Abstract
A growing number of older adults are undergoing liver transplantation (LT) in the US. In some settings, it is thought that adherence declines with age. This retrospective study examined adherence and clinical outcomes in older versus younger adult LT recipients. Medical records of adult LT recipients from 2009–2012 from a single urban center were reviewed. The medication level variability index (MLVI) was the pre-defined primary outcome, with nonadherence defined as MLVI >2.5. The secondary outcome was incidence of rejection. Outcomes were evaluated starting one year post-LT until 2015. 42/248 patients were ≥65 at transplant. Older adults had significantly better adherence than younger ones (65% ≥65 were adherent vs. 42% younger adults; Chi-Square two-tailed p=0.02). Survival analyses of rejection between age groups censored by time since transplant showed no difference among the four age groups (χ2 = 0.84, p=0.84). Older age was not found to be a risk factor for reduced adherence or graft rejection in patients surviving at least one year post-LT.
Keywords: medication adherence, biomarkers, graft rejection
INTRODUCTION
Liver transplantation (LT) in older adults (≥65 years old) in the United States is increasingly being performed, and this trend is expected to continue.1–3 A growing older population, expanded selection criteria for organ recipients, and advances in treatment for common causes of liver disease are all contributing factors.1,4 Increasing general life expectancy rates predispose more patients to ultimately develop decompensated liver disease or hepatocellular carcinoma, which are the major indications for LT. Because older adults are considered to have unique physiological and psychosocial characteristics, it becomes increasingly important to investigate outcomes and risk factors in this particular group of patients.5,6
Many factors, such as premorbid medical conditions, influence post-LT outcomes in adults.1,7 The degree of adherence to medical recommendations is amongst the most consistently reported determinants of posttransplant outcomes. Although not all studies demonstrate evidence of lower rates of adherence in older adults,8–10 many suggest that nonadherence rates increase with depression; lack of daily structure; social isolation; visual or auditory impairment; decreased physical health, cognitive function and memory; longer time since diagnosis; and increased number of medications, side effects, and doses per day.8,11–14 All of these are more common in older adults, and many are also present after liver transplant. Indeed, nonadherence to immunosuppressants occurs in 15–40% of adults 6 months to more than 5 years post-LT and one study of older adult (55+) kidney transplant recipients reported nonadherence in as many as 86% of patients 1 year after transplant.15–17
Older adults tend to present a higher surgical risk for transplantation due to medical co-morbidities. Therefore, they are generally considered to require more rigorous medical eligibility screening.1,2,7 While it is known that the higher surgical risk may lead to greater morbidity in the immediate post-operative period, it is not clear whether older patients are more or less adherent in the long term posttransplant period as compared with their younger peers. The answer to this question might be helpful to transplant centers contemplating decision-making parameters predicated upon recipient age, and may also prioritize resource allocation for psychosocial support for these recipients.
To examine the relative risk for nonadherence and poor outcomes in younger versus older adult recipients who are long-term survivors of LT, we conducted a retrospective chart review of adult LT recipients. Nonadherence to tacrolimus (TAC) immunosuppressive therapy was determined via a validated biomarker of serum drug level fluctuation, the medication level variability index (MLVI). We investigated whether older adults demonstrated differences in TAC adherence compared to younger adult LT recipients (pre-defined primary outcome measure) a year or more posttransplant.
METHODS
Patients and Procedures
All available medical records of adult liver transplant recipients from the Recanati Miller Transplantation Institute (RMTI) and Mount Sinai Medical Center between 2009 and 2013 were identified (n=423). The final sample consisted of 248 patients based on the following inclusion criteria: underwent LT between 2009–2013, were prescribed TAC for maintenance immunosuppression between 2009–2015, were at least 1 year post-LT, and had at least 3 outpatient post-LT TAC levels. A CONSORT-like diagram demonstrating the selection process is presented in Figure 1. A waiver of informed consent was approved by the Institutional Review Board at the Icahn School of Medicine at Mount Sinai.
Figure 1. Inclusion/Exclusion Criteria for the Study Patients.
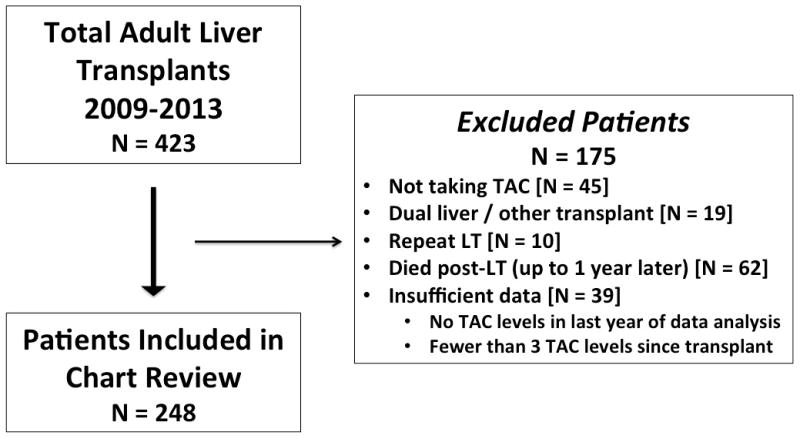
423 patient charts eligible for review. 175 excluded based on exclusion criteria. Complete chart reviews were not performed for excluded patients, but it is known that these patients did not differ significantly from those included in the study with respect to sex (χ2 = 0.57, two-tailed p value = 0.45) or age at time of transplant (t(422) = 1.49, p = 0.13). 248 patients included in final study population. N= number of patients; TAC = Tacrolimus; LT = liver transplant
Data Collection
Psychosocial and demographic variables were collected from two electronic medical record (EMR) systems: OTTR Solid Organ Transplant System (for patients undergoing LT prior to 2011) and EpicCare (for patients undergoing transplant in 2011 or later). Similar information was available from each EMR, and records from OTTR were linked to EpicCare records when the institution transitioned its EMR system.
The standard practice in our center is for all recipients to undergo an evaluation with a licensed clinical social worker prior to LT listing. For patients whose records were found in OTTR, social work evaluations were documented in a standard electronic template and included information on race, marital status (single, married, divorced/separated, widowed), employment status (working, not working, retired), type of insurance coverage (Medicare, Medicaid, private), presence of social support, history of psychiatric illness, history of substance abuse, and prior legal convictions. A similar, but not identical, template was used by the EpicCare platform. While it may have differed in presentation and placement in the more recent EMR format, the clinical content of the actual evaluation did not differ during the study period.
The review of medical records included sex, age at the time of transplant, indication for liver transplant, and primary reason for LT, and TAC levels drawn regularly for dose adjustment as a part of standard of care post-LT management.
Outcome Measures
The primary outcome was nonadherence in the period beginning one year after LT as measured by MLVI. To compute the MLVI, we followed the standard procedure described elsewhere 17: we calculated a standard deviation of each patient’s TAC levels measured over time as part of standard of care follow-up. A minimum of three levels from different time points was used to calculate the MLVI (all available levels were used if >3 were available). The MLVI has been independently validated as a predictor of poor post-LT outcomes in adult and pediatric LT recipients with levels ≥2.5 considered nonadherent and associated with allograft rejection.18,19 Outpatient serum TAC levels drawn at least one year post-LT were used to calculate MLVI scores. MLVI scores >2.5 have been shown to predict graft rejection in adult and pediatric LT recipients.18–20 Only outpatient values were used to calculate MLVI, as hospitalized patients are not responsible for their own medication management, and serum TAC levels would not, therefore, reflect individual adherence. A secondary outcome was biopsy-proven graft rejection.
Statistical Analyses
All analyses were conducted using IBM SPSS Statistics package, 20th edition. Descriptive statistics were used to characterize the sample. Predefined age groups followed UNOS categories: 18–34, 35–49, 50–64, ≥65 years old. Preliminary analyses were conducted to examine baseline difference on demographic variables between the age groups. The primary analysis looked at the MLVI as a dichotomy, comparing age groups and categorical outcomes (MLVI threshold and occurrence of rejection). To look at the relationship between time to rejection and age, we used a Kaplan-Meier survival analysis, although it may be somewhat limited by a small “n” in subgroups, especially upon long-term follow-up. In this approach, differences in follow-up are addressed by presenting “censored” or extrapolated findings. Analysis of variance using ANOVA was utilized to compare age groups and time of follow-up. A “p” value of less than 0.5 was chosen as the level of significance.
RESULTS
The mean age at transplant for the study cohort was 56.1 years (SD: 10.54, Range: 19–74). The groups differed in highest level of education attained, employment status, type of insurance, and sex (Table 1). Therefore, we examined whether these variables were associated with our primary outcome, MLVI. There were no significant Chi-Square associations between MLVI threshold and education, employment status, insurance, or sex. One-way ANOVAs revealed that MLVI measured continuously was not associated with any of these variables either. No significant differences existed in the baseline psychosocial variables amongst the four age groups. The absolute range of time since transplant was 1.34 – 5.78 years. The average time since transplant in all subjects was 3.61 years (3.63 years in younger adults and 3.49 years in older adults, t(247) = 1.05, p = 0.29). The mean number of tacrolimus levels used to calculate MLVI in patients who experienced rejection was 25.7 (SD = 19.0) and 17.5 (SD = 12.6) in patients who did not experience rejection. Data collection began after the first year, meaning that tacrolimus levels over an average of 2.61 years were used to calculate adherence. Time since transplant captured in data collection was similar amongst all groups (ANOVA, F (3, 244) = 0.38, p = 0.77).
Table 1.
Patient Demographics
| Younger Adults | Older Adults | |
|---|---|---|
| Sex* | ||
| Male | 137 | 21 |
| Female | 67 | 21 |
| Race | ||
| White | 83 | 18 |
| Hispanic | 42 | 13 |
| Black | 32 | 3 |
| Asian | 33 | 6 |
| Other | 15 | 0 |
| Marital Status | ||
| Married | 121 | 27 |
| Significant Other | 17 | 0 |
| Divorced/Separated | 15 | 5 |
| Single | 50 | 8 |
| Widowed | 1 | 2 |
| Highest level of completed education* | ||
| Graduate | 15 | 4 |
| College | 57 | 9 |
| High School | 102 | 14 |
| Less than high school | 17 | 11 |
| Employment* | ||
| No | 99 | 10 |
| Yes | 72 | 6 |
| Retired | 22 | 25 |
| Insurance* | ||
| Medicaid | 54 | 13 |
| Medicare | 39 | 19 |
| Private | 112 | 10 |
significant difference between two groups
Forty-two patients (17% of all LT recipients) were ≥65 years old at the time of transplant. The mean age of older adults was 68.7 years (SD 2.45, range 65–74 years); 21 older adult LT recipients (50%) were male. Outcomes and indications for transplant by age group are shown in table 2.
Table 2.
LT Outcomes, Indications and MLVI by Age Group
| Age | N | MLVI | Nonadherence (MLVI >2.5) | Indications for LT | |
|---|---|---|---|---|---|
| (yrs) | n (% total pop) | (mean, SD) | n (% age group) | Condition | n (% age group) |
| 18–34 | 13 (5.2%) | 2.36 (1.57) | 5 (38.5%) | Fulminant Hepatic Failure | 5 (38%) |
| Hepatitis C Virus | 2 (15%) | ||||
| Primary Sclerosing Cholangitis | 2 (15%) | ||||
| Hepatitis B virus | 1 (1%) | ||||
| Autoimmune Hepatitis | 1 (1%) | ||||
| Alcoholic Liver Disease | 1 (1%) | ||||
| Other | 1 (1%) | ||||
| 35–49 | 35 (19.4%) | 2.79 (1.44) | 16 (44.4%) | Hepatitis C Virus | 8 (23%) |
| Hepatitis B Virus | 7 (20%) | ||||
| Alcoholic Liver Disease | 5 (14%) | ||||
| Primary Sclerosing Cholangitis | 4 (11%) | ||||
| Fulminant Hepatic Failure | 3 (9%) | ||||
| Other | 3 (9%) | ||||
| Nonalcoholic Fatty Liver Disease | 2 (6%) | ||||
| Hepatocellular Carcinoma | 1 (3%) | ||||
| Primary Biliary Cirrhosis | 1 (3%) | ||||
| Alcoholic Liver Disease w/Hepatitis C Virus | 1 (3%) | ||||
| 50–64 | 158 (63.7%) | 3.00 (1.59) | 91 (57.6%) | Hepatitis C Virus | 79 (50%) |
| Hepatitis B Virus | 25 (16%) | ||||
| Hepatocellular Carcinoma | 11 (7%) | ||||
| Alcoholic Liver Disease with Hepatitis C Virus | 11 (7%) | ||||
| Fulminant Hepatic Failure | 6 (4%) | ||||
| Alcoholic Liver Disease | 6 (4%) | ||||
| Primary Biliary Cirrhosis | 5 (3%) | ||||
| Nonalcoholic Fatty Liver Disease | 5 (3%) | ||||
| Autoimmune Hepatitis | 4 (3%) | ||||
| Primary Sclerosing Cholangitis | 3 (2%) | ||||
| Other | 3 (2%) | ||||
| 65+ | 42 (16.9%) | 2.53 (1.50) | 15 (34.9%) | Hepatitis C Virus | 17 (40%) |
| Hepatocellular Carcinoma | 8 (19%) | ||||
| Non-Alcoholic Fatty Liver Disease | 6 (14%) | ||||
| Hepatitis B virus | 4 (10%) | ||||
| Primary Biliary Cirrhosis | 3 (7%) | ||||
| Alcoholic Liver Disease | 2 (5%) | ||||
| Alcoholic Liver Disease w/Hepatitis C Virus | 2 (5%) | ||||
The most common primary indications for LT in older adults were hepatitis C virus (HCV) (n=17), hepatocellular carcinoma (n=8), and non-alcoholic fatty liver disease (NAFLD) (n=6). Overall, nonadherence was high (50.8% of all subjects), though it is difficult to compare to other studies that measured adherence using different tools and over different periods of time.15–17 Despite the relatively high overall prevalence of nonadherence identified in the period beginning at least one year post-LT, older adults had significantly better rates of TAC adherence than younger adults as measured by the lower percentage of above-threshold MLVI scores in older adults (65% of recipients ≥65 were adherent vs. 42% of younger recipients; χ2 = 5.89, p=0.02).
When analyzed as a continuous variable, MLVI scores among the four groups were not significantly different (ANOVA, F (3, 244) = 2.24, p = 0.08). In addition, this analysis was repeated controlling for demographic variables (education, employment status, insurance, and sex); the model remained nonsignificant, F (3, 217) = 1.48, p = 0.18. Finally, we ran Kaplan-Meier survival analyses with rejection as the “status” variable and “age groups” as the factor. When censoring by time since transplant, there was no difference between the four age groups, χ2 = 0.84, p=0.84 (Figure 2).
Figure 2. Time-to-rejection analysis by age group.
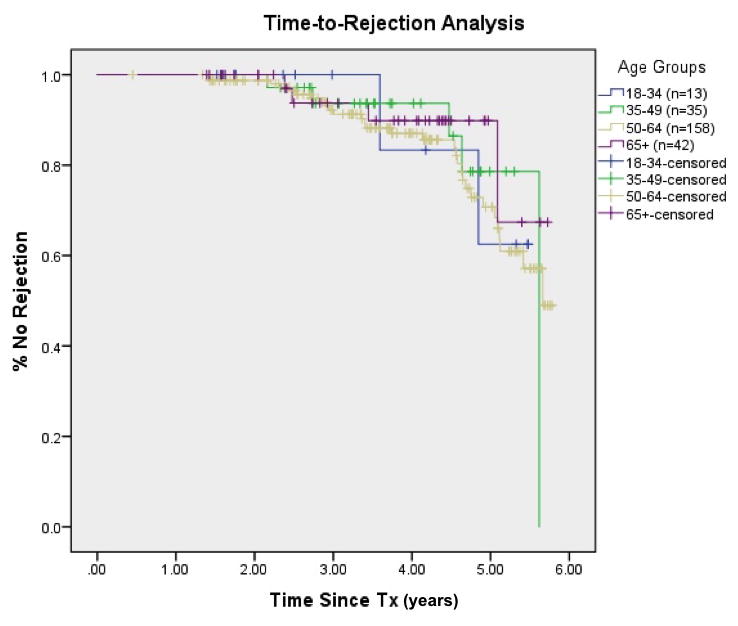
Kaplan-Meier survival analyses with rejection as the “status” variable and “age groups” as the factor. When censored by time since transplant, there was still no difference between the four age groups, χ2 = 0.84, p=0.84.
There was a modest positive correlation between MLVI and the number of levels used to calculate an MLVI score for each patient (Pearson’s correlation = 0.17, p < 0.01).
Of those patients who were excluded due to mortality within the first year after LT, older adults were overrepresented. Thirty-four percent of transplanted older adults died within 1 year, as compared to 16% of younger adults (Fisher’s Exact p<0.01). Complete chart reviews were not performed for excluded patients, but it is known that these patients did not differ significantly from those included in the study with respect to sex (χ2 = 0.57, two-tailed p value = 0.45) or age at time of transplant (t(422) = 1.49, p = 0.13).
Thirteen patients died in the follow-up period beyond one year post-LT, with no significant difference between older and younger adults (8 younger adults and 5 older adults, Fisher’s exact p = 0.05). Death after one year post-LT was not a reason for exclusion.
DISCUSSION
To our knowledge, this is the first study to look at older age as a potential risk factor for medication nonadherence in liver transplant recipients, and one of few studies to consider recipients older than 65. The fact that we found older age to be a protective factor for nonadherence, not a risk, has implications for transplant decision-making and resource allocation in transplant clinics. While, as expected, 7,21 older adults were a medically high-risk group (as they were more likely to die within the first year post-LT), they were not high-risk as far as adherence is concerned. Instead, we found that older liver transplant recipients displayed significantly better adherence to their immunosuppressant regimens than younger adults and no increased risk for rejection. These findings are consistent with other studies that indicate that older age is correlated with better adherence in a transplant setting,22–24 particularly when compared with young adult transplant recipients (<20 year old). However, since transplantation of older adults is a relatively new practice, these studies primarily include data from much younger patients than those examined in the current study and there is a relative dearth of data on older adult adherence in transplant settings. However, in non-transplant settings, older adults are often considered to be a high-risk group when it comes to adherence and chronic disease management.8,12,17,23,25 In one study showing a correlation between overall adherence to posttransplant regimens and increasing age, the “accidental noncompliers” in the study group were significantly older than those patients whose nonadherence was purposeful, though the “older” group had an average age of 44, much younger than the older adults considered in the current study.23
Our study groups, as would be expected, differed in characteristics that are closely tied to age, such as highest level of education attained, employment status, and insurance (in the United States, insurance carriers change with age as patients become eligible for Medicare). Such differences are expected when patients of different ages are compared. There was also a difference in sex, attributable to the fact that about 67% of our young adult recipients were males whereas only 50% were men in the adult cohort. The overall study population contained about 64% male recipients, which is consistent with national listing and transplant data (based on OPTN data as of May 1, 2016). The drop in males in the older age group may reflect some degree of sampling error, but is likely at least partially explained by the shorter life expectancy in American males.26 Additionally, among liver transplant recipients, more males have liver disease related to primary sclerosing cholangitis, alcohol, and hepatitis C complicated by a higher percentage of non-alcoholic fatty liver compared to females.
Our findings expand on earlier reports showing that those who survived were no more likely to experience graft rejection than younger adults, 4,27,28 and add that older patients have better adherence to their medication regimens compared to younger patients. Our data do not provide information regarding the medical selection of transplant candidates at our center. However, consistent with prior literature,1,4 in those older adults who were deemed medically eligible and fit for transplant, our results continue to support the idea that the long-term management of older adult transplant recipients need not involve a particularly higher concern for persistently increased needs for psychosocial supports compared to younger adult recipients.
Furthermore, the finding that the older adults experienced both the highest rates of adherence and the lowest rates of graft rejection is consistent with prior studies that consistently show that the MLVI predicts rejection in children and adults,18,29,30 although the association between this marker and rejection was not specifically examined in older adults prior to the present study.
If TAC levels used to calculate MLVI were a string of random numbers, it would be expected that the variability, and therefore the MLVI, would be smaller when more variables are available for the calculation. However, blood levels are not random numbers. We found a modest positive correlation (Pearson’s coefficient = 0.17) between the MLVI and the number of levels used to calculate it in our sample, establishing that it is not the case that more levels were associated with a lower MLVI. It is possible that patients with aberrant (too high or too low) results were tested more frequently, though this hypothesis cannot be conclusively stated from the current data.. Prior work using the MLVI as a measurement of adherence showed that the number of blood levels used to calculate the MLVI did not vary significantly between subjects found to be adherent vs. nonadherent, and so, in clinical settings, the MLVI is not affected by the number of input values used to calculate it.18 This insight also suggests that adherence to a recommendation to measure blood levels in transplant settings is not the same as adherence to the medication: patients do get tested, even when they are not taking their medications as prescribed.
One year post-LT was chosen as the designated starting point because prior studies show that immunosuppressant adherence within the first year is generally high, dropping off as more time elapses.23,31 Furthermore, graft rejections prior to the one year mark are not considered to be primarily related to nonadherence.31 Tacrolimus levels are measured frequently for dose-adjustment in the first few weeks and months post-LT, and resultant variability would falsely elevate the MLVI in a time period when the expected adherence rates in all patients would be similarly high, despite the possibility of drop offs in adherence later in the posttransplant course.
Strengths of this study include a sample reflective of “real world” patient selection (as opposed to prospective trials evaluating outcomes in a controlled study environment) and outcomes at a center with a relatively large population of older adult LT recipients and long-term follow-up data. Though the overall number of patients in this study is somewhat small, our data include a relatively large proportion of older adult LT recipients: 17% of study patients were 65+ years old at the time of transplant compared to 12.5% of all U.S. patients undergoing liver transplantation during our study period (based on OPTN data of liver transplant recipients from 2009–2013). The MLVI measure of adherence is another major strength of this study as it is a validated, objective biomarker of adherence. It has been validated in several independent cohorts of transplant recipients, across different ages and different organs; it is therefore the most widely studied objective adherence measure in transplant medicine.18,20,32–34 Furthermore, the use of state-mandated social work evaluations allowed access to demographic and psychosocial variables for all patients from standardized templates
There is no gold standard for adherence assessment in transplant settings. Besides the MLVI, other methods are sometimes used to assess adherence, such as self-reports, electronic monitoring devices, and pill counts. The reason there is no gold standard is that each method has its weaknesses and strengths, as reviewed elsewhere20. Our study used only the MLVI to assess adherence, and it is possible that, had we used another method, it would have resulted in different findings. One limitation to our study was its retrospective design, which did not allow for a prospective evaluation of endpoints. However, since evaluation processes are standardized in our center, the shortcomings of the retrospective design are mitigated to some extent, while also allowing us to examine an unbiased clinical sample. While our sample included a relatively large number of older adult recipients, the overall sample size was only 248 patients. In addition to the relatively small “n”, generalizability may be limited due to possible selection bias in that included patients may inherently differ compared to excluded patients (particularly those who lack sufficient tacrolimus levels to measure MLVI) and the fact that all patients were treated at a single, urban, expert tertiary care center with a rigorous medical and psychosocial evaluation and follow-up protocol. Furthermore, though all patients were required to have adequate social supports in place prior to transplant listing, the extent and quality of these supports is neither quantifiable nor available in patient records. The social supports requirement has its underpinnings in the theory that social support impacts a patient’s ability to adhere to his/her treatment plan as a whole (including doctor’s visits, medication regimens, dietary/behavioral modifications, etc.), yet we cannot assess a quantifiable relationship between adherence to tacrolimus and quality/extent of social support in the current study. Another limitation is that we do not have access to information predating subject enrollment. Therefore, we could have missed pre-enrollment nonadherence or adverse outcomes in any of the investigated groups.
Although prior research does document that adherence gets worse over time in transplant recipients, we still think that it is surprising that in our sample, it appears that nonadherence was worse between the ages of 50–64; young adults had better adherence. We are not sure what could explain this trajectory, other than the general deterioration of adherence noted elsewhere.7 Our primary interest, and focus, in this study was the very old age group. Secondary findings such as this one, although intriguing, will have to be replicated in studies that are directed to answer the question of progression of adherence in the less-than-very-old age group, before they are considered further.
Possible reasons for the higher rates of adherence seen in older adults in this study include: more extensive screening for medical clearance prior to transplant listing or closer post-LT follow up for older adults; possible age-related changes in immunity that make it more difficult to mount a rejection response; potentially better health literacy and health habits of adults who reach older age prior to requiring a transplant; more family/home health worker involvement in older adult post-LT care and possibly different attitudes toward transplant in patients of different ages. Although selection bias might also be an explanation (in that the most nonadherent patients might have died within the first year), we consider this unlikely because adherence in the first year after transplantation is actually considered to be better, rather than worse, as compared to later in the transplant survivor’s course. 31 For similar reasons, we hypothesize that the overrepresentation of older adults in patients excluded due to death within the first year after transplant is not related to worse adherence, but rather, to the increased medical risk that older patients may carry into transplant surgery and recovery. Although this study did not seek to identify explanations for differences in first year mortality between the groups, future studies may explore these aspects further.
In summary, we found that adults receiving a liver transplant at the age of 65 or older and surviving at least one year are a resilient group of patients who demonstrate better immunosuppressant adherence with no increased rejection risk as compared with younger adults. These results might have implications for resource allocation of ancillary services in transplant programs, and suggest that transplantation of older adults should not be withheld on the basis of age alone.
Acknowledgments
FUNDING
This work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (R01DK080740 to E.S.) and contributions by the Jean and Louis Dreyfus Foundation and the National Institute for Aging to the Medical Student Training in Aging Research (MSTAR) Program administered by the American Federation for Aging Research (AFAR) to E.L.
Footnotes
AUTHOR CONTRIBUTIONS:
All authors were meaningfully involved in the study design, data acquisition, data analysis and interpretation, and manuscript preparation. Emily Leven,1 participated in determining study concept and design, acquisition of data, analysis and interpretation of data, and preparation of manuscript.
Rachel Annunziato, PhD,2 participated in determining study concept and design, analysis and interpretation of data, and preparation of manuscript.
Jacqueline Helcer MS, MA,2 participated in determining study concept and design, acquisition of data, interpretation of data, and preparation of manuscript.
Sarah R. Lieber, MD,3 participated in determining study concept and design, acquisition of data, interpretation of data, and preparation of manuscript.
Christopher S. Knight,2 participated in determining study concept and design, acquisition of data, interpretation of data, and preparation of manuscript.
Catherine Wlodarkiewicz,4 participated in determining study concept and design, acquisition of data, interpretation of data, and preparation of manuscript.
Rainier P. Soriano, MD,5 participated in determining study concept and design, interpretation of data, and preparation of manuscript.
Sander S. Florman, MD,6 participated in determining study concept and design, interpretation of data, and preparation of manuscript.
Thomas D. Schiano, MD,7 participated in determining study concept and design, interpretation of data, and preparation of manuscript.
Eyal Shemesh, MD8 participated in determining study concept and design, interpretation of data, and preparation of manuscript.
CONFLICT OF INTEREST
To the best of our knowledge, no conflict of interest exists for any authors or contributors. Funding sources are listed below.
References
- 1.Keswani RN, Ahmed A, Keeffe EB. Older Age and Liver Transplantation: A Review. Liver Transpl. 2004;10(8):957–967. doi: 10.1002/lt.20155. [DOI] [PubMed] [Google Scholar]
- 2.Aduen JF, Sujay B, Dickson RC, Heckman MG, Hewitt WR, Stapelfeldt WH, et al. Outcomes After Liver Transplant in Patients Aged 70 Years or Older Compared With Those Younger Than 60 Years. Mayo Clin Proc. 2009;84(11):973–978. doi: 10.1016/S0025-6196(11)60667-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aloia TA, Knight R, Gaber AO, Ghobrial RM, Goss JA. Analysis of liver transplant outcomes for United Network for Organ Sharing recipients 60 years old or older identifies multiple model for end-stage liver disease-independent prognostic factors. Liver Transpl. 2010;16:950–959. doi: 10.1002/lt.22098. [DOI] [PubMed] [Google Scholar]
- 4.Cross TJ, Antoniades CG, Muiesan P, Al-Chalabi T, Aluvihare V, Agarwal K, et al. Liver transplantation in patients over 60 and 65 years: an evaluation of long-term outcomes and survival. Liver Transpl. 2007;13(10):1382–1388. doi: 10.1002/lt.21181. [DOI] [PubMed] [Google Scholar]
- 5.American Geriatrics Society Core Writing Group of the Task Force on the Future of Geriatric M. Caring for Older Americans: The Future of Geriatric Medicine. J Am Geriatr Soc. 2005;53(S6):S245–S256. doi: 10.1111/j.1532-5415.2005.53350.x. [DOI] [PubMed] [Google Scholar]
- 6.Lipshutz GS, Busuttil RW. Liver Transplantation in Those of Advancing Age: The Case for Transplantation. Liver Transpl. 2007;13:1355–1357. doi: 10.1002/lt.21089. [DOI] [PubMed] [Google Scholar]
- 7.Zetterman RK, Belle SH, Hoofnagle JH, Lawlor S, Wei Y, Everhart J, et al. Age and Liver Transplantation: A Report of the Liver Transplantation Database. Transplantation. 1998;66(4):500–506. doi: 10.1097/00007890-199808270-00015. [DOI] [PubMed] [Google Scholar]
- 8.Cooper C, Carpenter I, Katona C, Schroll M, Wagner C, Fialova D, et al. The AdHOC Study of older adults’ adherence to medication in 11 countries. Am J Geriatr Psychiatry. 2005;13(12):1067–1076. doi: 10.1176/appi.ajgp.13.12.1067. [DOI] [PubMed] [Google Scholar]
- 9.Park DC, Hertzog C, Leventhal H, Morrell RW, Leventhal E, Birchmore D, et al. Medication Adherence in Rheumatoid Arthritis Patients: Older Is Wiser. J Am Geriatr Soc. 1999;47(2):172–183. doi: 10.1111/j.1532-5415.1999.tb04575.x. [DOI] [PubMed] [Google Scholar]
- 10.Morrell RW, Park DC, Kidder DP, Martin M. Adherence to Antihypertensive Medications Across the Life Span. The Gerontologist. 1997;37(5):609–619. doi: 10.1093/geront/37.5.609. [DOI] [PubMed] [Google Scholar]
- 11.Krueger K, Berger B, Felkey B. Medication adherence and persistence: A comprehensive review. Adv Ther. 2005;22(4):313–356. doi: 10.1007/BF02850081. [DOI] [PubMed] [Google Scholar]
- 12.Schlenk EA, Dunbar-Jacob J, Engberg S. Medication non-adherence among older adults: a review of strategies and interventions for improvement. J Gerontol Nurs. 2004;30(7):33. doi: 10.3928/0098-9134-20040701-08. [DOI] [PubMed] [Google Scholar]
- 13.Tielen M, van Exel N, Maasdam L, Weimar W. Attitudes towards medication non-adherence in elderly kidney transplant patients: a Q methodology study. Nephrol Dial Transplant. 2011;26(5):1723–1728. doi: 10.1093/ndt/gfq642. [DOI] [PubMed] [Google Scholar]
- 14.Prendergast MB, Gaston RS. Optimizing Medication Adherence: An Ongoing Opportunity To Improve Outcomes After Kidney Transplantation. Clinical Journal of the American Society of Nephrology: CJASN. 2010;5(7):1305–1311. doi: 10.2215/CJN.07241009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burra P, Germani G, Gnoato F, Lazzaro S, Russo F, Cillo U, et al. Adherence in liver transplant recipients. Liver Transpl. 2011;17:760–770. doi: 10.1002/lt.22294. [DOI] [PubMed] [Google Scholar]
- 16.Mor E, Gonwa T, Husberg B, Goldstein R, Klintmalm G. Late-onset acute rejection in orthoptic liver transplantation- associated risk factors and outcomes. Transplantation. 1992;54:821–824. doi: 10.1097/00007890-199211000-00010. [DOI] [PubMed] [Google Scholar]
- 17.Russell CL, Cetingok M, Hamburger KQ, Owens S, Thompson D, Hathaway D, et al. Medication adherence in older renal transplant recipients. Clin Nurs Res. 2010;19(2):95–112. doi: 10.1177/1054773810362039. [DOI] [PubMed] [Google Scholar]
- 18.Supelana C, Annunziato RA, Schiano TD, Anand R, Vaidya S, Chuang K, et al. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014;20(10):1168–1177. doi: 10.1002/lt.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieber SR, Volk ML. Non-adherence and graft failure in adult liver transplant recipients. Dig Dis Sci. 2013;58(3):824–834. doi: 10.1007/s10620-012-2412-0. [DOI] [PubMed] [Google Scholar]
- 20.Shemesh E, Fine RN. Is calculating the standard deviation of tacrolimus blood levels the new gold standard for evaluating non-adherence to medications in transplant recipients? Pediatr Transplant. 2010;14(8):940–943. doi: 10.1111/j.1399-3046.2010.01396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sonny A, Kelly D, Hammel JP, Albeldawi M, Zein N, Cywinski JB. Predictors of poor outcome among older liver transplant recipients. Clin Transplant. 2015;29(3):197–203. doi: 10.1111/ctr.12500. [DOI] [PubMed] [Google Scholar]
- 22.Sketris I, Waite N, Grobler K, West M, Gerus S. Factor affecting compliance with cyclosporine in adult renal transplant patients. Transplant Proc. 1994;26:2538. [PubMed] [Google Scholar]
- 23.Greenstein S, Siegal B. Compliance and noncompliance in patients with a functioning renal transplant: a multicenter study. Transplantation. 1998;66(12):1718–1726. doi: 10.1097/00007890-199812270-00026. [DOI] [PubMed] [Google Scholar]
- 24.Rovelli M, Palmeri D, Vossler E, Bartus S, Hull D, Schweizer R. Noncompliance in organ transplant recipients. Transplant Proc. 1989;21:833. [PubMed] [Google Scholar]
- 25.Gellad WF, Grenard JL, Marcum ZA. A Systematic Review of Barriers to Medication Adherence in the Elderly: Looking Beyond Cost and Regimen Complexity. The American journal of geriatric pharmacotherapy. 2011;9(1):11–23. doi: 10.1016/j.amjopharm.2011.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Life expectancy at birth and 65 years of age, by sex: Organisation for Economic Co-operation and Development (OECD) countries. Vol. 2014 Centers for Disease Control; 2014. [Google Scholar]
- 27.Collins BH, Pirsch JD, Becker YT, Hanaway MJ, Van der Werf WJ, D’Alessandro AM, et al. Long-term results of liver transplantation in older patients 60 years of age and older. Transplantation. 70(5):780–783. doi: 10.1097/00007890-200009150-00012. [DOI] [PubMed] [Google Scholar]
- 28.Bilbao I, Dopazo C, Lazaro JL, Castells L, Escartin A, Lopez I, et al. Our experience in liver transplantation in patients over 65 yr of age. Clin Transplant. 22(1):82–88. doi: 10.1111/j.1399-0012.2007.00749.x. [DOI] [PubMed] [Google Scholar]
- 29.Christina S, Annunziato RA, Schiano TD, Anand R, Vaidya S, Chuang K, et al. Medication level variability index predicts rejection, possibly due to nonadherence, in adult liver transplant recipients. Liver Transpl. 2014;20(10):1168–1177. doi: 10.1002/lt.23930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stuber ML, Shemesh E, Seacord D, Washington J, 3rd, Hellemann G, McDiarmid S. Evaluating non-adherence to immunosuppressant medications in pediatric liver transplant recipients. Pediatr Transplant. 2008;12(3):284–288. doi: 10.1111/j.1399-3046.2008.00923.x. [DOI] [PubMed] [Google Scholar]
- 31.De Geest S, Burkhalter H, Bogert L, Berben L, Glass TR, Denhaerynck K, et al. Describing the evolution of medication nonadherence from pretransplant until 3 years post-transplant and determining pretransplant medication nonadherence as risk factor for post-transplant nonadherence to immunosuppressives: The Swiss Transplant Cohort Study. Transpl Int. 2014;27(7):657–666. doi: 10.1111/tri.12312. [DOI] [PubMed] [Google Scholar]
- 32.Shemesh E, Shneider BL, Savitzky JK, Arnott L, Gondolesi GE, Krieger NR, et al. Medication adherence in pediatric and adolescent liver transplant recipients. Pediatrics. 2004;113(4):825–832. doi: 10.1542/peds.113.4.825. [DOI] [PubMed] [Google Scholar]
- 33.Pollock-Bar Ziv SM, Finkelstein Y, Manlhiot C, Dipchand AI, Hebert D, Ng VL, et al. Variability in tacrolimus blood levels increases the risk of late rejection and graft loss after solid organ transplantation in older children. Pediatr Transplant. 2010;14(8):968–975. doi: 10.1111/j.1399-3046.2010.01409.x. [DOI] [PubMed] [Google Scholar]
- 34.Venkat VL, Nick TG, Wang Y, Bucuvalas JC. An objective measure to identify pediatric liver transplant recipients at risk for late allograft rejection related to non-adherence. Pediatr Transplant. 2008;12(1):67–72. doi: 10.1111/j.1399-3046.2007.00794.x. [DOI] [PubMed] [Google Scholar]


