Abstract
Fragile X syndrome results from the transcriptional silencing of a gene, Fmr1, that codes for an mRNA-binding protein (fragile X mental retardation protein, FMRP) present in neuronal dendrites. FMRP can act as a translational suppressor, and its own translation in dendrites is regulated by group I metabotropic glutamate receptors (mGluRs). Multiple lines of evidence suggest that mGluR-induced translation is exaggerated in Fragile X syndrome because of a lack of translational inhibition normally provided by FMRP. We characterized the role of FMRP in the regulation of mRNA granules, which sediment as a heavy peak after polysomes on sucrose gradients. In WT mouse brain, FMRP distributed with polysomes and granules. EM and biochemical analyses suggested that the granule fraction itself contained clusters of polysomes. In Fmr1 knockout brain, we observed a significant decrease in the amount of mRNA granules relative to WT mice. This difference appeared to be due to a role of FMRP in regulating the activation of granules during mGluR-induced translation; in vivo administration of the mGluR5 antagonist 2-methyl-6-(phenylethynyl)pyridine increased granule content in Fmr1 knockout mouse brain to levels comparable with those seen in WT brain. In accord with a role of mGluR5 in the regulation of ongoing translation in vivo, we observed that the phosphorylation of several initiation factors in response to application of the mGluR1/5 agonist S-3,5-dihydroxyphenylglycine in vitro was blocked by methyl-6-(phenylethynyl)pyridine. Together, these data suggest that although large, polysome-containing granules can form in the absence of FMRP, their use in response to mGluR-induced translation is exaggerated.
Keywords: fragile X syndrome, translation, 2-methyl-6-(phenylethynyl)pyridine
Fragile X syndrome (FXS) is the most prevalent inherited form of mental retardation. It is caused by the pathological expansion of a CGG trinucleotide repeat in the 5′-UTR of the Fmr1 gene that codes for the RNA-binding protein fragile X mental retardation protein (FMRP), resulting in its transcriptional silencing (for review, see ref. 1). The syndrome is characterized by a broad set of symptoms, including cognitive impairment, autistic behavior, anxiety and compulsive disorders, seizures, and physical manifestations such as macroorchidism (1, 2). Neuroanatomically, FXS is associated with an overabundance of long, thin, and tortuous dendritic spines in cortical regions (3, 4). This synaptic abnormality is observed also in the Fmr1 knockout (KO) mouse model of FXS (5), although the overall behavioral phenotype of these mice is less severe than expected (6).
The current view of the cellular role of FMRP is that it is involved in the transport and translational regulation of mRNAs critical for the development of neurons and activity-dependent plasticity of their synaptic connections (1, 7, 8). FMRP contains nuclear import and export sequences, and multiple mRNA-binding domains (see ref. 9 and references therein) that mediate interactions between FMRP, and ≈4% of mRNA in brain (10-14). FMRP can act as a translational repressor in vitro and in situ. Studies of the regulation of FMRP targets in Fmr1 KO mice suggest complex changes in translation (10, 14-16), including heightened translation of dendritic mRNAs, such as MAP1B (17, 18), that may contribute to alterations in spine morphology. FMRP binds and regulates the translation of its own mRNA as well (11). Stimulation of group I metabotropic glutamate receptors (mGluRs) induces rapid synthesis of FMRP in the synaptodendritic compartment (19, 20). This finding suggests that mGluR-induced translation of FMRP targets is limited at the synapse by a negative-feedback mechanism. In accord with this possibility, a form of translation-dependent long-term depression (LTD) induced by mGluRs is enhanced in Fmr1 KO mice (21). Stimulation of group I mGluRs also results in a translation-dependent elongation of dendritic spines resembling those seen in fragile X brain (22). These observations motivated the theory that, in FXS, exaggerated mGluR-dependent translation results in synaptic changes that are the proximal causes of cognitive impairment (8).
The presence of FMRP in polysomes and mRNA granules in brain suggests that it has a role in regulating complexes of translation machinery in dendrites. FMRP is associated with translating polysomes and with stalled polysomes (23-25), suggesting that it gates translation after the initiation step. Such a role of FMRP may be integral to the formation and function of large mRNA granules (heavy-sedimenting particles that contain multiple mRNAs), ribosomes, other mRNA-binding proteins, translation factors, and motor proteins (26-30). Granules are thought to provide a repertoire of mRNAs that are transported into dendrites in a translationally silent state for subsequent site-specific utilization at synapses undergoing protein synthesis-dependent changes (27, 31, 32). Some large mRNA granules contain aggregates of translationally inactive polysomes (27), whereas others appear to lack ribosomes (32). Prolonged depolarization of cortical neurons induces structural changes in granules that suggest that constituent polysomes are being derepressed (27). These changes are accompanied by a shift in the distribution of several dendritic mRNAs into lighter, actively translating polysome fractions. Recent studies identified FMRP as a component of large mRNA granules that also contain staufen 1 and other factors that are implicated in the transport of mRNAs into dendrites (28, 29). As a component of granules, FMRP may influence the activity-dependent translation of granule mRNAs by regulating preassembled polysomes.
In this study, we used density-gradient fractionation, Western blot analysis, and EM techniques to characterize and compare large mRNA granules in WT and Fmr1 KO mouse brain. Also, we studied the role of mGluRs in the regulation of granules in vivo by using the mGluR5-specific antagonist 2-methyl-6-(phenylethynyl)pyridine (MPEP). The data suggest that FMRP is involved in regulating mGluR-dependent translation from a large percentage of mRNA granules and that actively translating polysomes may be derived from translationally silent FMRP-containing granules in adult mouse brain.
Materials and Methods
Animals and Drug Injections. Male F1 hybrid mice (FVB/NJ X C57BL/6J) with the fmr1-tm1Cgr allele were used (6). Mice were injected i.p. with MPEP (35 mg/kg) 45 min before they were killed. Mice were anesthetized with halothane before decapitation and brain dissection.
Preparation of mRNA Granule and Polysome Profiles from Rat or Mouse Brain. Granules and other components of the translation machinery from whole brain were fractionated by adapting the protocol of Krichevsky and Kosik (27). Brains from adult WT or Fmr1 KO mice were rapidly harvested and lysed by Dounce homogenization (10-15 strokes) in 3.5 ml per brain (or, for adult rat, 6 ml per brain) ice-cold lysis-buffer containing 20 mM Tris·HCl (pH 7.4), 3 mM MgCl2, 10 mM NaCl, 2% sucrose, 0.3% Triton X 100, 100 μg/ml cycloheximide, protease inhibitors (leupetin, aprotinin, and PMSF), and 2 mM vanadyl ribonucleoside complex (VRC). A postmitochondrial supernatant was prepared by centrifuging the lysate at 10,000 × g for 10 min, and salt-adjusted (27). We loaded 20 A260 units of this cytoplasmic extract onto a linear (20-55%) sucrose gradient (10.5 ml) and centrifuged them at 200,000 × g for 2.25 h at 4°C in a SW41 swinging-bucket rotor. Fractions were obtained by upward displacement with 70% (wt/vol) sucrose and their absorbance at 254 nm monitored continuously by using an ISCO UA-6 UV monitor. mRNA granules were also prepared according to Kanai et al. (28), in which brains were first homogenized in a buffer containing 20 mM Hepes (pH 7.4), 140 mM potassium acetate, 1 mM magnesium acetate, and 1 mM EGTA, supplemented with protease and RNase inhibitors. A postmitochondrial supernatant (S2) prepared without detergent was laid over a 15-30% linear sucrose gradient (8 ml) with a final 2.5-ml cushion of 70%. S2 was centrifuged in the SW41 rotor at 200,000 × g for 2 h at 4°C. Half-milliliter fractions from the 70% sucrose cushion only were collected and assayed by Western blot analysis and EM.
Treatment of Brain Lysates. The cytoplasmic extracts described above were incubated with RNase A (100 μg/ml) for 30 min on ice. Alternatively, the cytosolic fraction was incubated at 37°C for 30 min in the presence of 100 μg/ml cyclohexamide before fractionation. Treatment of lysates with 30 mM EDTA was done at 4°C for 30 min.
Slice Cultures. Organotypic cultures of hippocampus were prepared as described (22). Drug treatments were initiated by media exchange. S-3,5-dihydroxyphenylglycine (DHPG) was added at 40 μM for 7.5 min. MPEP was added at 10 μM 20 min before incubation with DHPG or daily for 72 h.
Western Blot Analysis. Protein samples were precipitated with TCA, washed with acetone, then boiled in NuPage sample buffer, separated by SDS/PAGE, and transferred to PVDF membranes. Membranes were blocked, probed with antibodies, and developed by using the Western Breeze kit (Invitrogen). The antibodies against FMRP (mAb7G1-1, Developmental Studies Hybridoma Bank, University of Iowa, Iowa City), and ribosomal proteins were used at 1:500 dilutions. Rabbit polyclonal antibodies to ribosomal proteins L3, L4, and S20 were produced in house by immunizing with proteins from purified rat liver large and small ribosomal subunits. Antibodies to translation factors were purchased from Cell Signaling Technology (Beverly, MA).
EM. mRNA granules isolated by sucrose gradient centrifugation were fixed with 2.5% glutaraldehyde in a 0.1 M Na+ cacodylate buffer and pelleted. At The Scripps Electron Microscopy Core Facility, samples were contrasted with uranyl acetate or negatively stained. Samples were adsorbed to glow-discharged grids after resuspension or embedded and sectioned.
Results
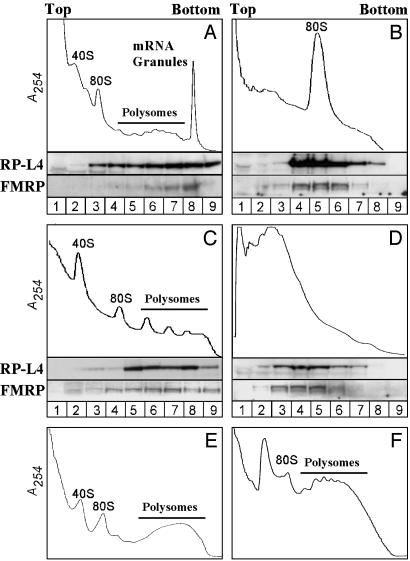
FMRP Is Present in a Heavy Sedimenting Peak That Contains mRNA Granules. To investigate the presence of FMRP in mRNA granules, lysates derived from PND13 rat brain were sedimented through a 25-55% linear sucrose gradient as described by Krichevsky and Kosik (27), with slight modification. As shown in Fig. 1A, FMRP was distributed predominantly in a heavy peak that sedimented below the polysomes, whereas the ribosomal protein L4 (RP-L4) was distributed more evenly along the gradient between 80S monosomes, polysomes, and the heavy peak. Krichevsky and Kosik (27) demonstrated that the heavy peak contained large, densely packed clusters of ribosomes that they refer to as “mRNA granules.”
Fig. 1.
FMRP cofractionates with polysomes and heavy mRNA granules in lysates of postnatal day 13 rat brain. (A) Absorbance profile (254 nm) of rat brain postmitochondrial fraction sedimented through a 20 -55% linear sucrose gradient in the presence of cyclohexamide. Western blots of samples taken throughout the gradient (numbered at the bottom) show that ribosomal protein L4 (RP-L4) and FMRP are in the polysomes and a heavier granule fraction. The heavy mRNA granule fraction was eliminated by treatment of the lysates with RNase A (B), heating at 37°C (C), or treatment with 30 mM EDTA (D) before fractionation. Granules were not observed in lysates of liver (E) or testis (F).
FMRP can associate with ribosomes through high-affinity interactions with the 60S subunit (33). To investigate whether FMRP is associated with ribosomes within the mRNA granules, we treated the cytosolic extract with RNase A to digest mRNA between ribosomes before sucrose fractionation. This digestion resulted in a loss of polysomes and mRNA granules, with a concomitant accumulation of 80S monosomes (Fig. 1B). Western blot analysis showed that, after RNase A treatment, FMRP cosedimented with the 80S monosomes (Fig. 1B), suggesting that FMRP was associated with ribosomes present in mRNA granules and polysomes, and that mRNA was not a prerequisite for this interaction. Thus, FMRP interacts with 80S monosomes already assembled within mRNA granules.
Further evidence for ribosomal association of FMRP in mRNA granules was obtained by incubating the cytosolic fraction at 37°C before the sucrose-gradient fractionation (Fig. 1C). This incubation resulted in a shift in the RNA distribution across the sucrose gradient. The heavy peak disappeared, and an increase in the polysome peaks was observed. Western blot analysis showed that FMRP was associated with these polysomes derived from the mRNA granules, as shown by the cosedimentation of FMRP with the RP-L4 (Fig. 1C). The granule peak was also affected by treatments that disrupt polysomes. Treatment of lysates with 30 mM EDTA disrupted polysomes and resulted in the accumulation of FMRP-containing complexes that sediment with the 60S ribosomal subunit (24, 33). As shown in Fig. 1D, this treatment also completely disrupted mRNA granules, with an increase of peaks that correspond to large and small ribosomal subunits.
The above observations suggested that polysomes are critical for the integrity of the heavy granule peak and that the cosedimentation of FMRP with mRNA granules involves its association with ribosomes. We assayed for heavy granules in nonneuronal tissues as well and in a neuronal cell line. Sucrose gradient profiles derived from lysates of liver and testes (Fig. 1 E and F, respectively) did not contain the heavy peak, nor did lysates of neuro2A cells (data not shown). Thus, the heavy granule peak is specific to brain tissue and not likely to be the product of an artifactual association of polysomes during preparation of lysates.
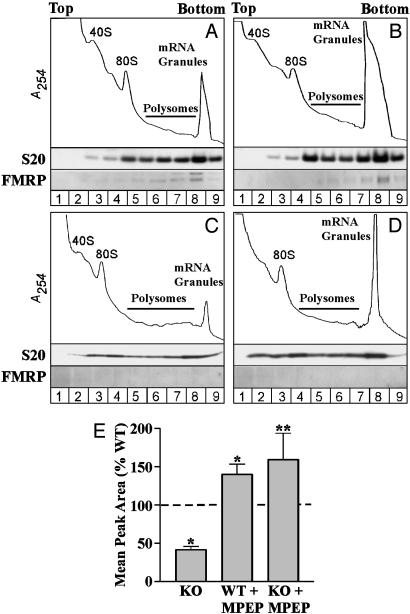
Fmr1 KO Mice Have a Reduced mRNA Granule Peak. Inasmuch as FMRP can suppress translation after polysome formation and multimerize with other proteins into macromolecular complexes, we investigated whether it is involved in granule formation. We prepared brain lysates from adult Fmr1 KO and WT mice and fractionated components of the translation machinery on sucrose gradients as described above. As shown in Fig. 2 (compare A and C), Fmr1 KO mice have a significantly reduced mRNA granule peak relative to WT animals (KO reduced to 41.3 ± 8% of WT; P < 0.001). Also, the polysome peak intensities, which are relatively low in adult animals under the lysis conditions used (23), were slightly increased in Fmr1 KO. These data suggest that lack of FMRP either prevents granule formation or derepresses mRNAs within granules, thereby promoting their translation in the form of less dense polysomes. Our studies with an mGluR antagonist (see below) support the latter view.
Fig. 2.
mRNA granules are reduced in Fmr1 KO mouse brain, relative to WT, and they are reconstituted by antagonism of mGluR5 in vivo. Absorbance profiles of mRNA granules resolved from WT and Fmr1 KO mice show that, relative to WT animals (A), Fmr1 KO mice (C) have a reduced mRNA granule peak. Injection of the mGluR5-specific antagonist MPEP before killing increased the mRNA granule peak in WT (B) and Fmr1 KO (D) mice. Results varied across experiments, but consistent reductions in the KO and increases with MPEP were observed in each experiment. (E) Bar graph showing the average granule peak area, expressed as a percentage of the WT, in Fmr1 KO mice and in both genotypes after injection of MPEP. Significant differences were observed in comparisons of Fmr1 KO (P < 0.01, n = 12) and MPEP-injected WT mice (P < 0.05, n = 6) versus WT (n = 17) and of Fmr1 KO mice with and without MPEP (P < 0.01, n = 5) (*, one-sample t test; **, two-tailed t test).
mGluR5 Activity Regulates the Amount of mRNA Granules in WT and Fmr1 KO Mice. Stimulation of mGluRs induces local translation, synthesis of FMRP (20), and dendritic trafficking of FMRP-containing granules (34). Moreover, mGluR-induced translation is involved in spine elongation (22) and in the stabilization of a form of LTD that is enhanced in Fmr1 KO mice (21). In light of these observations, we investigated the role of mGluRs in regulating mRNA granule levels in WT and Fmr1 KO mice. As shown in Fig. 2, injection of the mGluR5-specific antagonist MPEP (35 mg/kg) 45 min before killing increased the mRNA granule peak in both WT and Fmr1 KO mice (compare A with B, and C with D); similar effects were seen at a lower dose of MPEP (10 mg/kg; data not shown). On average, injection of MPEP increased the size of the granule peak in both Fmr1 KO and WT mice to ≈50% over uninjected WT controls (Fig. 2E).
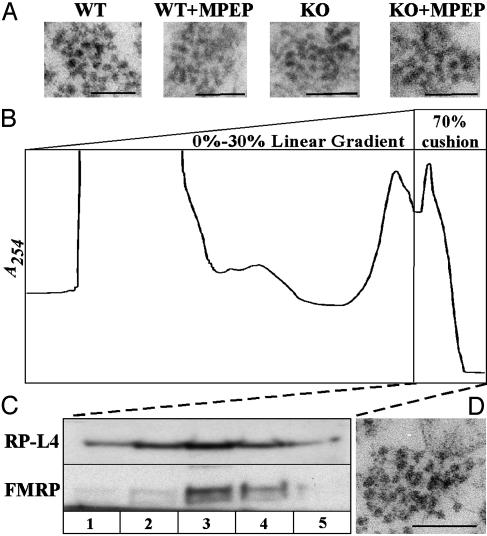
EM Examination of mRNA Granules from WT and Fmr1 KO Mice Reveals No Obvious Structural Differences. To investigate whether the lack of FMRP has an effect on mRNA granule structure, EM analyses of granules derived from WT, KO, and MPEP-injected animals of each genotype were conducted. Sucrose-gradient fractions corresponding to the heavy granule peak were pelleted, fixed, and sectioned for EM analysis. Sections through the granule pellet revealed that the heavy peak contains membranous material and polysome-containing structures but that much of the membranous material collects above the polysome structures, forming a discrete interface. Fig. 3A shows sections through the bottom of the granule pellet, which had an almost homogenous collection of polysome-containing structures in WT and Fmr1 KO granule fractions. Analysis of granules close to the base of the pellet for each genotype, with or without MPEP, revealed the presence of large, densely packed clusters of 10-20 ribosomes, ranging in size from ≈100 to 250 nm (Fig. 3). No obvious changes in the shape of these granules were observed between WT and Fmr1 KO animals, nor were there any obvious changes associated with the injection of MPEP in either genotype.
Fig. 3.
EM examination of granules resolved from WT and Fmr1 KO mouse brain. (A) Electron micrographs of granules collected from WT, KO, and MPEP-injected animals. (Scale bar, 100 nm.) mRNA granules are shown as large, densely packed clusters of 10 -20 ribosomes, ranging in size from 100 to 300 nm. (B) Velocity-gradient isolation of granules from brains lysed without detergent according to the method of Kanai et al. (28). Large particles that absorb at 254 nm penetrate a dense 70% sucrose cushion after separation from other translation components on a 15-30% gradient. (C) Western blot analysis of fractions from the 70% cushion show that FMRP cosediments with these large particles and the ribosomal protein L4. (D) EM analysis revealed large, densely packed clusters of ribosomes that ranged in size from 100 to 300 nm, resembling the granules resolved on 20 -55% linear gradients in the presence of detergent, as shown in A.
To characterize further the structure of granules and the presence of FMRP in granules, we used another, recently published sucrose gradient method to resolve granules from rodent brain lysates. Kanai et al. (28) described mRNA granules as large complexes (≈1,000S), resistant to treatment with 1% Triton X-100, that sedimented through a 0-30% linear sucrose gradient and entered a 70% cushion within 2 h of high-speed centrifugation. By using the same method, we observed two major RNA-containing peaks on either side of the interface between 30% and 70% sucrose (Fig. 3B). Western blot analysis of fractions collected from the 70% sucrose cushion revealed the cosedimentation of FMRP and RP-L4 with the fastest-migrating RNA peak (Fig. 3C). With increasing time of centrifugation, this RNA peak, FMRP, and RP-L4 comigrated through the 70% sucrose at the same rate (data not shown). EM analyses of the fractions isolated from this cushion (Fig. 3D) showed a homogenous population of large, densely packed clusters of ribosomes in the fast-migrating structures containing FMRP. The structures that we observed by using both methods for granule isolation had a regular, spherical appearance and resembled granules seen earlier by Krichevsky and Kosik (27). However, we did not see granules that appeared to be unfurled or opened up with ribosomes radiating out like beads on a string.
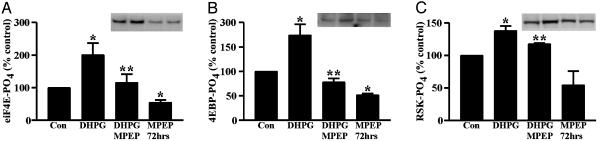
Stimulation of mGluR5 Induces Phosphorylation of Translation Initiation Factors. The above results suggested that granules can form in the absence of FMRP, but that their disassembly in response to ongoing mGluR-induced translation is exaggerated in Fmr1 KO animals. To characterize the effects of mGluR5 on translation further, we tested the effects of MPEP on the regulation of eukaryotic initiation factors (eIFs) in response to the mGluR1/5 agonist DHPG (40 mM). Hippocampal slice cultures from mouse brain were used so that strict control over treatment parameters and tissue processing could be maintained. As shown in Fig. 4, DHPG induced rapid and significant increases in the phosphorylation of the cap-binding protein, eIF4E, and a protein that binds eIF4E (eIF4E-BP) in its nonphosphorylated state to occlude interactions between eIF4E and the initiation scaffolding protein eIF4G. These changes are consistent with mGluRs having an activating effect on initiation of translation. Phosphorylation of the 90-kDa ribosomal S6 protein kinase (p90RSK) was also increased significantly. This kinase has been shown to translocate to polysomes upon stimulation of translation, and its phosphorylation leads to downstream changes in eIF2 that promote translation (35).
Fig. 4.
Modulation of translation factor phosphorylation by the mGluR5 antagonist, MPEP. Hippocampal slice cultures were treated with DHPG for 7.5 min with or without MPEP, or with MPEP alone for 72 h. DHPG increased the phosphorylation of eIF4E (A), eIF4E-BP (B), and the 90-kDa ribosome-specific kinase, p90RSK (C). (Insets) Blots show examples of changes in the phosphorylation of each protein. Their order follows the graph conditions. Phosphorylation levels in DHPG-treated slices were significantly different from untreated slices (P < 0.05) or those coincubated with MPEP (P < 0.05). MPEP alone significantly decreased phosphorylation of eIF4E and eIF4E-BP relative to controls. P < 0.05, for one-sample (*) and one-tailed (**) t test. All data reflect averages from four to seven independent experiments with internal replicates.
Coincubation of slices with MPEP blocked DHPG-induced phosphorylation of eIF4E and eIF4E-BP and attenuated p90RSK phosphorylation, suggesting that mGluR5 is responsible for most mGluR-induced translation in hippocampal slices. Prolonged incubation of slices with MPEP alone reduced eIF4E and eIF4E-BP phosphorylation below control levels, indicating that there is ongoing mGluR5-regulated translation in the culture system. Taken with the results of our in vivo studies on the effect of MPEP on granules, these data suggest that the reduction of granules in Fmr1 KO mice results from the lack of a suppressive influence on ongoing mGluR5-induced translation.
Discussion
In this article, we present evidence that the mRNA granule fraction is reduced significantly in Fmr1 KO mouse brain, which is thought to have exaggerated mGluR-dependent translation relative to WT brain (8). The granule peak was rapidly reconstituted in the Fmr1 KO animals upon injection (i.p.) of the mGluR5-specific antagonist, MPEP. This result suggests that suppression of mGluR-induced translation in vivo by FMRP stabilizes granules after they are formed. Moreover, it appeared that mRNA granules may serve as a reservoir of translationally arrested polysomes (27) containing FMRP that can be activated or derepressed, resulting in their redistribution to lighter polysome fractions. Overall, our data are consistent with reports showing that FMRP plays an important role in the regulation of local translation from mRNA granules (34), and they support the idea that mGluR-dependent translation is exaggerated in FXS (8, 21, 22).
The Presence of FMRP in mRNA Granules. Two different fractionation protocols yielded granules that cosedimented with FMRP and that had a similar structural appearance: that of a dense, spherical cluster of polysomes. The presence of FMRP in these granules depended on associations with polysomes. Upon disruption of polysomes, granules disappeared, and as reported (24, 33), FMRP was found primarily in the ribosomal fraction, consistent with previous demonstrations of an association of FMRP with the 60S ribosomal subunit (33).
Recent reports that FMRP associates with polysomes in brain have not detected a granule peak (23, 24). This result has led to the suggestion that granules may be an artifact of the culture preparation originally used to characterize them. However, many reports that characterize FMRP-containing complexes as polysomes or smaller entities also show evidence for FMRP in pellet fractions. Thus, it is possible that large mRNA granules with sedimentation properties similar to those observed here and elsewhere (27, 28, 32) might have been observed if broader sucrose gradients had been used or if fractionation methods had been altered to retain heavier material. The recent purification of FMRP-containing granules by two methods (28, 29) and colabeling of identified components in situ provides critical support for the existence of large mRNA granules and for FMRP as a component protein.
Our data provide additional support for the existence of granules in that their abundance is linked to the presence of FMRP and to the activity of receptors that elicit many phenomena that are altered in the absence of FMRP. Moreover, we could not detect granules in liver and testis. There is no a priori reason to predict that these tissues would be less subject to generating artifactual associations of translation machinery than brain. Also, note that the heavy granule peak is discrete, suggesting that granules may exhibit a degree of structural regularity. If granules were artifactual in origin, they might be expected to display a broader range of sizes.
The presence of FMRP in the granule fraction may reflect a number of roles of this mRNA-binding protein in the regulation of translation. Our data suggest that one role is to suppress granule mRNAs, regulating their translation in response to synaptic activity. Several lines of evidence indicate that FMRP is a translational suppressor of specific mRNAs (36, 37), and its presence in transport granules may ensure that mRNA cargos are not translated at inappropriate sites or times (34). Further characterization of effectors of FMRP-mediated suppression in granules, such as components of the microRNA pathway (38), will aid in evaluating this possibility. It has already been shown that the noncoding dendritic RNA, BC1, and several mRNAs presumably suppressed in dendrites by FMRP-BC1 interactions are present in granules (16, 28). FMRP could also regulate the composition of granules; it associates with other mRNA-binding proteins, and dimerization of FMRP with the human homolog of zipcode binding protein 1 (IMP1) on mRNA is sufficient to induce the formation of larger mRNA-containing granules (39). Last, the presence of NLS and NES sequences in FMRP suggests a role in mRNA transport. However, in situ hybridization analyses of the distributions of several mRNAs present in granules found no difference between WT and Fmr1 KO mice, suggesting that FMRP does not have a major role in transport of mRNAs packaged into granules (28, 40).
Reduction of the Heavy mRNA Granule Peak in Fmr1 KO Mice. We observed a reduction in the heavy mRNA granule peak resolved from brain lysates of Fmr1 KO mice relative to WT mice. This reduction could reflect a lack of FMRP-mediated translational suppression in the KO brain, and a commensurate shift of polysomes sequestered in a silent state into dense granules to a less dense fraction of translating polysomes. The alternative notion that FMRP may be a key structural component that is critical for the assembly of some granules seems to be less likely because, after treatment with the mGluR5 antagonist, granules in KO mice were as abundant as in WT mice. Moreover, the EM analyses did not reveal obvious structural differences in granules from Fmr1 KO, compared with WT mouse brain, and the granules that were present in these animals had similar sedimentation properties. These observations suggest that granules can form properly in Fmr1 KO mice but that they may be “unpackaged” at a faster rate than in WT brain.
The granule peak was not entirely absent in the KO brain. Studies of the relative distributions of mRNA-binding proteins found in granules (32, 34, 41) suggest that the mRNA granule pool is heterogeneous. If the sucrose-gradient-fractionation method resolves most heavy granules equally without disruption, then it is likely that most heavy granules contain FMRP.
Increased mRNA Granule Levels After in Vivo Injection of MPEP. Administration of MPEP to Fmr1 KO mice brought the low level of granules in these animals to levels approximately equal to those seen in WT mice injected with MPEP. This result is significant because it suggests that mGluR5-induced translation from granules is an ongoing process in mouse brain that occurs at a higher rate in the absence of FMRP. Our studies on translation-factor phosphorylation, as well as other reports on the regulation of translation machinery by mGluRs (42, 43), indicate that mGluRs have a strong activating effect on translation initiation. Thus, increased levels of granules after MPEP treatment may be due to an attenuation of translation-factor activity. In WT, changes in the distribution or posttranslational modification of FMRP may be involved also. It has been shown that stimulating mGluRs causes FMRP to uncouple from Fmr1 target mRNA (34) and that a fraction of phosphorylated FMRP is present in stalled polysomes (25). In the Fmr1 KO mouse, compensating activities of the structurally related FXRs or phosphorylation of eukaryotic elongation factor 2 (eEF2) may be responsible for stalling polysomes to regenerate granules (44). Phosphorylation of eEF2 mediates translational suppression in various circumstances, and it can be induced by the activity of NMDA-type glutamate receptors, which may dominate its regulation when mGluR signaling is inhibited.
Significance for Translational Regulation in FXS. The “mGluR theory” of FXS holds that exaggerated mGluR-dependent translation leads to changes in synaptic plasticity and structure that are the proximal causes of symptoms (8). These synaptic changes may include an exaggeration of LTD (21) and other events that have been linked to mGluR-dependent translation, such as control of intrinsic neuronal properties and seizure thresholds (45, †), spine elongation (22), and the priming of LTP consolidation (42, 46). Our data support this theory and suggest that, in particular, translation of mRNAs transported in the form of large, polysome-containing granules is exaggerated in FXS. Also, the ability of MPEP to restore the heavy granule fraction in Fmr1 KO mice supports the notion that antagonists of mGluRs may be efficacious in treating many symptoms of FXS (8).
How well antagonism of mGluRs can offset changes caused by a lack of FMRP will be determined in part by whether FMRP regulates mGluR-induced translation exclusively. Multiple receptors at glutamatergic synapses induce translation, and their signaling can converge onto common points of translation control. However, there is reason to believe that mechanisms for differential translation exist at synapses (47). At least three forms of long-term synaptic efficacy change require local translation for their consolidation: LTD, LTP, and potentiation induced with the brain-derived neurotrophic factor. These plastic changes differ in valence, induction and expression mechanism, associated spine-shape changes, and longevity. Thus, it is reasonable to assume that they require distinct translational responses. For example in hippocampus, where LTD is exaggerated in the Fmr1 KO mouse, no differences in LTP have been observed. Moreover, there is evidence that selective utilization of mRNAs in dendrites can arise from differences in receptor signaling and translation factor modulation.
If the set of mRNAs bound by FMRP were to overlap extensively with that regulated by mGluRs, then mGluR antagonists could impact a broad set of symptoms in FXS. Studies of the FMRP target mRNAs provide some basis to address this issue. Identification of mRNAs bound by FMRP in heavy granules, and the set of mRNAs selectively translated in response to mGluR activation may clarify this issue further. Last, the changes in mRNA granule peak size described here may provide a convenient biological marker with which to evaluate the effects of candidate therapeutic compounds on components of the translation machinery in Fmr1 KO mice.
Acknowledgments
We thank Dr. Robert Bauchwitz (Columbia University, New York) for providing hybrid (C57 X FVB) Fmr1 KO mice, Dr. Malcolm Wood for assistance with EM studies, and Katherine Woodard for excellent technical assistance. This research was supported by a grant from the Fragile X Research Foundation (to P.W.V.), National Institutes of Health Grants HD09635-31 (to G.M.E.) and HDL6550-23 (B.A.C.), and the Skaggs Institute for Chemical Biology (A.A.).
Author contributions: A.A., B.A.C., and P.W.V. designed research; A.A. and P.W.V. performed research; A.A. and P.W.V. analyzed data; and A.A., G.M.E., and P.W.V. wrote the paper.
Abbreviations: FMRP, fragile X mental retardation protein; DHPG, S-3,5-dihydroxyphenylglycine; MPEP, 2-methyl-6-(phenylethynyl)pyridine; eIF, eukaryotic initiation factor; FXS, fragile X syndrome; mGluR, metabotropic glutamate receptor; KO, knockout; LTD, long-term depression.
Footnotes
Chuang, S., Yan, Q., Bauchwitz, R. P. & Wong, R. K. S. (2004) Soc. Neurosci. Abstr. 228.5.
References
- 1.O'Donnell, W. T. & Warren, S. T. (2002) Annu. Rev. Neurosci. 25, 315-338. [DOI] [PubMed] [Google Scholar]
- 2.Hagerman, R. J., Amiri, K. & Cronister, A. (1991) Am. J. Med. Genet. 38, 283-287. [DOI] [PubMed] [Google Scholar]
- 3.Hinton, V. J., Brown, W. T., Wisniewski, K. & Rudelli, R. D. (1991) Am. J. Med. Genet. 41, 289-294. [DOI] [PubMed] [Google Scholar]
- 4.Irwin, S. A., Galvez, R. & Greenough, W. T. (2000) Cereb. Cortex 10, 1038-1044. [DOI] [PubMed] [Google Scholar]
- 5.Comery, T. A., Harris, J. B., Willems, P. J., Oostra, B. A., Irwin, S. A., Weiler, I. J. & Greenough, W. T. (1997) Proc. Natl. Acad. Sci. USA 94, 5401-5404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yan, Q. J., Asafo-Adjei, P. K., Arnold, H. M., Brown, R. E. & Bauchwitz, R. P. (2004) Genes Brain Behav. 3, 337-359. [DOI] [PubMed] [Google Scholar]
- 7.Antar, L. N. & Bassell, G. J. (2003) Neuron 37, 555-558. [DOI] [PubMed] [Google Scholar]
- 8.Bear, M. F., Huber, K. M. & Warren, S. T. (2004) Trends Neurosci. 27, 370-377. [DOI] [PubMed] [Google Scholar]
- 9.Adinolfi, S., Bagni, C., Musco, G., Gibson, T., Mazzarella, L. & Pastore, A. (1999) RNA 5, 1248-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Darnell, J. C., Jensen, K. B., Jin, P., Brown, V., Warren, S. T. & Darnell, R. B. (2001) Cell 107, 489-499. [DOI] [PubMed] [Google Scholar]
- 11.Schaeffer, C., Bardoni, B., Mandel, J. L., Ehresmann, B., Ehresmann, C. & Moine, H. (2001) EMBO J. 20, 4803-4813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alvarez, G., Ramos, M., Ruiz, F., Satrustegui, J. & Bogonez, E. (2003) J. Neurosci. Res. 73, 260-269. [DOI] [PubMed] [Google Scholar]
- 13.Hinds, H. L., Ashley, C. T., Sutcliffe, J. S., Nelson, D. L., Warren, S. T., Housman, D. E. & Schalling, M. (1993) Nat. Genet. 3, 36-43. [DOI] [PubMed] [Google Scholar]
- 14.Brown, V., Jin, P., Ceman, S., Darnell, J. C., O'Donnell, W. T., Tenenbaum, S. A., Jin, X., Feng, Y., Wilkinson, K. D., Keene, J. D., et al. (2001) Cell 107, 477-487. [DOI] [PubMed] [Google Scholar]
- 15.Miyashiro, K. Y., Beckel-Mitchener, A., Purk, T. P., Becker, K. G., Barret, T., Liu, L., Carbonetto, S., Weiler, I. J., Greenough, W. T. & Eberwine, J. (2003) Neuron 37, 417-431. [DOI] [PubMed] [Google Scholar]
- 16.Zalfa, F., Giorgi, M., Primerano, B., Moro, A., Di Penta, A., Reis, S., Oostra, B. & Bagni, C. (2003) Cell 112, 317-327. [DOI] [PubMed] [Google Scholar]
- 17.Lu, R., Wang, H., Liang, Z., Ku, L., O'Donnell, W. T., Li, W., Warren, S. T. & Feng, Y. (2004) Proc. Natl. Acad. Sci. USA 101, 15201-15206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang, Y. Q., Bailey, A. M., Matthies, H. J., Renden, R. B., Smith, M. A., Speese, S. D., Rubin, G. M. & Broadie, K. (2001) Cell 107, 591-603. [DOI] [PubMed] [Google Scholar]
- 19.Todd, P. K., Mack, K. J. & Malter, J. S. (2003) Proc. Natl. Acad. Sci. USA 100, 14374-14378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiler, I. J., Irwin, S. A., Klintsova, A. Y., Spencer, C. M., Brazelton, A. D., Miyashiro, K., Comery, T. A., Patel, B., Eberwine, J. & Greenough, W. T. (1997) Proc. Natl. Acad. Sci. USA 94, 5395-5400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber, K. M., Gallagher, S. M., Warren, S. T. & Bear, M. F. (2002) Proc. Natl. Acad. Sci. USA 99, 7746-7750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanderklish, P. W. & Edelman, G. M. (2002) Proc. Natl. Acad. Sci. USA 99, 1639-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khandjian, E. W., Huot, M. E., Tremblay, S., Davidovic, L., Mazroui, R. & Bardoni, B. (2004) Proc. Natl. Acad. Sci. USA 101, 13357-13362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stefani, G., Fraser, C. E., Darnell, J. C. & Darnell, R. B. (2004) J. Neurosci. 24, 7272-7276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceman, S., O'Donnell, W. T., Reed, M., Patton, S., Pohl, J. & Warren, S. T. (2003) Hum. Mol. Genet. 12, 3295-3305. [DOI] [PubMed] [Google Scholar]
- 26.Antic, D., Lu, N. & Keene, J. D. (1999) Genes Dev. 13, 449-461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krichevsky, A. M. & Kosik, K. S. (2001) Neuron 32, 683-696. [DOI] [PubMed] [Google Scholar]
- 28.Kanai, Y., Dohmae, N. & Hirokawa, N. (2004) Neuron 43, 513-525. [DOI] [PubMed] [Google Scholar]
- 29.Villace, P., Marion, R. M. & Ortin, J. (2004) Nucleic Acids Res. 32, 2411-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ohashi, S., Koike, K., Omori, A., Ichinose, S., Ohara, S., Kobayashi, S., Sato, T. A. & Anzai, K. (2002) J. Biol. Chem. 277, 37804-37810. [DOI] [PubMed] [Google Scholar]
- 31.Keene, J. D. & Tenenbaum, S. A. (2002) Mol. Cell 9, 1161-1167. [DOI] [PubMed] [Google Scholar]
- 32.Antic, D. & Keene, J. D. (1998) J. Cell Sci. 111, 183-197. [DOI] [PubMed] [Google Scholar]
- 33.Siomi, M. C., Zhang, Y., Siomi, H. & Dreyfuss, G. (1996) Mol. Cell. Biol. 16, 3825-3832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Antar, L. N., Afroz, R., Dictenberg, J. B., Carroll, R. C. & Bassell, G. J. (2004) J. Neurosci. 24, 2648-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Angenstein, F., Greenough, W. T. & Weiler, I. J. (1998) Proc. Natl. Acad. Sci. USA 95, 15078-15083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li, Z., Zhang, Y., Ku, L., Wilkinson, K. D., Warren, S. T. & Feng, Y. (2001) Nucleic Acids Res. 29, 2276-2283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Laggerbauer, B., Ostareck, D., Keidel, E. M., Ostareck-Lederer, A. & Fischer, U. (2001) Hum. Mol. Genet. 10, 329-338. [DOI] [PubMed] [Google Scholar]
- 38.Jin, P., Alisch, R. S. & Warren, S. T. (2004) Nat. Cell Biol. 6, 1048-1053. [DOI] [PubMed] [Google Scholar]
- 39.Rackham, O. & Brown, C. M. (2004) EMBO J. 23, 3346-3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steward, O., Bakker, C. E., Willems, P. J. & Oostra, B. A. (1998) NeuroReport 9, 477-481. [DOI] [PubMed] [Google Scholar]
- 41.Tiruchinapalli, D. M., Oleynikov, Y., Kelic, S., Shenoy, S. M., Hartley, A., Stanton, P. K., Singer, R. H. & Bassell, G. J. (2003) J. Neurosci. 23, 3251-3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Francesconi, W., Cammalleri, M. & Sanna, P. P. (2004) Brain Res. 1022, 12-18. [DOI] [PubMed] [Google Scholar]
- 43.Shin, C. Y., Kundel, M. & Wells, D. G. (2004) J. Neurosci. 24, 9425-9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Scheetz, A. J., Nairn, A. C. & Constantine-Paton, M. (2000) Nat. Neurosci. 3, 211-216. [DOI] [PubMed] [Google Scholar]
- 45.Sourdet, V., Russier, M., Daoudal, G., Ankri, N. & Debanne, D. (2003) J. Neurosci. 23, 10238-10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raymond, C. R., Thompson, V. L., Tate, W. P. & Abraham, W. C. (2000) J. Neurosci. 20, 969-976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vanderklish, P. W. & Edelman, G. M. (2005) Genes Brain Behav., in press. [DOI] [PubMed]