Abstract
Benzphetamine is a Schedule III anorectic agent that is a prodrug for d-amphetamine and d-methamphetamine and may have utility as an “agonist” medication for cocaine use disorder treatment. This study evaluated the pharmacokinetic-pharmacodynamic profile of benzphetamine using a drug discrimination procedure in rhesus monkeys. The potency and time course of cocaine-like discriminative stimulus effects were compared for benzphetamine (10–18 mg/kg, intramuscular (IM)) and d-amphetamine (0.032–0.32 mg/kg, IM) in monkeys (n=3–4) trained to discriminate IM cocaine (0.32 mg/kg) from saline in a two-key food-reinforced discrimination procedure. Parallel pharmacokinetic studies in the same monkeys determined plasma benzphetamine, d-methamphetamine and/or d-amphetamine levels for correlation with behavioral effects. d-Amphetamine produced dose-dependent, time-dependent, and full cocaine-like effects, i.e. ≥ 90% cocaine-appropriate responding,in all monkeys without altering response rates. The time course of d-amphetamine’s cocaine-like discriminative stimulus effects correlated with plasma d-amphetamine levels. Benzphetamine was 180-fold less potent than d-amphetamine and produced full cocaine-like effects in only 2 of 4 monkeys while significantly decreasing response rates. Benzphetamine administration increased plasma d-methamphetamine (peak at 100 min) and d-amphetamine (peak at 24 h) levels, but the time course of behavioral effects did not correlate with increased levels of benzphetamine, d-methamphetamine or d-amphetamine. These results suggest that benzphetamine yields d-amphetamine and d-methamphetamine as active metabolites in rhesus monkeys, but generation of these metabolites is not sufficient to account for benzphetamine behavioral effects. The incomplete cocaine substitution profile and protracted d-amphetamine plasma levels suggest that benzphetamine may still warrant further evaluation as a candidate pharmacotherapy for cocaine use disorder treatment.
Keywords: cocaine, discrimination, benzphetamine, rhesus monkey, pharmacokinetic, pharmacodynamic
1.0 Introduction
Cocaine use disorder remains a significant and global public health problem for which no Food and Drug Administration-approved pharmacotherapies exist (Acri and Skolnick, 2013). “Agonist”-based pharmacotherapies share pharmacodynamic mechanisms of action with the target abused drug, and this approach has shown potential for cocaine use disorder (for review, see (Herin et al., 2010, Negus and Henningfield, 2015, Stoops and Rush, 2013)). For example, chronic treatment with the monoamine transporter substrate d-amphetamine has demonstrated efficacy to decrease cocaine-maintained behaviors in both preclinical (Czoty et al., 2011, Negus, 2003, Thomsen et al., 2013) and human laboratory (Greenwald et al., 2010, Rush et al., 2010) studies, and in clinical trials (Grabowski et al., 2001, Mariani et al., 2012, Nuijten et al., 2016). However, the broad clinical deployment of d-amphetamine as a pharmacotherapy for cocaine use disorder treatment has been hindered by undesirable effects that include high abuse liability.
Prodrugs of agonist medications represent one potential mechanism to slow the onset of drug effects, prolong the duration of action, and potentially reduce abuse liability while retaining therapeutic effectiveness of the active metabolite (Balster and Schuster, 1973, Huttunen et al., 2011, Schindler et al., 2009). For example, the clinically available d-amphetamine prodrug lisdexamfetamine displays a slower onset and longer duration of action compared to d-amphetamine in humans (Jasinski and Krishnan, 2009a, b), rhesus monkeys (Banks et al., 2015), and rats (Rowley et al., 2012). In addition, lisdexamfetamine failed to maintain drug self-administration in rats (Heal et al., 2013), but retained some treatment efficacy to decrease cocaine vs. food choice in monkeys (Banks et al., 2015, Johnson et al., 2016). Overall, this body of literature supports the conceptual framework of d-amphetamine prodrug formulations as candidate medications for cocaine use disorder treatment.
Benzphetamine is a Schedue III anorectic agent that yields both d-amphetamine and d-methamphetamine as active metabolites in humans (Cody and Valtier, 1998). However, the relationship of benzphetamine behavioral effects to its pharmacokinetics is unknown. To address this issue, the present study used Pharmacokinetic-Pharmacodynamic (PK/PD) analysis (Negus and Banks, 2016) to compare benzphetamine cocaine-like discriminative stimulus effects to plasma benzphetamine, d-amphetamine, and d-methamphetamine levels in the rhesus monkeys. PK/PD analysis has been particularly useful for elucidating the role of active metabolites in the behavioral effects of prodrug formulations, such as lisdexamfetamine (Banks et al., 2015, Rowley et al., 2012). We hypothesized that benzphetamine would produce full substitution for cocaine with a time course that paralleled generation of d-amphetamine and/or d-methamphetamine as active metabolites. Drug discrimination and pharmacokinetic studies were also conducted with d-amphetamine for comparison to results with benzphetamine.
2.0 Methods
2.1 Subjects
Studies were conducted in 4 individually housed adult male rhesus monkeys (Macaca mulatta) that had an extensive experimental history behaving under the cocaine drug discrimination procedure (Banks, 2014, Banks et al., 2013, Banks et al., 2015). Monkeys could earn 1-g banana-flavored pellets (Grain-based Precision Primate Tablets; Test Diets, Richmond, IL) during daily experimental sessions (see below). In addition, monkeys received daily rations of food biscuits (Lab Diet High Protein Monkey Biscuits; PMI Feeds, St Louis, MO) and fresh fruit or vegetables delivered in the afternoons after behavioral sessions to minimize the effects of biscuit availability and consumption on food-maintained operant responding. Water was continuously available. A 12-h light/dark cycle was in effect (lights on from 0600 to 1800 h). Environmental enrichment, which consisted of movies displayed on a monitor in the housing room and foraging boards loaded with nuts, seeds or diced vegetables, was also provided after behavioral sessions. Facilities were licensed by the United States Department of Agriculture and accredited by the Association for Assessment and Accreditation of Laboratory Animal Care. The Institutional Animal Care and Use Committee approved all experimental and enrichment protocols. Animal research and husbandry were conducted according to the 8th edition of the Guide for the Care and Use of Laboratory Animals.
2.2 Pharmacodynamic studies
Pharmacodynamic studies were conducted using a two-key food-reinforced cocaine discrimination procedure that has been described previously in detail (Banks et al., 2013). Experimental sessions were conducted in each monkey’s home chamber. A custom operant response panel with three horizontally arranged square response keys was attached to the front of the home chamber, and only the left and right keys were used in the present studies. A pellet dispenser (Med Associates, ENV-203-1000, St. Albans, VT) was bolted to each panel. Equipment operation and data collection were accomplished with a Windows-based computer and MED-PC IV software (Med Associates).
Monkeys were trained to discriminate 0.32 mg/kg cocaine intramuscularly (IM) from saline. Training was conducted 5 days per week during daily sessions composed of multiple components. Each component consisted of a 5-min response period, during which the right and left response keys were transilluminated red and green, respectively, and monkeys could earn up to 10 food pellets by responding under a fixed-ratio (FR) 30 schedule of food presentation. Training sessions were composed of three components presented at 2-h intervals, and either saline or cocaine (0.32 mg/kg) was administered IM approximately 15 min prior to the start of each component. On training days, monkeys received a sequence of saline (S) and cocaine (C) injections in the order SSS, SSC, SCS, CSS, SCC, CSC, CCS, or CCC. These sequences were presented to engender daily experience with randomized sequences of saline-and cocaine-appropriate components. The 2h inter-component interval exceeded the time course of discriminative stimulus effects produced by the cocaine-training dose (Lamas et al., 1995), and thereby minimized effects of cocaine administered in earlier trials on performance during later trials on the same day. Following saline administration, only responding on the green key (the saline-appropriate key) produced food, whereas following 0.32 mg/kg cocaine administration, only responding on the red key (the cocaine-appropriate key) produced food. Responses on the inappropriate key reset the FR requirement on the appropriate key. The criterion for accurate discrimination was ≥85% injection-appropriate responding before delivery of the first reinforcer, ≥90% injection-appropriate responding for the entire component, and rates of responding ≥0.1 responses/s (sufficient to earn at least one pellet) for all components during 7 of 8 consecutive sessions.
Test sessions were identical to training sessions except that (a) responding on either key produced food, (b) monkeys received only one injection of vehicle or a test drug dose at the start of the session, and (c) 5-min response components began 10, 30, 56, 100, 180, 300, and 560 min after the injection to assess the time course of drug effects. If >50% cocaine-appropriate responding was still observed in any monkey after 560 min, then additional response components began after 24 h in all monkeys. The drugs and dose ranges tested were: benzphetamine (10–18 mg/kg) and d-amphetamine (0.032–0.32 mg/kg). Test sessions were generally conducted on Tuesdays and Fridays with training sessions conducted on Mondays, Wednesdays, and Thursdays. Test sessions were conducted only if performance during the previous two training sessions met the criteria for accurate discrimination (described above). Benzphetamine doses were tested twice, whereas d-amphetamine doses were tested once in each monkey. All studies with a drug were completed in a given monkey before testing the next drug in that monkey, and vehicle (saline) test sessions were conducted before or after evaluation of each test drug. The order of both drug doses and drugs was counterbalanced across monkeys.
2.3 Pharmacokinetic studies
After completion of behavioral studies, the same monkeys were used to evaluate pharmacokinetics of behaviorally active benzphetamine and d-amphetamine doses. Monkeys were transferred to primate restraint chairs and fitted with temporary intravenous catheters (24 gauge Exel safelet catheter, Fisher scientific, Pittsburg, PA) inserted into a saphenous vein. Blood samples (1–2 mLs) were collected in Vacutainer© tubes containing 3mg of sodium fluoride and 6mg sodium ethylenediaminetetraacetic acid before and 10, 30, 56, 100, 180, 300 min, 24 h, and 48 h after 18 mg/kg (IM) benzphetamine or 0.32 mg/kg d-amphetamine administration. Drugs were tested in a counterbalanced order across monkeys with at least two weeks between experiments. Samples were immediately centrifuged at 1000g for 10min. The plasma supernatant was transferred into a labeled storage tube and frozen at −80°C until analyzed. The identification and quantification of benzphetamine, d-methamphetamine, and d-amphetamine were accomplished using an API-5000 with a turbo V source for Turbolon Spray (Applied Biosystems, Foster City, CA) run in multiple reaction monitoring mode (MRM) and attached to a Waters Acquity UPLC system (Milford, MA) controlled by Analyst 1.4.2 software. The column used was a Phenomenex (Torrance, CA) Synergi Hydro-RP (50 × 2 mm, 4μm) with mobile phases consisting of A: 10 mM ammonium bicarbonate, pH 7.0, and B: Acetonitrile:Methanol (50:50) and a flow rate of 500 μL/min. Initial chromatography conditions consisted of 5% B held for 1 min and increased to 95% B over 1 min, and then held at 95% B for 2 min before returning to initial conditions. The first min of flow was diverted to waste while make-up solvent was pumped to the mass spectrometer. d-Amphetamine, d-methamphetamine, and benzphetamine were monitored in positive electrospray mode with an MRM transitions of 136.032 → 91.200, 149.805 → 91.000, and 226.010 → 90.900, repectively. Mass spectrometer parameters were as follows: CUR=10, GS1=50, GS2=60, IS=2000, TEM=600°C, CAD=10.
2.4 Data Analysis
For pharmacodynamics studies, the primary dependent measures were (1) percent cocaineappropriate responding (%CAR) (defined as (number of responses on the cocaine-associated key divided by the total number of responses on both the cocaine-and saline-associated keys)*100), and (2) rates of responding during each component. These dependent measures were then plotted as a function of time after drug or saline administration. Percent CAR and rates of responding were analyzed using linear mixed effect analysis with drug dose and time as the main fixed effects and subjects as the random effect (JMP Pro 12.1, SAS, Cary, NC). A significant drug×time interaction was followed by the Dunnett’s posthoc test for comparison to vehicle (saline) conditions within a given time point. Drug doses that produced ≥90% cocaine-appropriate responding were considered to produce full substitution.
For pharmacokinetic studies, the primary dependent measures were plasma levels of benzphetamine, d-methamphetamine, and d-amphetamine in ng/ml. These dependent measures were then plotted as a function of time. Pharmacokinetic parameters after 0.32 mg/kg d-amphetamine or 18 mg/kg benzphetamine administration were estimated using a one-compartment extravascular model with an assumption of no lag time for the intramuscular dose route (WinNonlin version 2.1, Pharmsight, Princeton, NJ).
Two approaches were used to compare the time course of drug effects in the cocaine discrimination procedure with the time course of plasma levels of benzphetamine, d-methamphetamine, and d-amphetamine. For both approaches, the plasma level of benzphetamine, d-methamphetamine, or d-amphetamine at each time point was plotted on the X-axis, and drug effect on either cocaine-appropriate responding or response rate was plotted on the Y-axis. The correlation between these two variables independent of time was evaluated by calculating the Pearson correlation coefficient (r) and the coefficient of determination (r2). Additionally, to incorporate the variable of time, the data within each graph were also assessed as hysteresis plots (Banks et al., 2015, Banks et al., 2016). For graphs relating plasma drug/metabolite levels to rate-decreasing behavioral effects, rate suppression was transformed to Percent Rate Suppression using the equation {1-[(saline control rate-test rate)/saline control rate])*100}.
2.5 Drugs
(−)-Cocaine HCl was supplied by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). d-Amphetamine hemifumarate and racemic benzphetamine HCl were purchased from Sigma-Aldrich (St. Louis, MO). All drugs were dissolved in sterile water. Drug doses were calculated and expressed using the salt forms listed above.
3.0 Results
3.1 Effects of Control Treatments and d-Amphetamine
On cocaine and saline training days preceding all test days, mean ± s.e.m. percent injection-appropriate responding was 99.9 ± 0.1% and 100 ± 0%, respectively. Rates of operant responding (mean ± s.e.m.) during cocaine and saline training components were 2.1 ± 0.4 and 2.3 ± 0.4 responses/sec, respectively. On test days, saline injections engendered primarily saline-appropriate responding (≤10% cocaine-appropriate responding) at all time points (Figure panels 1A and 2A) and had no effect on rates of operant responding (Figure panels 1B and 2B).
Figure 1.

Behavioral effects and pharmacokinetics of d-amphetamine in rhesus monkeys (n=3) trained to discriminate intramuscular cocaine (0.32 mg/kg) from saline. Panels A and B show potency and timecourse of cocaine-like discriminative stimulus (A) or rate-altering (B) effects following d-amphetamine (0.032–0.32 mg/kg, IM). Symbols above “S” and “C” represent means ± SEM for all training sessions preceding test sessions when the saline- and cocaine-associated keys were correct, respectively. Panel C shows plasma d-amphetamine levels as a function of time (log scale) following 0.32 mg/kg intramuscular d-amphetamine. Panel D shows the hysteresis loop for plasma d-amphetamine levels and percent cocaine-appropriate responding following 0.32 mg/kg intramuscular d-amphetamine. Arrows denote the temporal direction in the hysteresis loop. All points represent the mean ± SEM from 3 monkeys, except for Panel D where error bars are not shown for visual clarity. Filled symbols denote statistical significance (p<0.05) within a given time point during an experimental session where saline (dashed line) was administered. The R-squared value and p-value are reported within the axes.
Figure 2.

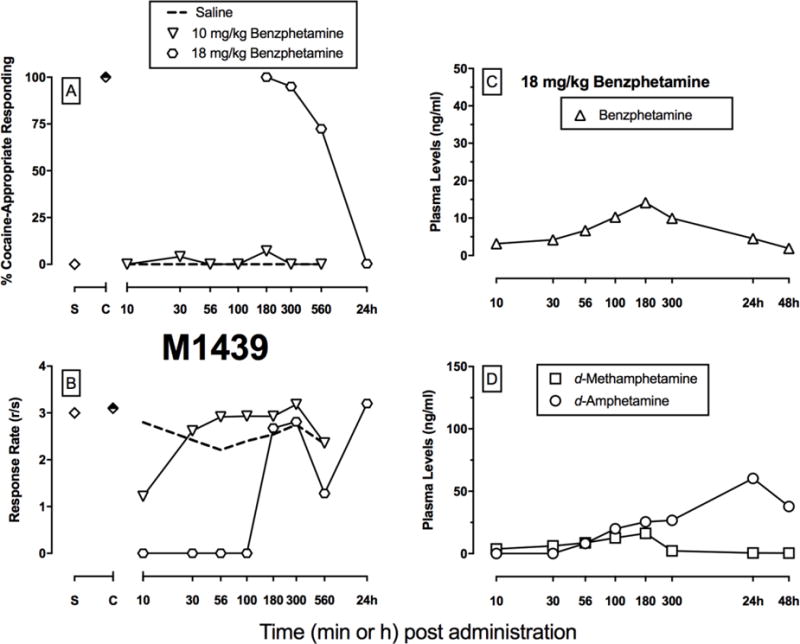
Behavioral effects and pharmacokinetics of benzphetamine in an individual rhesus monkey trained to discriminate intramuscular cocaine (0.32 mg/kg) from saline. Panels A and B show potency and timecourse of cocaine-like discriminative stimulus (A) or rate-altering (B) effects following benzphetamine (10–18 mg/kg, IM). Symbols above “S” and “C” represent means ± SEM for all training sessions preceding test sessions when the saline- and cocaine-associated keys were correct, respectively. Panel C shows plasma benzphetamine levels as a function of time (log scale) following 18 mg/kg intramuscular benzphetamine. Panel D shows plasma d-methamphetamine and d-amphetamine levels as a function of time (log scale) following 18 mg/kg intramuscular benzphetamine.
Figure 1A also shows that d-amphetamine produced dose- and time-dependent cocaineappropriate responding in all 3 monkeys (d-amphetamine dose: F3.54=7.59, p<0.0001). 0.1 mg/kg d-amphetamine produced full substitution in 2 monkeys, and 0.32 mg/kg d-amphetamine produced full substitution in all monkeys. These doses of d-amphetamine did not significantly alter rates of operant responding (Figure 1B). Figure 1C shows plasma d-amphetamine levels as a function of time after 0.32 mg/kg d-amphetamine administration. Plasma d-amphetamine levels peaked at 91.6 ng/mL with a time to maximum levels (Tmax) of 100 min; d-amphetamine levels were still detectable (~ 16 ng/ml) at 48 h post-administration. Figure 1D shows a significant positive correlation between the cocaine-like discriminative stimulus effects of 0.32 mg/kg intramuscular d-amphetamine and the time course of d-amphetamine plasma levels (R2=0.99, p<0.0001). This relationship was characterized by a narrow clockwise hysteresis loop.
3.2 Effects of benzphetamine
In contrast to d-amphetamine, benzphetamine did not produce a significant dose- or time-dependent increase in cocaine-appropriate responding up to a dose that significantly decreased rates of operant responding at the 10-min time point (time: F6,18=5.75, p=0.0017; interaction: F12,36=3.0, p=0.0053). As a result, individual subject data for benzphetamine in the discrimination procedure are shown in panels A and B in Figures 2–5. 18 mg/kg benzphetamine produced full substitution in 2 monkeys for at lease one time point, and the peak cocaine-like effects occurred at 100 and 180 min for these 2 monkeys (Monkeys M1488 and M1439, Figures 2–3). Maximal cocaine-appropriate responding was 88% at 30 min in the third monkey (M1479, Figure 4), and this was bracketed by periods when benzphetamine eliminated rates of operant responding. In the fourth monkey, cocaine-appropriate responding never exceeded 10% (Monkey M1357, Figure 5).
Figure 5.
Behavioral effects and pharmacokinetics of benzphetamine in an individual rhesus monkey trained to discriminate intramuscular cocaine (0.32 mg/kg) from saline. Otherwise, details are as described in Figure 2.
Figure 3.

Behavioral effects and pharmacokinetics of benzphetamine in an individual rhesus monkey trained to discriminate intramuscular cocaine (0.32 mg/kg) from saline. Otherwise, details are as described in Figure 2.
Figure 4.

Behavioral effects and pharmacokinetics of benzphetamine in an individual rhesus monkey trained to discriminate intramuscular cocaine (0.32 mg/kg) from saline. Otherwise, details are as described in Figure 2.
Figures 2–5 also show individual subject results for plasma benzphetamine (Panel C), d-methamphetamine (Panel D), and d-amphetamine (Panel D) levels as a function of time after 18 mg/kg benzphetamine. Plasma benzphetamine levels generally peaked at 180 min; benzphetamine levels were still detectable at low levels (~ 2 ng/mL) at 48 h. Plasma d-methamphetamine levels peaked between 56–180 min between monkeys; levels persisted at low (~ 1 ng/mL), but still detectable, levels 48 h post-benzphetamine administration. Plasma d-amphetamine levels generally peaked at 24 h; levels remained elevated after 48 h.
Figure 6 shows the PK/PD analysis of plasma benzphetamine (A, B), d-methamphetamine (C, D), and d-amphetamine (E, F) levels and either cocaine-like discriminative stimulus (top panels) or ratealtering (bottom panels) effects. There was not a positive correlation between plasma levels of any drug or metabolite and any behavioral effect, although there was a negative correlation between rate-decreasing effects and plasma d-amphetamine levels (i.e. greatest rate decreasing effects at lowest d-amphetamine levels) (Figure 6F). Counter-clockwise hysteresis loops related both plasma benzphetamine and d-methamphetamine levels to percent cocaine-appropriate responding; whereas, clockwise hysteresis loops related plasma benzphetamine and d-methamphetamine levels to percent rate suppression. Furthermore, a clockwise hysteresis loop was observed relating behavioral effects and plasma d-amphetamine levels after benzphetamine administration. However, these relationships were a poor fit as noted by the R2 values and none of the correlations were significant.
Figure 6.

Pharmacokinetic-Pharmacodynamic analysis of cocaine-like discriminative stimulus effects (A, C, E) and rate-altering effects (B, D, F) produced by intramuscular benzphetamine (18 mg/kg) administration in rhesus monkeys (n=4). Hysteresis loops for plasma venous levels of benzphetamine (A) and its metabolites (C: d-methamphetamine; E: d-amphetamine) and percent cocaine-appropriate responding are shown in the top panels. Hysteresis loops for plasma venous levels of benzphetamine (B) and its metabolites (D: d-methamphetamine; F: d-amphetamine) and rates of operant responding are shown in the bottom panels. Abscissae: All points represent the mean of four monkeys and error bars are not shown for visual clarity. Arrows denote the temporal direction in the hysteresis loop. The R-squared values are reported within the axes.
4.0 Discussion
The present study aim was to compare benzphetamine cocaine-like discriminative stimulus effects with plasma benzphetamine, d-amphetamine, and d-methamphetamine levels in the rhesus monkeys. There were three main findings. First, d-amphetamine produced full cocaine-like effects in all monkeys, whereas benzphetamine produced full cocaine-like effects in only 2 out of 4 monkeys. In addition, benzphetamine was both less potent and displayed a slightly slower onset of discriminative stimulus effects compared to d-amphetamine. Second, pharmacokinetic results demonstrated that benzphetamine administration was associated with the sequential emergence of both d-methamphetamine and d-amphetamine plasma levels, although d-amphetamine levels did not peak until 24h later. These results support the proposition that benzphetamine functions as a prodrug for both d-methamphetamine and d-amphetamine. Lastly, neither the cocaine-like discriminative stimulus effects nor the rate-decreasing effects of benzphetamine were positively correlated with plasma levels of benzphetamine, d-amphetamine, or d-methamphetamine. This finding suggests that neither the parent compound nor generation of d-amphetamine or d-methamphetamine as active metabolites is sufficient to account for benzphetamine behavioral effects. These results have implications for the use of benzphetamine as a candidate medication for treatment of cocaine use disorder.
4.1 Cocaine-like effects of benzphetamine
Agonist-like medications for substance use disorder treatment are founded upon the principle that the medication shares similar pharmacodynamic mechanisms with the target abused drug. The present results suggest that benzphetamine may function as a potential “agonist-like” medication for cocaine use disorder. First, the incomplete benzphetamine substitution profile for cocaine in the present study was consistent with incomplete substitution profile in rhesus monkeys trained to discriminate intragastric d-amphetamine (de la Garza and Johanson, 1987). Furthermore, benzphetamine produced partial substitution in humans trained to discriminate oral d-amphetamine (Chait and Johanson, 1988). In contrast, benzphetamine failed to substitute in pigeons trained to discriminate d-amphetamine (Evans and Johanson, 1987). However, the pretreatment time for the pigeon studies was 10 min and based on the present results, that may have contributed to the lack of effect. Second, the present results extended the previous benzphetamine study in monkeys by determining the time course of benzphetamine effects. Benzphetamine did not display a slower onset of cocaine-like effects compared to d-amphetamine as peak discriminative stimulus effects for both drugs occurred at the 30-min time point. Because benzphetamine failed to produce significant cocaine-like effects at the group analysis level, comparisons for duration of action are complicated by the lack of benzphetamine effect. However, in the 2 monkeys that benzphetamine did produce ≥90% cocaine-appropriate responding, cocaine-like effects were back to saline-like levels, ≤ 15% cocaine-appropriate responding, at 24h. Lastly, benzphetamine was 180-fold less potent than d-amphetamine in the 2 monkeys for which benzphetamine did produce ≥90% cocaineappropriate responding. The lower potency of benzphetamine compared to d-amphetamine in the present study was consistent with previous monkey (de la Garza and Johanson, 1987) and human results (Chait and Johanson, 1988). Overall, the present result support the potential of benzphetamine to produce pharmacodynamically similar effects to cocaine, albeit with significant individual subject variability.
4.2 Relationship between plasma drug levels and benzphetamine behavioral effects
Plasma d-amphetamine levels were related to d-amphetamine discriminative stimulus effects according to a narrow clockwise hysteresis loop. The clockwise hysteresis loop observed in the present study with d-amphetamine is consistent with and extends previous human laboratory PK/PD findings correlating plasma d-amphetamine levels with either subjective or cardiovascular effects (Angrist et al., 1987, Brauer et al., 1996). A clockwise hysteresis loop suggests the pharmacodynamic effect decreased as a function of time for a given drug level and may be indicative of acute tolerance (Louizos et al., 2014), although the narrow shape of the hysteresis loop suggests that the magnitude of acute tolerance was small. The exact mechanisms of any acute tolerance during the time course of d-amphetamine discriminative stimulus effects remains to be elucidated, but the consistency of the present d-amphetamine results provide an empirical foundation to correlate benzphetamine behavioral effects with plasma benzphetamine, d-methampehtamine, and d-amphetamine levels.
Benzphetamine administration resulted in detectable plasma d-methamphetamine and d-amphetamine levels over the 48-h monitoring period. These results in monkeys are consistent with and extend previous urinalysis and hair results in humans (Cody and Valtier, 1998) and plasma results in rats (Kikura and Nakahara, 1995) demonstrating that benzphetamine is metabolized to both d-methamphetamine and d-amphetamine. Plasma d-methamphetamine levels after benzphetamine administration were of similar magnitude and duration compared to those observed after intramuscular d-methamphetamine (0.32 mg/kg) alone in monkeys (Banks et al., 2016). In addition, plasma d-amphetamine levels after benzphetamine administration reached similar peak levels compared to those observed after d-amphetamine (0.32 mg/kg) alone in the present study, albeit with time course differences. Moreover, peak d-amphetamine levels were greater than peak d-methamphetamine levels in all 4 monkeys suggesting that metabolism of d-methamphetamine to d-amphetamine was not the only metabolic pathway generating d-amphetamine after benzphetamine administration. N-demethylation of benzphetamine to desmethylbenzphetamine has been proposed as one potential benzphetamine metabolite that could then be further metabolized to d-amphetamine in humans (Figure 7) (Cody and Valtier, 1998). The present benzphetamine pharmacokinetic results in monkeys provide additional empirical support for desmethylbenzphetamine as an intermediary benzphetamine metabolite to d-amphetamine.
Figure 7.

Main metabolic pathways of benzphetamine biotransformation to d-methamphetamine and d-amphetamine.
Despite benzphetamine producing plasma d-methamphetamine and d-amphetamine levels that were sufficient to produce behaviorally-active effects when these compounds were administered alone (Banks et al., 2016; present study), benzphetamine displayed an incomplete substitution profile for cocaine in the discrimination procedure. For example, the two monkeys (M1357 and M1488) that displayed the highest d-methamphetamine and d-amphetamine levels were also the two monkeys in which benzphetamine failed to produce full cocaine-like effects. Whether these discrimination results are the consequence of pharmacological activity of benzphetamine or a metabolite at the dopamine transporter or the temporal pattern of plasma drug levels remains to be fully elucidated. Furthermore, significant ratealtering effects were observed a time point (10 min) of low benzphetamine, d-methamphetamine, and d-amphetamine levels suggesting that some other benzphetamine metabolite contributed to this behavioral effect. For example, methylbenzene (i.e. toluene) might be one potential metabolic product from the biotransformation of benzphetamine to d-methampehtamine that could contribute to benzphetamine rate-altering effects (Cody and Valtier, 1998). Previous studies in mice have demonstrated that toluene produced rate-altering effects in a toluene discrimination procedure (Shelton, 2007). Overall, the present results suggest that benzphetamine has a complex metabolic pathway that complicates PK/PD analysis of its behaviorally-active metabolites.
4.3 Implications for benzphetamine as a candidate medication for cocaine use disorder
Prodrug formulations of monoamine releasers represent one drug development method for addressing abuse liability concerns of candidate “agonist-like” pharmacotherapies for substance use disorder treatment. Although benzphetamine produced full cocaine-like effects in a subset of monkeys and generated plasma d-amphetamine levels similar to d-amphetamine administration alone, there are two potential considerations regarding further evaluation of benzphetamine as a candidate pharmacotherapy for cocaine use disorder treatment. First, the incomplete substitution profile of benzphetamine for cocaine in monkeys and d-amphetamine in humans suggests that patient compliance may be an issue (Grabowski et al., 2004, Kreek et al., 2002). Thus, one potential disadvantage of prodrug formulations is the reliance on the research subject or patient to generate the active metabolite, and there is considerable biological/genetic variation in hepatic enzyme activity that may contribute to PK/PD variance (Huttunen et al., 2011). Furthermore, prodrug formulations may also generate metabolites other than the metabolite of interest, and these metabolites may have biological activity that might either 1) oppose effects of the metabolite of interest, or 2) produce undesirable behavioral effects. Second, the present benzphetamine results can be compared with another d-amphetamine prodrug, lisdexamfetamine, which has been evaluated under similar experimental procedures. Lisdexamfetamine requires enzymatic hydrolysis via red blood cells, not hepatic enzymes, for bioconversion to the active d-amphetamine metabolite and the amino acid L-lysine (Pennick, 2010). In contrast to benzphetamine, lisdexamfetamine displayed a slow onset and prolonged duration of action in the cocaine discriminaton procedure that was consistent in all monkeys (Banks et al., 2015). Furthermore, lisdexamfetamine generated peak plasma d-amphetamine levels (~ 350 ng/mL) that were approximately 3-fold higher and without a significant decrease in rates of operant responding compared to benzphetamine. Although lisdexamfetamine also retained treatment effectiveness to decrease cocaine vs. food choice in monkeys (Banks et al., 2015, Johnson et al., 2016), a pilot clinical trial with a moderate lisdexamfetamine dose did not significantly decrease cocaine use (Mooney et al., 2015). Although prodrug formulations offer the potential to reduce undesirable effects of the active metabolite, the clinical effectiveness of prodrug formulations as candidate pharmaotherapies for cocaine use disorder treatment remains to be fully elucidated.
Highlights.
d-amphetamine produced full cocaine-like stimulus effects in all monkeys
A clockwise hysteresis loop related d-amphetamine behavioral effects and levels
Benzphetamine produced full cocaine-like stimulus effects in 2 of 4 monkeys
Benzphetamine produced sequential d-methamphetamine and d-amphetamine plasma levels
Benzphetamine behavioral effects did not correlate with metabolite levels
Acknowledgments
We acknowledge the technical assistance of Crystal Reyns and Kevin Costa for coding the original behavioral program version.
Funding:
Research reported in this publication was supported by the National Institute on Drug Abuse of the National Institutes of Health under Award number R01DA026946. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest:
The authors declare no conflicts of interest.
References
- Acri J, Skolnick P. Pharmacotherapy of substance use disorders. In: Chamey D, Buxbaum J, Sklar P, Nestler EJ, editors. Neurobiology of mental illness. 4. London: Oxford University Press; 2013. pp. 761–71. [Google Scholar]
- Angrist B, Corwin J, Bartlik B, Cooper T. Early pharmacokinetics and clinical effects of oral D-amphetamine in normal subjects. Biol Psychiatry. 1987;22:1357–68. doi: 10.1016/0006-3223(87)90070-9. [DOI] [PubMed] [Google Scholar]
- Balster RL, Schuster CR. Fixed-interval schedule of cocaine reinforcement: effect of dose and infusion duration. J Exp Anal Behav. 1973;20:119–29. doi: 10.1901/jeab.1973.20-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML. Effects of the nicotinic acetylcholine receptor antagonist mecamylamine on the discriminative stimulus effects of cocaine in male rhesus monkeys. Exp Clin Psychopharmacol. 2014;22:266–73. doi: 10.1037/a0035274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Blough BE, Fennell TR, Snyder RW, Negus SS. Role of phenmetrazine as an active metabolite of phendimetrazine: Evidence from studies of drug discrimination and pharmacokinetics in rhesus monkeys. Drug Alcohol Depend. 2013;130:158–66. doi: 10.1016/j.drugalcdep.2012.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Hutsell BA, Blough BE, Poklis JL, Negus SS. Preclinical Assessment of Lisdexamfetamine as an Agonist Medication Candidate for Cocaine Addiction: Effects in Rhesus Monkeys Trained to Discriminate Cocaine or to Self-Administer Cocaine in a Cocaine Versus Food Choice Procedure. Int J Neuropsychopharmacol. 2015 doi: 10.1093/ijnp/pyv009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banks ML, Smith DA, Kisor DF, Poklis JL. Relationship between discriminative stimulus effects and plasma methamphetamine and amphetamine levels of intramuscular methamphetamine in male rhesus monkeys. Pharmacol Biochem Behav. 2016;141:58–65. doi: 10.1016/j.pbb.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brauer LH, Ambre J, De Wit H. Acute tolerance to subjective but not cardiovascular effects of d-amphetamine in normal, healthy men. J Clin Psychopharmacol. 1996;16:72–6. doi: 10.1097/00004714-199602000-00012. [DOI] [PubMed] [Google Scholar]
- Chait LD, Johanson CE. Discriminative stimulus effects of caffeine and benzphetamine in amphetamine-trained volunteers. Psychopharmacology. 1988;96:302–8. doi: 10.1007/BF00216054. [DOI] [PubMed] [Google Scholar]
- Cody JT, Valtier S. Detection of Amphetamine and Methamphetamine Following Administration of Benzphetamine. J Anal Toxicol. 1998;22:299–309. doi: 10.1093/jat/22.4.299. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gould RW, Martelle JL, Nader MA. Prolonged Attenuation of the Reinforcing Strength of Cocaine by Chronic d-Amphetamine in Rhesus Monkeys. Neuropsychopharmacology. 2011;36:539–47. doi: 10.1038/npp.2010.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Garza R, Johanson CE. Discriminative stimulus properties of intragastrically administered d-amphetamine and pentobarbital in rhesus monkeys. J Pharmacol Exp Ther. 1987;243:955–62. [PubMed] [Google Scholar]
- Evans SM, Johanson CE. Amphetamine-like effects of anorectics and related compounds in pigeons. J Pharmacol Exp Ther. 1987;241:817–25. [PubMed] [Google Scholar]
- Grabowski J, Rhoades H, Schmitz J, Stotts A, Daruzska LA, Creson D, et al. Dextroamphetamine for cocaine-dependence treatment: a double-blind randomized clinical trial. J Clin Psychopharmacol. 2001;21:522–6. doi: 10.1097/00004714-200110000-00010. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav. 2004;29:1439–64. doi: 10.1016/j.addbeh.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Greenwald MK, Lundahl LH, Steinmiller CL. Sustained Release d-Amphetamine Reduces Cocaine but not/‘Speedball/’-Seeking in Buprenorphine-Maintained Volunteers: A Test of Dual-Agonist Pharmacotherapy for Cocaine/Heroin Polydrug Abusers. Neuropsychopharmacology. 2010;35:2624–37. doi: 10.1038/npp.2010.175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heal DJ, Buckley NW, Gosden J, Slater N, France CP, Hackett D. A preclinical evaluation of the discriminative and reinforcing properties of lisdexamfetamine in comparison to d-amfetamine, methylphenidate and modafinil. Neuropharmacology. 2013;73:348–58. doi: 10.1016/j.neuropharm.2013.05.021. [DOI] [PubMed] [Google Scholar]
- Herin DV, Rush CR, Grabowski J. Agonist-like pharmacotherapy for stimulant dependence: preclinical, human laboratory, and clinical studies. Ann NY Acad Sci. 2010;1187:76–100. doi: 10.1111/j.1749-6632.2009.05145.x. [DOI] [PubMed] [Google Scholar]
- Huttunen KM, Raunio H, Rautio J. Prodrugs—from Serendipity to Rational Design. Pharmacological Reviews. 2011;63:750–71. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- Jasinski D, Krishnan S. Abuse liability and safety of oral lisdexamfetamine dimesylate in individuals with a history of stimulant abuse. J Psychopharmacol. 2009a;23:419–27. doi: 10.1177/0269881109103113. [DOI] [PubMed] [Google Scholar]
- Jasinski D, Krishnan S. Human pharmacology of intravenous lisdexamfetamine dimesylate: abuse liability in adult stimulant abusers. J Psychopharmacol. 2009b;23:410–8. doi: 10.1177/0269881108093841. [DOI] [PubMed] [Google Scholar]
- Johnson AR, Banks ML, Blough BE, Lile JA, Nicholson KL, Negus SS. Development of a translational model to screen medications for cocaine use disorder I: Choice between cocaine and food in rhesus monkeys. Drug Alcohol Depend. 2016;165:103–10. doi: 10.1016/j.drugalcdep.2016.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikura R, Nakahara Y. Hair analysis for drugs of abuse. XI. Disposition of benzphetamine and its metabolites into hair and comparison of benzphetamine use and methamphetamine use by hair analysis. Biol Pharm Bull. 1995;18:1694–9. doi: 10.1248/bpb.18.1694. [DOI] [PubMed] [Google Scholar]
- Kreek MJ, LaForge KS, Butelman E. Pharmacotherapy of addictions. Nat Rev Drug Discov. 2002;1:710–26. doi: 10.1038/nrd897. [DOI] [PubMed] [Google Scholar]
- Lamas X, Negus SS, Hall E, Mello NK. Relationship between the discriminative stimulus effects and plasma concentrations of intramuscular cocaine in rhesus monkeys. Psychopharmacology. 1995;121:331–8. doi: 10.1007/BF02246072. [DOI] [PubMed] [Google Scholar]
- Louizos C, Yanez JA, Forrest ML, Davies NM. Understanding the hysteresis loop conundrum in pharmacokinetic/pharmacodynamic relationships. J Pharm Pharm Sci. 2014;17:34–91. [PMC free article] [PubMed] [Google Scholar]
- Mariani JJ, Pavlicova M, Bisaga A, Nunes EV, Brooks DJ, Levin FR. Extended-Release Mixed Amphetamine Salts and Topiramate for Cocaine Dependence: A Randomized Controlled Trial. Biol Psychiatry. 2012;72:950–6. doi: 10.1016/j.biopsych.2012.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney ME, Herin DV, Specker S, Babb D, Levin FR, Grabowski J. Pilot study of the effects of lisdexamfetamine on cocaine use: A randomized, double-blind, placebo-controlled trial. Drug Alcohol Depend. 2015;153:94–103. doi: 10.1016/j.drugalcdep.2015.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS. Rapid Assessment of Choice between Cocaine and Food in Rhesus Monkeys: Effects of Environmental Manipulations and Treatment with d-Amphetamine and Flupenthixol. Neuropsychopharmacology. 2003;28:919–31. doi: 10.1038/sj.npp.1300096. [DOI] [PubMed] [Google Scholar]
- Negus SS, Banks ML. Pharmacokinetic-Pharmacodynamic (PKPD) Analysis with Drug Discrimination Current Topics in Behavioral Neuroscience. Cham: Springer International Publishing; 2016. pp. 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Henningfield J. Agonist Medications for the Treatment of Cocaine Use Disorder. Neuropsychopharmacology. 2015;40:1815–25. doi: 10.1038/npp.2014.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuijten M, Blanken P, van de Wetering B, Nuijen B, van den Brink W, Hendriks VM. Sustained-release dexamfetamine in the treatment of chronic cocaine-dependent patients on heroin-assisted treatment: a randomised, double-blind, placebo-controlled trial. The Lancet. 2016;387:2226–34. doi: 10.1016/S0140-6736(16)00205-1. [DOI] [PubMed] [Google Scholar]
- Pennick M. Absorption of lisdexamfetamine dimesylate and its enzymatic conversion to d-amphetamine. Neuropsychiatr Dis Treat. 2010;6:317–27. doi: 10.2147/ndt.s9749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley HL, Kulkarni R, Gosden J, Brammer R, Hackett D, Heal DJ. Lisdexamfetamine and immediate release d-amfetamine – Differences in pharmacokinetic/pharmacodynamic relationships revealed by striatal microdialysis in freely-moving rats with simultaneous determination of plasma drug concentrations and locomotor activity. Neuropharmacology. 2012;63:1064–74. doi: 10.1016/j.neuropharm.2012.07.008. [DOI] [PubMed] [Google Scholar]
- Rush CR, Stoops WW, Sevak RJ, Hays LR. Cocaine choice in humans during D-amphetamine maintenance. J Clin Psychopharmacol. 2010;30:152–9. doi: 10.1097/JCP.0b013e3181d21967. [DOI] [PubMed] [Google Scholar]
- Schindler CW, Panlilio LV, Thorndike EB. Effect of rate of delivery of intravenous cocaine on selfadministration in rats. Pharmacol Biochem Behav. 2009;93:375–81. doi: 10.1016/j.pbb.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton KL. Inhaled toluene vapor as a discriminative stimulus. Behav Pharmacol. 2007;18:219–29. doi: 10.1097/FBP.0b013e328157f460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoops WW, Rush CR. Agonist Replacement for Stimulant Dependence: A Review of Clinical Research. Curr Pharm Design. 2013;19:7026–35. doi: 10.2174/138161281940131209142843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen M, Barrett AC, Negus SS, Caine SB. Cocaine versus food choice procedure in rats: environmental manipulations and effects of amphetamine. J Exp Anal Behav. 2013;99:211–33. doi: 10.1002/jeab.15. [DOI] [PMC free article] [PubMed] [Google Scholar]


