeva, K. & Citovsky, V. (2004) Crit. Rev. Plant Sci. 23, 195–250. [Google Scholar]
- 9.Ueki, S. & Citovsky, V. (2001) Curr. Top. Plant Biol. 2, 167–179. [Google Scholar]
- 10.Kim, M., Canio, W., Kessler, S. & Sinha, N. (2001) Science 293, 287–289. [DOI] [PubMed] [Google Scholar]
- 11.Schaad, M. C. & Carrington, J. C. (1996) J. Virol. 70, 2556–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citovsky, V., Ghoshroy, S., Tsui, F. & Klessig, D. F. (1998) Plant J. 16, 13–20. [DOI] [PubMed] [Google Scholar]
- 13.Ueki, S. & Citovsky, V. (2002) Nat. Cell Biol. 4, 478–485. [DOI] [PubMed] [Google Scholar]