Abstract
Current approaches to derive oligodendrocytes from human pluripotent stem cells (hPSCs) need extended exposure of hPSCs to growth factors and small molecules, which limits their clinical application because of the lengthy culture time required and low generation efficiency of myelinating oligodendrocytes. Compared to extrinsic growth factors and molecules, oligodendrocyte differentiation and maturation can be more effectively modulated by regulation of the cell transcription network. In the developing central nervous system (CNS), two basic helix-loop-helix transcription factors, Olig1 and Olig2, are decisive in oligodendrocyte differentiation and maturation. Olig2 plays a critical role in the specification of oligodendrocytes and Olig1 is crucial in promoting oligodendrocyte maturation. Recently viral vectors have been used to overexpress Olig2 and Olig1 in neural stem/progenitor cells (NSCs) to induce the maturation of oligodendrocytes and enhance the remyelination activity in vivo. Because of the safety issues with viral vectors, including the insertional mutagenesis and potential tumor formation, non-viral transfection methods are preferred for clinical translation. Here we report a poly(β-amino ester) (PBAE)-based nanoparticle transfection method to deliver Olig1 and Olig2 into human fetal tissue-derived NSCs and demonstrate efficient oligodendrocyte differentiation following transgene expression of Olig1 and Olig2. This approach is potentially translatable for engineering stem cells to treat injured or diseased CNS tissues.
Keywords: nanoparticles, transfection, Olig1, Olig2, oligodendrocyte differentiation
Graphical abstract
Introduction
Existent approaches of directed differentiation require extended exposure of human pluripotent stem cells (hPSCs) to extrinsic factors, such as growth factors and small molecules; therefore they are challenging to implement for transplanted hPSCs in vivo [1-4]. Specifically, current protocols to derive oligodendrocytes from hPSCs are limited in application because of lengthy culture time required (80 to 200 days) and low generation efficiencies of mature oligodendrocytes [3-6]. There is an urgent need to develop more efficient methods to accelerate the differentiation and maturation timeline of hPSCs for regenerative therapy.
In comparison with the extrinsic factors supplemented in the medium, stem cell differentiation and maturation can be more efficiently modulated through regulating intrinsic factor expression, such as resetting the transcription network using transcription factors [7, 8]. In the developing central nervous system (CNS), two basic helix-loop-helix (bHLH) transcription factors, Olig1 and Olig2, are expressed in oligodendrocyte progenitor cells and myelinating oligodendrocytes; Olig2 is decisive for the specification of oligodendrocytes and Olig1 is essential in fostering oligodendrocyte differentiation and subsequent myelination primarily in the brain [9, 10]. Overexpression of Olig2 in neural stem/progenitor cells (NSCs) by viral vector has shown to promote oligodendrocyte differentiation and maturation and enhance remyelination activity in vivo [11, 12].
Currently viral vectors have been extensively used to mediate transfection of transcription factors to stem cells to control their differentiation and maturation [13]. However, these viral vectors have raised lots of safety concerns with the insertional mutagenesis and excessive inflammation and immune response [14]. Viral vector-mediated persistent expression of exogenous transcription factors may unfavorably affect the differentiated cell maturation and function [15, 16].
Numerous biomaterials have been investigated as potential non-viral gene delivery vectors [17-20]. As compared to viral vectors, biomaterial-based vectors are easier to manufacture and scale-up, but they are less efficient in mediating transgene expression. In particular, poly (β-amino ester)s (PBAEs) have been studied as polymeric gene carriers due to their structural versatility, biodegradability, and low cytotoxicity [21-24]. PBAEs have shown to condense plasmid DNA forming nanoparticles with relatively high transgene expression in several stem cell types [21, 25, 26]. Here we develop an efficient approach to expedite and enhance oligodendrocyte differentiation from human fetal tissue-derived NSCs through PBAE-DNA nanoparticle-mediated transient expression of Olig1 and Olig2 in hNSCs.
Results and Discussion
Highly Efficient PBAE-DNA Nanoparticle-Mediated Transfection of hNSCs
A series of PBAE polymers were synthesized following the method that we have previously reported using the monomers and the reaction scheme shown in Figure S1 [21, 22]. Briefly, a diacrylate backbone (B), an amino-alcohol side chain (S), and an amine containing end-capping (E) were conjugated through a two-step process in which the addition of the end-group followed the formation of a BS base-polymer. Polymers were named according to their “BSE” structure, where monomers forming the base-polymer BS were identified by the number of carbons in its hydrocarbon portion. For example, 536 refers to the polymer synthesized with B5, S3, and E6, where B5 corresponds to a backbone with 5 hydrocarbons between the acrylate groups and S3 to a side chain with 3 hydrocarbons between the amine and alcohol groups. The numbers assigned for end-capping monomers are merely sequential, arranged according to structural similarities among amine groups.
As previously demonstrated by us, single changes on the hydrocarbon content, and therefore hydrophobicity, of the BS base-polymer can significantly modify the polymer activity [21, 22]. Increase in PBAE hydrophobicity is associated with high gene expression, but only up to a certain limit, from which the increase in cytotoxicity becomes much higher than any additional increment in transfection efficiency. On the other hand, a balanced hydrophobicity/hydrophilicity between backbone and side chain monomers can afford high transfection efficiencies while preserving cell viability. In other words, optimized transfection and viability outcomes can be achieved, for example, by employing hydrophobic backbones (e.g. B5) combined with hydrophilic side chains (S3), or yet, intermediately-hydrophobic base diacrylates (e.g. B4) with hydrophobic side chains (S5). Further end-modification of PBAE polymers with secondary (E6) or tertiary amines (E7) groups enables high endosomal buffering, as opposed to the low buffering effect of primary amines (e.g. E4). High buffering capacity is a desirable characteristic, since it is necessary, even though not enough, to favor transfection efficiency [27]. Based on such findings and previous work with glial cells [21, 22], three top-performing PBAE formulations (447, 456, and 536) were chosen to achieve an optimal transfection condition with high level of transgene expression and low cytotoxicity for hNSCs.
Initial screens used an Olig2-GFP plasmid DNA dose of 0.3, 0.6, and 1 μg/cm2 and a selected range of PBAE/plasmid DNA ratios of 20, 30, and 40 w/w in order to identify top polymers from a group consisting PBAE 447, 456, and 536 (Figure 1). Among all condition tested, PBAE 536 at the polymer/DNA ratio of 20 w/w with the DNA dose of 1 μg/cm2, showed the highest transfection efficiency of 57.4 ± 2.4% and cell viability of 118.3 ± 5.1% relative to non-transfected cells on day 2 following transfection (Figure 1A). The transfected gene expression lasted for at least 10–14 days, and showed efficiency of 8.2 ± 0.6% with a cell viability of 93.6 ± 4.5% relative to non-transfected cells on day 9 (Figure 1B). Immunostaining analysis confirmed the expression of GFP was co-localized to Olig2 expression in the transfected hNSCs on both days 2 and 9 (Figure 1C and D). We have chosen PBAE 536 at the polymer/DNA ratio of 20 w/w and the DNA dose of 1 μg/cm2 for following studies.
Figure 1. Identification of a nanoparticle composition with high transfection efficiency and low cytotoxicity.
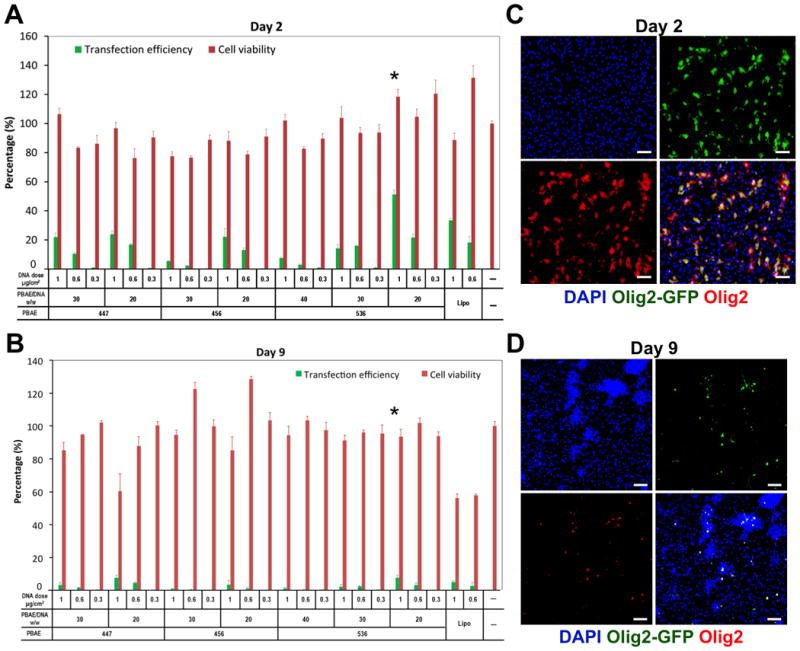
(A and B) Initial screening used Olig2-GFP plasmid DNA doses of 0.3, 0.6, and 1 μg/cm2 and an abbreviated range of PBAE/plasmid DNA ratios (20, 30, and 40 w/w) in order to identify top polymers among 447, 456, and 536 based on their transfection efficiencies and cytotoxicities on days (A) 2 and (B) 9. (C and D) Expression of transfected Olig2-GFP (green) and Olig2 (red) on days (C) 2 and (D) 9 of the tested PBAE 536 at the polymer/DNA ratio of 20 w/w and the DNA dose of 1 μg/cm2. Extensive colocalization of GFP and Olig2 were observed on days 2 and 9. DAPI was used to stain cell nuclei in blue. Scale bar = 100 μm.
Nanoparticle-Mediated Co-Delivery of Olig1 and Olig2 into hNSCs
Through the optimized transfection condition identified above, we successfully delivered Olig1, Olig2, and both Olig1 and Olig2 into hNSCs through nanoparticles containing plasmid encoding Olig1, plasmid encoding Olig2, and the mixture of these two plasmids (Olig1/2; 0.5 μg/cm2 Olig1 and 0.5 μg/cm2 Olig2-GFP), respectively (Fig. 2). Olig1 and Olig2 were transfected into hNSCs with an efficiency of 36.7 ± 6.8% and 33.3 ± 7.0%, respectively. Co-delivery of Olig1 and Olig2 was shown to be feasible through incorporation of these two plasmids into a single dose of PBAE nanoparticles. Olig1 and Olig2 were expressed in the Olig1/2-transfected hNSCs with the efficiencies of 31.5 ± 6.5% and 35.5 ± 7.0%, respectively. About 22.5 ± 5.0% cells simultaneously expressed Olig1 and Olig2 according to the co-localization of Olig1 and Olig2 in nuclei. Around 9.0 ± 3.0% cells only expressed Olig1 while 13.0 ± 5.0% cells only expressed Olig2.
Figure 2. Nanoparticle-mediated Olig1 and Olig2 expression on hNSCs.
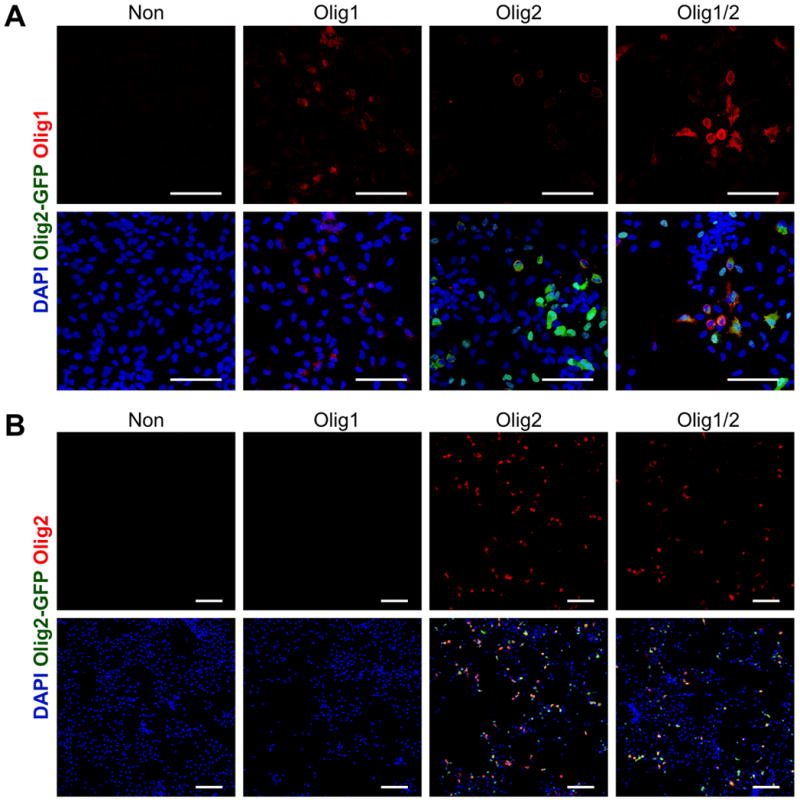
(A) Olig1 (red) expression in hNSCs after transfection with PBAE 536 nanoparticles containing plasmid encoding Olig1, plasmid encoding Olig2-GFP, or the mixture of these two plasmids (Olig1/2; 0.5 μg/cm2 Olig1 and 0.5 μg/cm2 Olig2-GFP, respectively) at the polymer/DNA ratio of 20 w/w and the DNA dose of 1 μg/cm2 on day 2. (B) Olig2 (red) expression in hNSCs after transfection with PBAE 536 nanoparticles containing plasmid encoding Olig1, plasmid encoding Olig2-GFP, or the mixture of these two plasmids on day 2. Non-transfected cells were used as a control. DAPI was used to stain cell nuclei in blue. Scale bar = 100 μm.
We investigated the effect of Olig1 and Olig2 expression on the long-term viability of hNSCs using AlamarBlue assay (Figure 3). On day 15, Olig1, Olig2, and Olig1/2- transfected cells showed viabilities of 102.8 ± 4.3%, 105.6 ± 4.8%, and 110.2 ± 5.6%, respectively, relative to non-transfected cells on day 2. There was no significant cell death following the transfection for 15 days. In addition, since the cells in all groups were cultured under an oligodendrocyte enrichment medium with a cocktail of growth factors including PDGF-AA, NT-3, and FGF-2, all of these cells did not show significant proliferation after day 5. These cells may prefer differentiation rather than proliferation due to the differentiation culture condition.
Figure 3. Long-term viability of hNSCs after nanoparticle-mediated transfection.
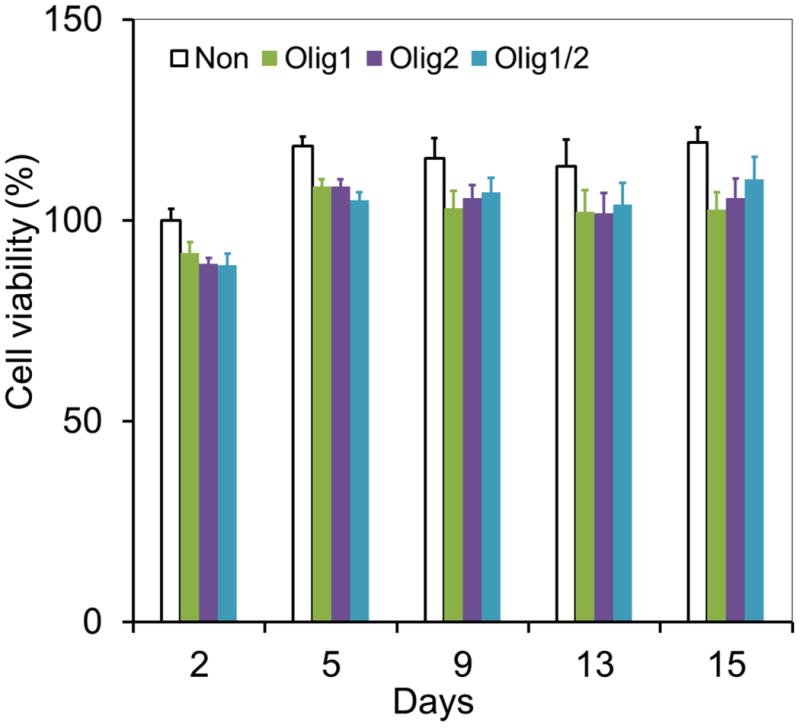
Viabilities of Olig1, Olig2, or Olig1/2-transfected hNSCs were inspected by AlamarBlue assay on days 2, 5, 9, 13, and 15, respectively (n = 4; *P < 0.05). Cells without transfection were used as a control.
Enhancing Oligodendrocyte Differentiation by Nanoparticle-mediated Olig1 and Olig2 Expression
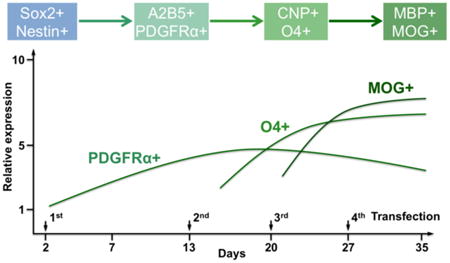
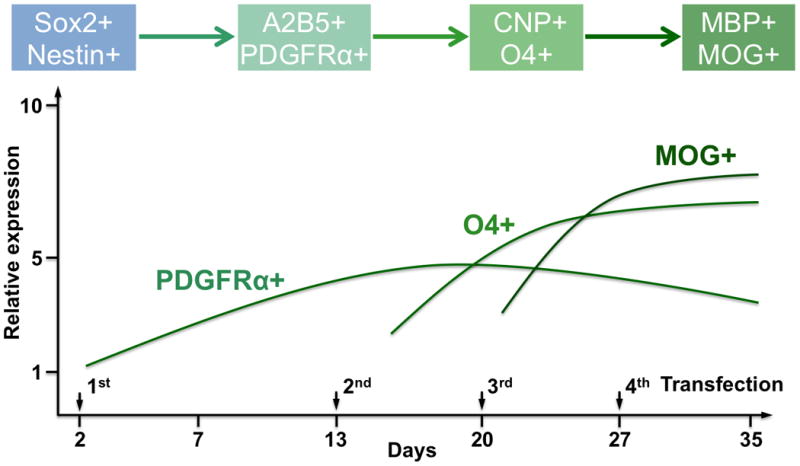
We have previously demonstrated that multiple-round transfections could enhance the expression of transcription factor, such as neurogenin 2 (Ngn2), in human fetal tissue-derived NSCs to improve their neuronal differentiation and maturation in vitro and at a brain lesion site after traumatic injury [21]. In this study, we followed the similar protocol and performed 4 rounds of transfection in hNSCs on Days 2, 13, 20, and 27, respectively, to extend the expressions of Olig1 and Olig2 (Figure 4A). We did not observe significant difference in term of transfection efficiency at different times following transfection.
Figure 4. Modulating oligodendrocyte differentiation from hNSCs through nanoparticle-mediated expression of Olig1 and Olig2.
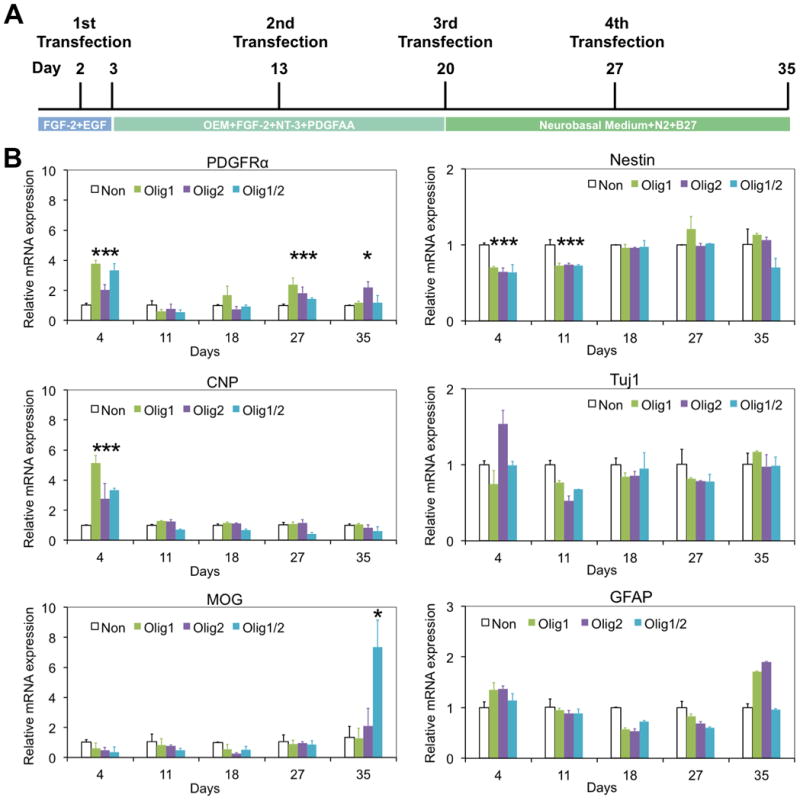
(A) Schematic of oligodendrocyte differentiation from hNSCs. Nanoparticle-mediated transfection was performed on days 2, 13, 20, and 27, respectively. (B) PDGFRα, CNP, MOG, nestin, Tuj1, and GFAP expression of Olig1, Olig2, and Olig1/2-transfected hNSCs on days 4, 11, 18, 27, and 35, respectively, investigated by quantitative real-time PCR assay. Cells without transfection were used as a control (n = 3; *P < 0.05).
First we examined the differential gene expression of hNSCs following transfection with Olig1, Olig2, or Olig1/2 through quantitative real-time PCR. Non-transfected cells were used as a control. As shown in Figure 4B, a specific gene for early oligodendrocyte progenitor cells (OPCs), platelet-derived growth factor receptor alpha (PDGFRα), was upregulated following Olig1, Olig2, and Olig1/2 transfection in hNSCs on day 4. Higher levels of PDGFRα were also observed in three transfected groups on day 27. One specific gene for late OPCs, 2, 3-cyclic nucleotide 3 phosphodiesterase (CNP), was also upregulated following Olig1, Olig2, and Olig1/2 transfection in hNSCs on day 4. While one of specific genes for myelinating oligodendrocyte, myelin oligodendrocyte glycoprotein (MOG) was enriched only in the Olig1/2-transfected cells on day 35. In addition, lower levels of nestin gene expression were observed in three transfected groups compared to the control group on both days 4 and 11. In term of neurogenic gene, Tuj1, and astrocytic gene, glial fibrillary acidic protein (GFAP), their expressions did not show clear trends among all four groups.
We then characterized cell phenotypes through immunostaining on both days 20 and 35. As shown in Figure 5, no significant differences were detected among all the groups in term of Tuj1+ neurons and GFAP+ astrocytes on day 20. In consideration of the oligodendrocyte lineage, approximately 30% cells in three transfected groups were bipolar PDGFRα+ OPCs, which were significantly more than those in the control group (Olig1/2: 31.7±7.5%, Olig1: 23.4±4.7%, Olig2: 29.7±6.0%, and Non: 9.2±1.4%; n=6; P<0.05). There were no significant differences in PDGFRα+ OPCs observed among Olig1, Olig2, and Olig1/2 transfected groups. Whereas the Olig1/2-expressing hNSCs produced the largest number of O4+ pre-myelinating oligodendrocytes with branched processes among all the groups on day 20 (Olig1/2: 23.8±4.8%, Non: 4.8±1.1%, Olig1: 8.9±1.8%, and Olig2: 15.9±3.2%; n=6; P<0.05). These results imply that nanoparticle-mediated Olig expression may expedite hNSC differentiation into OPCs and the combination of Olig1 and Olig2 may enhance oligodendrocyte differentiation more effectively than Olig1 or Olig2 only.
Figure 5. Enhancing oligodendrocyte differentiation by nanoparticle-mediated expression of Olig1 and Olig2.
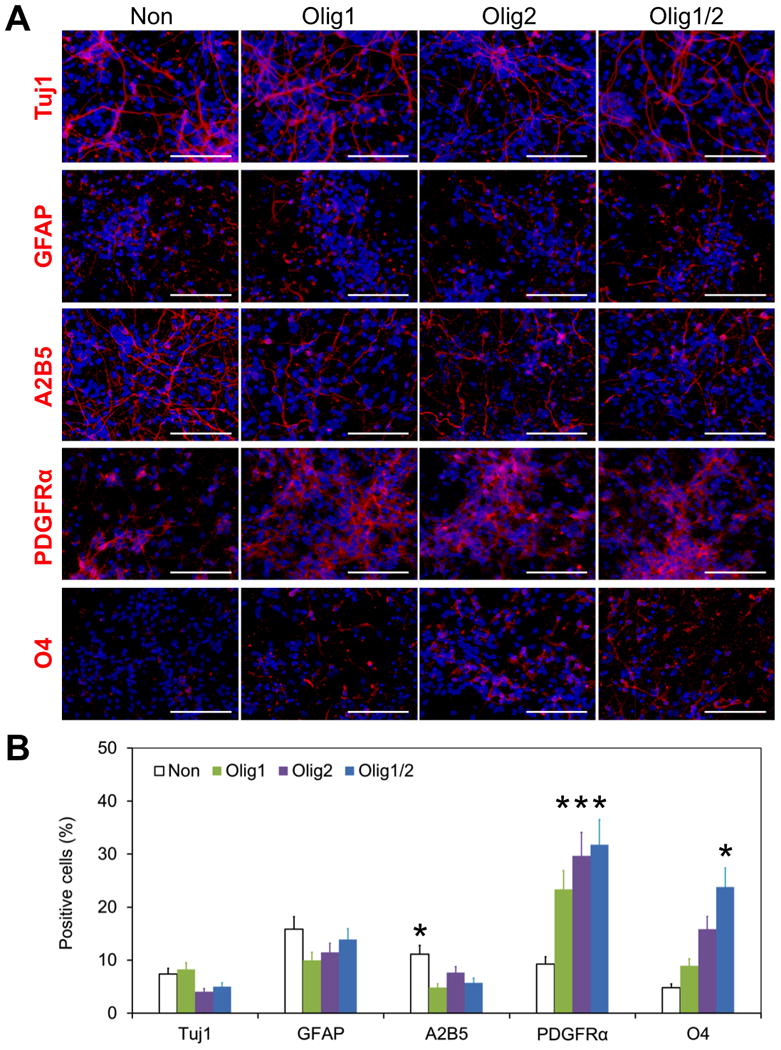
(A) Differentiation of Olig1, Olig2, and Olig1/2-transfected hNSCs on day 20. Cells were stained with Tuj1 (red), GFAP (red), A2B5 (red), PDGFRα (red), and O4 (red), respectively. Cells without transfection were used as a control. DAPI was used to stain cell nuclei in blue. Scale bar = 100 μm. (B) Quantitative analysis of the differentiation of hNSCs on day 20 (*P < 0.05). The positive cells for each marker were counted. At least 6 random fields per sample were taken for quantitative analysis.
After culture in a growth factor-free differentiation medium for additional two weeks, O4+ pre-myelinating oligodendrocytes displayed a significant increase by 3.2-fold, 3.2-fold, and 1.8-fold in Olig1, Olig2, and Olig1/2 transfected groups, respectively (Figure 6, n = 6; P < 0.05). Approximately 44% O4+ oligodendrocytes appeared in the Olig1/2-transfected group on day 35. In contrast, in the control group, only less than 5% O4+ cells appeared on day 35, which was similar to those on day 20 (4.8 ± 1.0% vs. 4.9 ± 1.0%; n = 6). There were about 50% PDGFRα+ OPCs in the control group with 5-fold increase from day 20 to day 35 (52.2 ± 10.4% vs. to 9.2 ± 1.4%; n = 6; P < 0.05). There was no significant difference among all the groups in term of PDGFRα+ cells on day 35. These results confirm that nanoparticle-mediated Olig1 and Olig2 expression can expedite the differentiation of hNSCs into O4+ oligodendrocytes.
Figure 6. Expediting oligodendrocyte differentiation by nanoparticle-mediated expression of Olig1 and Olig2.
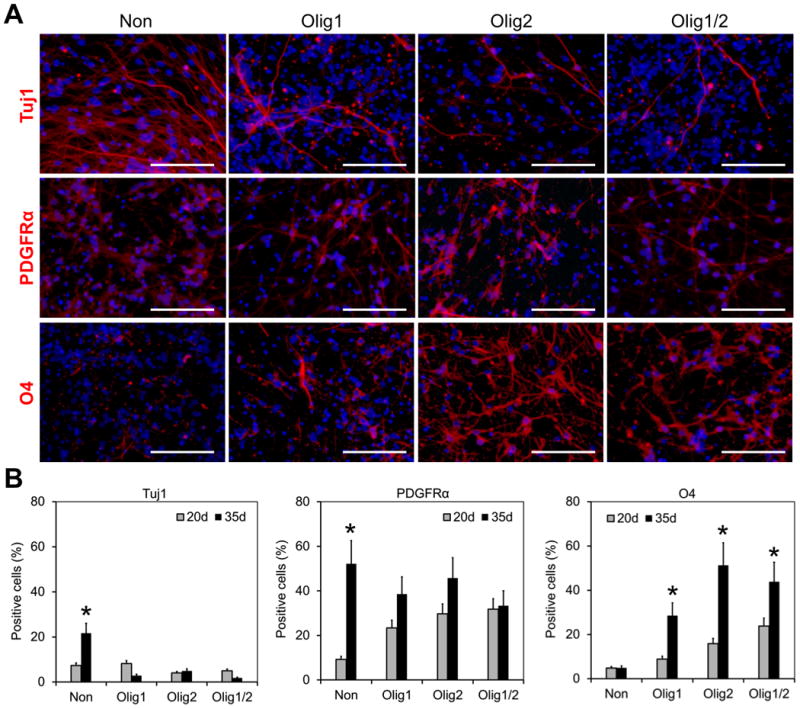
(A) Differentiation of Olig1, Olig2, and Olig1/2-transfected hNSCs on day 35. Cells were stained with Tuj1 (red), PDGFRα (red), and O4 (red), respectively. Cells without transfection were used as a control. DAPI was used to stain cell nuclei in blue. Scale bar = 100 μm. (B) Comparison of differentiation capacities of hNSCs on days 20 and 35 (*P < 0.05). The positive cells for each marker were counted. At least 6 random fields per sample were captured for quantitative analysis.
Additionally, about 25% cells in the control group are Tuj1+ neurons, which were significantly more than those in three transfected groups on day 35 (Non: 24.7 ± 4.9%, Olig1: 2.8 ± 1.0%, Olig2: 4.9 ± 2.0%, and Olig1/2: 1.8 ± 1.0%; n = 6; P < 0.05). Western blot analysis also confirmed the highest level of Tuj1 in the control group (Figure 7; n = 3; P < 0.05). These results suggest overexpression of Olig1 and Olig2 in hNSCs may inhibit neuronal differentiation of these transfected cells.
Figure 7. Western blot analysis of differentiation of transfected hNSCs on day 35.
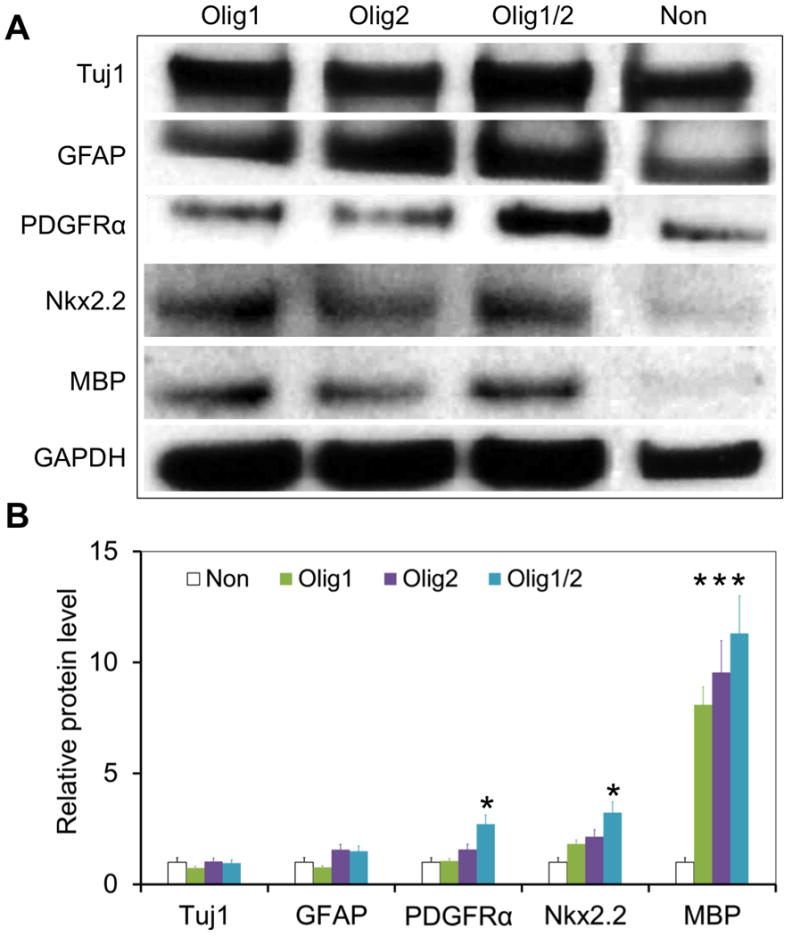
Cells without transfection were used as a control. Olig1/2-transfected hNSCs expressed the highest level of PDGFRα and Nkx2.2 among all the groups on day 35 (n = 3; *P < 0.05).
Higher levels of myelin basic protein (MBP) were observed in these three transfected groups compared to the control group on day 35 (Figure 7; n = 3; P < 0.05). Specifically, Olig1/2-transfected cells expressed the highest level of both PDGFRα and Nkx2.2 among all the groups (n = 3; P < 0.05), which further validates that the combination of Olig1 and Olig2 can more efficiently promote oligodendrocyte differentiation than a single factor.
Schematic summary of our approach on enhancing oligodendrocyte differentiation has shown in Figure 8. Using human fetal tissue-derived NSCs as a model, we have demonstrated that this non-viral, nanoparticle-mediated transcription activation approach is more effective in generating O4+ oligodendrocytes than a standard differentiation medium (44% vs. 5% in a 35-day differentiation culture). In addition, Franklin and colleagues have recently reported a high yield of oligodendrocytes and an acceleration of the overall differentiation program from hESCs at low and physiological (3%) oxygen levels, a mimic environment of the developing forebrain [28]. Culture our Olig1/2-transfected cells in this hypoxia condition may potentially further enhance the yield of O4+ cells. Furthermore, PDGFRα+ OPCs, isolated by fluorescence-activated cell sorting (FACS) using PDGFRα as a marker, have shown robust remyelination following their transplantation in the hypomyelinated shiverer mouse brain [29, 30]. In contrast, O4+ OPCs, which could be purified by FACS using O4 as a marker, not only have the comparable remyelination potential, but eliminate contaminant cells and minimize the tumorigenic potential following their transplantation [31]. FACS-purified O4+ cells also hold great potential for in vitro myelination assays and screen for myelinating compounds [32-34], thus making our approach a more clinically relevant option.
Figure 8. Schematic summary of enhancing oligodendrocyte differentiation by nanoparticle-mediated Olig expression.
Given that previous study showed efficient remyelination of transplanted OPCs in a shiverer mice [29], this model could be used for assessing the myelinating ability of nanoparticle-mediated Olig1/2-transfected hNSCs following transplantation. Our nanoparticle transfection approach may also be applied to deliver Olig genes to engineer endogenous glial cells at the lesion site for the treatment of brain injuries and diseases.
Beyond neural repair in brain tissue, Olig overexpression holds promise in improving spinal cord repair as well. Kim et al. have recently shown that injection of retroviruses encoding Olig1 and Olig2 in combination into the lesion site after contusive spinal cord injury increased the number of endogenous glial progenitor cells without tumor formation and enhanced specification and maturation of oligodendrocytes, leading to improved locomotor recovery. In contrast, Olig1 alone exhibited only modest effects and Olig2 induced the glioma formation in the injured spinal cord [35]. Compared to this retroviruses transduction method, our PBAE-DNA nanoparticle-mediated transfection may offer a safer alternative for in situ transcription modification to promote neural repair.
Conclusion
We demonstrated the effectiveness of PBAE-DNA nanoparticle-mediated overexpression of Olig1 and Olig2 in enhancing oligodendrocyte differentiation from hNSCs. Our approach can generate around 44% O4+ cells by Olig1/2-cotransfected cells in 35 days. This method provides a translatable approach to modulate oligodendrocyte differentiation and maturation, and has potential to improve the therapeutic outcomes of stem cell-based therapy.
Materials and Methods
Materials
Homo sapiens oligodendrocyte transcription factor 2 (Olig2, RG208209) and oligodendrocyte transcription factor 1 (Olig1, SC123482) were purchased from OriGene Technologies (Rockville, MD). Recombinant human fibroblast growth factor-2 (FGF-2), epidermal growth factor (EGF), platelet derived growth factor-AA (PDGF-AA), and neurotrophin 3 (NT-3) were obtained from Millipore (Billerica, MA). Poly (β-amino ester)s (PBAEs) were synthesized as we reported before (Figure S1)[21, 22].
Cell Culture
Human neural stem/progenitor cells (hNSCs), immortalized cells derived from ventral mesencephalon region of fetal brain tissue, were purchased from Millipore (Billerica, MA). Human NSCs were cultured in ReNcell Neural Stem Cell Medium (Millipore, Billerica, MA) with FGF-2 (20 ng/mL) and EGF (20 ng/mL) and used before Passage 5 in this study. In consideration of oligodendrocyte differentiation, hNSCs were cultured in Oligodendrocyte Enrichment Medium [OEM: DMEM/F12 with non-essential amino acid (1×), L-glutamine (2 mM), N21 medium supplement (1×), and a cocktail of growth factors (PDGF-AA, NT-3, and FGF-2; each at 20 ng/mL)] for 17 days. Then cells were continuously cultured in the ReNcell Neural Stem Cell Medium for 14 days.
Cell Transfection
PBAE structures (447, 456, and 536), polymer/plasmid DNA ratios (20, 30, and 40 w/w), and DNA doses (0.3, 0.6, and 1 μg/cm2) were screened to identify an optimized nanoparticle composition with high transfection efficiency and low cytotoxicity through GFP-tagged Olig2 plasmid DNA. The transfection protocol was followed as we previously reported [21, 22]. Olig2-GFP expression efficiencies were measured at days 2 and 9 after transfection, respectively, by a flow cytometry (Accuri C6, BD Biosciences, San Jose, CA) with Hypercyt high-throughput robotic sampler (Intellicyt, Albuquerque, NM). Cytotoxicities of nanoparticles were investigated by WST-1 assay at days 1 and 9 following cell transfection, respectively.
In addition, AlamarBlue assay (Invitrogen, Carlsbad, CA) was performed to examine transfected cell viability in a long-term culture [22]. In order to improve transgene expression, the optimized nanoparticle condition was selected for multiple transfections. The second, third, and forth transfections with the same protocol were conducted at days 13, 20, and 27, respectively.
Cell Differentiation
RNA Isolation and Quantitative Real-Time PCR
The differentiation of hNSCs after transfection with nanoparticles containing plasmid encoding Olig1, plasmid encoding Olig2, or the mixture of these two plasmids (Olig1/2) was examined by real-time PCR. RNA was isolated by the RNeasy Mini Kit (QIAGEN, Frederick, MD). Quantitative RNA was converted to single-stranded cDNA by a High Capacity cDNA Reverse Transcription Kit (Life Techonology, Carlsbad, CA). Primers used for real-time PCR were shown in Table S1.
Immunocytochemistry
The cells were fixed with 4% (w/w) paraformaldehyde at days 20 and 35, respectively. Cells were stained with primary antibodies, including Olig1, Olig2, Tuj1, GFAP, A2B5, PDGFRα, and O4 (Table S2) and then Cy3-conjugated affinity-purified secondary antibodies (Jackson ImmunoResearch, West Grove, PA). Cell nuclei were stained with 4′, 6-diamidino-2-phenylindole, dihydrochloride (DAPI, Molecular Probes, Eugene, OR). Then cell images were captured by Zeiss LSM 510 Meta Confocal Microscope (Thornwood, NY). At least 6 random fields per sample were taken for quantitative analysis. The percentage of positive cells in the population was calculated and compared among groups.
Western Blot
Western blot was performed as we previously reported [21]. Protein extracts were quantitated by BCA assay and blotted with antibodies including Tuj1, GFAP, PDGFRα, Nkx2.2, MBP, and GAPDH.
Statistical Analysis
Data were shown as mean ± S.D. (standard deviation). Data were analyzed by Student's t-test or one-way ANOVA (analysis of variance) followed by Tukey's post hoc test as needed. The values were considered significantly different at P < 0.05.
Supplementary Material
Figure S1. Monomers and reaction scheme used to synthesize PBAE library. One backbone monomer (B) was polymerized with one side chain monomer (S). The diacrylate B-S base polymer was then terminated with one end-capping monomer (E).
Figure S2. Transfection efficiencies of nanoparticle-mediated transfection on Day 2 (A) and Day 9 (B) examined by flow cytometry.
Table S1. Primers used in this study.
Table S2. Primary antibodies used in this study.
Statement of Significance.
Current approaches to derive oligodendrocytes from human pluripotent stem cells (hPSCs) need extended exposure of hPSCs to growth factors and small molecules, which limits their clinical application because of the lengthy culture time required and low generation efficiency of myelinating oligodendrocytes. We described a new approach to enhance oligodendrocyte differentiation through nanoparticle-mediated transcription modulation. We tested an effective transfection method using cell-compatible poly (β-amino ester) (PBAE)/DNA nanoparticles as gene carrier to deliver transcription factor Olig1 and Olig2 into human fetal tissue-derived neural stem/progenitor cells, and showed efficient oligodendrocyte differentiation following transgene expression of Olig1 and Olig2. We believe that this translatable approach can be applied to many other cell-based regenerative therapies as well.
Acknowledgments
This work was supported by the U.S. National Institute of Neurological Disorders and Stroke (R21NS085714 to H.-Q.M.), and National Institute of Biomedical Imaging and Bioengineering (5R01EB016721 to G.J.J.). X.L. acknowledges a postdoctoral fellowship from the Maryland Stem Cell Research Fund (2013MSCRF-00042169). We really appreciate Dr. Dacheng Ding at the Johns Hopkins Translational Tissue Engineering Center for assistance on the PCR experiment.
Footnotes
Author Contributions: X.L. contributed to the design, execution, and analysis of the described experiments. S.T. contributed to the polymer synthesis and nanoparticle screening. C.G.Z. contributed to the transfection screening studies. J.G. contributed to the discussion and analysis of transfection studies. V.K. and G.M. contributed to the discussion of the experimental design and interpretation of results. H.-Q.M. conceived the project and contributed to study design and result analysis. X.L. and H.-Q.M. prepared the manuscript with inputs from all authors.
Competing Financial Interests: The authors affirm no competing financial interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldskogius H, Berens C, Kanaykina N, Liakhovitskaia A, Medvinsky A, Sandelin M, Schreiner S, Wegner M, Hjerling-Leffler J, Kozlova EN. Regulation of boundary cap neural crest stem cell differentiation after transplantation. Stem Cells. 2009;27(7):1592–603. doi: 10.1002/stem.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xu L, Ryu J, Hiel H, Menon A, Aggarwal A, Rha E, Mahairaki V, Cummings BJ, Koliatsos VE. Transplantation of human oligodendrocyte progenitor cells in an animal model of diffuse traumatic axonal injury: survival and differentiation. Stem Cell Research & Therapy. 2015;6:93. doi: 10.1186/s13287-015-0087-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goldman SA, Kuypers NJ. How to make an oligodendrocyte. Development (Cambridge England) 2015;142(23):3983–95. doi: 10.1242/dev.126409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang S, Bates J, Li X, Schanz S, Chandler-Militello D, Levine C, Maherali N, Studer L, Hochedlinger K, Windrem M, Goldman SA. Human iPSC-derived oligodendrocyte progenitor cells can myelinate and rescue a mouse model of congenital hypomyelination. Cell Stem Cell. 2013;12(2):252–64. doi: 10.1016/j.stem.2012.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douvaras P, Fossati V. Generation and isolation of oligodendrocyte progenitor cells from human pluripotent stem cells. Nature Protocols. 2015;10(8):1143–54. doi: 10.1038/nprot.2015.075. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Jiang P, Deng W. OLIG gene targeting in human pluripotent stem cells for motor neuron and oligodendrocyte differentiation. Nature Protocols. 2011;6(5):640–55. doi: 10.1038/nprot.2011.310. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Pol SU, Haberman AK, Wang C, O'Bara MA, Sim FJ. Transcription factor induction of human oligodendrocyte progenitor fate and differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(28):E2885–94. doi: 10.1073/pnas.1408295111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Emery B, Lu QR. Transcriptional and epigenetic regulation of oligodendrocyte development and myelination in the central nervous system. Cold Spring Harbor Perspectives in Biology. 2015;7(9):a020461. doi: 10.1101/cshperspect.a020461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meijer DH, Kane MF, Mehta S, Liu H, Harrington E, Taylor CM, Stiles CD, Rowitch DH. Separated at birth? The functional and molecular divergence of OLIG1 and OLIG2. Nature Reviews Neuroscience. 2012;13(12):819–31. doi: 10.1038/nrn3386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dai J, Bercury KK, Ahrendsen JT, Macklin WB. Olig1 function is required for oligodendrocyte differentiation in the mouse brain. Journal of Neuroscience. 2015;35(10):4386–402. doi: 10.1523/JNEUROSCI.4962-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hwang DH, Kim BG, Kim EJ, Lee SI, Joo IS, Suh-Kim H, Sohn S, Kim SU. Transplantation of human neural stem cells transduced with Olig2 transcription factor improves locomotor recovery and enhances myelination in the white matter of rat spinal cord following contusive injury. BMC Neuroscience. 2009;10:117. doi: 10.1186/1471-2202-10-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Copray S, Balasubramaniyan V, Levenga J, de Bruijn J, Liem R, Boddeke E. Olig2 overexpression induces the in vitro differentiation of neural stem cells into mature oligodendrocytes. Stem Cells. 2006;24(4):1001–10. doi: 10.1634/stemcells.2005-0239. [DOI] [PubMed] [Google Scholar]
- 13.Braun SM, Pilz GA, Machado RA, Moss J, Becher B, Toni N, Jessberger S. Programming hippocampal neural stem/progenitor cells into oligodendrocytes enhances remyelination in the adult brain after injury. Cell Reports. 2015;11(11):1679–85. doi: 10.1016/j.celrep.2015.05.024. [DOI] [PubMed] [Google Scholar]
- 14.Yin H, Kanasty RL, Eltoukhy AA, Vegas AJ, Dorkin JR, Anderson DG. Non-viral vectors for gene-based therapy. Nature Reviews Genetics. 2014;15(8):541–55. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 15.Dai J, Bercury KK, Jin W, Macklin WB. Olig1 acetylation and nuclear export mediate oligodendrocyte development. Journal of Neuroscience. 2015;35(48):15875–93. doi: 10.1523/JNEUROSCI.0882-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mei F, Wang H, Liu S, Niu J, Wang L, He Y, Etxeberria A, Chan JR, Xiao L. Stage-specific deletion of Olig2 conveys opposing functions on differentiation and maturation of oligodendrocytes. Journal of Neuroscience. 2013;33(19):8454–62. doi: 10.1523/JNEUROSCI.2453-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzeng SY, Guerrero-Cázares H, Martinez EE, Sunshine JC, Quiñones-Hinojosa A, Green JJ. Non-viral gene delivery nanoparticles based on Poly(beta-amino esters) for treatment of glioblastoma. Biomaterials. 2011;32(23):5402–10. doi: 10.1016/j.biomaterials.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sunshine JC, Bishop CJ, Green JJ. Advances in polymeric and inorganic vectors for nonviral nucleic acid delivery. Therapeutic Delivery. 2011;2(4):493–521. doi: 10.4155/tde.11.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pickard MR, Barraud P, Chari DM. The transfection of multipotent neural precursor/stem cell transplant populations with magnetic nanoparticles. Biomaterials. 2011;32(9):2274–84. doi: 10.1016/j.biomaterials.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 20.Tseng TC, Hsieh FY, Dai NT, Hsu SH. Substrate-mediated reprogramming of human fibroblasts into neural crest stem-like cells and their applications in neural repair. Biomaterials. 2016;102:148–61. doi: 10.1016/j.biomaterials.2016.06.020. [DOI] [PubMed] [Google Scholar]
- 21.Li X, Tzeng SY, Liu X, Tammia M, Cheng YH, Rolfe A, Sun D, Zhang N, Green JJ, Wen X, Mao HQ. Nanoparticle-mediated transcriptional modification enhances neuronal differentiation of human neural stem cells following transplantation in rat brain. Biomaterials. 2016;84:157–66. doi: 10.1016/j.biomaterials.2016.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li X, Kozielski K, Cheng YH, Liu H, Zamboni CG, Green J, Mao HQ. Nanoparticle-mediated conversion of primary human astrocytes into neurons and oligodendrocytes. Biomaterials Science. 2016:1100–12. doi: 10.1039/c6bm00140h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tzeng SY, Green JJ. Subtle changes to polymer structure and degradation mechanism enable highly effective nanoparticles for siRNA and DNA delivery to human brain cancer. Advanced Healthcare Materials. 2013;2(3):468–80. doi: 10.1002/adhm.201200257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lynn DM, Anderson DG, Putnam D, Langer R. Accelerated discovery of synthetic transfection vectors: parallel synthesis and screening of a degradable polymer library. Journal of the American Chemical Society. 2001;123(33):8155–6. doi: 10.1021/ja016288p. [DOI] [PubMed] [Google Scholar]
- 25.Tzeng SY, Hung BP, Grayson WL, Green JJ. Cystamine-terminated poly(beta-amino ester)s for siRNA delivery to human mesenchymal stem cells and enhancement of osteogenic differentiation. Biomaterials. 2012;33(32):8142–51. doi: 10.1016/j.biomaterials.2012.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mangraviti A, Tzeng SY, Gullotti D, Kozielski KL, Kim JE, Seng M, Abbadi S, Schiapparelli P, Sarabia-Estrada R, Vescovi A, Brem H, Olivi A, Tyler B, Green JJ, Quinones-Hinojosa A. Non-virally engineered human adipose mesenchymal stem cells produce BMP4, target brain tumors, and extend survival. Biomaterials. 2016;100:53–66. doi: 10.1016/j.biomaterials.2016.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sunshine J, Green JJ, Mahon KP, Yang F, Eltoukhy AA, Nguyen DN, Langer R, Anderson DG. Small-molecule end-groups of linear polymer determine cell-type gene-delivery efficacy. Advanced Materials. 2009;21(48):4947–51. doi: 10.1002/adma.200901718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stacpoole Sybil RL, Spitzer S, Bilican B, Compston A, Karadottir R, Chandran S, Franklin Robin JM. High yields of oligodendrocyte lineage cells from human embryonic stem cells at physiological oxygen tensions for evaluation of translational biology. Stem Cell Reports. 2013;1(5):437–50. doi: 10.1016/j.stemcr.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sim FJ, McClain CR, Schanz SJ, Protack TL, Windrem MS, Goldman SA. CD140a identifies a population of highly myelinogenic, migration-competent and efficiently engrafting human oligodendrocyte progenitor cells. Nature Biotechnology. 2011;29(10):934–41. doi: 10.1038/nbt.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Douvaras P, Wang J, Zimmer M, Hanchuk S, O'Bara MA, Sadiq S, Sim FJ, Goldman J, Fossati V. Efficient generation of myelinating oligodendrocytes from primary progressive multiple sclerosis patients by induced pluripotent stem cells. Stem Cell Reports. 2014;3(2):250–9. doi: 10.1016/j.stemcr.2014.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roy NS, Cleren C, Singh SK, Yang L, Beal MF, Goldman SA. Functional engraftment of human ES cell-derived dopaminergic neurons enriched by coculture with telomerase-immortalized midbrain astrocytes. Nature Medcine. 2006;12(11):1259–68. doi: 10.1038/nm1495. [DOI] [PubMed] [Google Scholar]
- 32.Lee S, Leach MK, Redmond SA, Chong SYC, Mellon SH, Tuck SJ, Feng ZQ, Corey JM, Chan JR. A culture system to study oligodendrocyte myelination processes using engineered nanofibers. Nature Medicine. 2012;9(9):917–22. doi: 10.1038/nmeth.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Najm FJ, Madhavan M, Zaremba A, Shick E, Karl RT, Factor DC, Miller TE, Nevin ZS, Kantor C, Sargent A, Quick KL, Schlatzer DM, Tang H, Papoian R, Brimacombe KR, Shen M, Boxer MB, Jadhav A, Robinson AP, Podojil JR, Miller SD, Miller RH, Tesar PJ. Drug-based modulation of endogenous stem cells promotes functional remyelination in vivo. Nature. 2015;522(7555):216–20. doi: 10.1038/nature14335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei F, Fancy SPJ, Shen YAA, Niu J, Zhao C, Presley B, Miao E, Lee S, Mayoral SR, Redmond SA, Etxeberria A, Xiao L, Franklin RJM, Green A, Hauser SL, Chan JR. Micropillar arrays as a high-throughput screening platform for therapeutics in multiple sclerosis. Nature Medicine. 2014;20(8):954–60. doi: 10.1038/nm.3618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kim HM, Hwang DH, Choi JY, Park CH, Suh-Kim H, Kim SU, Kim BG. Differential and cooperative actions of Olig1 and Olig2 transcription factors on immature proliferating cells after contusive spinal cord injury. Glia. 2011;59(7):1094–106. doi: 10.1002/glia.21182. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Monomers and reaction scheme used to synthesize PBAE library. One backbone monomer (B) was polymerized with one side chain monomer (S). The diacrylate B-S base polymer was then terminated with one end-capping monomer (E).
Figure S2. Transfection efficiencies of nanoparticle-mediated transfection on Day 2 (A) and Day 9 (B) examined by flow cytometry.
Table S1. Primers used in this study.
Table S2. Primary antibodies used in this study.


