Abstract
Recent studies have suggested that dissociable processes featuring distinct types of inhibition support cognitive control in tasks requiring participants to override a prepotent response with a control-demanding alternative response. An open question concerns how these processes support cognitive flexibility in rule-switching tasks. We used a technique known as reach tracking to investigate how 5- to 8-year-olds (Experiment 1) and adults (Experiment 2) select, maintain, and switch between incompatible rule sets in a computerized version of the Dimensional Change Card Sort (DCCS). Our results indicate that rule switching differentially impacts two key processes underlying cognitive control in children and adults. Adult performance also revealed a strong response bias not observed in children, which complicated a direct comparison of switching between the age groups and reopens questions concerning the relation between child and adult performance on the task. We discuss these findings in the context of a contemporary model of cognitive control.
Keywords: cognitive control, cognitive development, Dimensional Change Card Sort (DCCS), reach tracking
1. Introduction
We live in a noisy world. To behave adaptively we must be able to select and maintain information relevant to our current goals and suppress irrelevant or misleading information. Given the stochastic nature of our environment, however, our goals are liable to change and information that was previously distracting or misleading can become relevant to the task at hand. Adaptive behavior therefore requires striking a balance between two competing demands. On one hand, we must be able to organize our attention, thought, and action around our current goals in order to counteract distracting or misleading information – a process referred to as goal shielding. On the other hand, we must be able to supplant that established structure when new goals emerge – a process known as switching (modified from the distinction by Goschke & Dreisbach, 2008).
In the developmental literature, this tension between goal shielding and switching is captured by performance on the Dimensional Change Card Sort (DCCS) (Zelazo, 2006; Zelazo, Frye, & Rapus, 1996; Zelazo, Müller, Frye, & Marcovitch, 2003). In the DCCS, children sort a series of cards that feature one of two bi-dimensional images (e.g., a blue boat or a red truck) into one of two trays. Attached to each tray is a target card that matches each of the sorting cards along one dimension but not the other (e.g., one target card would feature a red boat, while the other would feature a blue truck). In the pre-switch phase of the task, children are instructed to sort the cards according to one of the dimensions (e.g., shape). After sorting a number of cards according to the pre-switch dimension, children are presented with a new set of rules that requires them to sort the cards according to the alternate (post-switch) dimension (e.g., color). The majority of children 5 years of age and older adopt the new sorting strategy, while the majority of 3-year-olds perseverate and continue to sort the cards according to the rules presented in the pre-switch phase.
The DCCS is commonly used to assess the development of cognitive control (also referred to as executive function; Diamond, 2013) and shares a number of characteristics with other prominent measures of cognitive control such as the Stroop task (Stroop, 1935). In the Stroop task, participants identify what color of text a word is written in regardless of the word’s meaning. When the meaning of the word cues a different response than the color of its text (e.g., the word “BLUE” written in red text), participants must overcome their bias to classify the word according to its meaning. Similarly, in the DCCS participants must overcome a bias to sort the cards according to whichever strategy was first learned.
Two key differences separate these tasks. The Stroop task involves a response bias that is developed over years of experience with reading, while the response bias in the DCCS is acquired over the course of a small number of trials. The DCCS also requires participants to switch between different sets of rules, while the standard version of the Stroop task does not. The DCCS thus presents the opportunity to study how a response bias is established in the context of a competing sorting strategy (goal shielding), as well as how this tendency is replaced when a new sorting strategy is introduced (switching).
To outline our argument, we first introduce a prominent model of cognitive control that was developed to account for performance on inhibitory control tasks such as the Stroop (Botvinick, Braver, Barch, Carter, & Cohen, 2001; Cohen, Dunbar, & McClelland, 1990; Cohen & Huston, 1994; Shenhav, Botvinick, & Cohen, 2013). We then discuss recent research that has used a technique known as reach tracking to target how key processes featured in this model function in children and adults (Erb, Moher, Sobel, & Song, 2016; Erb, Moher, Song, & Sobel, 2017). We propose that reach tracking can be used to investigate how the tension between goal shielding and switching influences the functioning of these processes at different points in development. Finally, we investigate this claim in 5- to 8-year-olds (Experiment 1) and adults (Experiment 2).
1.1 Cognitive Control and Reach Tracking
Cohen and colleagues present a model of cognitive control in which performance on the Stroop task can be understood to involve two distinct processing pathways: an automatic pathway that is attuned to word meaning and a control-demanding pathway that can be directed to attend to text color (e.g., Botvinick et al., 2001; Cohen et al., 1990; Cohen & Huston, 1994; Shenhav et al., 2013). On incongruent trials (e.g., “BLUE” written in red text), the automatic pathway generates strong activation in favor of the response cued by word meaning while the control-demanding pathway generates relatively weak activation in favor of the response cued by text color. In order to ensure that the appropriate response is ultimately selected, cognitive control is required to sway response activations in favor of the response supported by the control-demanding pathway.
In the model, cognitive control is supported by three central components (Shenhav et al., 2013). The monitoring component (associated with the dorsal anterior cingulate cortex) registers conflict stemming from the co-activation of the competing responses. In light of this conflict, the specification component identifies the appropriate course of action given one’s current goal (e.g., responding according to text color in the Stroop task). The specification component has also been linked to a response threshold adjustment process involving a directed global form of inhibition in which signals of conflict from the monitoring component raise one’s threshold to initiate a response by temporarily halting motor output (Cavanagh et al., 2011; Frank, 2006; Munakata et al., 2011). This process has been proposed to allow additional time for the third component – the regulation component (associated with the lateral prefrontal cortex) – to implement a controlled response selection process by providing strong top-down support in favor of the appropriate pathway (Shenhav et al., 2013). In addition to increasing activation along the control-demanding pathway, top-down support from the regulation component has been proposed to suppress activation in the automatic pathway through a process of competitive inhibition involving lateral inhibitory connections between the pathways (Munakata et al., 2011).
One of the strengths of this model is that it provides a framework for considering how the components and processes underlying cognitive control function across different timescales. At the timescale of a single trial, the model offers an account of how conflict is detected, motor output is inhibited, the appropriate goal is specified, and top-down support is allocated. At the timescale of multiple trials, the model has been used to account for trial sequence effects in which qualities of one trial influence performance on a subsequent trial (e.g., Botvinick et al., 2001; Shenhav et al., 2013). At the timescale of years, one can consider how components of this model change across development. In the case of the Stroop task, for example, the pathway attuned to word meaning becomes automatized as children learn to read. This automation, in turn, increases the demands placed on cognitive control when the automatic response is inappropriate (i.e., incongruent trials).
In recent work, Erb and colleagues (Erb et al., 2016, 2017) have used a technique known as reach tracking to investigate how key processes underlying cognitive control function across different timescales in response conflict tasks such as the Stroop. In contrast to button-press measures of accuracy and response time, recording the path that a participant’s hand travels to reach a response target provides a detailed image of how processes across perception, cognition, and action unfold over time (Song & Nakayama, 2009). Erb et al. (2016, 2017) proposed that two of the measures afforded by reach tracking – initiation time (the time elapsed between stimulus onset and movement onset) and curvature (the degree to which a response deviates from a direct path to the selected target) – can be used to target the functioning of the response threshold adjustment process and controlled response selection process, respectively. On this view, initiation time indexes the degree of motoric stopping experienced before a movement is started, with higher response thresholds generating longer periods of motoric stopping and, consequently, longer initiation times. Curvature reflects the controlled response selection process by capturing the degree of competition between co-active responses over the course of the movement, with larger curvatures indicating greater pull toward a competing response before top-down support is recruited in favor of the appropriate response.
Consistent with their proposal, Erb et al. (2016) observed different patterns of effects in initiation time and reach curvature in the Stroop task and the Eriksen flanker task. Crucially, the effects observed in initiation time and curvature conformed to the same patterns of effects linked to the response threshold adjustment process and controlled response selection process in previous electrophysiology and functional neuroimaging research (Kerns et al., 2004; Shenhav et al., 2013; Sheth et al., 2012). In a subsequent study targeting the development of cognitive control, Erb et al. (2017) found that reach curvature but not initiation time revealed age-related gains in flanker task performance between childhood and adulthood, suggesting that the response threshold adjustment process and controlled response selection process follow different developmental trajectories.
1.2 Linking this Model of Cognitive Control to the DCCS: The Current Study
The tasks used in the reach tracking studies reviewed above featured preexisting response biases and did not require participants to switch between different rule sets. Consequently, these tasks did not enable the researchers to investigate the cognitive and developmental dynamics underlying the tension between goal shielding and switching. In the current study we address this gap by using manual reach tracking to investigate how children and adults establish, maintain, and then supplant a response bias in a computerized version of the DCCS.
How might the model of cognitive control introduced above be applied to the DCCS? Let us assume that competing sorting strategies in the DCCS (e.g., matching by color or shape) can be mapped onto dissociable processing pathways in the same manner that text color and word meaning are in the Stroop task. In contrast to the Stroop task, however, these pathways are roughly equal in strength before the task begins.1 Given that the sorting and target cards present conflicting sorting strategies, goal shielding can be expected to occur over the course of the pre-switch phase as top-down support strengthens the pathway corresponding to the appropriate response (the pre-switch pathway) relative to the competing (post-switch) pathway.
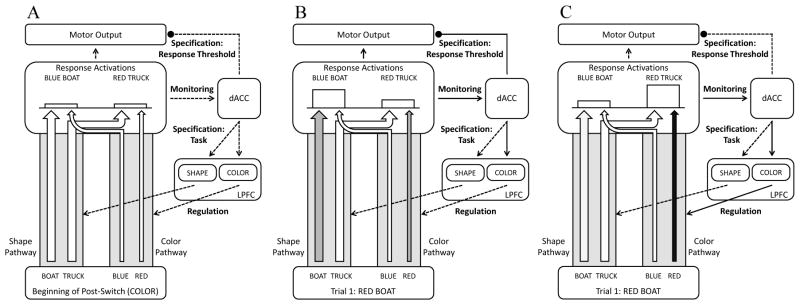
Immediately following a rule switch, processing will be biased in favor of the strengthened pre-switch pathway (see Figure 1). Increased activity along the post-switch pathway will generate conflict between the co-active responses, resulting in higher response thresholds and longer initiation times. Temporarily putting the brake on behavior is insufficient to ensure that the correct response is selected, however. In order to resolve conflict between the competing responses, participants must recruit top-down support to sway response activations in favor of the relatively weak post-switch pathway. The faster that participants can engage the controlled response selection process and override conflict between the competing responses, the more direct their reach movements will be toward the appropriate response.
Figure 1.
Illustration of DCCS model when sorting by color in the post-switch phase. The degree of activity along the shape and color pathways is illustrated by arrow darkness with darker arrows indicating greater activation. Dashed lines indicate non-active links, while solid lines indicate active links. Arrows indicate excitatory links, while circles indicate inhibitory links. (A) After sorting according to shape in the pre-switch phase, the shape pathway is strengthened relative to the color pathway (illustrated by thicker arrows). (B) Presentation of the stimulus results in activation along both of the pathways, generating competing response activations favoring the incorrect response cued by shape. The resulting conflict is registered by the monitoring component of cognitive control associated with the dorsal anterior cingulate cortex (dACC). In response to this conflict, the specification component (i) raises one’s threshold to initiate a response by temporarily inhibiting motor output and (ii) identifies the appropriate sorting strategy for the current task (e.g., color). (C) The regulation component associated with the lateral prefrontal cortex (LFPC) then implements a controlled response selection process by providing top-down support in favor of the appropriate pathway, which is thought to decrease activity in the competing pathway through lateral inhibitory connections (not depicted). Adapted from Shenhav et al. (2013).
On this view, preschoolers’ difficulty with the DCCS reflects the inherent tension between goal shielding and switching, with goal shielding during the pre-switch phase increasing the demands placed on cognitive control in the post-switch phase. This account predicts that reducing the need for goal shielding in the pre-switch phase should decrease the demands placed on cognitive control when a switch is required. This prediction is supported by a number of studies featuring modified versions of the DCCS. For example, in the redundant DCCS (Zelazo et al., 2003, Experiment 9), the sorting and target cards are identical in the pre-switch phase of the task and, consequently, both sorting strategies generate the same response. The sorting cards are then replaced before the post-switch phase so that the color and shape strategies conflict with one another, as in the standard DCCS. In contrast to the standard DCCS, however, a majority of 3-year-olds successfully adopt the post-switch sorting strategy in the redundant DCCS. This finding indicates that removing the need for goal shielding in the pre-switch phase helps to decrease the demands placed on cognitive control when a switch is required.
Further evidence in favor of this account comes from examination of versions of the DCCS in which the dimensions of interest (e.g., color and shape) are distributed across multiple objects (e.g., Diamond, Carlson, & Beck, 2005; Kloo & Perner, 2005; Kloo, Perner, Aichorn, & Schmidhuber, 2010). For example, Diamond and colleagues (2005) presented 2.5- to 3.5-year-olds with sorting and target cards that featured one of two black shapes in the foreground and one of two different colors in the background. The researchers found that children were significantly more likely to successfully switch on the distributed version of the task relative to the standard integrated version. These results suggest that separating the dimensions reduced the demands placed on goal shielding, as within-stimulus conflict has been found to interfere with performance more than between-stimulus conflict in tasks such as the Stroop (MacLeod, 1998).
The model of DCCS performance reviewed above raises two central questions concerning the dynamics of rule switching. First, how does the degree of competition between the pre- and post-switch pathways change across the post-switch phase? If a participant is capable of recruiting sufficient top-down control to overcome the bias established in the pre-switch phase, it can be expected that competition among the pathways will decrease over the post-switch trials as top-down control is repeatedly recruited in favor of the post-switch pathway. However, at present it is unclear how rapidly the relative strength of these pathways is adjusted at different points in development.
Second, how do changes in the relative strength of the pre- and post-switch pathways influence the response threshold adjustment process and controlled response selection process? Reach movements can be expected to become more direct as the post-switch pathway is strengthened in the trials following a rule switch. It is not necessarily the case that initiation times will also decrease as the post-switch pathway is strengthened, however. This is because the response threshold adjustment process is sensitive to co-activation between the pathways, regardless of which pathway is relatively stronger. This suggests that increasing the strength of the post-switch pathway will not result in lower initiation times if the degree of co-activation between the pathways remains high. Thus, initiation times may not decrease across the post-switch trials in a manner proportional to reach curvatures.
In the following experiments, we use the measures of initiation time and curvature to target how the response threshold adjustment process and controlled response selection process are impacted by rule switching in the DCCS. Given that the majority of children 5 years of age and older routinely adopt the post-switch sorting strategy (e.g., Zelazo et al., 2003), focusing on children 5 to 8 years of age allowed us to investigate developmental gains in rule switching beyond those reflected in response accuracy. Further, previous research indicates that adults’ response times in a computerized version of the DCCS reveal switch costs analogous to those observed in preschoolers’ accuracy, suggesting continuity in task performance across development (Diamond & Kirkham, 2005). Thus, the age groups selected in the current study allowed us to examine how key processes underlying cognitive control contribute to the developmental gains in rule switching observed between childhood and adulthood.
2. Experiment 1
The current experiment featured a computerized version of the DCCS that was adapted for use with reach tracking. Children responded in the task by reaching to touch response targets on a digital display that was mounted upright on the table in front of them. In contrast to the standard DCCS, rule reminders were not presented before each trial and feedback was provided in the form of an auditory tone after each trial. Participants completed two rounds of the task with different sorting and target images, allowing each child to switch from sorting by color to sorting by shape and vice versa.
2.1 Method
2.1.1 Participants
Forty-four right-handed children 5 to 8 years of age (M = 82.3 months, SD = 12.6, 21 females) with normal reaching behavior and normal or corrected-to-normal vision participated in the experiment. Participants were recruited from a list of birth records or through contact at a local children’s museum. All testing took place at Brown University and the Institutional Review Board at Brown University approved the protocol.
2.1.2 Apparatus
The experiment was conducted using a rear-mounted projector to display the task on a Plexiglass screen that was arranged upright on a table approximately 48 centimeters in front of the participant. Reach movements and response selections were measured at a rate of approximately 160 Hz with an electromagnetic position and orientation recording system (Liberty, Polhemus). In order to measure hand position, a small motion-tracking marker was secured to participants’ right index finger with a Velcro strap. The task was programmed in MATLAB (Mathworks).
2.1.3 Procedure
Before beginning the experimental trials, participants completed a 9-point calibration sequence. After calibration, participants were presented with 16 trials of a baseline task. On each trial of the baseline task, a blue star or an orange smiley face appeared toward the top left or right of the screen, respectively. Movements in the baseline trials and the experimental trials were initiated from a Styrofoam starting block located 27 cm in front of the screen, aligned with the center of the display. The task was designed so that a trial would not initiate until the subject’s finger was resting on the starting block for 1 second. If the participant’s hand moved from this location before the onset of the stimulus array, the task was paused and did not resume until the participant’s hand returned to the starting block for 1 second.
Following calibration and the baseline trials, all participants performed two rounds of the DCCS. Each round featured different images. One round featured a blue truck and a red boat as target images, and a blue boat and a red truck as test images. The other featured a green plus sign and a yellow heart as target images, and a yellow plus sign and a green heart as test images. All images were presented within a square approximately 5 cm by 5 cm with a grey background.
Participants switched from sorting by color to shape in one round and from shape to color in the other. The order that participants completed the rounds was counterbalanced, as were the images used in each round. For example, some participants started the first round by sorting blue boats and red trucks according to color, while others started the first round by sorting yellow plus signs and green hearts according to shape.
Each round consisted of 20 pre-switch and 20 post-switch trials. On each trial, the target images appeared toward the top left and right of the screen, followed by a cue in the center of the screen that appeared one second before stimulus onset (see Figure 2). Once the sorting image appeared at the bottom center of the screen, participants had 3.5 seconds to make a response by touching one of the two target locations on the screen. Participants initiated the next trial by returning their finger to the starting block.
Figure 2.
Illustration of a trial in the computerized DCCS task. Following a cue, an image appeared at the bottom center of the display. Participants responded by reaching to touch one of the targets at the top of the display. The image in this example is sorted by color.
Before beginning the experimental trials, participants were introduced to the task. The experimenter explained that the participants would be playing a matching game in which two pictures would appear at the top of the screen, followed by another picture at the bottom center of the screen. The experimenter then introduced the images that would be used in that round. The target images and one of the test images appeared on the screen and the experimenter explained to the participant that they would be playing either the shape or the color game (e.g., “We are going to play the shape game. In the shape game, we match the pictures by their shape.”). The experimenter then illustrated the rule for sorting the test image (e.g., “If the picture down here is a boat [gesturing to the bottom center of the screen], then touch the boat up here [gesturing to the top left of the screen].”).
Next, the experimenter introduced the other test image and illustrated the rule for sorting it (e.g., “If this picture is a truck [gesturing to the bottom of center of the screen], then touch the truck up here [gesturing to the top right of the screen].”). The experimenter instructed the participant to not touch the picture at the bottom center of the screen but only the picture that it matched at the top of the screen. The experimenter then repeated the rules and asked the participant if he or she was ready to play the game. Before beginning the pre-switch trials, the experimenter offered one final rule reminder (e.g., “Remember, we are playing the shape game”).
After the block of 20 pre-switch trials concluded the experimenter explained that the previous game was finished and that the participant would play a different matching game next. The experimenter explained the second game in the same manner as the first (e.g., “Now, we are going to play the color game. We aren’t playing the shape game anymore. In the color game, we match the pictures by their color. If this picture is red [gesturing to the bottom center of the screen], then touch the red one up here [gesturing to the top left of the screen]. If this picture is blue [gesturing to the bottom of center of the screen], then touch the blue one up here [gesturing to the top right of the screen].”). Once again, the experimenter asked if the participant was ready to play the game and provided one final reminder before the task began: “Remember, we are playing the color game.” After completing one round of the task, participants were given a brief break before the second round started. The second round followed the same procedure as the first, but with the opposite sorting order and different stimuli.
Before the task began, participants were informed that they had limited time to provide a response and were instructed to sort the images quickly. In the rare instances in which participants’ responses were not provided within this time limit, a low tone sounded and the experimenter explained to the participants that they had taken too long to provide a response. Additionally, in the event that an incorrect response was provided, a low tone sounded and the experimenter reminded the participant which game to play (e.g., “Remember, we are playing the shape game. Let’s try again!”).
2.1.4 Data Processing
The data processing methods used in the current study were largely adapted from Moher and Song (2013). The data from each participant was analyzed offline using custom MATLAB (Mathworks) software. Three-dimensional resultant speed scalars were created for each trial using a differentiation procedure in MATLAB. These scalars were then submitted to a second order, low-pass Butterworth filter with a cutoff of 10 Hz. Movement onset was calculated as the first point on each trial after stimulus onset at which hand movement speed exceeded 25.4 cm/s. Each individual trial was visually inspected as in previous work (e.g., Song & Nakayama, 2006, 2007); for trials in which the default threshold clearly missed part of the movement or included substantial movement back to the starting point, thresholds were adjusted manually to more appropriate levels for that trial. Movement initiation time was defined as the time elapsed between stimulus onset and movement onset.
Trajectories for calculating curvature were measured in two-dimensional xy spaced by calculating a line from the start to the end point of the movement, and measuring the orthogonal deviation of the actual movement from that line at each sample throughout the movement. Curvature was defined as the maximum point of deviation in centimeters divided by the length of the line from the start to the end points of the movement in centimeters (see, e.g., Desmurget, Jordan, Prablanc, & Jeannerod, 1997; Moher & Song, 2013; Song & Nakayama, 2006).
2.2 Results
Only accurate trials not preceded by an error were included in the following analyses for each of the measures except error rate. In addition to initiation time and curvature, we also measured movement time (the time elapsed between movement onset and response completion) and total time (the time elapsed between stimulus onset and response completion). Preliminary analyses revealed that the effects of order (Order 1: color first in Round 1, shape first in Round 2 vs. Order 2: shape first in Round 1, color first in Round 2) and round (Round 1 vs. Round 2) did not substantially alter the effect of phase (Pre-Switch vs. Post-Switch) on these measures (see Supplementary Materials). We therefore collapsed across order and round to analyze the effect of phase with a series of repeated measures ANOVAs.
2.2.1 Average Performance across Pre- and Post-Switch Blocks
Error rate, initiation time, curvature, movement time, and total time all revealed significant main effects of phase, all p-values < .001, all ηp2-values > .41, with more errors, larger curvatures, and slower initiation times, movement times, and total times in the post-switch phase relative to the pre-switch phase (see Figure 3). To evaluate the effect of age on switching, we computed difference scores for each participant using average pre- and post-switch performance on each measure. We then correlated these differences scores with age in months. The correlation between age and the error rate difference score was significant, r(43) = −.41, p < .01, with the difference between pre- and post-switch error rates decreasing with age (see Figure 4).2 The corresponding correlations for initiation times, reach curvatures, movement times, and total times did not approach significance, all p-values > .50. The lack of correlation between these measures and age may reflect a trade-off effect with error rates. Given that younger children were more likely to make an error than older children, a greater proportion of trials were excluded in the analysis of reach curvatures for younger children. Consequently, a larger proportion of high conflict trials may have been included in the average reach curvatures of older children, potentially obscuring developmental differences.
Figure 3.
Children’s average performance on measures of (A) error rate, (B) initiation time, (C) curvature, (D) movement time, and (E) total time for pre-switch and post-switch trials. Error bars indicate standard errors.
Figure 4.
Scatterplot of error difference scores (post-switch minus pre-switch) by age. One outlier is not displayed.
2.2.2 Performance Across Pre- and Post- Switch Trials
Next, we evaluate how performance changed over the course of the pre- and post-switch blocks. We computed average values for each of the twenty pre- and post-switch trials for each measure by collapsing across the first and second rounds of trials (see Figure 5). We then used linear mixed effects regression to analyze how performance in the pre- and post-switch blocks differed as a function of trial number. This method allowed us to model discrete and continuous fixed effects while simultaneously controlling for individual subject variation (Baayen, Davidson, & Bates, 2008). Once again, only accurate trials not preceded by an error were included in the following analyses for the measures of initiation time, curvature, movement time, and total time.
Figure 5.
Children’s average performance across the pre-switch and post-switch trials, collapsed across rounds and orders for measures of (A) error rate, (B) initiation time, (C) curvature, (D) movement time, and (E) total time. Pre-switch trials = 1–20; Post-switch trials = 21–40. Error bars indicate standard errors.
The analyses were carried out in R (R Core Team, 2014) using lmer, part of the lme4 package (Bates, Maechler, Bolker, & Walker, 2014). The full model featured two discrete fixed effects (phase and order) and one continuous fixed effect (trial number of block; 1–20). The discrete fixed effects were deviation coded (contrasts = −0.5, 0.5, with the order starting with color and the pre-switch phase coded as negative) and the continuous fixed effect was centered (M = 0). To account for variation in performance in individual participants, random intercepts, slopes, and interactions were included for all within-subject factors (phase and trial number). Due to occasional convergence difficulties, random effects were not allowed to co-vary by subject, following the suggestion of Barr, Levy, Scheepers, and Tily (2013). This simplification allows mixed effects models to converge without increasing the risk of Type I error. Following Barr et al. (2013), p-values were computed by nested model comparison. For each of the measures discussed below, independent mixed effects models were fit using the same fixed and random effects structure described above.
Given that the average error rate on each trial number could only be one of three values for each participant – wrong on both rounds (0), correct on one round (.5), or correct on both rounds (1) – it was inappropriate to analyze error rates with linear mixed effects regression. However, Figure 5A indicates that error rates were uniformly low over the pre-switch trials. Immediately following a rule switch, error rates increased substantially and then decreased steadily over the post-switch trials. This pattern of results indicates that the strength of the previously relevant pre-switch sorting strategy decreased over a relatively protracted period in the post-switch phase.
2.2.2.1 Initiation Time
Initiation times revealed two main effects and no interactions. Initiation times decreased over the course of the twenty trials in each block (β = −0.003, SE = 0.001, t = 2.73, χ2(1) = 7.15, p < 0.01) and were elevated on post-switch relative to pre-switch trials (β = 0.125, SE = 0.021, t = 6.01, χ2 (1) = 27.32, p < 0.001) (see Figure 5B). The interaction between trial number and phase did not approach significance (β < 0.001, SE = 0.002, t = 0.38, χ2 (1) = 0.16, p = .69). The lack of an interaction between trial number and phase indicates that the effect of switching was constant across the post-switch trials.
2.2.2.2 Curvature
Reach curvatures revealed two main effects and one two-way interaction. Curvatures decreased over the course of the twenty trials in each block (β = −0.004, SE = 0.001, t = 5.72, χ2 (1) = 25.98, p < 0.001) and were elevated on post-switch relative to pre-switch trials (β = 0.064, SE = 0.010, t = 6.36, χ2 (1) = 29.69, p < 0.001) (see Figure 5C). A significant interaction between trial number and phase was also observed (β = −0.005, SE = 0.001, t = 4.18, χ2 (1) = 15.48, p < 0.001), indicating that the effect of trial number was larger in the post-switch than the pre-switch trials. This finding indicates that the effect of switching decreased across the post-switch trials.
2.2.2.3 Movement Time
Movement times revealed two main effects and one two-way interaction. Movement times decreased over the course of the twenty trials in each block (β = −0.006, SE = 0.001, t = 6.14, χ2 (1) = 28.26, p < 0.001) and were elevated on post-switch relative to pre-switch trials (β = 0.093, SE = 0.015, t = 6.11, χ2 (1) = 27.88, p < 0.001) (see Figure 5D). A significant interaction between trial number and phase was also observed (β = −0.007, SE = 0.002, t = 4.16, χ2 (1) = 15.23, p < 0.001). As in reach curvatures, this interaction effect indicates that the effect of switching decreased across the post-switch trials.
2.2.2.4 Total Times
Total times revealed two main effects and one two-way interaction. Total times decreased over the course of the twenty trials in each block (β = −0.009, SE = 0.002, t = 5.59, χ2 (1) = 24.42, p < 0.001) and were elevated on post-switch relative to pre-switch trials (β = 0.218, SE = 0.029, t = 7.47, χ2 (1) = 37.24, p < 0.001) (see Figure 5E). A significant interaction between trial number and phase was also observed (β = −0.008, SE = 0.002, t = 3.53, χ2 (1) = 11.38, p < 0.001). In light of the initiation time and movement time results reviewed above, this interaction effect can be understood to reflect the effect of switching on movement times decreasing across the post-switch trials.
2.3 Discussion
Experiment 1 revealed prolonged competition between the pre- and post-switch response strategies following a rule switch in children 5 to 8 years of age. Higher error rates, initiation times, reach curvatures, movement times, and total times were observed on post-switch relative to pre-switch trials. Error rates, reach curvatures, and movement times decreased significantly more over the post-switch than the pre-switch trials, indicating that switch costs decreased over the post-switch block. In contrast to these measures, initiation time did not reveal an interaction between trial number and phase, indicating that the switch costs observed in initiation time were relatively stable across the post-switch block. This distinction between initiation times and reach curvatures provides further support for the claim that these two measures are differentially sensitive to two dissociable processes underlying cognitive control (see Erb et al., 2016, 2017).
To relate these findings to the model of cognitive control discussed in the introduction, we interpret initiation time and reach curvature to reflect the response threshold adjustment process and the controlled response selection process, respectively. Following a rule switch, activation along the pre- and post-switch pathways generates competing response activations, resulting in higher response thresholds with longer periods of motoric stopping and, consequently, longer initiation times. To resolve conflict between the co-active responses in favor of the appropriate sorting strategy, sufficient top-down support from the regulation component must be recruited before an error is committed. Over the course of the post-switch trials, the relative strength of the pre-switch pathway decreases and reaches become increasingly direct to the appropriate post-switch target. However, the pathways remain sufficiently co-activated to maintain heightened thresholds across the post-switch block and, consequently, initiation times do not recover to pre-switch levels to the same extent as reach curvatures.
The lack of a significant interaction between trial number and phase in initiation time raises a number of questions regarding how children’s response thresholds recover following a rule switch. Although the interaction did not approach significance in the current experiment (p = .69), we expect that post-switch initiation times would decrease once competition between the pre- and post-switch pathways became sufficiently low. Future research could evaluate the time course of this recovery by increasing the number of post-switch trials presented.
Significant age-related gains in performance were only observed in error rate. Overall, 40 children committed at least one error in a post-switch block. Reach curvatures on error trials were quite small (M = 0.11, SD = 0.10) compared to average reach curvatures on accurate post-switch trials (M = 0.19, SD = 0.08). Additionally, reach curvatures on post-switch error trials were comparable to accurate pre-switch trials (M = 0.14, SD = 0.05). These findings suggest that the majority of children’s errors stemmed from difficulties specifying the appropriate sorting strategy. In other words, children appear to have fallen into a pre-switch mindset and failed to notice that their unfolding action was inappropriate. The negative relation between age and error rates suggests that older children were better able to actively maintain the post-switch sorting strategy in memory. Future research could explore this possibility in greater depth by investigating the role of self-directed speech or working memory capacity on children’s post-switch error rates.
It is important to note that the task used in the current experiment differs from the standard DCCS in a number of ways, which may have contributed to the relatively high error rate in children 5 to 8 years of age. In the standard DCCS, no feedback is presented in the post-switch phase but children are reminded of the appropriate response strategy before each trial. In the current study, children received feedback on every trial but were only reminded of the appropriate response strategy following an error. Thus, it is possible that the memory demands of the current study led to higher error rates than would be expected in the standard DCCS.
The results of the current study raise a number of questions concerning how adult performance on the DCCS relates to that of children. In Experiment 2, we investigate the extent to which the model of cognitive control used to account for children’s performance in the current experiment can also be used to account for adult performance. Of particular interest is whether conflict will differentially effect initiation times and reach curvatures in adults as in children.
3. Experiment 2
Previous research by Diamond and Kirkham (2005) suggested that preschoolers’ difficulties with switching in the DCCS continue on into adulthood, albeit in the form of elevated response times rather than explicit errors. They presented adults with a computerized version of the DCCS that required participants to classify stimuli by pressing one of two buttons. The researchers found that response times were significantly higher on the first two post-switch trials than the last two pre-switch trials. The results of Experiment 1 suggest that this comparison may have overestimated switch costs given that performance tends to improve over both the pre-switch and the post-switch phase. Further, another study investigating adult performance of the DCCS by Hindy and Spivey (2008) found that adults exhbibited an advantage for sorting by color in the task, suggesting that a direct comparison of child and adult switch costs may be more complicated than previously acknowledged. Thus, the current study aims to clarify the relation between child and adult performance of the DCCS by presenting adults with the same task used in Experiment 1.
3.1 Method
3.1.1 Participants
Thirty-four right-handed adults (M = 19.5 years, SD = 1.3, 23 females) with normal reaching behavior and normal or corrected-to-normal vision participated in the experiment. Participants received course credit for their participation. The protocol was approved by the Institutional Review Board at Brown University.
3.1.2 Apparatus, Procedure, and Data Processing
The apparatus, procedure, and data processing described in Experiment 1 were used in the current experiment.
3.2 Results
As in Experiment 1, only accurate responses not preceded by an error are included in the following analyses. Error rates were at floor (occurring on less than 0.5% of trials) and were not analyzed further. In contrast to Experiment 1, preliminary analyses revealed substantial effects of order (Order 1: color first in round 1, shape first in round 2 vs. Order 2: shape first in round 1, color first in round 2) and round (Round 1 vs. Round 2) on phase (Pre-Switch vs. Post-Switch). Consequently, the following analyses were conducted using a series of 2 (order) x 2 (round) x 2 (phase) ANOVAs. The observed interactions among order, round, and phase also prevented us from conducting the linear mixed effects regression analyses used to evaluate children’s performance in Experiment1.
3.2.1 Initiation Time
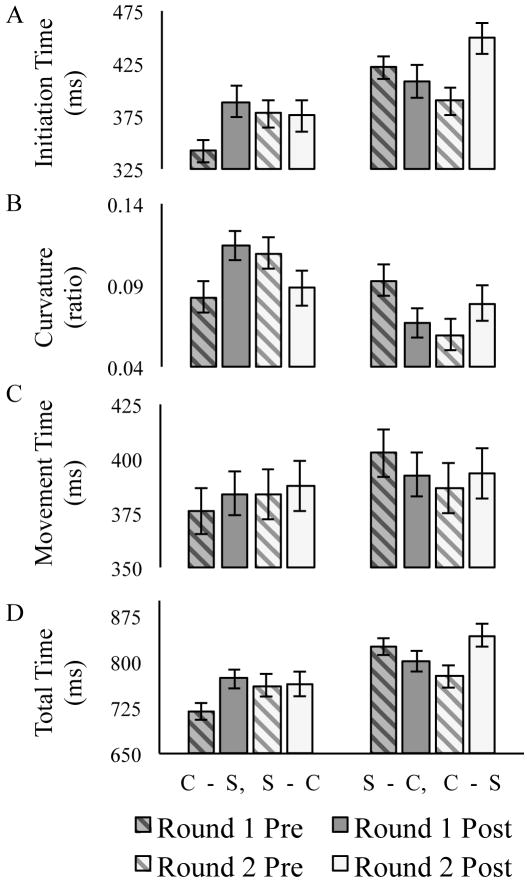
The average initiation time for each round and phase is presented in Figure 6A for both of the orders. The ANOVA on initiation times revealed a significant main effect of phase, with longer initiation times on post-switch than pre-switch trials, F(1, 32) = 21.58, p < .001, ηp2 = 0.40, and a main effect of order, F(1, 32) = 6.96, p = .013, ηp2 = 0.18, with faster initiation times in participants who started sorting by color in the first round (Order 1) relative to those who started sorting by shape in the first round (Order 2). A significant three-way interaction among round, phase, and order was also observed, F(1, 32) = 50.65, p < .001, ηp2 = 0.61. Follow-up tests revealed that the effect of phase was significant when switching from color to shape (Round 1 in Order 1, Round 2 in Order 2), F(1, 33) = 74.26, p < .001, ηp2 = 0.69, but not when switching from shape to color (Round 2 in Order 1, Round 1 in Order 2), F(1, 33) = 1.27, p = .27, ηp2 = 0.04.
Figure 6.
Adults’ average performance on measures of (A) initiation time, (B) curvature, (C) movement time, and (D) total time for Pre-Switch (striped) and Post-Switch (solid) trials in Rounds 1 (dark) and 2 (light). Order 1 (C-S, S-C = Color First) is presented in the leftmost bars and Order 2 (S-C, C-S = Shape First) in the rightmost bars. Error bars indicate standard errors.
3.2.2 Curvature
The average reach curvature for each round and phase is presented in Figure 6B for both of the orders. The ANOVA on reach curvatures revealed a significant main effect of order, with larger reach curvatures observed in participants who received Order 1 relative to those who received Order 2, F(1, 32) = 5.30, p = .028, ηp2 = 0.14. The interaction among round, phase, and order was also significant, F(1, 32) = 25.41, p < .001, ηp2 = 0.44. Follow-up tests revealed that the effect of phase was significant when switching from color to shape (Round 1 in Order 1, Round 2 in Order 2), F(1, 33) = 21.21, p < .001, ηp2 = 0.39, with reach curvatures increasing from the pre-to the post-switch phase. The effect of phase was also significant when switching from shape to color (Round 2 in Order 1, Round 1 in Order 2), F(1, 33) = 7.02, p = .01, ηp2 = 0.17, with reach curvatures decreasing from the pre- to the post-switch phase.
3.2.3 Movement Time
The average movement time for each round and phase is presented in Figure 6C for both of the orders. The ANOVA on movement times revealed an interaction among round, phase, and order, F(1, 32) = 4.68, p = .038, ηp2 = 0.13. Follow-up tests revealed that the effect of phase was significant when switching from color to shape (Round 1 in Order 1, Round 2 in Order 2), F(1, 33) = 4.59, p = .04, ηp2 = 0.12, but not when switching from shape to color (Round 2 in Order 1, Round 1 in Order 2), F(1, 33) = 0.41, p = .53, ηp2 = 0.01.
3.2.4 Total Time
The average total time for each round and phase are presented in Figure 6D for both of the orders. The ANOVA on total times revealed a significant main effect of phase, with longer total times on post-switch than pre-switch trials, F(1, 32) = 12.50, p < .01, ηp2 = 0.28, and a significant main effect of order, with faster total times observed in participants who started sorting by color in the first round (Order 1) relative to those who started sorting by shape in the first round (Order 2), F(1, 32) = 7.76, p < .01, ηp2 = 0.20. The interaction among round, phase, and order was also significant, F(1, 32) = 53.39, p < .001, ηp2 = 0.62. Follow-up tests revealed that the effect of phase was significant when switching from color to shape, F(1, 33) = 59.83, p < .001, ηp2 = 0.64, but not when switching from shape to color, F(1, 33) = 1.32, p = .26, ηp2 = 0.04.
3.3 Discussion
In contrast to the results of Experiment 1, adult performance of the DCCS revealed a number of interactions among phase, order, and round. Reach curvatures were significantly larger when participants sorted by shape regardless of phase, while initiation times, movement times, and total times increased significantly when participants switched from color to shape but not from shape to color. These results indicate that adults had an advantage for sorting by color that was not as pronounced in children in the current study.
Despite the complexity of the current experiment’s results, adult performance of the DCCS can be understood to reflect the same basic components, processes, and pathways used to account for children’s performance in Experiment 1. The color advantage observed in adults can be interpreted to reflect a stronger or more direct processing pathway for color relative to shape. This processing advantage altered the demands placed on adult cognitive control by introducing a new source of conflict. In Experiment 1, conflict was predominantly tied to switching. In the current experiment, conflict also occurred when participants sorted by the subdominant shape-based strategy. This is illustrated, for example, by the finding that initiation times were significantly longer in the pre-switch phase of round one when adults sorted by shape as opposed to color, t(32) = 5.11, p < .001, d = 1.75 (see Figure 6A), indicating that sorting by a subdominant strategy generated conflict and, consequently, resulted in increased response thresholds.
As in Experiment 1, initiation times and reach curvatures revealed different effects of switching, with more pronounced switch costs in initiation times than reach curvatures. This difference is most clearly illustrated by comparing the effects of switching from shape to color on each measure. While reach curvatures decreased when participants switched from shape to color, initiation times remained elevated. This finding indicates that sorting by shape in the pre-switch phase resulted in increased activity along the shape pathway in the post-switch phase. Although this activation was not sufficient to counteract the strength of the color pathway (as indicated by reach curvatures), it was sufficient to maintain heightened response thresholds (as indicated by initiation times). Thus, the current experiment provides further support for the claim that initiation times reflect a response threshold adjustment process that is sensitive to general co-activation between the competing pathways, while reach curvatures reflect a controlled response selection process that is sensitive to the relative strength of each pathway.
It is important to note that the color advantage observed in the current experiment can be interpreted in a number of ways. As noted above, the advantage can be understood to reflect a stronger or more direct processing pathway for color relative to shape. Alternatively, it may have reflected the relative discriminability of the specific color and shape combinations selected for the current study. Given that the advantage was more pronounced in adults than in children, our data suggest that it stemmed at least in part from age-related differences in how the stimuli were processed. However, it should also be noted that 6- and 7-year-olds demonstrated a color advantage in a rule-switching study by Ellefson, Shapiro, and Chater (2006), suggesting that the processing advantage can be observed in children under certain conditions.
The color advantage observed in adult performance of the DCCS does not appear to be specific to the materials used in the current study. Hindy and Spivey (2008) also observed a color advantage in adults in a mouse-tracking version of the DCCS, while Diamond and Kirkham (2005) observed descriptively faster response times when participants sorted according to color (M = 571 ms) relative to shape (M = 605 ms) in the pre-switch phase. Taken together, these findings suggest that adults have a general advantage for sorting by color in the DCCS when the stimuli are comparable to those used in the standard DCCS with children.
4. General Discussion
The current study used reach tracking to investigate how children and adults switch between incompatible sorting strategies in the DCCS. In light of a prominent model of cognitive control developed by Cohen and colleagues (e.g., Botvinick et al., 2001; Cohen et al., 1990; Cohen & Huston, 1994; Shenhav et al., 2013), we proposed that reaching behavior would reflect the relative strength of two pathways, each corresponding to one of the incompatible sorting strategies. Further, we proposed that initiation time and reach curvature would be sensitive to two dissociable processes featured in the model: a response threshold adjustment process involving the inhibition of motor output and a controlled response selection process involving top-down support from the regulation component of cognitive control. The results of both experiments supported these claims.
In Experiment 1, children’s post-switch reach curvatures revealed prolonged competition between the sorting strategies, indicating that goal shielding in the pre-switch phase increased the demands placed on cognitive control in the post-switch phase. Over the course of the post-switch trials, competition between the sorting strategies decreased and reach movements became more direct. Children’s initiation times were also elevated in the post-switch phase. However, in contrast to reach curvatures, the rate with which initiation times decreased over the post-switch block did not differ from that of the pre-switch block. This finding indicates that, although reach movements became more direct over the post-switch trials, the degree of co-activation between the pathways was sufficient to maintain heightened response thresholds across the post-switch block.
A color advantage was observed in adults’ performance in Experiment 2, which resulted in elevated levels of conflict when participants sorted by the subdominant shape-based strategy. Reach curvatures were significantly larger when participants sorted by shape regardless of phase, indicating that the relative strength of the two pathways remained stable over the course of the experiment. In other words, the processing advantage for color was sufficiently strong that sorting by shape in the pre-switch phase did not substantially alter the relative strength of the pathways. Initiation times, on the other hand, did not significantly decrease when participants switched from shape to color, indicating that the shape and color pathways remained at least minimally co-activated in the post-switch phase. Consistent with the results of Experiment 1, these findings indicate that switch costs in the DCCS are more temporally extended in initiation times than reach curvatures.
The results of the current study make a number of important contributions to our current understanding of how performance on the DCCS develops across the lifespan. First, our findings indicate that previous research by Diamond and Kirkham (2005) likely overestimated switch costs in adults by failing to control for how performance improves within the pre- and post-switch phases. Second, our results highlight the importance of considering how the same task can place different demands on children and adults as a result of age-related changes in the functioning of key components and processes underlying performance. In Experiment 2, a processing advantage for color fundamentally altered the demands placed on cognitive control by introducing an additional source of conflict that was not tied to switching. This finding suggests that future studies seeking to directly compare child and adult switch costs in the DCCS should take steps to reduce or eliminate this advantage by, for example, altering the salience of the color and shape dimensions.
Finally, our results indicate that the model of cognitive control developed by Cohen and colleagues (e.g., Shenhav et al., 2013) provides a compelling account of the dynamics underlying DCCS performance. On this view, children who fail the standard DCCS are unable to recruit sufficient top-down support to sway response activations in favor of the post-switch strategy. Manipulations that reduce the need for goal shielding in the pre-switch phase decrease the demands placed on the controlled response selection process in the post-switch and, consequently, a greater proportion of children can recruit sufficient top-down support to respond adaptively (Diamond et al., 2005; Kloo & Perner, 2005; Kloo et al., 2010; Zelazo et al., 2003).
It is important to note that many different factors may contribute to a child’s capacity to recruit sufficient top-down support in the standard DCCS, including working memory capacity (Morton & Munakata, 2002), attentional control (Kirkham, Cruess, & Diamond, 2003), and representational complexity (Zelazo et al., 2003). An ongoing discussion in the DCCS literature concerns how each of these factors contributes to the transition children make from perseverating to switching. Although the current study was not designed to arbitrate among competing theories of preservation, our results (i) provide insight into the cognitive mechanisms that underlie DCCS performance and (ii) present a common framework in which to ground these theories.
Indeed, the account of DCCS performance presented in the current study shares a number of similarities with existing computational models of the task, including the connectionist model developed by Morton and Munakata (2002) and the Dynamic Neural Field model developed by Buss and Spencer (2014). One virtue of the model adopted in the current study is that it provides a framework for considering how dissociable processes underlying cognitive control are reflected in distinct behavioral measures and impacted differentially by rule switching. That said, we expect that existing models of DCCS performance could be adapted to account for the results of the current study. Given the richness of the data provided by reach tracking and other related techniques, we believe continuous behavioral measures will serve an important role in further developing and testing models that seek to account for how processes across perception, action, and cognition unfold to support adaptive thought and behavior at different points in development.
5. Conclusion
The current study built on previous research with children and adults indicating that reach tracking can be used to target how key processes underlying cognitive control function across different timescales (Erb et al., 2016, 2017). While these past studies focused on tasks that required participants to overcome preexisting response biases, the current study explored the tension between goal shielding and switching by investigating how a response bias is established and subsequently overcome in the DCCS, a rule-switching task commonly used to study cognitive flexibility in children. Our findings indicate that the model of cognitive control developed by Cohen and colleagues (Botvinick et al., 2001; Cohen et al., 1990; Cohen & Huston, 1994; Shenhav et al., 2013) presents a compelling account of how the cognitive dynamics underlying rule switching in the DCCS unfold at different points in development. Our findings also provide further support for the claim that initiation time and reach curvature can be used to target dissociable processes featured within the model. Thus, the current study contributes to a growing body of research highlighting the potential of using continuous behavioral measures to study the mind in action (for reviews, see Freeman, Dale, & Farmer, 2011; Song & Nakayama, 2008; Spivey, 2008).
Supplementary Material
Acknowledgments
This project was supported by NSF 1223777 to D.M.S & NIGMS-NIH IDeA P20GM103645 to J.H.S. Special thanks to Neal P. Fox for assistance in analyzing the data in Experiment 1.
Footnotes
This notion is supported by research indicating that children’s performance is equivalent regardless of whether the pre-switch dimension is color or shape (Zelazo et al., 2003).
One outlier was excluded from this analysis. Including the outlier data resulted in a slightly stronger correlation, r(44) = −.46, p < .01.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baayen RH, Davidson DJ, Bates DM. Mixed-effects modeling with crossed random effects for subjects and items. Journal of Memory and Language. 2008;59(4):390–412. doi: 10.1016/j.jml.2007.12.005. [DOI] [Google Scholar]
- Barr DJ, Levy R, Scheepers C, Tily HJ. Random effects structure for confirmatory hypothesis testing: Keep it maximal. Journal of Memory and Language. 2013;68(3):255–278. doi: 10.1016/j.jml.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates D, Maechler M, Bolker B, Walker S. lme4: Linear mixed effects models using Eigen and S4. R package version 1.1–7. 2014 < http://CRAN.R-project.org/package=lme4>.
- Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295X.108.3.624. [DOI] [PubMed] [Google Scholar]
- Buss AT, Spencer JP. The emergent executive: A dynamic field theory of the development of executive function. Monographs of the Society for Research in Child Development. 2014;79(2):vii-103. doi: 10.1002/mono.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Wiecki TV, Cohen MX, Figueroa CM, Samanta J, Sherman SJ, Frank MJ. Subthalamic nucleus stimulation reverses mediofrontal influence over decision threshold. Nature Neuroscience. 2011;14(11):1462–1467. doi: 10.1038/nn.2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JD, Dunbar K, McClelland JL. On the control of automatic processes: A parallel distributed processing account of the Stroop effect. Psychological Review. 1990;97(3):332–361. doi: 10.1037/0033-295X.97.3.332. [DOI] [PubMed] [Google Scholar]
- Cohen JD, Huston TA. Progress in the use of interactive models for understanding attention and performance. In: Umiltà C, Moscovitch M, editors. Attention and Performance. XV. Cambridge, MA: MIT Press; 1994. pp. 453–476. [Google Scholar]
- Desmurget M, Jordan M, Prablanc C, Jeannerod M. Constrained and unconstrained movements involve different control strategies. Journal of Neurophysiology. 1997;77(3):1644–1650. doi: 10.1152/jn.1997.77.3.1644. [DOI] [PubMed] [Google Scholar]
- Diamond A. Executive functions. Annual Review of Psychology. 2013;64:135–168. doi: 10.1146/annurev-psych-113011-143750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Carlson SM, Beck DM. Preschool children’s performance in task switching on the dimensional change card sort task: Separating the dimensions aids the ability to switch. Developmental Neuropsychology. 2005;28(2):689–729. doi: 10.1207/s15326942dn2802_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Kirkham N. Not quite as grown-up as we like to think parallels between cognition in childhood and adulthood. Psychological Science. 2005;16(4):291–297. doi: 10.1111/j.0956-7976.2005.01530.x. doi:0.1111/j.0956-7976.2005.01530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb CD, Moher J, Sobel DM, Song JH. Reach tracking reveals dissociable processes underlying cognitive control. Cognition. 2016;152:114–126. doi: 10.1016/j.cognition.2016.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erb CD, Moher J, Song JH, Sobel DM. Reach tracking reveals dissociable processes underlying inhibitory control in 5- to 10-year-olds and adults. Developmental Science. 2017 doi: 10.1111/desc.12523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farmer TA, Cargill SA, Hindy NC, Dale R, Spivey MJ. Tracking the continuity of language comprehension: Computer mouse trajectories suggest parallel syntactic processing. Cognitive Science. 2007;31(5):889–909. doi: 10.1080/03640210701530797. [DOI] [PubMed] [Google Scholar]
- Frank MJ. Hold your horses: A dynamic computational role for the subthalamic nucleus in decision making. Neural Networks. 2006;19(8):1120–1136. doi: 10.1016/j.neunet.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Freeman J, Dale R, Farmer T. Hand in motion reveals mind in motion. Frontiers in Psychology. 2011;2:59. doi: 10.3389/fpsyg.2011.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goschke T, Dreisbach G. Conflict-triggered goal shielding response conflicts attenuate background monitoring for prospective memory cues. Psychological Science. 2008;19(1):25–32. doi: 10.1111/j.1467-9280.2008.02042.x. [DOI] [PubMed] [Google Scholar]
- Hindy NC, Spivey MJ. Motor dynamics of task switching. Proceedings of the 30th Annual Conference of the Cognitive Science Society; 2008. Jul, pp. 2474–2479. [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303(5660):1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kirkham NZ, Cruess L, Diamond A. Helping children apply their knowledge to their behavior on a dimension-switching task. Developmental Science. 2003;6(5):449–467. [Google Scholar]
- Kloo D, Perner J. Disentangling dimensions in the dimensional change card-sorting task. Developmental Science. 2005;8(1):44–56. doi: 10.1111/j.1467-7687.2005.00392.x. [DOI] [PubMed] [Google Scholar]
- Kloo D, Perner J, Aichhorn M, Schmidhuber N. Perspective taking and cognitive flexibility in the Dimensional Change Card Sorting (DCCS) task. Cognitive Development. 2010;25(3):208–217. doi: 10.1016/j.cogdev.2010.06.001. [DOI] [Google Scholar]
- MacLeod CM. Training on integrated versus separated Stroop tasks: The progression of interference and facilitation. Memory & Cognition. 1998;26(2):201–211. doi: 10.3758/BF03201133. [DOI] [PubMed] [Google Scholar]
- Moher J, Song JH. Context-dependent sequential effects of target selection for action. Journal of Vision. 2013;13(8):10, 1–13. doi: 10.1167/13.8.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton JB, Munakata Y. Active versus latent representations: A neural network model of perseveration, dissociation, and decalage. Developmental Psychobiology. 2002;40(3):255–265. doi: 10.1002/dev.10033. [DOI] [PubMed] [Google Scholar]
- Munakata Y, Herd SA, Chatham CH, Depue BE, Banich MT, O’Reilly RC. A unified framework for inhibitory control. Trends in Cognitive Sciences. 2011;15(10):453–459. doi: 10.1016/j.tics.2011.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth SA, Mian MK, Patel SR, Asaad WF, Williams ZM, Dougherty DD, Bush G, Eskandar EN. Human dorsal anterior cingulate cortex neurons mediate ongoing behavioural adaptation. Nature. 2012;488(7410):218–221. doi: 10.1038/nature11239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Role of focal attention on latencies and trajectories of visually guided manual pointing. Journal of Vision. 2006;6(9):982–995. doi: 10.1167/6.9.11. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Automatic adjustment of visuomotor readiness. Journal of Vision. 2007;7(5):2, 1–9. doi: 10.1167/7.5.2. [DOI] [PubMed] [Google Scholar]
- Song JH, Nakayama K. Hidden cognitive states revealed in choice reaching tasks. Trends in Cognitive Sciences. 2009;13(8):360–366. doi: 10.1016/j.tics.2009.04.009. [DOI] [PubMed] [Google Scholar]
- Spivey M. The continuity of mind. Oxford University Press; 2008. [Google Scholar]
- Stroop JR. Studies of interference in serial verbal reactions. Journal of Experimental Psychology. 1935;18(6):643–662. doi: 10.1037/h0054651. [DOI] [Google Scholar]
- Zelazo PD. The Dimensional Change Card Sort (DCCS): A method of assessing executive function in children. Nature Protocols. 2006;1:297–301. doi: 10.1038/nprot.2006.46. [DOI] [PubMed] [Google Scholar]
- Zelazo PD, Frye D, Rapus T. An age-related dissociation between knowing rules and using them. Cognitive Development. 1996;11(1):37–63. doi: 10.1016/S0885-2014(96)90027-1. [DOI] [Google Scholar]
- Zelazo PD, Müller U, Frye D, Marcovitch S. The development of executive function in early childhood. Monographs of the Society for Research in Child Development. 2003;68(3):93–119. doi: 10.1111/j.0037-976X.2003.00266.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.