Abstract
Cholestasis is associated with accumulation of bile acids and lipids, and liver injury. The constitutive androstane receptor (CAR) and pregnane X receptor (PXR) are xenobiotic nuclear receptors that coordinate protective hepatic responses to potentially toxic stimuli, including bile acids. We investigated the role of these receptors in the regulation of bile acid and lipid metabolism in a bile duct ligation (BDL) model of cholestasis applied to receptor knockout mice. Hepatic damage from bile acid accumulation was increased in both CAR knockout (CARKO) and PXR knockout mice, but bile acid concentrations were lower in CARKO mice. High-density lipoprotein (HDL) cholesterol was elevated in CARKO mice, and serum total cholesterol increased less in CARKO or PXR knockout mice than WT mice after BDL. Gene expression analysis of the BDL knockout animals demonstrated that, in response to cholestasis, PXR and CAR both repressed and induced the specific hepatic membrane transporters Oatp-c (organic anion transporting polypeptide C) and Oatp2 (Na+-dependent organic anion transporter 2), respectively. Induction of the xenobiotic transporter multidrug resistance protein 1 in cholestasis was independent of either PXR or CAR, in contrast to the known pattern of induction of multidrug resistance protein 1 by xenobiotics. These results demonstrate that CAR and PXR influence cholesterol metabolism and bile acid synthesis, as well as multiple detoxification pathways, and suggest their potential role as therapeutic targets for the treatment of cholestasis and lipid disorders.
Keywords: bile acid, cholestasis, high-density lipoprotein, cholesterol
Cholestatic liver disorders are characterized by impaired hepatocellular secretion of bile, resulting in accumulation of bile acids, bilirubin, and cholesterol. Bile acids are the major products of cholesterol catabolism in the liver and act as physiological detergents that facilitate absorption, transport, and distribution of lipid-soluble fats and vitamins, and excretion of lipids. However, hydrophobic bile acids are inherently cytotoxic, and accumulation in the liver results in progressive liver disease with eventual cirrhosis. Formation and elimination of bile acids must therefore be tightly regulated and is closely linked to cholesterol metabolism. This balance is achieved by feed-forward induction of genes responsible for bile acid detoxification and/or excretion and suppression of genes such as cholesterol 7α-hydroxylase (CYP7A1) (1).
Nuclear hormone receptors function as ligand-activated transcription factors with critical roles in diverse cellular processes such as mammalian development, differentiation, and metabolism (2). Although the pregnane X receptor (PXR; NR1I2) (3) and the constitutive androstane receptor (CAR; NR1I3) (4) are known to act as sensors for toxic xenobiotics (foreign compounds) (5, 6), a role for these receptors in the homeostasis of toxic endobiotics (endogenously synthesized compounds) is being increasingly recognized. Bile acids are ligands for PXR, and it has been suggested that PXR plays an essential role in mediating a protective feed-forward response mechanism in cholestasis (7, 8). Rifampin, a potent human PXR activator, has been used to treat the pruritus that is often associated with cholestatic liver disease, an action thought to be due to increased bile acid metabolism (9, 10). In addition, activation of PXR reduces liver injury produced by i.p. injection of lithocholic acid (LCA), the most cytotoxic of the bile acids (7, 8). However, recent work with bile duct ligated (BDL) mice has shown that feed-forward adaptation is effectively engaged despite the lack of accumulation of secondary bile acids such as LCA in this model (11), suggesting that other bile acids or receptors must be involved in this response. There is no evidence to date that endogenous bile acids are ligands for CAR. However, recent studies of PXR/farnesoid X receptor (FXR) double knockout mice fed a cholic acid-rich diet demonstrate both up-regulation of CAR and the CAR target gene Cyp2b10 (12). Additionally, detoxification of LCA by sulfation has been shown to be CAR-dependent in mice (13). Although CAR has these characterized roles in bile acid detoxification, it is not known whether CAR has a role in regulation of bile acid synthesis or lipid metabolism.
Previous in vivo studies of bile acid-induced liver injury typically involve administration of cholic acid (12), or the secondary bile acid LCA (7, 8), which is normally formed in the gut by bacterial dehydroxylation of chenodeoxycholic acid. However, individual bile acids vary significantly in their ligand affinity for nuclear receptors such as PXR or FXR (NR1H4) (8, 14). In clinical cholestasis, multiple primary bile acids accumulate, whereas LCA does not significantly accumulate in biliary obstruction (11), raising doubts about its in vivo relevance in cholestatic disease. In contrast, the BDL mouse represents a pathophysiological model of cholestasis caused by gallstone obstruction, malignancy, or accidental surgical interruption. As such, it is an effective substrate to study the in vivo interplay between PXR, CAR, and FXR and adaptive responses of the liver (11).
In the present study, we have sought to define the relative importance of PXR and CAR in providing hepato-protection against the liver injury that follows BDL and relate this outcome to altered patterns of protective gene expression. Because these receptors regulate a number of common target genes (including the P450 enzymes Cyp3a11 and Cyp2b10), phase II enzymes [such as GSTs, dehydroepiandrosterone sulfotransferase (STD), and glucurosyltransferases], and membrane transporters, it is necessary to include studies of the effect of BDL on both single and double knockouts to fully characterize the functional role of these receptors in the cholestatic response. We show that, as well as contributing to hepato-protection in cholestasis, PXR and CAR regulate basolateral transporters, including organic anion transporting polypeptide C (Oatp-c; Slc21a6) and Na+-dependent organic anion transporter 2 (Oatp2; Slc21a5), suppress key genes involved in bile acid synthesis, and influence the serum lipid profile. Deletion of CAR reduces bile acid accumulation in this model of cholestasis, suggesting that this nuclear receptor may represent a therapeutic target for patients with cholestatic liver diseases or lipid disorders.
Materials and Methods
Animal Studies. PXR knockout (PXRKO) and CAR knockout (CARKO) mice were crossed to generate double null mice (2XENKO) and genotyped as described (15, 16). The WT, single knockout, and double knockout mice used in these studies were of a mixed 129/Sv and C57BL/6 background. Eight- to ten-week-old male mice were used for all experiments. The animals were housed in an animal facility under a standard 12-h light/dark cycle, fed a commercial pellet diet, and allowed food and water ad libitum, except when they were fasted for 6 h before blood sampling. The use of mice in this study has complied with all relevant federal guidelines and institutional policies.
BDL. Mice were anesthetized (ketamine 100 mg/kg and xylazine 20 mg/kg, administered i.p.) and aseptically subjected to double ligation of the common bile duct below the bifurcation and single ligation above the pancreas. Sham operations were performed as described (11). Animals (n = 5–6 per group) were killed by exsanguination under anesthesia 6 days after BDL or sham operation. Blood samples (35 μl) were collected into heparinized capillary tubes from the tail vein of anesthetized mice before the initial surgical procedure on day 0, and again on day 6 immediately before killing. Total cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, and glucose were measured from these samples by using the Cholestech LDX Analyzer (Cholestech, Hayward, CA). Additional serum was collected at the time of killing to measure alanine aminotransferase (ALT) and bilirubin as a measure of liver injury and cholestasis, respectively. ALT and bilirubin were determined by the Department of Clinical Chemistry, Institute for Clinical Pathology and Medical Research, Western Sydney Area Health Service, by using automated procedures. Serum, urine, and liver samples were also frozen at –70°C for bile acid analysis, and liver was snap frozen in liquid nitrogen for subsequent RNA extraction.
Assessment of Liver Histology. Consecutive sections of liver (4 μm thick) from paraffin-embedded liver were cut for hematoxylin and eosin staining and Gomori's Trichrome staining for evaluation of liver injury and necrosis. Areas of bile infarct and hepatic necrosis were evaluated in five fields (×10 magnification) by using the optimas 6.5 image analysis software (Media Cybernetics, Silver Spring, MD).
Quantitation of Bile Acids. Individual bile acids in liver, serum, urine, and bile were quantified by high performance LC/MS by using authentic bile acid standards and deuterated internal standards. To a 50-μl sample of serum or urine, 20.5 ng of assay internal standard (cholic 2,2,4,4-d4 acid) was added, and for bile, 20-μl aliquots were used with 8.2 ng of assay internal standard. One hundred fifty microliters of methanol was then added to the sample for protein precipitation, and the mixture was vortexed. After centrifugation at 2,000 × g for 10 min, 150 μl of the supernatant was added to 50 μl (40 mM) of ammonium acetate (pH 4.5). Samples were stored at 4°C in the autosampler, and 20-μl aliquots were injected for analysis. For liver samples, bile acids were extracted by using liquid–liquid extraction as described (11). Chromatographic separations and quantitative determinations of bile acids from all samples were also carried out as described (11). However, a modified mobile phase gradient for α- and β-muricholic acid (α- and β-MCA) was used. The mobile phase consisted of solvent A (water), solvent B (methanol), and solvent C (100 mM ammonium Acetate, pH 4.5) delivered as a gradient: 0- to 7-min solvent B 60%; 7- to 12-min solvent B 90%, and 12- to 20-min solvent B 60% with solvent C constant at 10% at a flow rate of 0.2 ml/min. Total serum bile acid concentrations were determined by using a commercial 3α-hydroxysteroid dehydrogenase assay (Randox, Antrim, U.K.).
Statistical Analysis. Treatment groups were compared by using factorial ANOVA, and post hoc analysis was performed by using the Fisher's exact test. These results are presented as mean ± SD. Results are considered significant when P < 0.05.
Expression-Level Analysis. Total RNA was extracted from frozen tissue samples or cells by using TRIzol reagent (Invitrogen) according to the manufacturer's directions. RNA was then treated with DNase I (Ambion, Austin, TX) at 37°C for 30 min, followed by inactivation at 75°C for 5 min. cDNA was synthesized from total RNA with the SuperScript First-Strand Synthesis System (Invitrogen) and random hexamer primers. The real-time PCR measurement of individual cDNAs was performed by using either Taqman or SYBR green dye technology to measure duplex DNA formation using an ABI Prism 7700 or 7900 Sequence Detection System (PE Applied Biosystems). The sequences of the primer sets used for each gene studied are available upon request. For each biological sample, qPCR reactions were performed in triplicate, and expression was normalized to m36B4 expression.
Results
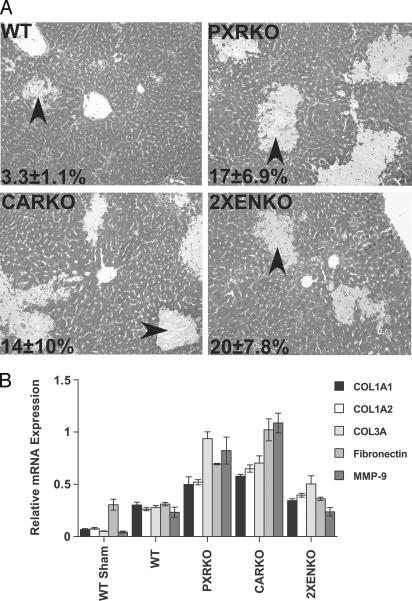
Deletion of PXR or CAR results in severe bile acid-induced liver injury. Ligation of the common bile duct is associated with intrahepatic bile acid overload and consequent liver injury. In all mice with BDL, bile infarcts were observed histologically, as reported in other mouse strains (Fig. 1A) (17). The areas of necrosis and bile infarction were significantly increased in both PXR and CAR knockout mice after BDL, although there was no additional increase in liver necrosis in the 2XENKO group compared with the other null genotypes (Fig. 1 A). Expression of collagen 3A, fibronectin, and matrix metalloproteinase 9 (MMP9), genes involved in fibrosis and tissue remodeling, were substantially higher in PXRKO and CARKO BDL mice compared with WT BDL (Fig. 1B). However, in the 2XENKO BDL mice, induction of collagen 3A was reduced, and no additional induction of fibronectin or MMP9 was seen relative to WT BDL (Fig. 1B), suggesting that there may be less injury response in this genotype. The mortality rates (including deaths and euthanized animals) for BDL genotypes were 15% for WT BDL, 22% for CARKO, and 23% for PXRKO mice. Surprisingly, there were no deaths among the 2XENKO BDL mice.
Fig. 1.
Deletion of PXR or CAR results in exacerbation of bile acid-induced liver injury. (A) Livers were removed from mice 6 days after BDL, and liver paraffin sections were stained with Gomori's Trichrome to evaluate liver injury and necrosis (n = 4–6 per group). Areas of bile infarction and hepatic necrosis (arrows) were evaluated in five microscopy fields (×10 magnification), quantified as a percentage of total area as indicated on the representative photomicrographs, and found to be increased in knockout mice relative to WT mice after BDL. PXRKO, P < 0.05; CARKO, P < 0.05; 2XENKO mice, P < 0.01. (B) Effects of BDL on hepatic expression of genes involved in fibrosis and tissue remodeling. Relative mRNA expression of collagen 1A1 (COL1A1), collagen 1A2 (COL1A2), collagen 3A (COL3A), fibronectin, and matrix metalloproteinase 9 (MMP9) in liver were examined by real-time RT-PCR and normalized for U36B4 expression.
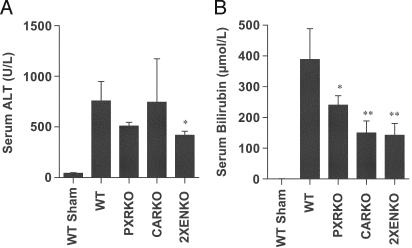
BDL-Induced Hepatopathology and Cholestasis. Serum ALT was significantly elevated in all BDL animals, consistent with the expected cholestatic injury, but the mean ALT was significantly lower in the 2XENKO group than other genotypes after BDL (Fig. 2A). Similarly, although serum total bilirubin was significantly elevated in all BDL animals compared with shams, bilirubin was lower after BDL in all nuclear receptor-null mice than WT mice (Fig. 2B). Surprisingly, these results and the mortality data suggest that nuclear receptor deletion may confer some protection in this model of cholestasis.
Fig. 2.
Evidence of liver injury as determined by serum biochemistry. Serum was collected 6 days after BDL or sham operation in WT, CARKO, PXRKO, and 2XENKO mice (n = 4–6 per group). ALT was significantly elevated in all BDL animals compared with shams, but was lower in 2XENKO than WT mice (P < 0.05). Total serum bilirubin was also significantly elevated in all BDL animals compared with shams. However, serum bilirubin was lower in all knockouts than WT mice after BDL; *, P < 0.05; **, P < 0.01 relative to WT BDL.
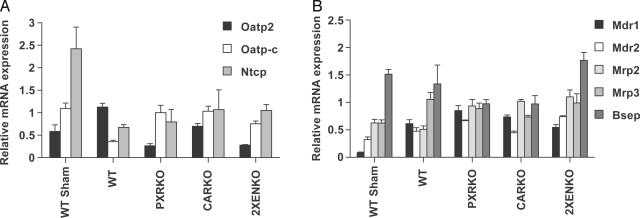
Expression of Genes Associated with Bile Acid Transport. Decreased expression of the hepatic basolateral membrane transporters sodium-taurocholate cotransporting polypeptide (Ntcp; Slc10a1) and Oatp-c is an early defense mechanism by which liver cells shut off bile acid and bilirubin influx during cholestasis (18). After BDL, mRNA expression of both genes was repressed in WT animals (Fig. 3A), and Ntcp was down-regulated in all genotypes, consistent with the proposed bile acid-mediated induction of the FXR/SHP (small heterodimer partner, NR1I0) pathway to repress Ntcp by means of hepatocyte nuclear factor 1α (19). In contrast, there was complete loss of repression of Oatp-c in the PXRKO and CARKO genotypes after BDL, indicating codependence on both receptors. This finding is important, because Oatp-c has been implicated in hepatic uptake of bilirubin by means of the sinusoidal surface of the hepatocyte (20), and we found lower serum bilirubin concentrations in the knockout mice, which would be consistent with increased hepatic uptake of bilirubin. The related Na+-dependent organic anion transporter 2 (Oatp2) gene was induced 2-fold in WT BDL animals but failed to be induced in either PXR or CAR-null mice, which may be beneficial to the hepatocyte because Oatp2 also mediates bile acid influx.
Fig. 3.
Expression of genes associated with bile acid transport. Livers were removed from WT, CARKO, PXRKO and 2XENKO mice (n = 4–6 per group) 6 days after BDL or sham operation. (A) Relative expression of mRNA for basolateral (sinusoidal) transporters. (B) Relative expression of mRNA for ABC membrane transporters.
Adaptive overexpression of canalicular ATP-binding cassette (ABC) transporters, which pump bile salts, bilirubin diglucuronides, glutathione conjugates, and various organic ions into bile, has been demonstrated in humans with cholestatic liver disease (21) and BDL mice (17), so we evaluated the role of PXR and CAR in mediating these potentially protective changes (Fig. 3B). Multidrug resistance-associated protein-2 (Mrp2; ABCC2) was not induced after BDL in the WT mouse, but modest induction was seen in the knockout mice. This finding may be consistent with a postulated role for PXR in Mrp2 repression (22), or alternatively could represent an adaptive response to increased concentrations of bilirubin in the hepatocyte. Multidrug resistance-associated protein 3 (Mrp3; ABCC3) is a basolateral transporter that has been proposed to have a role as a bile acid efflux system in cholestasis (17). Mrp3 mRNA expression was induced after BDL, but this induction was reduced in the CARKO mice, which may increase the hepatocyte's exposure to bile acids in this genotype (Fig. 3B). Multidrug resistance proteins 1 and 2 (Mdr1 and -2; ABCB1 and -B4) mRNA expression was significantly induced after BDL, but, surprisingly, induction was independent of either PXR or CAR (Fig. 3B). Although Mdr1 can be induced by PXR (23, 24), the present results show that toxic endobiotics such as bile acids can induce these transporters by an alternative pathway.
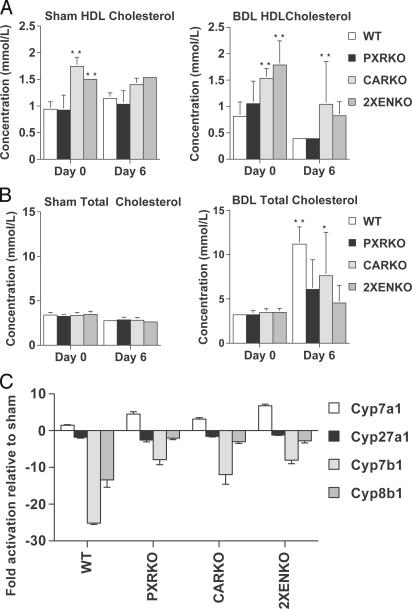
Lipids. Cholestatic liver disease in humans is associated with lipid abnormalities, including high total serum cholesterol and low HDL cholesterol (25). We therefore measured serial lipid concentrations to evaluate the roles of PXR and CAR in lipid homeostasis. Serum triglyceride concentrations were similar in all genotypes. However, HDL cholesterol was higher in sham CARKO and 2XENKO than WT or PXRKO mice (P < 0.0001), and decreased on day 6 after BDL in all genotypes (Fig. 4A). However, despite some variability, HDL remained significantly higher in CARKO than WT mice on day 6 after BDL (P = 0.005). Serum total cholesterol was similar in all genotypes of sham and BDL day 0 mice (Figs. 4B), and increased significantly by a mean of 7.9 ± 0.6 mmol/liter (P = 0.00) in WT BDL animals by day 6. However, after BDL, the magnitude of the increase in total cholesterol was reduced in CARKO (4.2 ± 1.5 mmol/liter; P = 0.046) mice, and was not significant in PXRKO (2.8 ± 0.9 mmol/liter) or 2XENKO (1.1 ± 0.5 mmol/liter) mice (Fig. 4B). Therefore, deletion of either PXR or CAR attenuates the expected increase in serum total cholesterol in cholestasis, and CAR deletion also raises HDL, resulting in a more favorable overall lipid profile.
Fig. 4.
Serum lipids and expression of bile acid synthesis genes after BDL. (A) Serum HDL cholesterol is increased in CARKO and 2XENKO mice. Blood samples were collected from the tail veins of anesthetized mice before BDL or sham operation on day 0, and again on day 6 immediately before killing. HDL cholesterol was significantly higher in CARKO and 2XENKO mice than WT mice (**, P < 0.01). (B) Changes in total serum cholesterol after BDL or sham operation. Total serum cholesterol increased significantly after BDL in WT (**, P < 0.01) and CARKO (*, P = 0.046) mice but not PXRKO or 2XENKO mice. (C) Relative expression of mRNA in liver for genes involved in bile acid synthesis after BDL. Data are expressed as the fold change relative to sham-operated animals.
Expression of Genes Involved in Bile Acid Synthesis. Bile acid synthesis from cholesterol is tightly regulated to ensure that sufficient amounts of cholesterol are catabolized to maintain homeostasis and to provide emulsification in the intestine. The roles of PXR and CAR in regulation of this pathway have not been clearly defined, so we quantified expression of all major genes involved in bile acid synthesis in liver. Cyp7a1 catalyzes the rate-limiting first step of the classical bile acid synthetic pathway and was significantly induced in PXR and CAR-null mice compared with WT mice after BDL (Fig. 4C). Although Cyp7b1 and Cyp8b1 were markedly repressed in WT mice after BDL, there was significant loss of this repression in the null genotypes, suggesting that PXR and CAR coordinately act to repress bile acid synthesis at several levels (Fig. 4C). Therefore, the overall effect from deletion of PXR or CAR in cholestasis is a relative increase in expression of the major enzymes Cyp7a1, Cyp7b1, and Cyp8b1, which would be expected to increase the rate of conversion of cholesterol to bile acids.
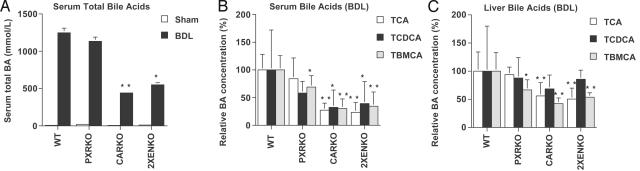
Bile Acid Concentrations Are Reduced in CAR-Null BDL Animals. Serum total bile acid concentrations were similar in all sham animals and increased 50- to 100-fold in all genotypes after BDL (Fig. 5A). In WT BDL mice, significant increases were seen in the primary bile acids, including conjugates of chenodeoxycholic acid, cholic acid, and hydroxylated bile acids (α-MCA and β-MCA) (data not shown and reported in ref. 11). However, in the CARKO and 2XENKO BDL mice, there was a significant reduction in both serum total bile acid (Fig. 5A) and individual bile acid concentrations (Fig. 5B), relative to WT BDL. Similar results were also observed in liver for tauro-cholic acid (TCA) and tauro-β-muricholic acid (Tβ-MCA), but not taurochenodeoxycholic acid (TCDCA) (Fig. 5C). In the PXRKO mice, a relative decrease in the hydroxylated bile acid Tβ-MCA was observed in serum and liver (Figs. 5 B and C). Concentrations of secondary bile acids, including LCA, were low in all groups after BDL, consistent with interruption of the enterohepatic circulation. In bile, the CARKO results were similar to those in liver, but the 2XENKO BDL group differed substantially from other genotypes, with high concentration of all bile acids (Fig. 6, which is published as supporting information on the PNAS web site). Surprisingly, therefore, deletion of CAR in cholestasis significantly reduces the accumulation of bile acids in serum, liver, and bile. This finding could be potentially beneficial in the context of cholestasis, where bile acid overload is the primary cause of both systemic symptoms and hepatotoxicity.
Fig. 5.
Primary bile acid concentrations are reduced in CAR-null BDL animals. (A) Total serum bile acids were measured in WT, CARKO, PXRKO, and 2XENKO mice (n = 4–6 per group) after BDL or sham operation. Total serum bile acids increased significantly after BDL in all genotypes (P < 0.001), but were reduced relative to WT BDL in CARKO and 2XENKO BDL mice. (B) Individual serum bile acids were quantitated by LC/MS 6 days after BDL. Serum TCA, Tβ-MCA, and TCDCA were significantly reduced in CARKO and 2XENKO mice, and Tβ-MCA was reduced in PXRKO relative to WT BDL. (C) Individual hepatic bile acids were quantitated by LC/MS 6 days after BDL. TCA and Tβ-MCA were significantly reduced in CARKO and 2XENKO mice, and Tβ-MCA was significantly reduced in PXRKO relative to WT BDL. *, P < 0.05; **, P < 0.01 for all panels.
Discussion
This work utilizes BDL in WT and knockout animals to increase endogenous primary bile acids to model the role of PXR and CAR in mediating responses to cholestasis and biliary obstruction. These data demonstrate that loss of either PXR or CAR increases the liver's vulnerability to bile acid-induced liver injury, and that neither receptor is sufficient to compensate for the loss of the other, although they have some functional overlap. However, despite an increase in hepatic necrosis, we found that double knockout mice showed an improvement in mortality and markers for liver injury, including serum ALT and bilirubin, and a decrease in hepatic expression of genes involved in fibrosis and remodeling, suggesting that deletion of these nuclear receptors in cholestasis may not be as harmful as expected.
The loss of CAR has a dramatic effect on bile acid concentrations in this model of obstructive cholestasis. Both the CARKO and 2XENKO mice had substantially reduced serum total bile acids compared with WT mice after BDL, with concurrent decreases in all major individual bile acids. These findings are unlikely to result from a bile acid transporter defect, which would lead to hepatic accumulation of bile acids; indeed, we also found a relative decrease in TCA and Tβ-MCA in liver. We found no evidence of increased urinary bile acid excretion, and increased sulfation or glucuronidation of bile acids is also unlikely to account for the relative differences, because total serum bile acid concentrations decreased to a similar extent. These findings are therefore most consistent with a reduction in overall bile acid synthesis in CAR-null genotypes.
However, despite the relative decrease in bile acid concentrations, we have not found a corresponding decrease in expression of genes involved in bile acid synthesis in CARKO mice. Conversely, all knockout animals had relative induction of Cyp7a1, and loss of repression of Cyp7b1 and Cyp8b1, which would be expected to increase bile acid synthesis. This result suggests that, in cholestasis, PXR and CAR play a role in coordinate repression of these major genes involved in bile acid synthesis. However, deletion of CAR must have other effects on the cholesterol and bile acid metabolic pathways that results in the observed decrease in bile acid concentrations despite induction of these genes.
A relative decrease in cholesterol as a substrate for bile acid synthesis could explain the lower bile acid concentrations in CARKO BDL mice. We observed attenuation of the expected increase in serum total cholesterol in both PXRKO and CARKO mice after BDL, and an increase in HDL cholesterol in CARKO mice, resulting in a substantially more favorable lipid profile in this genotype. However, PXRKO mice did not have the same relative decrease in bile acid concentrations, despite also having lower serum total cholesterol than WT BDL mice, so this finding may not fully explain the differences in bile acids. Alternatively, cholesterol could be diverted into oxysterol production rather than down the classic bile acid synthetic pathway, because induction of CH25H was observed in all BDL animals, despite repression of Cyp27a1. This explanation would account for the presence of increased hepatic necrosis despite lower bile acid concentrations, because oxysterols are cytotoxic and may induce apoptosis (26). Repression of Cyp7b1 is generally suggestive of a decrease rather than an increase in oxysterol concentrations (27); however, a marked loss of repression of Cyp7b1 was observed in knockout animals and could be consistent with a relative increase in substrate for this enzyme.
These findings raise the intriguing possibility that a CAR antagonist may potentially have beneficial effects in the context of hypercholesterolemia or early cholestasis, where a reduction of bile acid concentrations to within normal limits may prevent progressive liver disease, despite some loss of other protective mechanisms. We did find an increase in hepatic necrosis in the nuclear receptor-null mice, although mortality and other end-points were improved in the double knockouts, and both PXR and CAR have been implicated in mediating a number of adaptive responses to bile acid overload (7, 8, 12, 28). In the present work, we describe several additional findings that further elucidate the role of PXR and CAR in detoxification and transport of bile acids, bilirubin, and xenobiotics.
We found lower concentrations of the major 6β-hydroxylated bile acid Tβ-MCA in both PXR and CAR-null mice compared with WT mice after BDL. This finding is significant because ring hydroxylation of bile acids renders them less hydrophobic, and therefore less toxic and more accessible to excretory transporters (29). Our results suggest that loss of PXR or CAR impairs this important mechanism for bile acid detoxification, and the relative preservation of hepatic TCDCA that we observed may be explained by reduced conversion of chenodeoxycholic acid to β-MCA. It is noteworthy that CYP3A4, a PXR and CAR target gene, mediates the equivalent 6α-hydroxylation of bile acids in humans (7, 30), suggesting the importance of these receptors in feed-forward bile acid metabolism.
Hepatic ABC transporters play a crucial role in regulating hepatocellular bile acid concentrations because they mediate both uptake and efflux mechanisms, and adaptive changes in expression of these transporters have been demonstrated in cholestasis (17, 21). Decreased expression of the two major basolateral uptake systems, Ntcp and Oatp-c, is an early defense mechanism to reduce bile acid influx into hepatocytes in cholestasis (19), and is seen in WT BDL animals. Our data show that bile acid-induced repression of Oatp-c depends on both PXR and CAR. This finding is important, because failure to down-regulate Oatp-c may allow transport of bilirubin from serum into the liver by means of the sinsusoidal surface of hepatocytes (20). This bilirubin influx would increase liver damage while decreasing serum bilirubin levels. It has previously been proposed that CAR mediates bilirubin induction of Oatp-c and other enzymes that induce bilirubin clearance (31). The present data suggest that, in cholestasis-induced hyperbilirubinemia, bilirubin clearance from serum is delayed in WT animals, because of bile acid-induced down-regulation of Oatp-c mediated by PXR and CAR.
The Mdr1 gene product P-glycoprotein transports hydrophobic xenobiotics and peptides from the inside to the outside of cells. Induction of Mdr1 is therefore an important cellular protective mechanism, and induction by xenobiotics has been shown to be PXR-dependent in human hepatocytes and in mice (23, 24). However, we have shown that induction of Mdr1 in response to cholestasis occurs independently of PXR, suggesting that endogenous ligands such as bile acids are capable of inducing this important transporter by alternative pathways. It is therefore an important priority to identify whether other nuclear receptors are involved in regulation of Mdr1, because regulation of this transporter will have significant implications for chemotherapy treatments and drug interactions.
In summary, we have used a BDL model of cholestasis to show that PXR and CAR have crucial roles in the regulation of lipid and bile acid homeostasis. CARKO mice have a marked relative reduction in bile acid concentrations after BDL, despite a relative increase in expression of major genes involved in bile acid synthesis. Serum lipids are also favorably affected by nuclear receptor deletion, with attenuation of the expected increase in total cholesterol in both genotypes and increased HDL cholesterol in CARKO. Loss of either receptor increases the liver's vulnerability to cholestasis because of a loss of adaptive mechanisms, including loss of repression of Oatp-c and reduced 6β-hydroxylation of bile acids. However, induction of Mdr1 in cholestasis is independent of both PXR and CAR. These findings have important therapeutic implications for lipid disorders and cholestatic liver diseases and suggest the potential for selective CAR antagonists as a future treatment strategy.
Supplementary Material
Acknowledgments
R.M.E. is an Investigator of the Howard Hughes Medical Institute at The Salk Institute and March of Dimes Chair in Molecular and Developmental Biology. This work was supported by project grants from the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, by Nuclear Receptor Signaling Atlas (NURSA) Orphan Receptor Grant U19DK62434, and by the National Health and Medical Research Council of Australia (NHMRC). Additional support was given by the Howard Hughes Medical Institute. C.A.M.S. was a recipient of financial support from the Clinical Hepatology Trust Fund, Westmead Hospital, a Westmead Millennium Foundation Initiating Grant, New Zealand Gastroenterology Society/Ferring Pharmaceuticals Research Fellowship, and an NHMRC of Australia Postgraduate Medical Research Scholarship.
Author contributions: C.A.M.S., C.L., and M.D. designed research; C.A.M.S., C.L., S.A.C., J.G.A.A., and M.D. performed research; C.A.M.S., C.L., S.A.C., J.S., D.D.M., and M.D. contributed new reagents/analytic tools; C.A.M.S., C.L., S.A.C., J.S., and M.D. analyzed data; and C.A.M.S., C.L., R.M.E., and M.D. wrote the paper.
Abbreviations: ALT, alanine aminotransferase; β-MCA, β-muricholic acid; BDL, bile duct ligation; CAR, constitutive androstane receptor; CARKO, CAR knockout; Cyp7a1, cholesterol 7α-hydroxylase; LCA, lithocholic acid; Mdr1, multidrug resistance protein 1; Ntcp sodium-taurocholate cotransporting polypeptide; Oatp-c organic anion transporting polypeptide C; PXR, pregnane X receptor or steroid and xenobiotic receptor; PXRKO, PXR knockout; Tβ-MCA, tauro-β-muricholic acid; TCA, tauro-cholic acid; TCDCA, taurochenodeoxycholic acid; 2XENKO, PXR and CAR knockout; FXR, farnesoid X receptor; HDL, high-density lipoprotein; ABC, ATP-binding cassette.
References
- 1.Chiang, J. Y. (2002) Endocr. Rev. 23, 443–463. [DOI] [PubMed] [Google Scholar]
- 2.Blumberg, B. & Evans, R. M. (1998) Genes Dev. 12, 3149–3155. [DOI] [PubMed] [Google Scholar]
- 3.Kliewer, S. A., Moore, J. T., Wade, L., Staudinger, J. L., Watson, M. A., Jones, S. A., McKee, D. D., Oliver, B. B., Willson, T. M. & Zetterstrom, R. H., (1998) Cell 92, 73–82. [DOI] [PubMed] [Google Scholar]
- 4.Choi, H. S., Chung, M., Tzameli, I., Simha, D., Lee, Y. K., Seol, W. & Moore, D. D. (1997) J. Biol. Chem. 272, 23565–23571. [DOI] [PubMed] [Google Scholar]
- 5.Sonoda, J., Rosenfeld, J. M., Xu, L., Evans, R. M. & Xie, W. (2003) Curr. Drug Metab. 4, 59–72. [DOI] [PubMed] [Google Scholar]
- 6.Goodwin, B., Hodgson, E., D'Costa, D. J., Robertson, G. R. & Liddle, C. (2002) Mol. Pharmacol. 62, 359–365. [DOI] [PubMed] [Google Scholar]
- 7.Xie, W., Radominska-Pandya, A., Shi, Y., Simon, C. M., Nelson, M. C., Ong, E. S., Waxman, D. J. & Evans, R. M. (2001) Proc. Natl. Acad. Sci. USA 98, 3375–3380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Staudinger, J. L., Goodwin, B., Jones, S. A., Hawkins-Brown, D., MacKenzie, K. I., LaTour, A., Liu, Y., Klaassen, C. D., Brown, K. K., Reinhard, J., et al. (2001) Proc. Natl. Acad. Sci. USA 98, 3369–3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bachs, L., Pares, A., Elena, M., Piera, C. & Rodes, J. (1992) Gastroenterology 102, 2077–2080. [DOI] [PubMed] [Google Scholar]
- 10.Wietholtz, H., Marschall, H. U., Sjovall, J. & Matern, S. (1996) J. Hepatol. 24, 713–718. [DOI] [PubMed] [Google Scholar]
- 11.Stedman, C., Robertson, G., Coulter, S. & Liddle, C. (2004) J. Biol. Chem. 279, 11336–11343. [DOI] [PubMed] [Google Scholar]
- 12.Guo, G. L., Lambert, G., Negishi, M., Ward, J. M., Brewer, H. B., Jr., Kliewer, S. A., Gonzalez, F. J., Sinal, C. J., Ourlin, J. C., Lasserre, F., et al. (2003) J. Biol. Chem. 278, 45062–45071. [DOI] [PubMed] [Google Scholar]
- 13.Saini, S. P., Sonoda, J., Xu, L., Toma, D., Uppal, H., Mu, Y., Ren, S., Moore, D. D., Evans, R. M. & Xie, W. (2004) Mol. Pharmacol. 65, 292–300. [DOI] [PubMed] [Google Scholar]
- 14.Lew, J. L., Zhao, A., Yu, J., Huang, L., De Pedro, N., Pelaez, F., Wright, S. D. & Cui, J. (2004) J. Biol. Chem. 279, 8856–8861. [DOI] [PubMed] [Google Scholar]
- 15.Xie, W., Barwick, J. L., Downes, M., Blumberg, B., Simon, C. M., Nelson, M. C., Neuschwander-Tetri, B. A., Brunt, E. M., Guzelian, P. S. & Evans, R. M. (2000) Nature 406, 435–439. [DOI] [PubMed] [Google Scholar]
- 16.Wei, P., Zhang, J., Egan-Hafley, M., Liang, S. & Moore, D. D. (2000) Nature 407, 920–923. [DOI] [PubMed] [Google Scholar]
- 17.Wagner, M., Fickert, P., Zollner, G., Fuchsbichler, A., Silbert, D., Tsybrovskyy, O., Zatloukal, K., Guo, G. L., Schuetz, J. D., Gonzalez, F. J., et al. (2003) Gastroenterology 125, 825–838. [DOI] [PubMed] [Google Scholar]
- 18.Trauner, M. & Boyer, J. L. (2003) Physiol. Rev. 83, 633–671. [DOI] [PubMed] [Google Scholar]
- 19.Jung, D. & Kullak-Ublick, G. A. (2003) Hepatology 37, 622–631. [DOI] [PubMed] [Google Scholar]
- 20.Cui, Y., Konig, J., Leier, I., Buchholz, U. & Keppler, D. (2001) J. Biol. Chem. 276, 9626–9630. [DOI] [PubMed] [Google Scholar]
- 21.Zollner, G., Fickert, P., Silbert, D., Fuchsbichler, A., Marschall, H. U., Zatloukal, K., Denk, H. & Trauner, M. (2003) J. Hepatol. 38, 717–727. [DOI] [PubMed] [Google Scholar]
- 22.Kast, H. R., Goodwin, B., Tarr, P. T., Jones, S. A., Anisfeld, A. M., Stoltz, C. M., Tontonoz, P., Kliewer, S., Willson, T. M. & Edwards, P. A. (2002) J. Biol. Chem. 277, 2908–2915. [DOI] [PubMed] [Google Scholar]
- 23.Maglich, J. M., Stoltz, C. M., Goodwin, B., Hawkins-Brown, D., Moore, J. T. & Kliewer, S. A. (2002) Mol. Pharmacol. 62, 638–646. [DOI] [PubMed] [Google Scholar]
- 24.Synold, T. W., Dussault, I. & Forman, B. M. (2001) Nat. Med. 7, 584–590. [DOI] [PubMed] [Google Scholar]
- 25.Longo, M., Crosignani, A., Battezzati, P. M., Squarcia Giussani, C., Invernizzi, P., Zuin, M. & Podda, M. (2002) Gut 51, 265–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tabas, I. (2002) J. Clin. Invest. 110, 905–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Norlin, M. & Chiang, J. Y. (2004) Biochem. Biophys. Res. Commun. 316, 158–164. [DOI] [PubMed] [Google Scholar]
- 28.Zhang, J., Huang, W., Qatanani, M., Evans, R. M. & Moore, D. D. (2004) J. Biol. Chem. 279, 49517–49522. [DOI] [PubMed] [Google Scholar]
- 29.Hofmann, A. F. (1988) in The Liver: Biology and Pathobiology, eds. Arias, I. M., Jacoby, W. B., Popper, H., Schachter, D. & Shafritz, D. A. (Raven, New York), pp. 553–572.
- 30.Araya, Z. & Wikvall, K. (1999) Biochim. Biophys. Acta 1438, 47–54. [DOI] [PubMed] [Google Scholar]
- 31.Huang, W., Zhang, J., Chua, S. S., Qatanani, M., Han, Y., Granata, R. & Moore, D. D. (2003) Proc. Natl. Acad. Sci. USA 100, 4156–4161. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.