Abstract
Purpose
NRG Oncology RTOG 9202 was a randomized trial testing long-term adjuvant androgen deprivation (LTAD) versus initial androgen deprivation only (STAD) with external beam radiation therapy (RT) in mostly high-risk and some intermediate-risk prostate cancer patients. RTOG 9408 found an overall survival (OS) advantage in patients with cT1b-T2b disease and prostate-specific antigen (PSA) <20 ng/mL, with benefit observed mostly among intermediate-risk patients. It was still unknown whether intermediate-risk patients would experience an additional survival benefit with LTAD; thus, we performed a secondary analysis to explore whether LTAD had any incremental benefit beyond STAD among the intermediate-risk subset of RTOG 9202. The study endpoints were OS, disease-specific survival (DSS), and PSA failure (PSAF).
Methods and Materials
An analysis was performed for all patients enrolled in RTOG 9202 defined as intermediate-risk (cT2 disease, PSA<10 ng/mL, and Gleason score = 7 or cT2 disease, PSA 10-20 ng/mL, and Gleason score <7). This review yielded 133 patients: 74 (STAD) and 59 (LTAD). The Kaplan-Meier method was used to estimate OS; the cumulative incidence approach was used to estimate DSS and PSAF. A 2-sided test was used, with significance level defined to be .05.
Results
With over 11 years of median follow-up, 39 STAD patients were alive and 33 LTAD patients were alive. There was no difference in OS (10-year estimates, 61% STAD vs 65% LTAD; P=.53), DSS (10-year DSS, 96% vs 97%; P=.72), or PSAF (10-year PSAF, 53% vs 55%; P=.99) between groups.
Conclusion
LTAD did not confer a benefit in terms of OS, DSS, or PSAF rates in the intermediate-risk subset in this study. Whereas the subset was relatively small, treatment assignment was randomly applied, and a trend in favor of LTAD would have been of interest. Given the small number of disease-specific deaths observed and lack of benefit with respect to our endpoints, this secondary analysis does not suggest that exploration of longer hormonal therapy is worth testing in the intermediate-risk prostate cancer subset.
Introduction
Testosterone blockade has long been established as an integral part of prostate cancer therapy, particularly advanced-stage disease (1, 2). The rationale to investigate prolonged ADT came primarily from research in animal models, where it was shown that maximal tumor cytoreduction with the use of hormonal therapy provided improved radiation therapy (RT) outcomes attributed to supra-additive effects between these 2 treatments (3). In the 1990s, reversible androgen suppression with the use of luteinizing hormone-releasing hormone analogues and oral antiandrogen agents was shown to induce apoptotic regression in androgen-responsive cancers, potentially improving the prospects of local control and the duration of survival free of metastatic disease (4). Among patients with locally advanced disease, phase 3 clinical trials showed that when added to RT, long-term androgen deprivation (LTAD) improved overall survival (OS) (5, 6) but also increased toxic effects, including erectile dysfunction and myocardial infarction (7).
Short-term androgen deprivation therapy (STAD) could potentially mitigate these toxic effects. In 2003, Hanks et al published the first-analysis results of NRG Oncology RTOG 9202, which randomized 1554 patients with T2c-4 prostate adenocarcinoma to 4 months of neoadjuvant and concomitant androgen blockade followed by 2 years of additional blockade versus no further therapy (8). These results demonstrated a benefit for all study endpoints except OS, with the exception of the subset of patients who had a Gleason score of 8 to 10, who did show a benefit in OS. Long-term (10-year) follow-up confirmed the 5-year results (9). In 2011, Jones et al (10) published the results of RTOG 9408, which indicated that for intermediate-risk disease, 4 months of neoadjuvant and concomitant androgen blockade improved survival in comparison with no androgen deprivation therapy. This study included mostly patients with intermediate-risk disease. RTOG 9408 found an OS advantage in patients with T1b-T2b disease with prostate-specific antigen (PSA) ≤20 ng/mL, with the bulk of the benefit observed among intermediate-risk patients. Whereas STAD was validated in RTOG 9408, it is not known whether patients in the intermediate-risk subset would experience an additional survival benefit with longer hormonal therapy. The question that has not been addressed is whether or not patients with intermediate-risk disease would derive equal benefit from STAD compared with LTAD. Whereas there has been no randomized trial to date comparing these 2 therapies, a significant subset of intermediate-risk patients was included in the NRG Oncology RTOG 9202 cohort that may be able to address this question. In this secondary analysis of NRG Oncology RTOG 9202, we evaluate whether the subset of patients classified as intermediate-risk who were included in this study derived any incremental benefit of long-term androgen deprivation beyond 6 months of androgen blockade.
Methods and Materials
NRG Oncology RTOG 9202 included prostate cancer patients with T2c-T4 prostate cancer with no extra pelvic lymph node involvement and PSA <150 ng/mL. All institutions obtained institutional review board approval before patient recruitment, and all patients signed approved informed consent forms before trial enrollment. All patients received 4 months of goserelin and flutamide before and during RT. They were randomized to no further ADT (short-term ADT [STAD] RT) or 24 months of goserelin (long-term ADT [LTAD] RT), as previously described (Fig. E1; available online at www.redjournal.org) (8, 9). RT consisted of 45 Gy to the pelvic nodes and 65 to 70 Gy to the prostate. In our secondary analysis report (SAR), we obtained information from patients who were enrolled in NRG Oncology RTOG 9202 defined as having intermediate-risk disease using the following parameters: (T2 and PSA <10 ng/mL and Gleason score = 7) or (T2 and PSA 10-20 ng/mL and Gleason score <7).
Statistical methods
The primary study endpoint was OS, with the survival time defined as the time interval from the date of randomization to the date of death or the date of last follow-up visit (censored). With regard to the second study endpoint, disease-specific survival (DSS), the failure event was defined as a death resulting from prostate cancer, treatment toxicity, or unknown causes with distant metastasis or bone metastasis within any time. Finally, the third study endpoint, PSA failure (PSAF), was defined as occurring after 3 consecutive PSA rises after a nadir had been reached, the administration of hormone therapy for a rising PSA level, or a posttreatment PSA nadir level >4.0 ng/mL. The date of biochemical failure was halfway between the nadir and the date of first rise or any rise great enough to provoke the initiation of therapy (per ASTRO Consensus Definition) (11). The Kaplan-Meier method was used to estimate the survival rate for OS, with the log-rank test used to compare the difference between 2 treatment arms (12, 13). The cumulative incidence approach was used to estimate the rates of DSS and PSAF, with the Gray test used to test the significance between the 2 treatment arms (14). Two-sided tests were used at a significance level of .05.
Results
A total of 1554 patients were entered. The median follow-up time for all surviving patients was 11.31 and 11.27 years for the 2 arms (9). There were a total of 133 patients in the intermediate-risk subgroup defined by the SAR: 74 in the STAD + RT arm and 59 in the LTAD + RT arm. Table 1 describes the patients’ pretreatment characteristics. No marked differences were observed in patient or tumor pretreatment characteristics. Comparisons between the Gleason scores assigned by the institution and the central review have previously been described (8, 9). A comparison between the Gleason scores assigned by the institution and the central review specifically for the intermediate-risk patient subgroup of NRG Oncology RTOG 9202 is listed in Table 2. Table 3 summarizes the quality control review and morbidity data for both RT and ADT (STAD-RT and LTAD-RT) for the studied intermediate-risk subgroup of patients in NRG Oncology RTOG 9202. The median follow-up time was 11 years for the included patients. At the time of the SAR, 39 patients were alive in the STAD group and 33 were alive in the LTAD group.
Table 1.
Pretreatment characteristics
| Characteristic | STAD (n=74) | LTAD (n=59) |
|---|---|---|
| Age (y), P=.27* | ||
| Mean | 70.1 | 71.0 |
| SD | 6.3 | 6.0 |
| Median | 71 | 73 |
| Minimum-Maximum | 49-81 | 49-80 |
| Q1-Q3 | 67-74 | 68-75 |
| Race, P=1.00† | ||
| White | 63 (85.1%) | 50 (84.7%) |
| Hispanic | 3 (4.1%) | 0 (0.0%) |
| African American | 7 (9.5%) | 9 (15.3%) |
| Native Hawaiian or other Pacific Islander | 1 (1.4%) | 0 (0.0%) |
| KPS, P=.60† | ||
| 70 | 1 (1.4%) | 0 (0.0%) |
| 80 | 4 (5.4%) | 3 (5.1%) |
| 90 | 38 (51.4%) | 28 (47.5%) |
| 100 | 31 (41.9%) | 28 (47.5%) |
| PSA, P=.06† | ||
| <10 ng/mL | 27 (36.5%) | 12 (20.3%) |
| 10-20 ng/mL | 47 (63.5%) | 47 (79.7%) |
| Gleason score, P=.12‡ | ||
| 2-5 | 25 (33.8%) | 24 (40.7%) |
| 6 | 22 (29.7%) | 23 (39.0%) |
| 7 | 27 (36.5%) | 12 (20.3%) |
| Intercurrent disease, | (n=72) | (n=58) |
| P=1.00† | ||
| No | 19 (26.4%) | 15 (25.9%) |
| Yes | 53 (73.6%) | 43 (74.1%) |
| Clinical stage | ||
| T2 | 74 (100.0%) | 59 (100.0%) |
| Pathologic nodal status, | ||
| P=.26† | ||
| N0 | 9 (12.2%) | 4 (6.8%) |
| N1 | 1 (1.4%) | 0 (0.0%) |
| NX | 64 (86.5%) | 55 (93.2%) |
Abbreviations: KPS = Karnofsky performance status; PSA = prostate-specific antigen; Q1 = first quartile; Q3 = third quartile; SD = standard deviation.
Wilcoxon rank-sum test.
Fisher exact test: for race, white versus others; for KPS, 100 versus others; for nodal status, NX versus others.
Pearson χ2 test.
Table 2.
NRG Oncology RTOG 9202 Gleason score provided by participating institutions compared with Gleason score from central review: intermediate-risk patients
| Gleason score, provided by institutions | Central review | Gleason score | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| 8–10 | 7 | 6 | 2–5 | Unknown | Total | |
| 8–10 | 0 | 0 | 0 | 0 | 0 | 0 |
| 7 | 2 | 25 | 2 | 0 | 10 | 39 |
| 6 | 2 | 17 | 12 | 0 | 14 | 45 |
| 2–5 | 0 | 7 | 17 | 6 | 19 | 49 |
| Unknown | 0 | 0 | 0 | 0 | 0 | 0 |
| Total | 4 | 49 | 31 | 6 | 43 | 133 |
Table 3.
NRG Oncology RTOG 9202 quality control review and morbidity: intermediate-risk patients
| Factor | STAD-RT
|
LTAD-RT
|
||
|---|---|---|---|---|
| n | % | n | % | |
| Assessable patients | 74 | 59 | ||
| Radiation quality review: unacceptable major variation | 3 | 4.1 | 1 | 1.7 |
| Androgen deprivation quality review: unacceptable major variation | 0 | 0.0 | 4 | 6.7 |
| Acute radiation toxicity grade | ||||
| 3 | 6 | 8.1 | 2 | 3.4 |
| 4 | 1 | 1.4 | 1 | 1.7 |
| 5 | 0 | 0.0 | 0 | 0.0 |
| Androgen deprivation toxicity grade | ||||
| 3 | 4 | 5.4 | 3 | 5.1 |
| 4 | 0 | 0.0 | 0 | 0.0 |
| 5 | 0 | 0.0 | 0 | 0.0 |
| Late gastrointestinal radiation toxicity grade | ||||
| 3 | 0 | 0.0 | 2 | 3.4 |
| 4 | 0 | 0.0 | 1 | 1.7 |
| 5 | 0 | 0.0 | 0 | 0.0 |
The result of the primary study endpoint shows no difference in OS (10-year estimates: 61% STAD [95% CI: 50–73] vs 65% LTAD [95% CI: 52–77], P=.53) between the STAD and LTAD groups, shown in Figure 1. There were a total of only 4 failures in the STAD group and 3 in the LTAD group, with a 10-year DSS of 96% (95% CI: 91–100) in STAD and 97% (95% CI: 92–100) in LTAD (P=.72), shown in Figure 2. As previously described, DSS was defined as a death resulting from prostate cancer, treatment toxicity, or unknown causes of death with reported progressive prostate disease before death; whereas other causes of death were classified into cardiovascular, respiratory, neurologic, gastrointestinal, genitourinary, or other (8). In this study, PSAF occurred in 38 patients in the STAD group and 33 in the LTAD group, with 10-year PSAF rates of 53% (95% CI: 41-65) and 55% (95% CI: 42-68), respectively (P=.99), shown in Figure 3.
Fig. 1.
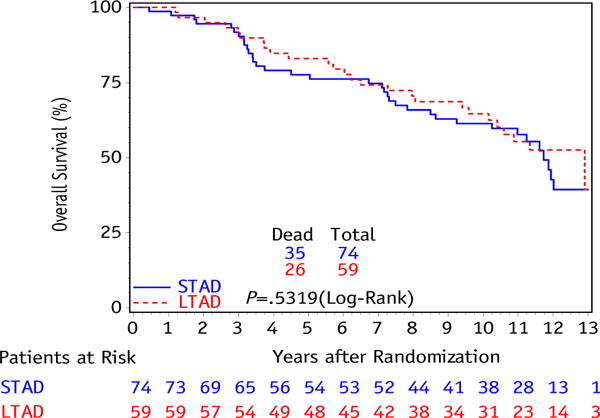
Kaplan-Meier curve for overall survival between initial androgen deprivation only (STAD) and long-term adjuvant androgen deprivation (LTAD) for intermediate-risk patient subset.
Fig. 2.
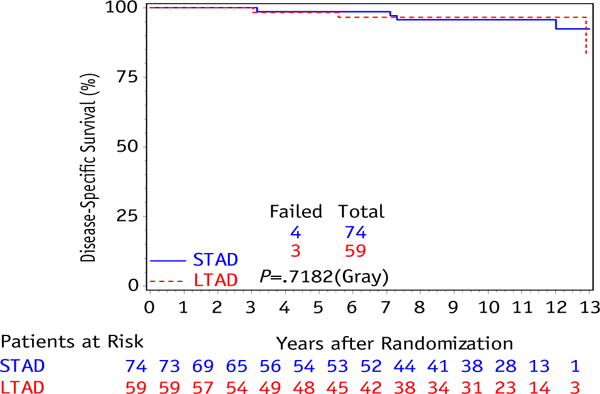
Cumulative incidence curve for disease-specific survival between initial androgen deprivation only (STAD) and long-term adjuvant androgen deprivation (LTAD) for intermediate-risk patient subset.
Fig. 3.
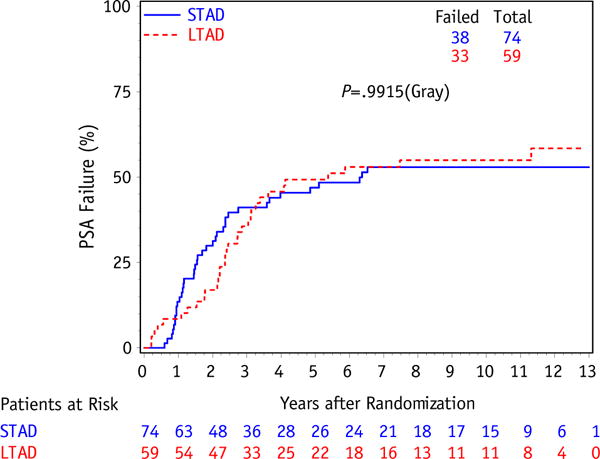
Cumulative incidence curve for PSA failure between initial androgen deprivation only (STAD) and long-term adjuvant androgen deprivation (LTAD) for intermediate-risk patient subset.
Discussion
Hormonal therapy has long been a mainstay of therapy for intermediate and advanced prostate cancer. Given that men are living longer because they receive more effective therapy, more and more late effects are being realized, and every effort must be taken to limit the impact of these toxicities. The most logical place to start is to further evaluate which patients may in fact be overtreated. In this secondary analysis, there was no statistically significant difference between LTAD and STAD for intermediate-risk patients. A recently reported phase 3 trial, RTOG 9910, randomized patients with intermediate-risk prostate cancer to 8 weeks of neoadjuvant ADT followed by concurrent RT and ADT versus 28 weeks of neoadjuvant ADT versus 8 weeks of RT plus ADT (15). This study demonstrated no additional benefit for additional neoadjuvant therapy. Although this particular study design was dissimilar to NRG Oncology RTOG 9202 in terms of the timing of hormonal therapy (pre-RT vs post-RT), it furthers the idea that there may be a limit to the additional benefit of ADT. Of note, this is a retrospective secondary analysis of a prospective randomized trial and thus possesses inherent biases and limitations based on the retrospective nature of the study. Furthermore, given that this analysis specifically examines the intermediate-risk subset of the trial population, additional biases and limitations of the study include a relatively small sample size, the use of older intermediate-risk classification (8, 9), and lack of differentiation between Gleason score 3 + 4 or 4 + 3 patterns among the Gleason score 7 subset, and thereby the inclusion of both low-intermediate and high-intermediate risk group patients in our analysis. A randomized study to directly evaluate the incremental benefit of STAD versus LTAD in patients who fall into the intermediate-risk subcategory has been suggested, but these data, along with RTOG 9910, offer enough statistically sound data to indicate that any further investigation may be of limited value and that there is an adequately supported conclusion from the above referenced data that hormonal therapy beyond 4 to 6 months does not confer any additional benefit in this setting.
In conclusion, NRG Oncology RTOG 9202 included a subset of patients with intermediate-risk prostate cancer; these intermediate-risk patients were analyzed to explore whether hormonal therapy duration beyond short-term use could potentially have resulted in an additional survival benefit. LTAD did not confer a benefit in terms of OS, DSS, and PSAF rates in the subset of patients analyzed in this study with intermediate-risk prostate cancer. This is despite the 5-year and 10-year results, in which there were improvements seen in all efficacy endpoints except OS with the unselected (intermediate-risk and high-risk) study population of NRG Oncology RTOG 9202 (8, 9). Whereas the subset was relatively small, the treatment assignment was randomly applied, and a trend in favor of longer hormonal therapy would have been of interest. Given the limited number of disease-specific deaths observed and the lack of a benefit with respect to any of the study endpoints, this secondary analysis does not suggest that exploration of LTAD is worth testing prospectively in the intermediate-risk subset of prostate cancer patients. As noted in the 10-year follow-up results of NRG Oncology RTOG 9202 by Horwitz et al (9), with respect to the lack of OS advantage in the patients with lower Gleason scores (Gleason scores ≤7, representing >70% of the NRG Oncology RTOG 9202 study population and the study patient subgroup from which the intermediate-risk patients were identified for our secondary analysis), potential explanations for the lack of significance in disease-specific events seen in our secondary analysis were that given the number of patients and the follow-up interval was not yet long enough, we observed only a very few disease-specific events. Horwitz et al noted that NRG Oncology RTOG 9202 had enough follow-up time to enable the observation of a survival advantage in the high-grade patients because their life expectancy would be less, but there may not be enough follow-up time to enable a difference in the low-grade patients to be seen (9). We thereby conclude, on the basis of this secondary analysis and on the above-referenced data, that LTAD is suitable only in the high-risk group of patients. Furthermore, we conclude that given that no benefit was seen in any of our study endpoints, exploration of longer duration hormonal therapy is not worth testing in the prospective randomized study setting in the intermediate-risk prostate cancer subset.
Supplementary Material
Summary.
This was a secondary analysis of the intermediate-risk patient subset treated on RTOG 9202 to evaluate whether long-term adjuvant androgen deprivation versus initial androgen deprivation only with external beam radiation therapy would result in an additional survival benefit. There was no benefit in overall survival, disease-specific survival, or prostate-specific antigen failure in the subset of patients analyzed, and thus does not suggest that the exploration of longer hormonal therapy is worth testing in this intermediate-risk subset.
Acknowledgments
Supported by grants U10CA21661, U10CA180868, U10CA180822, and U10CA37422 from the National Cancer Institute; also funded, in part, under grant 4100057652 with the Pennsylvania Department of Health. The Department specifically disclaims responsibility for any analyses, interpretations, or conclusions.
Footnotes
Presented at the 55th Annual Meeting of the American Society for Radiation Oncology, September 23, 2013, Atlanta, GA.
Conflict of interest: Dr Sandler reports grants from NRG Oncology during the conduct of the study; consulting/advisory fees from Janssen, Medivation, Ferring, NANT Health, and Clovis Oncology, outside the submitted work. The other authors report no conflict of interest.
Supplementary material for this article can be found at www.redjournal.org.
References
- 1.Huggins C, Stevens RE, Jr, Hodges CV. The effects of castration on advanced carcinoma of the prostate gland. Arch Surg. 1941;43:209–223. [Google Scholar]
- 2.Zagars GK, Johnson DE, von Eschenbach AC, et al. Adjuvant estrogen following radiation therapy for stage C adenocarcinoma of the prostate: Long-term results of a prospective randomized study. Int J Radiat Oncol Biol Phys. 1988;14:1085–1091. doi: 10.1016/0360-3016(88)90383-5. [DOI] [PubMed] [Google Scholar]
- 3.Zietman AL, Prince EA, Nakfoor BM, et al. Androgen deprivation and radiation therapy: Sequencing studies using the Shionogi in vivo tumor system. Int J Radiat Oncol Biol Phys. 1997;38:1067–1070. doi: 10.1016/s0360-3016(97)00309-x. [DOI] [PubMed] [Google Scholar]
- 4.Griffiths K, Eaton CL, Harper ME, et al. Hormonal treatment of advanced disease: Some newer aspects. Semin Oncol. 1994;21:672–687. [PubMed] [Google Scholar]
- 5.Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma - Long-term results of phase III RTOG 85-31. Int J Radiat Oncol. 2005;61:1285–1290. doi: 10.1016/j.ijrobp.2004.08.047. [DOI] [PubMed] [Google Scholar]
- 6.Bolla M, Gonzalez D, Warde P, et al. Improved survival in patients with locally advanced prostate cancer treated with radiotherapy and goserelin. N Engl J Med. 1997;337:295–300. doi: 10.1056/NEJM199707313370502. [DOI] [PubMed] [Google Scholar]
- 7.Keating NL, O’Malley AJ, Smith MR. Diabetes and cardiovascular disease during androgen deprivation therapy for prostate cancer. J Clin Oncol. 2006;24:4448–4456. doi: 10.1200/JCO.2006.06.2497. [DOI] [PubMed] [Google Scholar]
- 8.Hanks GE, Pajak TF, Porter A, et al. Phase III trial of long-term adjuvant androgen deprivation after neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate: The Radiation Therapy Oncology Group Protocol 92-02. J Clin Oncol. 2003;21:3972–3978. doi: 10.1200/JCO.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 9.Horwitz EM, Bae K, Hanks GE, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: A phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–2504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 10.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365:107–118. doi: 10.1056/NEJMoa1012348. [DOI] [PubMed] [Google Scholar]
- 11.Consensus statement: Guidelines for PSA following radiation therapy. American Society for Therapeutic Radiology and Oncology consensus panel. Int J Radiat Oncol Biol Phys. 1997;37:1035–1041. [PubMed] [Google Scholar]
- 12.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 13.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 14.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 15.Pisansky TM, Hunt D, Gomella LG, et al. Duration of androgen suppression before radiotherapy for localized prostate cancer: Radiation Therapy Oncology Group randomized clinical trial 9910. J Clin Oncol. 2015;33:332–339. doi: 10.1200/JCO.2014.58.0662. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


