Abstract
BACKGROUND
Inorganic arsenic at high and prolonged doses is highly neurotoxic. Few studies have evaluated whether long-term, low-level arsenic exposure is associated with neuropsychological functioning in adults.
OBJECTIVES
To investigate the association between long-term, low-level inorganic arsenic exposure and neuropsychological functioning among American Indians aged 64–95.
METHODS
We assessed 928 participants in the Strong Heart Study by using data on arsenic species in urine samples collected at baseline (1989–1991) and results of standardized tests of global cognition, executive functioning, verbal learning and memory, fine motor functioning, and speed of mental processing administered during comprehensive follow-up evaluations in 2009–2013. We calculated the difference in neuropsychological functioning for a 10% increase in urinary arsenic with adjustment for sex, age, education, and study site.
RESULTS
The sum of inorganic and methylated arsenic species (ΣAs) in urine was associated with limited fine motor functioning and processing speed. A 10% increase in ΣAs was associated with a 0.10 (95% CI −0.20, −0.01) decrease on the Finger Tapping Test for the dominant hand and a 0.13 decrease (95% CI −0.21, −0.04)) for the non-dominant hand. Similarly, a 10% increase in ΣAs was associated with a 0.15 (95% CI −.29, .00) decrease on the Wechsler Adult Intelligence Scale—Fourth Edition Coding Subtest. ΣAs was not associated with other neuropsychological functions.
CONCLUSIONS
Findings indicate an adverse association between increased urinary arsenic fine motor functioning and processing speed, but not with other neuropsychological functioning, among elderly American Indians.
Keywords: Arsenic, low-level exposure, American Indians, neuropsychological functioning, Strong Heart Study
INTRODUCTION
Inorganic arsenic is a naturally occurring element that is toxic to virtually all life forms in high or prolonged doses. Acute, high-level exposure to inorganic arsenic has been shown to cause severe neuropsychological impairment in humans (Bolla-Wilson and Bleecker 1987). More recent work has confirmed the adverse effects of high-level arsenic exposure on neurological and cognitive functioning in both humans and rodents, the biological mechanisms of which include altered neurological signaling, synaptic plasticity, and behavioral deficits (Tyler and Allan 2014). Moderate to high levels of exposure through drinking water levels of >50 μg/L can increase risk of cancer (Smith et al. 2002), low birth weight (Hopenhayn et al. 2003), and cardiovascular disease (Moon et al. 2012). Although less studied, long-term, low-level inorganic arsenic exposure—e.g., through drinking water levels of 10–20 μg/L over 15 years—is associated with elevated mortality rates for circulatory disease (Meliker et al. 2007; Medrano et al. 2010), and with neuropsychological impairment (Gong et al. 2011; O’Bryant et al. 2011; Tyler and Allan 2014).
Some rural and minority populations in the United States (US) might be at increased risk for negative health outcomes associated with such long-term, low-level exposure. The current US Environmental Protection Agency (EPA) standard for arsenic levels in drinking water is 10 μg/L, revised in 2001 from 50 μg/L (DHHS 2007). In the western US, rocks and soil are more likely to contain moderate to high levels of naturally occurring inorganic arsenic than in other regions of the country (Welch et al. 2000). Arsenic leaches from the soil into groundwater (Peters et al. 1996), so that arsenic concentrations in water from rural wells can be at or above federal standards in several US regions (Navas-Acien et al. 2009). Most rural American Indian communities are located in the western US, and many rely heavily on well water for daily consumption. These communities therefore experience a relatively high risk of long-term, low-level arsenic exposure.
The Strong Heart Study (SHS) was the largest epidemiologic study ever conducted with American Indian communities. From 1989 to 1999, SHS researchers collected longitudinal data on risk factors, incidence, prevalence, and mortality associated with cardiovascular disease in a cohort drawn from 13 American Indian tribes and communities in the western US (Arizona, Oklahoma, North Dakota, and South Dakota) (Lee et al. 1990; Navas-Acien et al. 2009). Recent studies using data on this unique cohort have associated long-term, low to moderate inorganic arsenic exposure with elevations in mortality due to cancer (García-Esquinas et al. 2013), prevalence of diabetes and albuminuria (Gribble et al. 2012; Zheng et al. 2013), and incidence of cardiovascular disease (Moon et al. 2013). However, no studies have investigated this cohort to determine the relationship between chronic, low to moderate arsenic exposure and neuropsychological outcomes.
We combined data from the SHS and an ancillary study, the Cerebrovascular Disease and its Consequences in American Indians (CDCAI) study (Suchy-Dicey et al. 2016), to examine the association of urinary arsenic concentrations measured at baseline (1989–1991) in 928 American Indian adults aged 45–74 years with neuropsychological functioning measured 20 years later. We investigated how this association might differ according to demographic characteristics including age, education, and sex.
METHODS
Study Setting and Sample
The SHS collected data in three phases (Phase I: 1989–1991, Phase II: 1993–1994, Phase III: 1998–1999). During Phase I, 4,549 tribal members aged 45–74 years were enrolled and examined: 1,500 in the American Southwest, 1,527 in the Central Plains, and 1,522 in the Northern Plains. Among eligible community members, participation rates were 72% at the Southwest center, 62% at the Central Plains center, and 55% at the Northern Plains center. Trained local staff collected data on participants’ medical history; family history of related illness; diet, alcohol and tobacco consumption; physical activity; and socioeconomic status. Objective measurements included body fat, waist circumference, body mass index (BMI), blood pressure, blood tests relevant to diabetes and cardiovascular disease, and urinary creatinine and albumin (see Lee et al. 1990 for a full discussion of methods). In 2009, urinary arsenic concentrations were measured in 3,973 Phase I participants who had stored urine samples available and whose demographic information was complete (Moon et al. 2013).
From 2009 to 2013, the CDCAI study, designated as the “Strong Heart Stroke Study” by participating communities and field centers, collected data pertinent to vascular and structural brain disease from surviving SHS participants. The study goals were to examine the prevalence of and risk factors for brain disease among American Indian adults aged 64–95 years. Trained local staff successfully contacted 1,664 (85%) of 1,956 surviving participants from all three study sites. More than half of those contacted (1,033; 62%) were eligible and agreed to participate in the new study. Among 631 participants who did not participate, 201 (32%) did not meet eligibility criteria (see below), 169 (27%) refused, and 261 (41%) became deceased or unable to attend the study visit because of physical limitations between the beginning of recruitment and the start of data collection (Figure 1). Exclusion criteria included prior surgery for a cerebral aneurysm; an internal metal or electrical device such as a pacemaker, metal prosthesis, or cochlear implant; weight ≥ 158 kg; and inability to complete study procedures. Study procedures included magnetic resonance imaging (MRI) of the brain and completion of a neuropsychological battery measuring general cognitive functioning, speed of mental processing, phonemic verbal fluency, immediate and delayed verbal memory, and fine motor functioning (see Suchy-Dicey et al. 2016 for a full discussion of methods). The sample for the current analysis consisted of 928 participants in the CDCAI study whose urinary arsenic levels were measured in 1989–1991.
Figure 1.
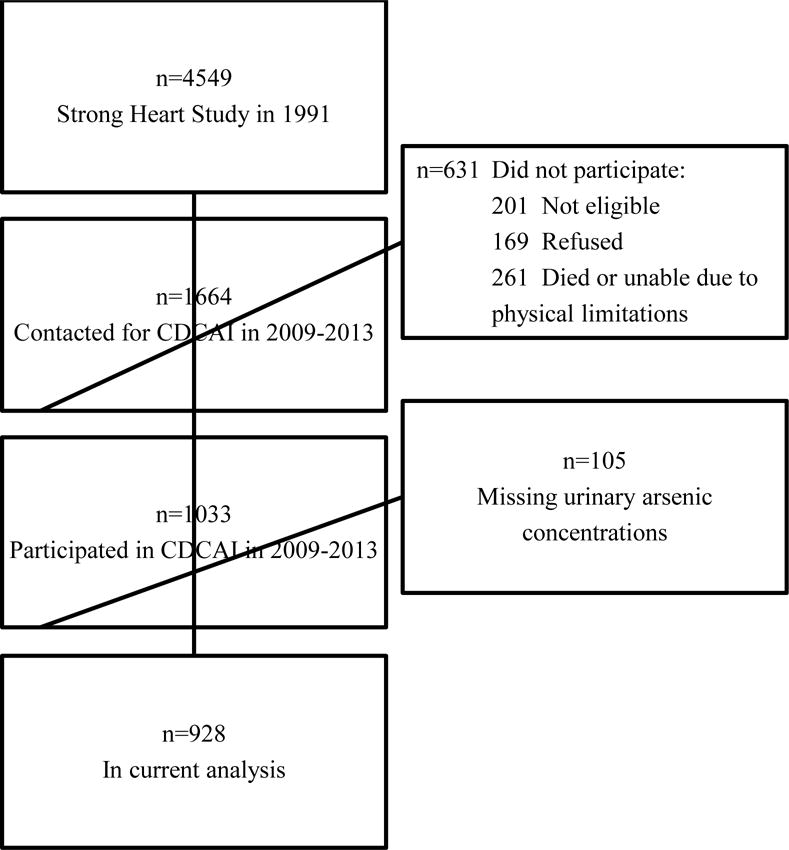
Sample selection flow-chart
Urinary Arsenic Concentration
Arsenic exposure is commonly assessed by measuring inorganic and methylated arsenic species in urine. Methylated arsenic species refer to monomethylarsonate (MMA) and dimethyarsinate (DMA). During Phase I of the SHS, spot urine samples were collected by using polypropylene tubes, then frozen within two hours, shipped on dry ice to the Penn Medical Laboratory (Hyattsville, Maryland) and the MedStar Health Research Institute (Washington, D.C.), and stored at −80 °C. In 2009, concentrations of arsenic species (inorganic arsenic, MMA, DMA, arsenobetaine, and other arsenic cations) were measured by the Trace Element Laboratory in Graz, Austria, by using inductively coupled plasma mass spectrometry (ICPMS) and highperformance liquid chromatography (Scheer et al. 2012). Urinary cadmium was also measured using ICPMS. The detection limit for all arsenic species was 0.1 μg/L. Values for samples that fell below this limit were replaced with or 0.07 μg/L. Urine samples with arsenic species below the limit of detection included 5.3% for inorganic arsenic, 0.7% for MMA, and <0.1% for DMA. For urine cadmium, 0.1% were below the detection limit. At the time of collection, a blinded quality control analysis of 47 duplicate urine aliquots stored in different vials found an intraclass correlation coefficient of 0.99 for total arsenic concentrations and arsenic species (Gribble et al. 2012). The inter-assay coefficient of variation based on an inhouse urine sample analyzed in each analytical run, together with the study samples, was 4.4% for total arsenic, 6.0% for inorganic arsenic, 6.5% for MMA, 5.9% for DMA, and 6.5% for arsenobetaine and other cations (Scheer et al. 2012). Our analysis focused on inorganic and methylated arsenic as a biomarker of inorganic arsenic exposure. Organic arsenic species from seafood were low in the SHS population, as seafood intake is rare in the study communities (Navas-Acien et al. 2009). Prior research with the SHS sample demonstrated long-term constancy in urinary arsenic concentrations and excretory patterns (intra-class correlation correlation = 0.80), supporting the use of urinary arsenic as a biomarker of long-term arsenic exposure (Navas-Acien et al. 2009; Moon et al. 2013).
Neuropsychological Functioning
Neuropsychological tests administered by the CDCAI study included the Modified Mini Mental State Examination (3MS), the California Verbal Learning Test—Second Edition Short Form (CVLT-II SF), the Controlled Oral Word Association test (COWA), the Finger Tapping Test (FTT), and the Wechsler Adult Intelligence Scale—Fourth Edition (WAIS-IV) Coding Subtest. The brief neuropsychological battery was selected for the CDCAI to investigate cognitive correlates of cerebrovascular disease, include instruments that were used in other similar, large studies of MRI-defined vascular brain injury, and which could be administered rapidly in community settings (Arnold et al. 2005; Rosamond et al. 1999). Field staff was trained in standardized assessment, scoring was quality controlled with feedback given to site staff, and protocol was standardized with the same administration across all sites (Suchy-Dicey et al. 2016).
The 3MS is a measure of global cognitive functioning that is widely used to screen for neuropsychological impairment (Spreen and Strauss 1998). The CVLT-II SF is a verbal learning and memory test in which participants try to learn a list of nine words in three categories over four learning trials and then recall the list at different time points (short and long delay free recall) (Delis et al. 2000). The COWA is a measure of phonemic verbal fluency, which also involves executive functions; participants generate as many words as they can that start with the letters F, A, or S within one minute (Benton et al. 1989). The FTT assesses fine motor functioning, specifically motor speed and lateralized coordination, as a measure of manual dexterity (Halstead 1947). Using one hand at a time, participants tap their index finger on a lever as quickly as possible for 10 seconds; a counting device records the number of taps. We included the FTT as a measure of lateralized brain dysfunction, computing the average for five trials. The WAIS-IV Coding Subtest is a measure of processing speed requiring the coding of symbols paired with specific numbers. Participants receive a score based on the number of correct responses within a two-minute period (Wechsler 2008).
Participant characteristics
Sociodemographic variables included sex, age at the time that the neuropsychological examinations were conducted (2009–2013), highest educational level (none, 8th grade, 9th–11th grade, high school graduate, 1–3 years of college, ≥ 4 years of college), and study center (Southwest, Central Plains, or Northern Plains).
Statistical Analysis
We calculated descriptive statistics by using median and interquartile range for continuous variables and percentages for categorical variables. To assess the potential for survival bias, we used the Kruskal Wallis test to compare arsenic levels according to vital status as of December 31, 2008, and CDCAI study participation. No individual data on vital status were available for 2009 or later. To further assess selection bias, we used t-tests and chi-square tests to compare demographic characteristics of CDCAI study participants to the characteristics of the original SHS cohort.
We used linear regression with robust standard errors to examine the association between urinary arsenic concentrations and neuropsychological functioning measured approximately 20 years later. We evaluated inorganic and methylated arsenic species separately and in combination as our main variable to reflect inorganic arsenic exposure. To account for urine dilution, we divided urinary arsenic concentrations by urinary creatinine concentrations and expressed the results in units of μg/g creatinine. Due to the skewed nature of the data, we log transformed the arsenic/creatinine ratio variable for use in regression models. Inferential results are presented as the difference in neuropsychological functioning associated with a 10% increase in urinary arsenic concentration, with 95% confidence intervals. Our goal was to capture the association between urinary arsenic concentration and neuropsychological functioning by any mechanism; therefore, regression models were only adjusted for known confounders of this pathway such as sex, age, education, and study center. We excluded adjustment variables such as diabetes, hypertension, and other conditions that could be mediators on this causal pathway. Due to potential confounding by other known environmental exposures, we also performed a sensitivity analysis that adjusted for urinary cadmium concentration. After exposure, inorganic arsenic is metabolized into mono and dimethyl arsenic compounds (MMA and DMA), which are eliminated through the urine together with inorganic arsenic. The relative proportion of each species over their sum (inorganic arsenic [iAs] %, MMA% and DMA%) in urine are commonly used as biomarkers for arsenic metabolism. We ran analyses for the association between arsenic metabolism biomarkers and neuropsychological functioning.
In further sensitivity analyses, we examined if the association between urinary arsenic concentration and neuropsychological functioning differed according to sex, age, education, and by arsenic metabolism biomarker strata (relative proportion of each arsenic metabolite over their sum, iAs%, MMA%, DMA%). We fit models for each strata separately to describe any observed differences between the strata and then tested for statistical significance of the differences by including an interaction term in the model including all strata combined. All analyses were conducted by using Stata 12 (StataCorp LP, College Station, TX), and an alpha level of 0.05 was considered statistically significant.
RESULTS
Participant Characteristics
The median age of participants in our study sample was 72 years (interquartile range: 68–76) and the median elapsed between the initial spot urine sample and the neuropsychological measurements was 21 years (interquartile range: 20–22). Roughly one-third of participants came from each of the three study centers; 69% were female; and 65% had completed at least a high school education.
Several characteristics differed across quartiles of arsenic concentrations (Table 1). As in a previous study (Navas-Acien et al. 2009), arsenic concentrations were highest among participants in the Southwest, moderate in Northern Plains, and lowest in Central Plains. Among participants with the highest arsenic exposure, 81% were female and 22% had completed 8th grade or less, whereas among those with the lowest exposure, 58% were female and only 6% had an 8th grade education or less. CDCAI study participants had a median urinary arsenic level of 8.8 μg/g creatinine (IQR 5.5–13.9), compared to 8.4 μg/g creatinine (IQR 5.1–13.8) in SHS cohort members who were alive as of January 1, 2009, but did not participate in the CDCAI study, and 11.4 μg/g creatinine (IQR 6.7–18.1) in SHS cohort members who died before 2009 (p < 0.001). Compared to SHS cohort members who did not participant in the CDCAI study, CDCAI study participants were younger (mean 52, SD 8 years vs. mean 58, SD 6 years; p < 0.001), had more education (mean 12, SD 3 years vs. mean 11, SD 3 years; p < 0.001), and were more likely to be female (69% vs 57%; p < 0.001). Descriptive statistics for the neuropsychological tests are presented in Table 2.
Table 1.
Participant characteristics according to quartile of urinary arsenic
| Urinary Arsenic1
|
||||
|---|---|---|---|---|
| Low | Low-Moderate | Moderate-High | High | |
| Characteristic | 1.71–5.51 μg/g | 5.52–8.76 μg/g | 8.77–13.84 μg/g | 13.85–109.1 μg/g |
| (n=232) | (n=233) | (n=231) | (n=232) | |
| Age, median years (IQR) | 72 (68–77) | 71 (68–76) | 73 (68–77) | 72 (68–76) |
| Time elapsed between arsenic and neuropsychological measurements, median years (IQR) | 21(20–22) | 21 (20–22) | 21 (20–22) | 21 (20–22) |
| Study center | ||||
| Southwest U.S. | 7% | 22% | 37% | 58% |
| Central Plains U.S. | 67% | 38% | 19% | 6% |
| Northern Plains U.S. | 26% | 39% | 44% | 36% |
| Female | 58% | 64% | 72% | 81% |
| Education completed | ||||
| None to 8th grade | 6% | 9% | 20% | 22% |
| 9th–11th grade | 19% | 17% | 18% | 27% |
| 12th grade | 25% | 36% | 32% | 23% |
| 1–3 years of college | 33% | 26% | 20% | 22% |
| 4 years or more of college | 18% | 11% | 9% | 7% |
Sum of inorganic and methylated arsenic species measured in urine arsenic / urine creatinine ratio (μg/g); IQR = interquartile range
Table 2.
Descriptive statistics for neuropsychological functioning
| Neuropsychological Test | N | Median | IQR |
|---|---|---|---|
| 3MS | 917 | 90 | 84 – 94 |
| CVLT-II SF, Short Delay Free Recall | 875 | 6 | 5 – 7 |
| CVLT-II SF, Long Delay Free Recall | 877 | 6 | 5 – 7 |
| COWA | 913 | 22 | 15 – 31 |
| FTT, Dominant hand | 918 | 33 | 26 – 41 |
| FTT, Non-dominant hand | 904 | 33 | 27 – 38 |
| WAIS-IV Coding | 903 | 43 | 34 – 53 |
IQR = interquartile range; 3MS= Modified Mini Mental State; CVLT-II SF = California Verbal Learning Test – 2nd edition Short Form; COWA = Controlled Oral Word Association test; FTT=Finger Tapping Test; WAIS-IV Coding=Wechsler Adult Intelligence Scale – 4th edition Coding subtest
Urinary Arsenic Concentration and Neuropsychological Functioning
Results for the association between neuropsychological functioning and urinary arsenic concentration measured as the sum of inorganic and methylated arsenic species (ΣAs) are presented in Table 3. When we examined the difference (Δ) in neuropsychological functioning for a 10% increase in ΣAs, we found no association for the 3MS (Δ −.04, 95% CI [−.13, .04]), the CVLT-II SF short or long delay free recall (Δshort −.01, 95% CI [−.03, .01]; Δlong −.01, 95% CI [− .03, .01]), or the COWA (Δ −.04, 95% CI [−.15, .07]). Higher ΣAs was marginally associated with poorer performance on the WAIS-IV Coding subtest (Δ −.15, 95% CI [−.29, .00], p=0.05). A 10% increase in ΣAs was associated with significantly decreased neuropsychological functioning for the FTT for both the dominant (Δ −.10, 95% CI [−.20, −.01]) and non-dominant hand (Δ −.13, 95% CI [−.21, −.04]). Results were similar when inorganic arsenic, MMA, and DMA were examined separately (Supplemental Table 1). Sensitivity analyses that adjusted for urinary cadmium concentration were similar.
The association between neuropsychological functioning and urinary arsenic concentration was similar when stratified by sex and education (Table 3). In addition to a lack of statistical significance, there was no consistent pattern for the observed differences across the neuropsychological tests. In contrast, for the age-stratified analyses, we observed a consistent pattern across most neuropsychological tests; the mid-range age group (70–74 years) showed a larger decrease in neuropsychological functioning for a 10% increase in urinary arsenic concentration compared to the lowest (64–69 years) and highest (75+ years) age groups. The difference across the age strata for the association between neuropsychological functioning and urinary arsenic concentration was significant for the COWA (p=0.01) and marginally significant for the 3MS (p=0.05).
Table 3.
Adjusted1 association between urinary arsenic2 and neuropsychological functioning, overall and stratified by age, education, and sex
| Difference in neuropsychological functioning for a 10% increase in urinary arsenic (95% CI)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall | Sex | Age in years | Completed education | ||||||
|
|
|
||||||||
| Neuropsychological Test | Female | Male | 64–69 | 70–74 | 75+ | < HS | HS | >HS | |
| 3MS | −.04 (−.13, .04) |
−.08 (−.19, .02) |
.07 (−.10, .25) |
.09 (−.04, .22) |
−.21 (−.38, −.04) |
−.02 (−.18, .14) |
−.12 (−.30, .06) |
−.05 (−.17, .07) |
.03 (−.11,−.17) |
| p = 0.32 | p = 0.24 | p = 0.05 | p = 0.38 | ||||||
| CVLT-II SF, Short Delay Free Recall | −.01 (−.03, .01) |
−.01 (−.03, .01) |
−.01 (−.04, .02) |
.00 (−.03, .03) |
−.01 (−.04, .02) |
−.01 (−.05, .02) |
−.01 (−.05, .02) |
.01 (−.02, .04) |
−.02 (−.05, .01) |
| p = 0.39 | p = 0.35 | p = 0.82 | p = 0.66 | ||||||
| CVLT-II SF, Long Delay Free Recall | −.01 (−.03, .01) |
−.01 (−.04, .02) |
.00 (−.05, .05) |
−.01 (−.04, .03) |
−.00 (−.03, .04) |
−.03 (−.06, .01) |
−.02 (−.06, .01) |
.00 (−.03, .04) |
−.01 (−.04, .02) |
| p = 0.35 | p = 0.53 | p = 0.41 | p = 0.85 | ||||||
| COWA | −.04 (−.15, .07) |
−.07 (−.20, .05) |
.05 (−.17, .29) |
.03 (−.16, .22) |
−.23 (−.41, −.05) |
.06 (−.12, .25) |
−.01 (−.18, .16) |
−.16 (−.38, .05) |
.02 (−.17, .21) |
| p = 0.49 | p = 0.05 | p = 0.01 | p = 0.63 | ||||||
| FTT, Dominant hand | −.10 (−.20, −.01) |
−.09 (−.20, .02) |
−.15 (−.34, .05) |
−.03 (−.17, .12) |
−.20 (−.38. −.03) |
−.08 (−.25, .08) |
−.02 (−.18, .14) |
−.23 (−.41, −.05) |
−.09 (−.24, .06) |
| p = 0.03 | p = 0.19 | p = 0.32 | p = 0.42 | ||||||
| FTT, Non-dominant hand | −.13 (−.21, −.04) |
−.09 (−.18, .00) |
−.23 (−.40, .06) |
−.01 (−.15, .12) |
−.21 (−.34, −.07) |
−.15 (−.30, −.01) |
−.09 (−.23, .05) |
−.23 (−.39, −.08) |
−.08 (−.21, .06) |
| p < 0.01 | p = 0.08 | p = 0.10 | p = 0.76 | ||||||
| WAIS-IV Coding | −.15 (−.29, .00) |
−.18 (−.35, −.01) |
−.05 (−.35, .24) |
−.01 (−.28, .26) |
−.38 (−.63, −.12) |
−.04 (−.26, .18) |
−.03 (−.25, .19) |
−.11 (−.41, .18) |
−.29 (−.52, −.05) |
| p = 0.05 | p = 0.20 | p = 0.11 | p = 0.32 | ||||||
Adjusted for age, study center, sex, and education;
Sum of inorganic and methylated arsenic species measured in urine arsenic / urine creatinine ratio (μg/g); CI = confidence interval; HS = high school graduate; 3MS= Modified Mini Mental State; CVLT-II SF = California Verbal Learning Test – 2nd edition Short Form; COWA = Controlled Oral Word Association test; FTT=Finger Tapping Test; WAIS-IV Coding=Wechsler Adult Intelligence Scale – 4th edition Coding subtest
For arsenic metabolism biomarkers, a 5% increase in iAs% was associated with lower neuropsychological functioning for several tests, including 3MS (Δ −.87, 95% CI [−1.66, −.09]), CVLT-II SF short and long delay free recall (Δshort −.17, 95% CI [−.30, −.04]; Δlong −.20, 95% CI [− .36, −.04]), and WAIS-IV Coding (Δ −1.10, 95% CI [−2.03, −.18], Supplementary Table 2). The correlation between the concentration of each arsenic species and its respective metabolism biomarker was 0.47 for iAS and iAs%, 0.32 for MMA and MMA%, and 0.09 for DMA and DMA%. In analyses of arsenic concentrations by strata of arsenic metabolism biomarkers, there were no major differences in the association between arsenic and neuropsychological functioning by arsenic metabolism biomarker levels (Supplementary Table 3).
DISCUSSION
Our findings reveal a significant relationship between long-term, low-level inorganic arsenic exposure measured at baseline and poor outcomes in the neuropsychological domain of fine motor functioning measured 20 years later. Although we report that only one domain in our neuropsychological assessment appears sensitive to arsenic exposure, these findings contribute to other recent research on the effects of long-term, low to moderate arsenic exposure (20–50 μg/L in drinking water) on cognition in children and adults. We also found an association between a higher proportion of iAs% in urine and several neuropsychological domains, including global cognitive functioning, fine motor functioning, and processing speed. However, little is known on the association between arsenic metabolism and neuropsychological functioning, as no studies have evaluated the association between arsenic metabolism biomarkers and multiple neuropsychological domains. Lastly, we observed a consistent pattern for the mid-range age group (70–74 years) across most neuropsychological tests, which showed a larger decrease in neuropsychological functioning for a 10% increase in urinary arsenic concentration compared to the lowest (64–69 years) and highest (75+ years) age groups. Although survivor bias may be present because arsenic increases mortality in this population (Moon et al. 2013), the observed pattern supports that slower methylation of inorganic arsenic results in worse neuropsychological function, affecting multiple domains.
In children aged 5–13 years, poor cognitive development, lower IQ, and impaired verbal learning and memory have been associated with both low and moderate levels of arsenic exposure in study populations in Mexico, Bangladesh, and the US (Wright et al. 2006; Rosado et al. 2007; Hamadani et al. 2011). In adults, chronic, low-level arsenic exposure has been linked to lower scores on tests of general cognitive functioning, immediate memory, and executive functioning (e.g., the Mini-Mental State Examination and the Repeatable Battery for the Assessment of Neuropsychological Status) (Gong et al. 2011; O’Bryant et al. 2011). One study among Hispanics and non-Hispanic Whites aged 40–96 years in rural west Texas (a Project FRONTIER study) revealed marked neuropsychological impairment in participants who lived in areas with higher arsenic concentrations in groundwater, as determined by Geographic Information System (GIS) arsenic data (O’Bryant et al. 2011). Given the strong associations noted, the authors suggested a link between Alzheimer’s disease and long-term, low-level arsenic exposure through drinking water.
Although the association between impaired motor functioning and exposure to heavy metals such as manganese, mercury, and lead is well documented (Echeverria et al. 2005; Mansouri and Cauli 2009; Hernández-Bonilla et al. 2011; Zoni and Lucchini 2013), few studies have explicitly examined the association between motor functioning and chronic, low-level arsenic exposure. One longitudinal study among children aged 8–11 years in Bangladesh found an inverse association between such exposure and motor functioning (Parvez et al. 2011). Although that study, like ours, measured urinary arsenic, the investigators assessed motor functioning by using the Bruininks-Oseretsky test, 2nd edition. This comprehensive measure, specific to children, assesses a wide range of motor skills. By contrast, the FTT that we used assesses fine motor functioning, specifically motor speed and lateralized coordination, and is primarily a measure of manual dexterity. Our work therefore makes a valuable contribution to an understudied but critical area by reporting the effects of arsenic in a very different population: rural American Indian adults.
Even though numerous studies have found significant associations between chronic, low-level arsenic exposure and measures of verbal learning and memory (Wright et al. 2006; Rosado et al. 2007; Gong et al. 2011; Hamadani et al. 2011; O’Bryant et al. 2011), we noted only a nonsignificant trend associating arsenic levels with scores on the CVLT-II SF long delay free recall. The present findings of no association might point to differences in exposure assessments as compared to other studies, as well as our own study’s methodological limitations. For one, discrepancies between our study and those of Project FRONTIER (Gong et al. 2011; O’Bryant et al. 2011) may be related to how arsenic exposure was assessed (urinary arsenic versus GIS-arsenic). Measurement of individual arsenic species in urine is the most reliable way to assess arsenic exposure (Scheer et al. 2012), therefore our results may represent a more accurate exposure assessment. However, with regard to methodological limitations, participants in the CDCAI study had significantly lower urinary arsenic levels than participants in the SHS. Thus, because arsenic levels were highest in SHS participants who died before the CDCAI study commenced, our results might suffer from survival bias. One previous study has shown that chronic exposure to modest arsenic levels is associated with higher incidence and mortality rates for cardiovascular disease (Moon et al. 2013). Our results might therefore be biased toward the null, compromising our efforts to detect an effect. Furthermore, neuropsychological functioning was not measured during Phase I of the SHS, so we have no longitudinal data for a direct test of the effect of arsenic exposure on the development of neuropsychological impairment. It is possible that despite the prospective nature of the study, we cannot distinguish between current and past effects of arsenic exposure due to the stability in arsenic exposure over time. Although urinary arsenic was measured only at baseline and could have changed over the course of 20 years, prior research in the SHS cohort, as well as a subset with repeated sampling, demonstrated stable urinary arsenic concentrations and excretory patterns between 1989 and 1999 (Navas-Acien et al. 2009; Moon et al. 2013).
Despite these limitations, our study adds to the literature by presenting the first examination of the neuropsychological effects of arsenic exposure over an extended period of time. Because we assessed surviving SHS cohort members after a lapse of 20 years, our results might reflect neuropsychological health effects that manifest slowly and cannot be detected with cross-sectional data. Additionally, ours is the first analysis of the relationship between urinary inorganic arsenic levels and neuropsychological functioning in American Indians, and one of the few studies on this topic in any adult population in the US. Lastly, although the mediation effects of cardiovascular disease and its risk factors on the association between arsenic and neuropsychological functioning are beyond the scope of this article, they point to promising pathways for future research.
CONCLUSIONS
Although we found an association between baseline urinary arsenic levels and fine motor functioning measured 20 years later among rural American Indians, much more research is needed to understand the neuropsychological outcomes of chronic, low-level arsenic exposure in this population. The trends that we observed in other neuropsychological measures underscore the importance of baseline measures of both arsenic exposure and neuropsychological functioning and careful study designs to account for the more immediate effects of arsenic. Although we were unable to exclude the role of chance in these observed associations, we also are unable to rule out the possibility that arsenic exposure has subtle, adverse effects on health. Lifetime accumulation of neurological damages might pose special threats to Native culture and American Indian communities, where intergenerational transmission of languages and other types of endangered cultural knowledge rely heavily on elders’ capacities in memory and language use (McGregor 2005; Berardo et al. 2014). Future research should conduct more extensive assessments of the relationship between neuropsychological functioning and arsenic exposure in American Indian communities.
Supplementary Material
Highlights.
We study the effects of arsenic on neuropsychological functioning among older American Indians.
We assess urinary arsenic concentrations in Strong Heart Study participants from 1991 and results of follow-up standardized neuropsychological tests 20 years later.
We find that limited fine motor functioning and processing speed are associated with low level arsenic exposure.
Acknowledgments
This study was supported by research grants (R01 HL090863, R01 HL093086, R01 HL109282, R01 HL109284, R01 HL109301, R01 HL109315, and R01 HL109319) and cooperative agreement grants (U01 HL41642, U01 HL41652, U01 HL41654, U01 HL65520, and U01 HL65521) from the National Heart, Lung, and Blood Institute, and a grant from the National Institute of Environmental Health Sciences (R01 ES021367). The preparation of the manuscript was supported in part by the National Institute on Aging (5P30AG15292; S.M. Manson, PI) through the Native Elder Research Center, which is jointly administered by the University of Washington and the University of Colorado Denver. Dr. Carroll was supported in part by a Ford Foundation Post-Doctoral Fellowship administered by the National Academy of Sciences. The authors would like to thank all Strong Heart Study and Cerebrovascular Disease and its Consequences in American Indians study participants, staff, and investigators, as well as the Native Elder Research Center faculty, trainees, and staff for their help in refining this article to its present form. The authors also thank Raymond M. Harris, PhD, for editing assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
The authors have obtained all required tribal, Institutional Review Board, and human subjects approvals. The authors declare that the work described in this article has been carried out in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans, and meets the uniform requirements for manuscripts submitted to biomedical journals. The authors further declare that they have no competing financial interests.
References
- Arnold AM, Psaty BM, Kuller LH, Burke GL, Manolio TA, Fried LP, Robbins JA, Kronmal RA. Incidence of cardiovascular disease in older Americans: The Cardiovascular Health Study. J Am Geriatr Soc. 2005;53:211–218. doi: 10.1111/j.1532-5415.2005.53105.x. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamsher K, Sivan A. Multilingual Aphasia Examination. AJA Associates, Inc.; Iowa City, IA: 1989. [Google Scholar]
- Berardo M, Peter L, Hirata-Edds T. Language loss and language revitalization in American Indian communities. In: Ross JI, editor. American Indians at Risk. Greenwood Press; Santa Barbara, CA: 2014. pp. 187–203. [Google Scholar]
- Bolla-Wilson K, Bleecker ML. Neuropsychological impairment following inorganic arsenic exposure. J Occup Med. 1987;29:500–503. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fourth Report on Human Exposure to Environmental Chemicals. U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; Atlanta, GA: p. 2009. URL: http://www.cdc.gov/exposurereport/ [Accessed 18 December 2016] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, Ober BA. California Verbal Learning Test - Second Edition (CVLT II) Psychological Corporation; San Antonio, TX: 2000. [Google Scholar]
- Echeverria D, Woods JS, Heyer NJ, Rohlman DS, Farin FM, Bittner AC, Li T, Garabedian C. Chronic low-level mercury exposure, BDNF polymorphism, and associations with cognitive and motor function. Neurotoxicol Teratol. 2005;27:781–796. doi: 10.1016/j.ntt.2005.08.001. [DOI] [PubMed] [Google Scholar]
- García-Esquinas E, Pollán M, Umans JG, Francesconi KA, Goessler W, Guallar E, Howard B, Farley J, Best LG, Navas-Acien A. Arsenic exposure and cancer mortality in a US-based prospective cohort: The Strong Heart Study. Cancer Epidem Biomar. 2013;22:1944–1953. doi: 10.1158/1055-9965.EPI-13-0234-T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong G, Hargrave KA, Hobson V, Spallholz J, Boylan M, Lefforge D, O’Bryant SE. Low-level groundwater arsenic exposure impacts cognition: A Project FRONTIER study. J Environ Health. 2011;74:16–22. [PubMed] [Google Scholar]
- Gribble MO, Howard BV, Umans JG, Shara NM, Francesconi KA, Goessler W, Crainiceanu CM, Silbergeld EK, Guallar E, Navas-Acien A. Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am J Epidemiol. 2012;176:865–874. doi: 10.1093/aje/kws153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstead WC. Brain and Intelligence: A Quantitative Study of the Frontal Lobes. University of Chicago Press; Chicago: 1947. [Google Scholar]
- Hamadani JD, Tofail F, Nermell B, Gardner R, Shiraji S, Bottai M, Arifeen SE, Huda SN, Vahter M. Critical windows of exposure for arsenic-associated impairment of cognitive function in pre-school girls and boys: A population-based cohort study. Int J Epidemiol. 2011;40:1593–1604. doi: 10.1093/ije/dyr176. [DOI] [PubMed] [Google Scholar]
- Hernández-Bonilla D, Schilmann A, Montes S, Rodríguez-Agudelo Y, Rodríguez-Dozal S, Solís-Vivanco R, Ríos C, Riojas-Rodríguez H. Environmental exposure to manganese and motor function of children in Mexico. Neurotoxicology. 2011;32:615–621. doi: 10.1016/j.neuro.2011.07.010. [DOI] [PubMed] [Google Scholar]
- Hopenhayn C, Ferreccio C, Browning SR, Huang B, Peralta C, Gibb H, Hertz-Picciotto I. Arsenic exposure from drinking water and birth weight. Epidemiology. 2003;14:593–602. doi: 10.1097/01.ede.0000072104.65240.69. [DOI] [PubMed] [Google Scholar]
- Lee ET, Welty TK, Fabsitz R, Cowan LD, Le NA, Oopik AJ, Cucchiara AJ, Savage PJ, Howard BV. The Strong Heart Study: A study of cardiovascular disease in American Indians: Design and methods. Am J Epidemiol. 1990;132:1141–1155. doi: 10.1093/oxfordjournals.aje.a115757. [DOI] [PubMed] [Google Scholar]
- Mansouri MT, Cauli O. Motor alterations induced by chronic lead exposure. Environ Toxicol Phar. 2009;27:307–313. doi: 10.1016/j.etap.2009.01.003. [DOI] [PubMed] [Google Scholar]
- McGregor D. Coming full circle: Indigenous knowledge, environment, and our future. Am Indian Quart. 2005;28:385–410. [Google Scholar]
- Medrano MA, Boix R, Pastor-Barriuso R, Palau M, Damián J, Ramis R, Del Barrio JL, Navas-Acien A. Arsenic in public water supplies and cardiovascular mortality in Spain. Environ Res. 2010;110:448–54. doi: 10.1016/j.envres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- Meliker JR, Wahl RL, Cameron LL, Nriagu JO. Arsenic in drinking water and cerebrovascular disease, diabetes mellitus, and kidney disease in Michigan: A standardized mortality ratio analysis. Environ Health. 2007;6:1–11. doi: 10.1186/1476-069X-6-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon K, Guallar E, Navas-Acien A. Arsenic exposure and cardiovascular disease: An updated systematic review. Curr Atheroscler Rep. 2012;14:542–555. doi: 10.1007/s11883-012-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moon KA, Guallar E, Umans JG, Devereux RB, Best LG, Francesconi KA, Goessler W, Pollak J, Silbergeld EK, Howard BV, Navas-Acien A. Association between exposure to low to moderate arsenic levels and incident cardiovascular disease: A prospective cohort study. Ann Intern Med. 2013;159:649–659. doi: 10.7326/0003-4819-159-10-201311190-00719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navas-Acien A, Umans JG, Howard BV, Goessler W, Francesconi KA, Crainiceanu CM, Silbergeld EK, Gualiar E. Urine arsenic concentrations and species excretion patterns in American Indian communities over a 10-year period: The Strong Heart Study. Environ Health Perspect. 2009;117:1428–1433. doi: 10.1289/ehp.0800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Bryant SE, Edwards M, Menon CV, Gong G, Barber R. Long-term low-level arsenic exposure is associated with poorer neuropsychological functioning: A Project FRONTIER study. Int J Environ Res Public Health. 2011;8:861–874. doi: 10.3390/ijerph8030861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F, Wasserman GA, Factor-Litvak P, Liu X, Slavkovich V, Siddique AB, Sultana R, Islam T, Levy D. Arsenic exposure and motor function among children in Bangladesh. Environ Health Perspect. 2011;119:1665–1670. doi: 10.1289/ehp.1103548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters GR, McCurdy RF, Hindmarsh JT. Environmental aspects of arsenic toxicity. Cr Rev Cl Lab Sc. 1996;33:457–493. doi: 10.3109/10408369609080055. [DOI] [PubMed] [Google Scholar]
- Rosado JL, Ronquillo D, Kordas K, Rojas O, Alatorre J, Lopez P, Garcia-Vargas G, Del Carmen Caamaño M, Cebrián ME, Stoltzfus RJ. Arsenic exposure and cognitive performance in Mexican schoolchildren. Environ Health Perspect. 2007;115:1371–1375. doi: 10.1289/ehp.9961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosamond WD, Folsom AR, Chambless LE, Wang C-H, McGovern PG, Howard G, Copper LS, Shahar E. Stroke Incidence and Survival Among Middle-aged Adults. Stroke. 1999;30:736–743. doi: 10.1161/01.str.30.4.736. [DOI] [PubMed] [Google Scholar]
- Scheer J, Findenig S, Goessler W, Francesconi KA, Howard BV, Umans JG, Pollak J, Tellez-Plaza M, Silbergeld EK, Guallar E. Arsenic species and selected metals in human urine: Validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal Method. 2012;4:406–413. doi: 10.1039/C2AY05638K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Lopipero PA, Bates MN, Steinmaus CM. Arsenic epidemiology and drinking water standards. Science. 2002;296:2145–2146. doi: 10.1126/science.1072896. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary. Oxford University Press; New York: 1998. [Google Scholar]
- Suchy-Dicey A, Shibata D, Best LG, Verney SP, Longstreth WT, Lee ET, Okin PM, Devereux R, O’Leary M, Ali T, Jensen PN, Muller C, Nelson LA, Rhoades E, Madhyastha T, Grabowski TJ, Beauchamp N, Umans JG, Buchwald D. Cranial magnetic resonance imaging (MRI) in elderly American Indians: Design, methods, and implementation of the Cerebrovascular Disease and its Consequences in American Indians Study. Neuroepidemiology. 2016;47:67–75. doi: 10.1159/000443277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler CR, Allan AM. The effects of arsenic exposure on neurological and cognitive dysfunction in human and rodent studies: A review. Curr Environ Health Rep. 2014;1:132–147. doi: 10.1007/s40572-014-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Fourth Edition (WAIS-IV) NCS Pearson; San Antonio, TX: 2008. [Google Scholar]
- Welch AH, Watkins SA, Helsel DR, Focazio MJ. Arsenic in groundwater resources of the United States. US Geological Survey Fact Sheet 063-00. 2000 Available: http://pubs.usgs.gov/fs/2000/fs063-00/ [accessed 8 November 2015]
- Wright RO, Amarasiriwardena C, Woolf AD, Jim R, Bellinger DC. Neuropsychological correlates of hair arsenic, manganese, and cadmium levels in school-age children residing near a hazardous waste site. Neurotoxicology. 2006;27:210–216. doi: 10.1016/j.neuro.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Zheng LY, Umans JG, Tellez-Plaza M, Yeh F, Francesconi KA, Goessler W, Silbergeld EK, Guallar E, Howard BV, Weaver VM, Navas-Acien A. Urine arsenic and prevalent albuminuria: Evidence from a population-based study. Am J Kidney Dis. 2013;61:385–394. doi: 10.1053/j.ajkd.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoni S, Lucchini RG. Manganese exposure: Cognitive, motor and behavioral effects on children: A review of recent findings. Curr Opin Pediatr. 2013;25:255–60. doi: 10.1097/MOP.0b013e32835e906b. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


