Abstract
Thrombin (factor IIa) and factor Xa (FXa) are key enzymes at the junction of the intrinsic and extrinsic coagulation pathways and are the most attractive pharmacological targets for the development of novel anticoagulants. Twenty non-amidino N2-thiophencarbonyl- and N2-tosyl anthranilamides 1–20 and six amidino N2-thiophencarbonyl- and N2-tosylanthranilamides 21–26 were synthesized to evaluate their activated partial thromboplastin time (aPTT) and prothrombin time (PT) using human plasma at a concentration of 30 µg/mL in vitro. As a result, compounds 5, 9, and 21–23 were selected to study the further antithrombotic activity. The anticoagulant properties of 5, 9, and 21–23 significantly exhibited a concentration-dependent prolongation of in vitro PT and aPTT, in vivo bleeding time, and ex vivo clotting time. These compounds concentration-dependently inhibited the activities of thrombin and FXa and inhibited the generation of thrombin and FXa in human endothelial cells. In addition, data showed that 5, 9, and 21–23 significantly inhibited thrombin catalyzed fibrin polymerization and mouse platelet aggregation and inhibited platelet aggregation induced by U46619 in vitro and ex vivo. Among the derivatives evaluated, N-(3′-amidinophenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide (21) was the most active compound.
Keywords: N2-Arylcarbonyl/sulfonylanthranilamides, prothrombin time, activated partial thromboplastin time, Thrombin, Factor Xa, U46619
1. Introduction
Thromboembolic disorders, such as cerebral thrombosis, deep vein thrombosis, unstable angina, myocardial infarction, and thromboembolic stroke; are one of the major disorders that cause morbidities and mortalities worldwise [1]. Several anticoagulants, such as heparins and vitamin K antagonists, have known to be effective in the treatment and prevention of venous thromboembolic diseases [2]. However, there are significant shortcomings, such as monitoring, inconvenience of drug administration, bleeding for heparins, and such as considerable drug and food interactions for vitamin K antagonists, therefore their clinical application was restricted [3].
Thrombin (factor IIa, FIIa) and factor Xa (FXa) occupy central positions in the blood coagulation cascade and play prominent role in various thromboembolic complications. Thrombin promotes blood clot formation by the conversion of fibrinogen to insoluble fibrin and activating platelets [4]. Inhibition of thrombin attenuates formation of fibrin, reduces thrombin generation, and may limit platelet aggregation. FXa is a trypsin like serine protease which plays a pivotal role in the sequence of blood coagulation events. By directly binding to FXa active site, FXa inhibitors lead to interruption of the intrinsic and extrinsic coagulation cascade pathways and thus to inhibition of thrombin formation and thrombus development.
Heparin inhibits both thrombin and factor Xa indirectly via complex formation with modulation of the activity of the serine protease inhibitor antithrombin III [5]. Fondaparinux sodium (Arixtra®), synthetic heparin pentasaccharide, acts as antithrombin III (AT-III) mediated selective inhibition of FXa, distinct from heparin. Its pharmacokinetic properties is nearly complete bioavailability subcutaneously to allow for a simple, fixed-dose, a prolonged half-life, without the need for monitoring [6,7]. Unexpectedly, fondaparinux sodium is inconvenient for long-term use and requires dose adjustment. If the therapeutic range and coagulation monitoring are necessary, it can be monitored with FXa levels by a chromogenic assay, particularly in hemorrhage and renal insufficiency, and it may also require the control of platelet contents [8].
The first synthetic direct thrombin inhibitor was ximelagatran, but it was not approved for medicinal use because of its hepatotoxicity. The direct thrombin inhibitor, dabigatran etexilate mesylate (Pradaxa®), argatroban, and synthetic 20-amino acid peptide, bivalirudin are approved for use on the market [9]. Apixaban (Eliquis®) and rivaroxaban (Xarelto®) are oral direct FXa inhibitors. But, while these anticoagulants carry similar side effects to warfarin, such as risk for gastrointestinal bleeding and intracranial hemorrhage, international normalized ratio (INR) and PT monitoring are not required.
The structures of thrombin and FXa show similarities in their active sites and for this reason, attempts have been made to develop novel synthetic compounds that exert a dual inhibition of the two main enzymes of the coagulation cascade. A combination of thrombin and FXa inhibitory activity in a single, synthetic, orally bioavailable, small molecular weight compound as a novel approach to antithrombotic therapy should therefore result in potent anticoagulant with potentially superior features over currently available therapies [10].
The first compounds to demonstrate dual inhibitor properties were BIBM 1015 and tanogitran (BIBT986) which belong to the methylbenzimidazole series [5]. Hexadecasaccharide (e.g., SR123781) [11,12] is a synthetic heparin mimetic that inhibits both FXa and thrombin via antithrombin III that was advanced to phase I clinical trials. This dual inhibitor demonstrated superior antithrombotic properties in three rat models of thrombosis following intravenous administration compared to selective factor Xa inhibitor, synthetic pentasaccharide fondaparinux (Arixtra®) [13]. In addition, novel anti-FXa pentasaccharides coupled to active site thrombin inhibitors to obtain dual inhibitory effects have also been reported [14,15]. However, problems with thrombin-rebound remain with these mimetics, which appear to result from the inability to inhibit clot-bound thrombin.
EP 217609 is a synthetic parenteral anticoagulant with a dual-action that combines a α-NAPAP analog (direct thrombin inhibitor) and a fondaparinux analog (indirect factor Xa inhibitor) in a single molecule without the complications of thrombin rebound [16]. In recent years several groups [17,18,19,20] have reported the development of dual FXa and thrombin inhibitors and proposed a stronger anticoagulant activity for such compounds compared to monoselective inhibitors.
Furthermore, some patients with cardiovascular disease have indications for anticoagulant and dual antiplatelet therapy. It is estimated that between 5 and 10 percent of patients scheduled for coronary artery stenting, and who thus require dual antiplatelet therapy, are receiving oral anticoagulant, most often for atrial fibrillation [21]. The concomitant use of dual antiplatelet therapy and oral anticoagulant is referred to as triple oral antithrombotic therapy (triple therapy).
We previously reported the synthesis and anticoagulant and antiplatelet activities of N3-aroylbenzamide derivatives. Two amidine compounds, N-(3′-amidino-, and N-(4′-amidinophenyl)-3-(thiophen-2′′-ylcarbonylamino)benzamide exhibited weak thrombin, FXa, and U46619 inhibitory activities [22]. Anthranilamide derivatives have been studied as FXa inhibitors [23,24,25,26], therefore anthranilic acid was considered as central ring of diamide to improve the activity. We now report the synthesis and evaluation of the thrombin, FXa, and U46619 inhibitory activities of non-amidino N2-thiophenecarbonyl- and N2-tosylanthranilamides and amidino N2-thiophenecarbonyl- and N2-tosylanthranilamides.
2. Results and Discussion
2.1. Chemistry
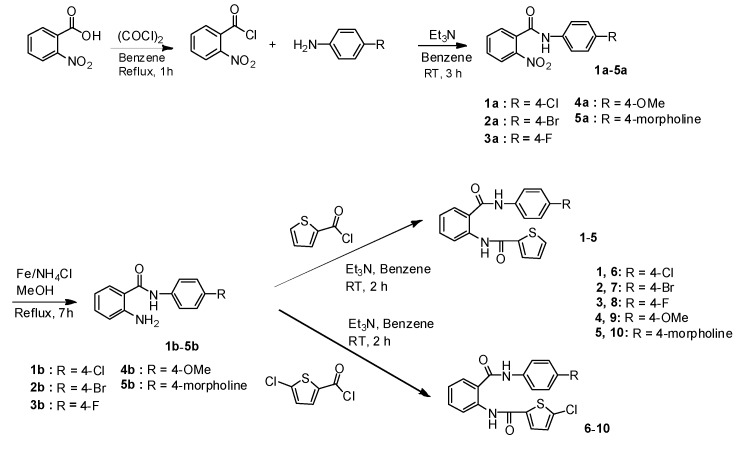
All synthetic methods used in this study are shown in Scheme 1, Scheme 2, Scheme 3 and Scheme 4. Scheme 1 and Scheme 2 showed the synthesis of non-amidine derivatives and Scheme 3 and Scheme 4 showed the synthesis of amidine derivatives. Scheme 1 illustrated the synthesis of N2-(thiophenecarbonyl)anthranilamide derivatives 1–10, which were prepared according to synthetic procedures for N3-(thiophenecarbonyl)benzamide derivatives [22]. The non-amidine type inhibitor, rivaroxaban, apixaban, and edoxaban, possess a small lipophilic substituent, such as a methoxy or chloro or morpholine group. The 2-nitrobenzoyl chloride was coupled with 4-substituted anilines (4-chloro, 4-bromo-, 4-fluoro-, 4-methoxy-, and 4-morpholinoaniline) as small lipophilic compounds to give 1a–5a and these nitro amides were reduced with Fe and NH4Cl to provide the corresponding amino amides 1b–5b. Intermediates 1b–5b were then coupled with thiophene-2-carbonyl chloride and 5-chlorothiophene-2-carbonyl chloride to furnish N2-(thiophene-2-carbonyl)anthranilamides 1–5 and N2-(5-chlorothiophene-2-carbonyl)anthranilamides 6–10, respectively.
Scheme 1.
Synthesis of N2-(thiophenecarbonyl)anthranilamides derivatives 1–10.
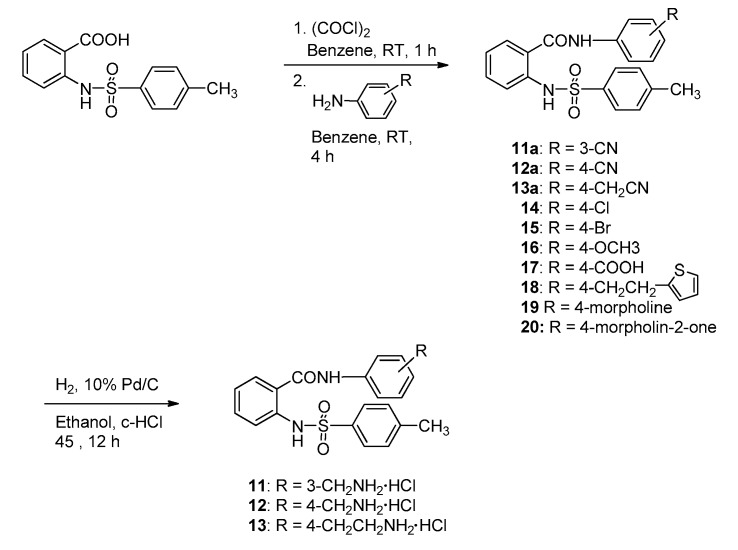
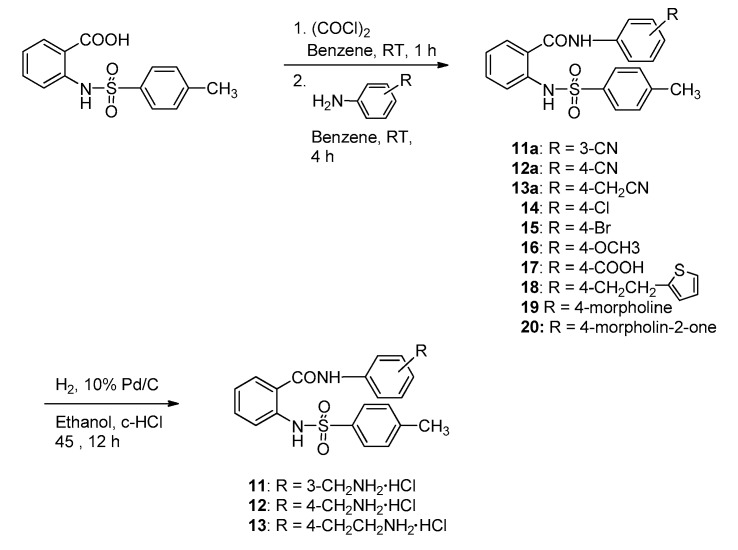
Scheme 2.
Synthetic procedures of N2-tosylanthranilamide derivatives 11–20.
Scheme 3.
Synthetic procedures of amidino N2-(thiophenecarbonyl)anthranilamide derivatives (21–23).
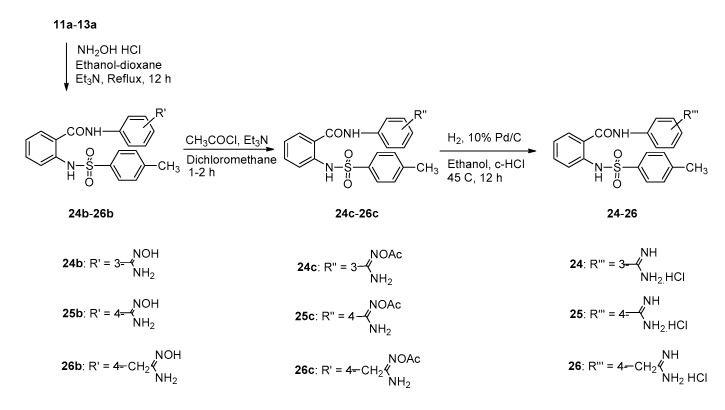
Scheme 4.
Synthetic procedures of amidino N2-tosylanthranilamide derivatives (24–26).
The introduction of sulfonamide instead of one carboxamide in the linker of diamide may give rise to differences in the possible hydrogen-binding interaction between the ligand and the residues in the thrombin/FXa active site. The N2-arylsulfonamide series 11–20 were synthesized to compare with the activity of N2-arylcarboxamide series 1–10. N2-tosylanthranilamides were synthesized as beginning compounds for development of N2-arylsulfonamide derivatives.
The preparative route to N2-tosylanthranilamide derivatives 11–20 are shown in Scheme 2. Anthranilic acid was reacted with p-tosyl chloride to give N-tosylbenzoic acid, followed by chlorination with oxalyl chloride to give N-tosylbenzoyl chloride. This acid chloride was coupled with 3-cyano-, 4-cyano-, 4-cyanomethyl-, 4-chloro-, 4-bromo-, 4-methoxy-, 4-carboxy-, 4-(2-(2-thienyl)ethyl)-, 4-morpholino-, and 4-(2-oxomorpholino)aniline to give N2-tosylanthranilamide derivatives 11a–13a and 14–20, respectively. To consider the pharmacokinetic problems we replaced the amidine group with less basic residues, aminoalkyl groups. The cyano group containing compounds 11a–13a were catalytically hydrogenated in the presence of 10% Pd/C and c-HCl at 45 °C to give aminoalkyl amides 11–13.
Scheme 3 illustrated the synthetic procedures of amidino N2-thiophenecarbonyl anthranilamide derivatives 21–23. The amidine group forms a bidentate salt bridge interaction with carboxylic acid of Asp189 in the S1 site of FXa. The high polar amidine-derived inhibitors demonstrated potent in vivo anticoagulant and antithrombotic activity. The first amide bond was obtained from acylation of 2-nitrobenzoyl chloride with 3-cyano-, 4-cyano-, and 4-cyanomethyl-aniline to afford 21a–23a.
2-Aminobenzamides 21b–23b were synthesized from reduction of 2-nitrobenzamides 21a–23a with Fe and NH4Cl. The treatment of 21b–23b with thiophene-2-carbonyl chloride prepared the N2-(thiophene-2-carbonyl)-N-(3-cyano- or -4-cyanophenyl)benzamides 21c and 22c, and the N2-(thiophene-2-carbonyl)-N-(4-cyanobenzyl)benzamide 23c, which were treated with hydroxylamine HCl and triethylamine to give amidoximes 21d–23d. The N–O bond of amidoximes 21d–23d was reduced to amidinium chloride 21–23 via the catalytic hydrogenation of the O-acetyl amidoximes 21e–23e over 10% Pd/C in the presence of c-HCl at 60 psi, 45 °C for 2 h. The four proton peaks of amidine HCl salt in 21 were determined as two broad singlets at 9.08 and 9.36 ppm, and the four proton peaks of amidine HCl salt in 22 and 23 were observed as one broad singlets at 9.36 and 8.92 ppm in the 1H-NMR spectra, respectively. In the HMBC spectrum, two amide NH proton peaks of 22 were distinguished by observing the correlation of CO (167.5 ppm) and NH (10.77 ppm) of the -NHCO group and the correlation of CO (164.9 ppm) and NH (11.53 ppm) of the -CONH group, respectively.
Scheme 4 illustrated the synthetic procedures of amidino N2-tosylanthranilamide derivatives 24–26. Compounds 11a–13a were refluxed with hydroxylamine hydrochloride and triethylamine in ethanol to provide the amidoxime derivatives 24b–26b to form amidinium chloride derivatives 24–26 via the catalytic hydrogenation of the O-acetyl amidoximes 24c–26c in 10% Pd/C and c-HCl at 60 psi, 45 °C for 12 h.
2.2. Biology
2.2.1. Effects of Non-Amidino N2-Aroylanthranilamides on In Vitro Activated Partial Thromboplastin Time (aPTT), Prothrombin Time (PT), In Vivo Tail Bleeding Time, and Ex Vivo Clotting Time
Many FXa and thrombin inhibitors containing an amidine group displayed insufficient absorption when administered orally, the reason is caused by strongly basic amidine group [27]. To improve oral bioavailability, synthetic projects seem to be changed to synthesis of non-amidines having weak basic groups. Rivaroxaban and apixaban are non-amidine direct FXa inhibitors, and argatroban and dabigatran eterxilate are non-amidine direct thrombin inhibitors.
The anticoagulant effects of twenty non-amidino N2-thiophenecarbonyl anthranilamides 1–10 and N2-tosylanthranilamides 11–20 were screened in aPTT and PT assays using human plasma at a concentration of 30 µg/mL in vitro and demonstrated in Table 1. As shown in Table 1, N-(4-morpholinophenyl)-2-(thiophen-2-ylcarbonyl)benzamide (5), N-(4-fluorophenyl)-2-(5-chloro thiophen-2-ylcarbonyl)benzamide (8), and N-(4-methoxyphenyl)-2-(5-chlorothiophen-2-ylcarbonyl)benzamide (9) displayed very high aPTT values (60.6, 59.3, and 65.1 s), respectively. Any N2-tosylanthranilamides 11–20 did not exhibit the prolongation of PT and aPTT. This result demostrate that both carboxamide linker and thiophene ring are necessary for anticoagulant activity. For the further antithrombotic experiments, thiophene compound 5 and 5-chlorothiophene compound 9 were investigated on anticoagulant activities (in vitro and ex vivo) and on tail bleeding time (in vivo) on mouse. These two compounds were selected for further evaluations as were the ones which showed an aPPT time higher than 60 s.
Table 1.
Anticoagulant activities of non-amidine compounds 1–20 at 30 µg/mL.
| Compound No. | aPPT (s) | PT (s) | Compound No. | aPTT (s) | PT (s) |
|---|---|---|---|---|---|
| DMSO 1 | 39.0 | 11.1 | 11 | 40.2 | 11.8 |
| 1 | 44.5 | 14.1 | 12 | 41.2 | 11.7 |
| 2 | 37.4 | 13.2 | 13 | 42.3 | 10.9 |
| 3 | 41.6 | 11.8 | 14 | 38.6 | 13.6 |
| 4 | 41.7 | 11.6 | 15 | 35.8 | 11.3 |
| 5 | 60.6 | 12.8 | 16 | 36.2 | 11.0 |
| 6 | 39.9 | 12.2 | 17 | 41.5 | 11.7 |
| 7 | 44.9 | 12.2 | 18 | 38.4 | 11.0 |
| 8 | 59.3 | 12.7 | 19 | 35.8 | 10.7 |
| 9 | 65.1 | 12.5 | 20 | 33.6 | 10.8 |
| 10 | 53.3 | 14.7 | Heparin 2 | 85 (30 µM) | 34 (30 µM) |
1 Dimethyl sulfoxide (DMSO) was used as the negative control. 2 The molecular weight of heparin used as positive control is 3500. aPTT: activated partial thromboplastin time, PT: prothrombin time.
According to the Table 2, aPTT in the saline-treated group was 23.6 ± 0.6 s (mean ± standard error of mean (SEM), n = 5) and aPTT of compounds 5, 9, and heparin was respectively prolonged as 33.2 ± 0.5 , 37.6 ± 0.3 and 53.3 ± 0.5 s at dose 20 µM. Although compounds 5 and 9 showed weaker activities than those of heparin, aPTT was significantly prolonged by 5 and 9 at concentrations of over 20 µM as compared to the saline-treated group. Unlike the methoxy compound 9, the morpholine compound 5 showed PT prolongation at concentrations 20 µM and above as compared to the saline-treated group.
Table 2.
In vitro anticoagulant activities of 5 and 9.
| Compound No. | Dose | aPTT (s) | PT (s) | PT (INR) |
|---|---|---|---|---|
| Control | saline | 23.6 ± 0.6 | 12.4 ± 0.4 | 1.00 |
| 5 | 5 µM | 23.7 ± 0.2 | 12.2 ± 0.4 | 0.96 |
| 10 µM | 24.2 ± 0.8 | 12.6 ± 0.6 | 1.04 | |
| 20 µM | 33.2 ± 0.5 * | 14.0 ± 0.3 * | 1.34 * | |
| 30 µM | 40.4 ± 0.6 * | 15.2 ± 0.5 * | 1.63 * | |
| 40 µM | 58.5 ± 0.5 * | 18.5 ± 0.4 * | 2.61 * | |
| 50 µM | 60.5 ± 0.7 * | 19.1 ± 0.6 | 2.78 * | |
| 9 | 5 µM | 23.2 ± 0.4 | 12.0 ± 0.2 | 0.92 |
| 10 µM | 23.4 ± 0.5 | 12.2 ± 0.5 | 0.96 | |
| 20 µM | 37.6 ± 0.3 * | 12.0 ± 0.2 | 0.92 | |
| 30 µM | 44.1 ± 0.4 * | 11.6 ± 0.6 | 0.85 | |
| 40 µM | 65.6 ± 0.6 * | 12.7 ± 0.5 | 1.06 | |
| 50 µM | 63.2 ± 0.8 * | 13.0 ± 0.8 | 1.12 | |
| Heparin 1 | 20 µM | 53.3 ± 0.5 * | 21.6 ± 0.4 * | 3.79 * |
| 30 µM | 62.5 ± 0.8 * | 27.2 ± 0.6 * | 6.59 * |
1 The molecular weight of heparin used as positive control in our experiments is 3500. Each value represents the means ± standard error of mean (SEM) (n = 5). * p < 0.05.
We can remember that a prolongation of aPTT indicates the inhibition of intrinsic pathway, and a prolongation of PT indicates the inhibition of extrinsic pathway including common pathway or not. Therefore, the prolongation of both aPTT and PT in compound 5 means inhibition of the extrinsic and intrinsic and/or common pathway. The prolongation of aPTT in 9 suggests inhibition of intrinsic pathway and/or common pathway.
The most active compounds 5 and 9 in aPTT in vitro evaluations will be evaluated in the tail bleeding times in vivo model. The circulating blood volume for mice is averagely 72 mL/kg [28]. Because the weight of used mouse is averagely 27 g, the molecular weights of 5 and 9 are 407.49 and 386.85 and the blood volume is averagely 2 mL, the amount of target compounds (24.4, 32.6, 40.8 µg/mouse for 5 and 23.2, 30.9, 38.7 µg/mouse for 9) injected provided a maximum concentration of 30, 40, or 50 µM in the peripheral blood.
As shown in Table 3, compounds 5 and 9 significantly prolonged the tail bleeding times in at concentrations 24.4 and 23.2 µg/mouse and above as compared to the control, respectively.
Table 3.
In vivo Bleeding time of 5 and 9.
| Compound No. | Dose | Tail Bleeding Time (s) |
|---|---|---|
| Control | saline | 30.8 ± 0.8 |
| 5 | 24.4 µg/mouse | 37.4 ± 1.0 * |
| 32.6 µg/mouse | 53.7 ± 1.2 * | |
| 40.8 µg/mouse | 61.8 ± 1.2 * | |
| 9 | 23.2 µg/mouse | 48.2 ± 1.6 * |
| 30.9 µg/mouse | 62.2 ± 1.4 * | |
| 38.7 µg/mouse | 65.4 ± 1.6 * | |
| Heparin 1 | 140.0 µg/mouse 210.0 µg/mouse |
51.2 ± 1.2 * |
| 68.4 ± 1.2 * |
1 The molecular weight of heparin used as positive control in our experiments is 3500. Each value represents the means ± SEM (n = 5). * p < 0.05.
The aPTT values were significantly prolonged by both 5 and 9 at concentration 24.4 and 23.2 µg/mouse and above ex vivo clotting times, while the prolongation in PT was found in compound 5. (Table 4).
Table 4.
Ex vivo clotting time of 5 and 9.
| Compound No. | Dose | aPTT (s) | PT (s) | PT (INR) |
|---|---|---|---|---|
| Control | saline | 32.2 ± 0.8 | 12.8 ± 0.4 | 1.00 |
| 5 | 24.4 µg /mouse | 38.4 ± 0.8 * | 14.8 ± 0.4 * | 1.42 * |
| 32.6 µg/mouse | 51.2 ± 1.4 * | 17.4 ± 0.8 * | 2.09 * | |
| 40.8 µg/mouse | 60.8 ± 1.2 * | 17.2 ± 0.7 * | 2.03 * | |
| 9 | 23.2 µg/mouse | 42.2 ± 0.6 * | 12.6 ± 0.8 | 0.96 |
| 30.9 µg/mouse | 57.4 ± 1.2 * | 13.0 ± 0.6 | 1.04 | |
| 38.7 µg/mouse | 61.2 ± 1.0 * | 12.4 ± 0.8 | 0.93 | |
| Heparin 1 | 140.0 µg/mouse | 63.4 ± 0.9 * | 25.4 ± 0.4 * | 5.18 * |
| 210.0 µg/mouse | 75.4 ± 1.2 * | 31.4 ± 0.6 * | 8.62 * |
1 The molecular weight of heparin used as positive control in our experiments is 3500. Each value represents the means ± SEM (n = 5). * p < 0.05.
In summary, aPTT (in vitro and ex vivo) of 9 was longer than those of 5 suggesting that methoxy group of 9 is more effective for anticoagulant activity than morpholine group of 5, while 5-chloro group of 5-chlorothiophene was seemed not to influence on the anticoagulant activity.
To obtain more active compounds, six amidino N2-aroylantranilamide derivatives (21–26) derivatives were synthesized and also screened in aPTT and PT assays using human plasma at a concentration of 30 µg/mL in vitro and were demonstrated in Table 5. In case of N2-thiophene compounds, both aPTT and PT of 3-amidino- and 4-amidinomethyl- compounds 21 and 23 were longer than 4-amidino compound 22. In reverse, among N2-tosyl derivatives 24–26, 4-amidino derivative 25 showed longer aPTT than 3-amidino- and 4-amidinomethyl- compounds 24 and 26.
Table 5.
Anticoagulant activities of amidine compounds 21–26 at 30 µg/mL.
| Compound No. | aPTT (s) | PT (s) | Compound No. | aPTT (s) | A PT (s) |
|---|---|---|---|---|---|
| DMSO 1 | 39.0 | 11.1 | 24 | 41.7 | 11.5 |
| 21 | 74.3 | 22.0 | 25 | 63.1 | 12.8 |
| 22 | 50.7 | 19.9 | 26 | 40.5 | 11.7 |
| 23 | 65.8 | 20.9 | Heparin 2 | 34 (30 µM) | 85 (30 µM) |
1 DMSO was used as the negative control. 2 The molecular weight of heparin used as positive control in our experiments is 3500. aPTT: activated partial thromboplastin time, PT: prothrombin time.
From these aPTT and PT results, 21–23 were further investigated on anticoagulant activities (in vitro and ex vivo) and on tail bleeding time (in vivo) on mouse. These three compounds were selected for further evaluations as were the ones which showed both aPPT and PT time higher than 50 s and 15 s, respectively.
As shown in Table 6, aPTT in the saline-treated group was 23.6 ± 0.6 s (mean ± SEM, n = 5) and aPTT showed 38.5 ± 0.4 s, 30.2 ± 0.3 s, 38.5 ± 0.7 s, and 53.3 ± 0.5 s at dose 20 µM in compounds 21–23 and heparin, respectively. aPTT of compounds 21 and 23 was significantly prolonged at concentrations of 10 µM and above, and 22 at concentrations of 20 µM and above as compared to the saline-treated group. In addition, 21–23 significantly showed PT prolongation (16.7 ± 0.5, 13.9 ± 0.3, 14.2 ± 0.5 s) and INR (2.04, 1.32, and 1.38) at concentrations of 20 µM and above as compared to the saline-treated group (12.4 ± 0.4 s). These results in this study showing prolongation of aPTT and PT of N2-thiophenecarbonyl anthranilamides 21–23 suggest inhibition of both extrinsic and intrinsic, and/or common pathway.
Table 6.
In vitro anticoagulant activity of amidino N2-thiophene compounds 21–23.
| Compound No. | Dose | aPTT (s) | PT (s) | PT (INR) |
|---|---|---|---|---|
| Control | saline | 23.6 ± 0.6 | 12.4 ± 0.4 | 1.00 |
| 21 | 5 µM | 24.1 ±0.6 | 12.8 ± 0.7 | 1.08 |
| 10 µM | 30.8 ± 0.5 * | 14.5 ± 0.5 * | 1.46 * | |
| 20 µM | 38.5 ± 0.4 * | 16.7 ± 0.5 * | 2.04 * | |
| 30 µM | 52.7 ± 0.7 * | 19.3 ± 0.6 * | 2.89 * | |
| 40 µM | 71.4 ± 0.6 * | 22.7 ± 0.7 * | 4.27 * | |
| 50 µM | 75.2 ± 0.5 * | 23.5 ± 0.5 * | 4.64 | |
| 22 | 5 µM | 22.9 ± 0.5 | 12.2 ± 0.4 | 0.96 |
| 10 µM | 23.7 ± 0.6 | 12.7 ± 0.4 | 1.06 | |
| 20 µM | 30.2 ± 0.3 * | 13.9 ± 0.3 * | 1.32 * | |
| 30 µM | 32.8 ± 0.5 * | 16.1 ± 0.7 * | 1.87 * | |
| 40 µM | 48.6 ± 0.4 * | 20.8 ± 0.5 * | 3.46 * | |
| 50 µM | 51.4 ± 0.7 * | 21.4 ± 0.6 * | 3.70 * | |
| 23 | 5 µM | 24.2 ± 0.2 | 12.1 ± 0.6 | 0.94 |
| 10 µM | 30.2 ± 0.5 * | 12.5 ± 0.4 | 1.02 | |
| 20 µM | 38.5 ± 0.7 * | 14.2 ± 0.5 * | 1.38 * | |
| 30 µM | 43.2 ± 0.4 * | 17.4 ± 0.4 * | 2.25 * | |
| 40 µM | 63.8 ± 0.6 * | 20.1 ± 0.6 * | 3.19 * | |
| 50 µM | 64.1 ± 0.4 * | 20.2 ± 0.5 * | 3.23 * | |
| Heparin 1 | 20 µM | 53.3 ± 0.5 * | 21.6 ± 0.4 * | 3.79 * |
| 30 µM | 62.5 ± 0.8 * | 27.2 ± 0.6 * | 6.59 * |
1 The molecular weight of heparin used as positive control in our experiments is 3500. Each value represents the means ± SEM (n = 5). * p < 0.05.
The most active compounds 21–23 in aPTT in vitro evaluations will be evaluated in the tail bleeding times in vivo model. As shown in Table 7, tail bleeding times of compounds 21 and 22 were significantly prolonged in at concentrations of 24.1 µg/mouse and above, and by compound 23 in at concentrations of 24.9 µg/mouse and above as compared to the control, respectively.
Table 7.
In vivo bleeding time of amidino N2-thiophene compounds 21–23.
| Compound No. | Dose | Tail Bleeding Time (s) |
|---|---|---|
| Control | saline | 30.8 ± 0.8 |
| 21 | 24.1 µg/mouse | 49.2 ± 1.2 * |
| 32.1 µg/mouse | 68.4 ± 1.0 * | |
| 40.1 µg/mouse | 70.4 ± 1.6 * | |
| 22 | 24.1 µg/mouse | 33.1 ± 1.0 * |
| 32.1 µg/mouse | 46.2 ± 0.6 * | |
| 40.1 µg/mouse | 48.3 ± 1.2 * | |
| 23 | 24.9 µg/mouse | 42.3 ± 0.6 * |
| 33.2 µg/mouse | 56.4 ± 1.6 * | |
| 41.5 µg/mouse | 58.2 ± 1.4 * | |
| Heparin 1 | 140.0 µg/mouse | 51.2 ± 0.8 * |
| 210.0 µg/mouse | 68.4 ± 1.2 * |
1 The molecular weight of heparin used as positive control in our experiments is 3500. Each value represents the means ± SEM (n = 5). * p < 0.05.
As shown Table 8, both aPTT and PT were dose-dependently prolonged by both 21 and 22 at concentration of 24.1 µg/mouse and above, and by 23 at concentrations of 24.9 µg/mouse and above ex vivo clotting times.
Table 8.
Ex vivo clotting time of amidino N2-thiophene compounds 21–23.
| Compound No. | Dose | aPTT (s) | PT (s) | PT (INR) |
|---|---|---|---|---|
| Control | Saline | 32.2 ± 0.8 | 12.8 ± 0.4 | 1.00 |
| 21 | 24.1 µg/mouse | 48.8 ± 1.0 * | 19.4 ± 0.8 * | 2.71 * |
| 32.1 µg/mouse | 65.8 ± 0.8 * | 21.2 ± 0.6 * | 3.36 * | |
| 40.1 µg/mouse | 69.4 ± 1.0 * | 23.7 ± 1.2 * | 4.39 * | |
| 22 | 24.1 µg/mouse | 37.2 ± 1.2 * | 15.7 ± 0.6 * | 1.63 * |
| 32.1 µg/mouse | 47.5 ± 0.6 * | 19.8 ± 0.8 * | 2.85 * | |
| 40.1 µg/mouse | 49.6 ± 0.8 * | 20.1 ± 0.8 * | 2.95 * | |
| 23 | 24.9 µg/mouse | 39.8 ± 0.8 * | 16.1 ± 0.5 * | 1.73 * |
| 33.2 µg/mouse | 54.2 ± 1.2 * | 18.2 ± 0.7 * | 2.33 * | |
| 41.5 µg/mouse | 56.4 ± 1.8 * | 19.4 ± 0.6 | 2.71 * | |
| Heparin1) | 140.0 µg/mouse | 63.4 ± 0.9 * | 25.4 ± 0.4 * | 5.18 * |
| 210.0 µg/mouse | 75.4 ± 1.2 * | 31.4 ± 0.6 * | 8.62 * |
1 The molecular weight of heparin used as positive control in our experiments is 3500. Each value represents the means ± SEM (n = 5). * p < 0.05.
2.2.2. Thrombin and Factor Xa (FXa) Activity
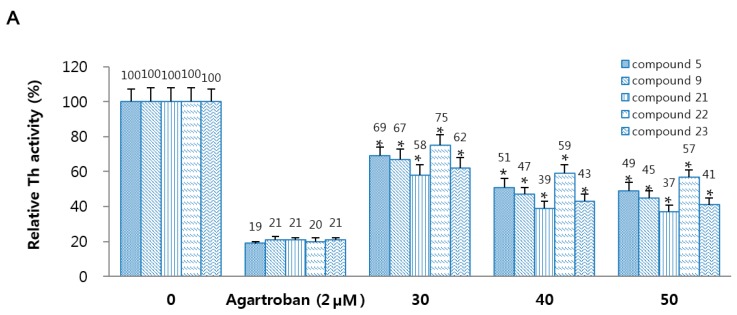
To determine the fundamental mechanism of 5, 9 and 21–23, the inhibitory activities of 5, 9 and 21–23 on the thrombin and FXa were investigated. According to the Figure 1A, compounds 5, 9, and 21–23 showed in a dose-dependent inhibition of the activity of thrombin. In addition, treatment with 5, 9, and 21–23 displayed in a dose-dependent inhibition of amidolytic activity of FXa, indicating direct inhibition of FXa activity. Agartroban and rivaroxaban were used as a positive control, respectively (Figure 1B).
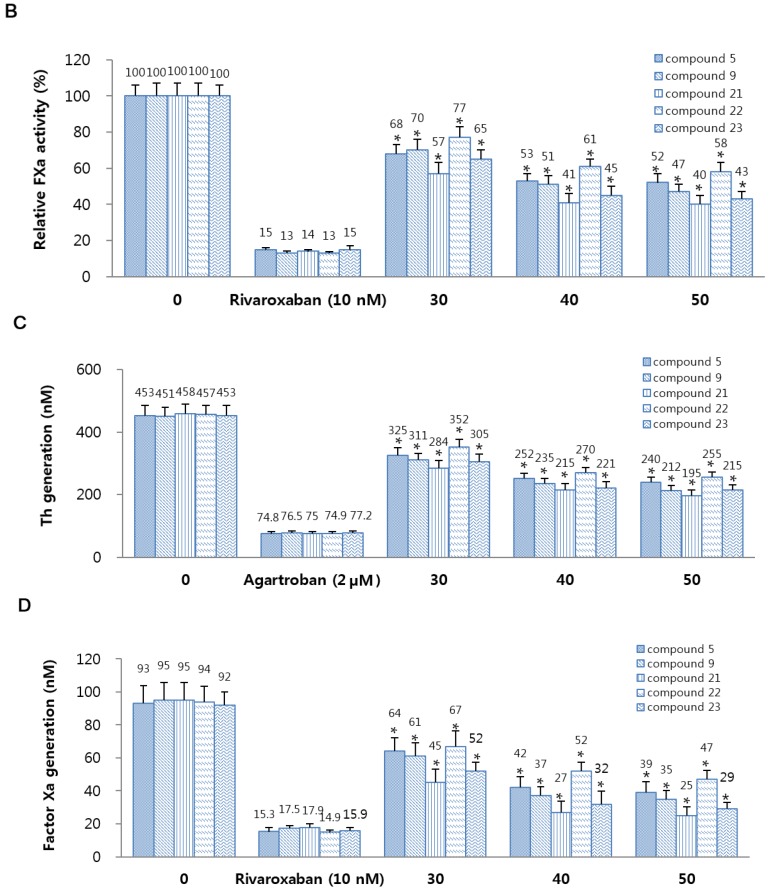
Figure 1.
Effects of 5, 9, and 21–23 on inhibitory activity and generation of thrombin and factor Xa. (A) Inhibitory activity of thrombin (Th) by 5 or 9 or 21–23 was evaluated using a chromogenic assay. (B) Inhibitory activity of FXa by 5 or 9 or 21–23 was investigated using a chromogenic assay, as. Argatroban (A,C) or rivaroxaban (B,D) were used as positive control. (C) Human umbilical vein endothelial cells (HUVEC) monolayers were pre-incubated with FVa (100 pM), FXa (1 nM), and the indicated concentrations of 5 or 9 or 21–23 for 10 min. Prothrombin was added to a final concentration of 1 µM and prothrombin activation was determined 10 min later. (D) HUVECs were pre-incubated with the indicated concentrations of 5 or 9 or 21–23 for 10 min. After TNF-α stimulated HUVECs were incubated with FVIIa (10 nM) and FX (175 nM), FXa generation was determined. Data represent the mean ± standard error of mean (SEM) of three independent experiments. * p < 0.05.
2.2.3. Thrombin and Factor Xa (FXa) Generation
According to findings reported by Sugo et al. [29], the endothelial cells are known to support the prothrombin activation by FXa. In this study, human umbilical vein endothelial cells (HUVECs) were pre-incubated with FVa and FXa in the presence of CaCl2 and the addition of prothrombin resulted in generation of thrombin (Figure 1C). In a previous study, Rao et al. [30] reported that the endothelium provides the functional equivalent of pro-coagulant phospholipids and supports activation of FXa, and, in TNF-α stimulated HUVECs, activated of FX by FVIIa occurred in a tissue factor (TF) expression-dependent manner. Thus, we determined the effects of 5, 9, and on activation of FX by FVIIa. Pre-incubation with 5, 9, and 21–23 resulted in dose-dependent inhibition of FX activation by FVIIa (Figure 1D). Therefore, these results indicated that 5, 9, and 21–23 can inhibit generation of thrombin and FXa.
2.2.4. Effects of 5, 9, and 21–23 on Thrombin-Catalyzed Fibrin Polymerization and Platelet Aggregation
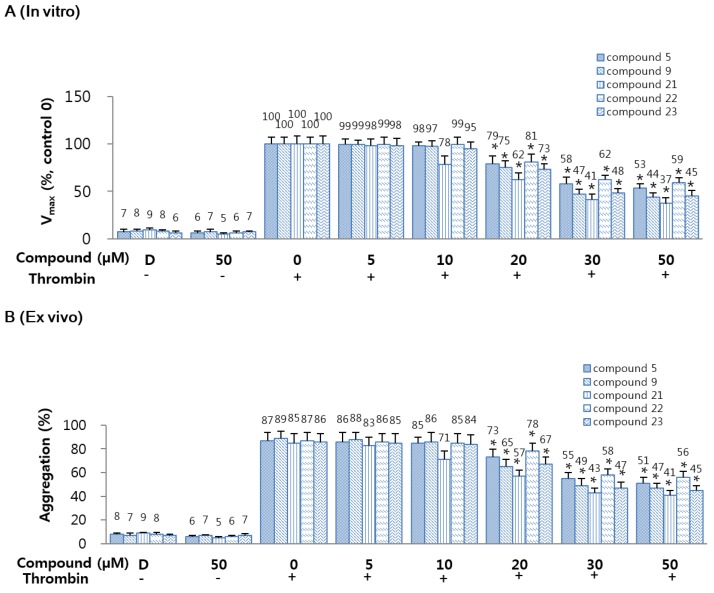
The effects of 5 or 9 or 21–23 on thrombin-catalyzed fibrin polymerization in human plasma were monitored as changes in absorbance at 360 nm. These results display that incubation of 5 or 9 or 21–23 with human plasma resulted in a significant decrease in the maximal rate of fibrin polymerization (Figure 2A). To delete the effect of sample pH, all samples were diluted with 50 mM tris-buffer saline (TBS) (pH 7.4). The effect of dimethyl sulfoxide (DMSO) on human plasma was also evaluated, however, coagulation properties were unaffected. In order to exclude the possibility that the decrease of fibrin polymerization could be due to a direct effect on thrombin leading to a decrease in fibrin generation rather than polymerization of fibrin formed, reptilase-catalyzed polymerization assay was performed. Results showed that 5, 9, and 21–23 induced a significant decrease in reptilase-catalyzed polymerization [31]. To determine the antiplatelet activities of compounds 5 or 9 or 21–23, thrombin-catalyzed platelet aggregation assay was performed and the results were shown in Figure 2B. Compounds 5 or 9 or 21–23 significantly and dose-dependently inhibited mouse platelet aggregation induced by thrombin (final concentration: 3 U/mL).
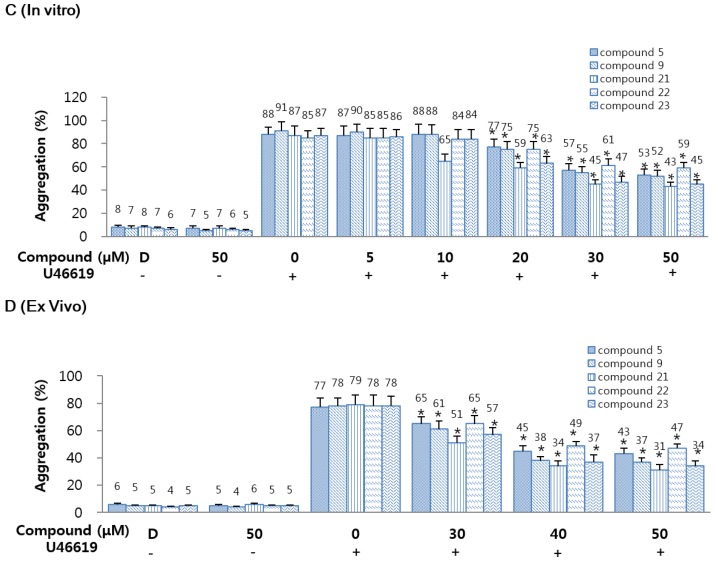
Figure 2.
Effects of 5, 9, and 21–23 on fibrin polymerization in human plasma. (A) Thrombin-catalyzed fibrin polymerization at the indicated concentrations of 5 or 9 or 21–23 was monitored using a catalytic assay. The results are Vmax values expressed as percentages versus controls; (B) The effect of 5 or 9 or 21–23 on mouse platelet aggregation induced by 3 U/mL thrombin; (C) The effect of each compound on human platelet aggregation induced by 2 mM U46619; (D) The indicated concentration of each compound in dimethyl sulfoxide (DMSO) was injected intravenously. The effects of each compound on mouse platelet aggregation induced by 2 µM U46619 were monitored ex vivo. D: 0.2% DMSO. Data represent the mean ± SEM of three independent experiments performed. * p < 0.05.
2.2.5. Effects of 5, 9, and 21–23 on U46619 Catalyzed Platelet Aggregation
To determine the antiplatelet activities of 5 or 9 or 21–23, a U46619 catalyzed platelet aggregation assay was carried out. As shown in Figure 2C, compounds 5 or 9 or 21–23 significantly and dose-dependently inhibited human platelet aggregation induced by U46619 (final concentration: 2 µM). These in vitro results were confirmed in an ex vivo platelet aggregation assay (intravenous injection, Figure 2D). As a result, 5 or 9 or 21–23 significantly and dose-dependently inhibited platelet aggregation induced by U46619 (final concentration: 2 µM). Until now, most of compounds containing amidine group have been known as FXa inhibitor, but non-amidines 5 and 9, and amidines 21–23 exhibited the potential as platelet aggregation inhibitor.
2.2.6. Cellular Viability
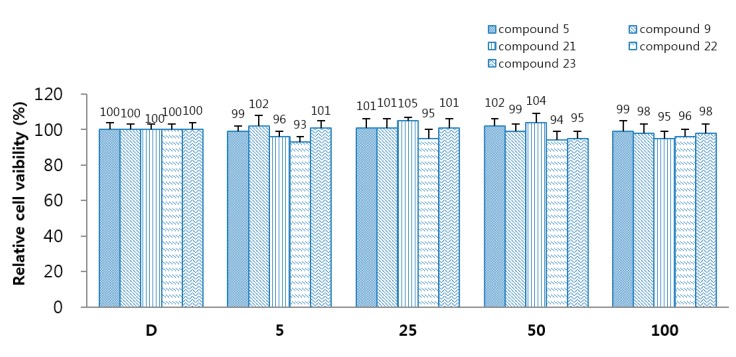
To identify the cellular viability of 5, 9, and 21–23, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed in HUVECs treated with 5, 9, and 21–23 for 24 h. Compounds 5, 9, and 21–23 did not influence cell viability at concentration up to 100 µM (Figure 3). It is safe to treat with a dose higher than the effect dose that does not show cytotoxicity even at a concentration three times higher than the effect concentration.
Figure 3.
Effects of 5, 9, and 21–23 on cellular viability were determined by MTT assay. The data represent the mean ± SEM of three independent experiments performed in triplicate. D: 0.2% DMSO is the control.
3. Materials and Methods
3.1. Chemistry
3.1.1. Reagents and Instruments
The reagents were commercially purchased from Sigma-Aldrich (St. Louis, MO, USA) or TCI (Tokyo, Japan). If necessary, solvents were purified and/or dried prior to use. All anhydrous reactions were carried out under a dry atmosphere of nitrogen. Melting points (m.p.) were measured on Thomas-Hoover melting point apparatus (Thomas Scientific, Swedesboro, NJ, USA) and not corrected. 1H, 13C-NMR and HMBC spectra were measured on a Varian 400 MHz spectrometer (Agilent Technologies, Santa Clara, CA, USA) in DMSO-d6, CDCl3, or (CD3)2CO. Chemical shifts (δ) are in ppm relative to tetramethylsilane, and coupling constants (J) are in Hz. DIP-MS (EI) was measured on an Agilent 7890A-5975C GC/MSD (Agilent Technologies). GC/MS (EI) was determined on a SHIMADZU QP 2010 model (Shimadzu, Kyoto, Japan) and FAB-MS was determined on a JEOL JMS-700 Mstation (JEOL, Tokyo, Japan). Fraction collection was performed on an EYELA fraction collector DC-1500 (Tokyo Rikakikai, Tokyo, Japan). An analytical TLC was performed on pre-coated silica gel 60 F254 plates (Merck, Kenilworth, NJ, USA). Solvent systems for TLC and column chromatography were ethyl acetate/n-hexane mixtures and 10% methanol in dichloromethane. Column chromatography was carried out on Merck silica gel 9385 (Merck).
3.1.2. General Synthetic Procedures for 1a–5a
The oxalyl chloride (38.92 mmol) and triethylamine (32.93 mmol) were added to a solution of 2-nitrobenzoic acid (29.94 mmol) in anhydrous dichloromethane (50 mL) at room temperature. The reaction mixture was refluxed for 1 h and the unreacted oxalyl chloride and solvent were removed under reduced pressure and 2-nitrobenzoyl chloride was used to next reaction without purification. To a solution of 3-aminophenylcyanide or 4-aminophenylcyanide or 4-aminobenzyl cyanide (7.19 mmol) in anhydrous dichloromethane (30 mL) was added 2-nitrobenzoyl chloride (8.99 mmol) and triethylamine (7.19 mmol). The reaction mixture was stirred at room temperature for 3 h and added with water, extracted with dichloromethane (3 × 30 mL), dried with anhydrous magnesium sulfate and filtrated. The filtrate was concentrated under reduced pressure to afford the oily residue, which was separated by column chromatography to afford pure white or pale yellow compounds 1a–5a.
N-(4′-Chlorophenyl)-2-nitrobenzamide (1a). Yield: 85%; m.p.: 184–186 °C (186–187 °C [32]).
N-(4′-Bromophenyl)-2-nitrobenzamide (2a) [33,34]. Yield: 86%; m.p.: 199–201 °C (202–205 °C [35]).
N-(4′-Fluorophenyl)-2-nitrobenzamide (3a). Yield: 78%; m.p.: 165–169 °C (167–171 °C [36]).
N-(4′-Methoxyphenyl)-2-nitrobenzamide (4a) [37]. Yield: 82%; m.p.: 153–154 °C.
N-(4′-Morpholinophenyl)-2-nitrobenzamide (5a). Yield: 76%; m.p.: 220–221 °C; 1H NMR (400 MHz, DMSO-d6) δ: 3.03 (4H, t, J = 4.8 Hz, H3′′ + H5′′), 3.71 (4H, t, J = 4.8 Hz, H2′′ + H6′′), 6.91 (2H, d, J = 9.0 Hz, H3′ + H5′), 7.49 (2H, d, J = 9.0 Hz, H2′ + H6′), 7.68–7.71 (2H, m, H4 + H6), 7.82 (1H, dt, J = 8.0, 1.1 Hz, H5), 8.09 (1H, d, J = 7.6 Hz, H3), 10.42 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 48.9 (C3′′, C5′′), 66.0 (C2′′, C6′′), 115.4 (C2′, C6′), 120.8 (C3′, C5′), 124.2 (C1), 129.3 (C3), 130.8 (C6), 131.0 (C1′), 132.8 (C4), 134.0 (C5), 146.6 (C2), 147.8 (C4′), 163.4 (C=O); GC-MS (EI) m/z: 327 [M]+.
3.1.3. General Synthetic Procedures for 1b–5b
Compounds 1a–5a (3.37 mmol) were dissolved in methanol (30 mL) and stirred with ammonium chloride (33.7 mmol) and iron powder (6.03 mmol) and then refluxed for 7 h. The reaction mixture was added with water and extracted with dichloromethane (3 × 40 mL), treated with anhydrous magnesium sulfate and filtrated. The filtrate was evaporated to prepare the oily residue, which was recrystallized with ethyl acetate and n-hexane mixture to afford pure white or pale yellow compounds 1b–5b.
2-Amino-N-(4′-chlorophenyl)benzamide (1b). Yield: 63%; m.p.: 148–150 °C (147 °C [38]).
2-Amino-N-(4′-bromophenyl)benzamide (2b). Yield: 70%; m.p.: 143–145 °C (139–144 °C [39]).
2-Amino-N-(4′-fluorophenyl)benzamide (3b). Yield: 62%; m.p.: 118–120 °C (122 °C [38]).
2-Amino-N-(4′-methoxyphenyl)benzamide [38] (4b). Yield: 80%; m.p.: 118–119 °C (121 °C [38]).
2-Amino-N-(4′-morpholinophenyl)benzamide (5b). Yield: 97%; m.p.: 167–168 °C; 1H NMR (400 MHz, DMSO-d6 ) δ: 3.06 (4H, t, J = 4.8 Hz, H3′′ + H5′′), 3.74 (4H, t, J = 4.8 Hz, H2′′ + H6′′), 6.29 (2H, s, NH2), 6.57 (1H, dt, J = 7.7, 1.1 Hz, H5), 6.73 (1H, dd, J = 8.2, 1.0 Hz, H3), 6.91 (2H, d, J = 9.0 Hz, H3′ + H5′), 7.17 (1H, dt, J = 8.4, 1.5 Hz, H4), 7.56 (2H, d, J = 9.0 Hz, H2′ + H6′), 7.59 (1H, dd, J = 7.8, 1.8 Hz, H6), 9.80 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 39.9 (C3′′, C5′′), 65.1 (C2′′, C6′′), 114.5 (C1), 115.2 (C2′, C6′), 115.6 (C3), 116.3 (C5), 121.7 (C3′, C5′), 129.5 (C6), 131.4 (C1′), 131.8 (C4), 147.4 (C4′), 149.6 (C2), 167.4 (C=O); GC-MS (EI) m/z: 297 [M]+.
3.1.4. General Synthetic Procedures for 1–10
Thiophene-2-carbonyl chloride and 5-chlorothiophene-2-carbonyl chloride were prepared according to the synthetic procedures for 1a–5a. Compounds 1b–5b (0.62 mmol) were dissolved in anhydrous benzene (30 mL) and then triethylamine (0.62 mmol) was slowly added and thiophene-2-carbonyl chloride (0.62 mmol) or 5-chlorothiophene-2-carbonyl chloride (0.62 mmol) was added. The reaction mixture was reacted at room temperature for 2 h and the following procedures were same as procedures for 1a–5a. Compounds 1–10 were recrystallized with ethyl acetate and n-hexane mixture to obtain as pure white or pale yellow compounds.
N-(4′-Chlorophenyl)-2-(thiophen-2′′-ylcarbonylamino)benzamide (1).Yield: 79%; m.p.: 197–199 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.25 (1H, dd, J = 4.9, 3.8 Hz, H4′′), 7.29 (1H, dt, J = 7.7, 1.1 Hz, H5), 7.43 (2H, d, J = 8.8 Hz, H3′ + H5′), 7.61 (1H, dt, J = 8.0, 1.2 Hz, H4), 7.74–7.78 (3H, m, H2′ + H6′ + H5′′), 7.88–8.02 (2H, m, H3 + H6), 8.32 (1H, d, J = 8.0 Hz, H3′′), 10.63 (1H, s, NHCO), 11.54 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 121.6 (C3), 122.5 (C2′, C6′), 123.0 (C1), 123.4 (C5), 127.9 (C4′), 128.4 (C3′, C5′), 128.6 (C6), 128.8 (C4′′), 129.0 (C5′′), 132.2 (C4), 132.3 (C3′′), 137.5 (C2′′), 138.1 (C1′), 139.5 (C2), 159.4 (CONH), 167.3 (NHCO); DIP-MS (EI): m/z 356 [M]+; HRMS (FAB): m/z calcd for C18H14ClN2O2S [M + H]+ 357.0465, found 357.0478.
N-(4′-Bromophenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide (2). Yield: 75%; m.p.: 210–212 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.25 (1H, dd, J = 4.9, 3.8 Hz, H4′′), 7.30 (1H, dt, J = 7.8, 1.1 Hz, H5), 7.56 (2H, d, J = 8.9 Hz, H3′ + H5′), 7.61 (1H, dt, J = 8.0, 1.2 Hz, H4), 7.71 (2H, d, J = 8.9 Hz, H2′ + H6′), 7.75 (1H, dd, J = 3.7, 1.0 Hz, H5′′), 7.88–8.03 (2H, m, H3 + H6), 8.30 (1H, d, J = 8.2 Hz, H3′′), 10.64 (1H, s, NHCO), 11.51 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 116.0 (C4′), 121.6 (C3), 122.9 (C2′, C6′), 123.1 (C1), 123.4 (C5), 128.4 (C6), 128.8 (C4′′), 129.0 (C5′′), 131.5 (C3′, C5′), 132.2 (C4), 132.3 (C3′′), 138.0 (C2′′), 138.1 (C1′), 139.5 (C2), 159.4 (CONH), 167.3 (NHCO); DIP-MS (EI): m/z 400 [M − 1]+, 402 [M + 1]+; HRMS (FAB): m/z calcd for C18H14BrN2O2S [M + H]+ 400.9959, found 400.9973.
N-(4′-Fluorophenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide (3). Yield: 73%; m.p.: 215–216 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.19–7.26 (3H, m, H3′ + H5′+ H4′′), 7.29 (1H, dt, J = 7.7, 1.1 Hz, H5), 7.61 (1H, dt, J = 8.0, 1.0 Hz, H4), 7.73–7.75 (3H, m, H2′ + H6′ + H5′′), 7.89 (1H, dd, J = 5.0, 1.1 Hz, H6), 7.92 (1H, dd, J = 7.8, 1.2 Hz, H3), 8.36 (1H, d, J = 7.6 Hz, H3′′), 10.58 (1H, s, NHCO), 11.68 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 115.9 (J = 22.3 Hz, C3′, C5′), 122.0 (C3), 123.3 (C1), 123.7 (J = 8.2 Hz, C2′, C6′), 124.1 (C5), 129.1 (C6), 129.4 (C4′′), 129.6 (C5′′), 133.0 (C4, C3′′), 135.5 (J = 3.0 Hz, C1′), 139.0 (C2′′), 140.2 (C2), 159.4 (J = 239.4 Hz, C4′), 160.0 (CONH), 167.9 (NHCO); DIP-MS (EI): m/z 340 [M]+; HRMS (FAB): m/z calcd for C18H14FN2O2S [M + H]+ 341.0760, found 341.0781.
N-(4′-Methoxyphenyl)-2-(thiophen-2′′-ylcarbonylamino)benzamide (4). Yield: 77%; m.p.: 170–172 °C; 1H NMR (400 MHz, DMSO-d6) δ: 3.73 (3H, s, CH3), 6.96 (2H, d, J = 9.0 Hz, H3′ + H5′), 7.31–7.23 (2H, m, H5 + H4′′), 7.59–7.64 (3H, m, H4 + H2 + H6′), 7.73 (1H, dd, J = 3.7, 0.6 Hz, H5′′), 7.89 (1H, dd, J = 5.0, 0.6 Hz, H6), 7.94 (1H, d, J = 6.9 Hz, H3), 8.43 (1H, d, J = 8.2 Hz, H3′′), 10.45 (1H, s, NHCO), 11.92 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 55.2 (CH3), 113.8 (C3′, C5′), 120.9 (C3), 122.1 (C1), 122.9 (C2′, C6′), 123.2 (C5), 128.4 (C6), 128.6 (C4′′), 128.8 (C5′′), 131.3 (C1′), 132.2 (C4), 132.3 (C3′′), 138.5 (C2′′), 139.6 (C2), 156.1 (C4′), 159.3 (CONH), 167.0 (NHCO); DIP-MS (EI): m/z 352 [M]+; HRMS (FAB): m/z calcd for C19H17N2O3S [M + H]+ 353.0960, found 353.0979.
N-(4′-Morpholinophenyl)-2-(thiophen-2′′′-ylcarbonylamino)benzamide (5). Yield: 76%; m.p.: 246–247 °C; 1H NMR (400 MHz, DMSO-d6) δ: 3.09 (4H, t, J = 4.8 Hz, H3′′ + H5′′), 3.74 (4H, t, J = 4.8 Hz, H2′′ + H6′′), 6.96 (2H, d, J = 9.0 Hz, H3′ + H5′), 7.22–7.30 (2H, m, H5 + H4′′′), 7.57–7.61 (3H, m, H4 + H2′ + H6′), 7.72 (1H, dd, J = 3.7, 0.6 Hz, H5′′′), 7.90 (1H, dd, J = 4.9, 0.5 Hz, H6), 7.93 (1H, d, J = 7.3 Hz, H3), 8.43 (1H, d, J = 8.1 Hz, H3′′′), 10.39 (1H, s, NHCO), 11.97 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 48.7 (C3′′, C5′′), 66.1 (C2′′, C6′′), 115.2 (C3′, C5′), 120.8 (C3), 122.0 (C1), 122.4 (C2′,C6′), 123.1 (C5), 128.4 (C6), 128.6 (C4′′), 128.8 (C5′′), 130.3 (C1′), 132.2 (C4), 132.3 (C3′′), 138.6 (C2′′), 139.6 (C2), 148.1 (C4′), 159.3 (CONH), 166.9 (NHCO); DIP-MS (EI): m/z 407 [M]+; HRMS (FAB): m/z calcd for C22H22N3O3S [M + H]+ 408.1382, found 408.1368.
N-(4′-Chlorophenyl)-2-((5-chlorothiophen-2′′-yl)carbonylamino)benzamide (6). Yield: 60%; m.p.: 207–209 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.29 (1H, d, J = 4.0 Hz, H4′′), 7.32 (1H, dt, J = 7.7, 1.1 Hz, H5), 7.43 (2H, d, J = 8.9 Hz, H3′ + H5′), 7.60 (1H, dt, J = 8.0, 1.2 Hz, H4), 7.64 (1H, d, J = 4.1 Hz, H6), 7.75 (2H, d, J = 8.9 Hz, H2′ + H6′), 7.88 (1H, dd, J = 7.8, 1.0 Hz, H3), 8.17 (1H, d, J = 8.2 Hz, H3′′), 10.63 (1H, s, NHCO), 11.44 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 122.2 (C3), 122.4 (C2′, C6′), 123.9 (C1), 124.2 (C5), 127.8 (C4′), 128.6 (C3′, C5′), 128.7 (C6), 129.1 (C4′′), 132.2 (C4, C3′′), 134.2 (C2′′), 137.4 (C5′′), 137.7 (C1′), 138.7 (C2), 158.4 (CONH), 167.1 (NHCO); DIP-MS (EI): m/z 390 [M − 1]+; HRMS (FAB): m/z calcd for C18H13Cl2N2O2S [M + H]+ 391.0075, found 391.0089.
N-(4′-Bromophenyl)-2-((5-chlorothiophen-2′′-yl)carbonylamino)benzamide (7). Yield: 41%; m.p.: 198–199 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.28 (1H, d, J = 4.1 Hz, H4′′), 7.32 (1H, dt, J = 7.7, 1.1 Hz, H5), 7.55 (2H, d, J = 8.9 Hz, H3′ + H5′), 7.60 (1H, dt, J = 8.0, 1.2 Hz, H4), 7.64 (1H, d, J = 4.1 Hz, H6), 7.70 (2H, d, J = 8.9 Hz, H2′ + H6′), 7.88 (1H, dd, J = 7.8, 1.3 Hz, H3), 8.18 (1H, dd, J = 8.2, 0.8 Hz, H3′′), 10.60 (1H, s, NHCO), 11.42 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 115.9 (C4′), 122.1 (C3), 122.8 (C2′, C6′), 123.9 (C1), 124.2 (C5), 128.7 (C6), 129.0 (C4′′), 131.5 (C3′, C5′), 132.1 (C4, C3′′), 134.1 (C2′′), 137.4 (C5′′), 138.0 (C1′), 138.6 (C2), 158.4 (CONH), 167.1 (NHCO); DIP-MS (EI): m/z 434 [M − 1]+, 436 [M + 1]+; HRMS (FAB): m/z calcd for C18H13BrClN2O2S [M + H]+ 434.9570, found 434.9549.
N-(4′-Fluorophenyl)-2-((5-chlorothiophen-2′′-yl)carbonylamino)benzamide (8). Yield: 73%; m.p.: 215–216 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.22 (2H, t, J = 8.9 Hz, H3′ + H5′), 7.27–7.34 (2H, m, H5 + H4′′), 7.64–7.57 (2H, m, H4 + H6), 7.73 (2H, dd, J = 9.1, 5.1 Hz, H2′ + H6′), 7.91 (1H, dd, J = 7.9, 1.1 Hz, H3), 8.24 (1H, d, J = 8.1 Hz, H3′′), 10.57 (1H, s, NHCO), 11.60 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 115.2 (J = 22.3 Hz, C2′, C6′), 121.8 (C3), 123.0 (J = 8.1 Hz, C3′, C5′), 123.5 (C1), 123.8 (C5), 128.6 (C6), 128.9 (C4′′), 132.2 (C4), 134.1 (C2′′), 134.8 (J = 2.2 Hz, C1′), 137.7 (C5′′), 138.6 (C2), 158.3 (CONH), 158.6 (J = 239.5 Hz, C4′), 167.0 (NHCO); DIP-MS (EI): m/z 374 [M]+; HRMS (FAB): m/z calcd for C18H13ClFN2O2S [M + H]+ 375.0370, found 375.0388.
N-(4′-Methoxyphenyl)-2-((5-chlorothiophen-2′′-yl)carbonylamino)benzamide (9). Yield: 77%; m.p.: 190–191 °C; 1H NMR (400 MHz, DMSO-d6) δ: 3.75 (3H, s, CH3), 6.95 (2H, d, J = 8.8 Hz, H3′ + H5′), 7.26–7.32 (2H, m, H5 + H4′′), 7.56–7.65 (4H, m, H4 + H6 + H2′ + H6′), 7.94 (1H, d, J = 7.7 Hz, H3), 8.31 (1H, d, J = 8.3 Hz, H3′′), 10.45 (1H, s, NHCO), 11.87 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 55.4 (CH3), 114.0 (C2′, C6′), 121.6 (C3), 123.0 (C3′, C5′), 123.1 (C1), 123.8 (C5), 128.7 (C6), 128.8 (C4′′), 129.1 (C1′), 131.5 (C3′′), 132.3 (C4), 134.4 (C2′′), 138.2 (C5′′), 139.9 (C2), 156.3 (C4′), 158.5 (CONH), 167.1 (NHCO); DIP-MS (EI): m/z 386 [M]+; HRMS (FAB): m/z calcd for C19H16ClN2O3S [M + H]+ 387.0457, found 387.0470.
N-(4′-Morpholinophenyl)-2-((5-chlorothiophen-2′′′-yl)carbonylamino)benzamide (10). Yield: 80%; m.p.: 230–231 °C; 1H NMR (400 MHz, DMSO-d6) δ: 3.08 (4H, t, J = 4.8 Hz, H3′′ + H5′′), 3.74 (4H, t, J = 4.8 Hz, H2′′ + H6′′), 6.96 (2H, d, J = 9.1 Hz, H3′ + H5′), 7.23–7.32 (2H, m, H5 + H4′′′), 7.51–7.64 (4H, m, H4 + H6 + H2′ + H6′), 7.92 (1H, dd, J = 7.9, 1.1 Hz, H3), 8.32 (1H, d, J = 8.3 Hz, H3′′′), 10.39 (1H, s, NHCO); 13C NMR (100 MHz, DMSO-d6) δ: 51.6 (C3′′, C5′′), 68.9 (C2′′, C6′′), 118.0 (C3′, C5′), 124.1 (C3), 125.2 (C2′, C6′), 125.6 (C1), 126.4 (C5), 131.2 (C6), 131.5 (C4′′), 131.7 (C3′′), 133.2 (C4), 135.0 (C2′′), 137.0 (C1′), 140.9 (C5′′), 141.6 (C2), 150.9 (C4′), 161.1 (CONH), 169.6 (NHCO); DIP-MS (EI): m/z 441[M]+; HRMS (FAB): m/z calcd for C22H21ClN3O3S [M + H]+ 442.0992, found 442.0977.
3.1.5. General Synthetic Procedures for 11a–13a
To a solution of 4-(methylphenylsulfonamido)benzoyl chloride (2, 2.06 mmol) in anhydrous benzene (30 mL) was added 3- or 4-aminobenzonitrile (220 mg, 1.87 mmol), 4-aminobenzylcyanide (265 mg, 1.87 mmol) and triethylamine (0.1 mL). The following procedures were same as procedures for 1a–5a. Compounds 11a–13a were recrystallized with dichloromethane and n-hexane mixture to obtain as a white powder.
N-(3’-Cyanophenyl)-2-(4”-methylphenylsulfonamido)benzamide (11a). Yield: 75%; m.p.: 216–218 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.23 (3H, s, CH3), 7.21 (2H, d, J = 8.4 Hz, H3′′ + H5′′), 7.23 (1H, t, J = 7.6 Hz, H5), 7.36 (1H, d, J = 8.0 Hz, H3), 7.48 (1H, t, J = 7.6 Hz, H4), 7.54–7.65 (4H, m, H6 + H5′ + H2′′ + H6′′), 7.69 (1H, d, J = 7.2 Hz, H4′), 7.88 (1H, d, J = 7.6 Hz, H6′), 8.08 (1H, s, H2′), 10.26 (1H, s, NHSO2), 10.53 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.6 (CH3), 112.1 (C3′), 119.3 (CN), 122.9 (C3), 124.1 (C1), 125.9 (C5), 127.6 (C6), 127.8 (C6′), 128.3 (C2′′, C6′′), 129.8 (C5′), 130.4 (C3′′, C5′′), 130.8 (C2′), 133.1 (C4), 136.6 (C4′), 137.1 (C1′′), 137.4 (C4′′), 140.0 (C1′), 144.3 (C2), 167.5 (C=O); DIP-MS (EI): m/z 391 [M]+.
N-(4′-Cyanophenyl)-2-(4”-methylphenylsulfonamido)benzamide (12a). Yield: 70%; m.p.: 253–255 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.22 (3H, s, CH3), 7.12–7.26 (3H, m, H5 + H3′′ + H5′′), 7.33 (1H, d, J = 7.6 Hz, H3), 7.47 (1H, t, J = 7.6 Hz, H4), 7.56 (2H, d, J = 7.6 Hz, H2′′ + H6′′), 7.67 (1H, d, J = 7.2 Hz, H6), 7.77–7.87 (4H, m, H2′ + H3′ + H5′ + H6′), 10.16 (1H, s, NHSO2), 10.59 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.6 (CH3), 106.4 (C4′), 119.7 (CN), 121.2 (C2′, C6′), 123.1 (C3), 125.2 (C1), 126.4 (C5), 127.5 (C6), 128.0 (C2′′, C6′′), 130.4 (C3′′, C5′′), 133.1 (C3′, C5′), 133.8 (C4), 137.2 (C1′′, C4′′), 143.6 (C1′), 144.3 (C2), 167.6 (C=O); DIP-MS (EI): m/z 391 [M]+.
N-(4′-Cyanomethylphenyl)-2-(4”-methylphenylsulfonamido)benzamide (13a). Yield: 68%; m.p.: 142–146 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.27 (3H, s, CH3), 4.02 (2H, s, CH2), 7.20–7.29 (3H, m, H5 + H3′′ + H5′′), 7.36 (2H, d, J = 8.8 Hz, H3′ + H5′), 7.45–7.53 (2H, m, H3 + H4), 7.67 (2H, d, J = 8.4 Hz, H2′′ + H6′′), 7.76 (1H, dd, J = 8.0, 1.2 Hz, H6), 10.39 (1H, s, NHSO2), 10.59 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.0 (CH3), 21.9 (CH2), 119.3 (CN), 121.4 (C2′, C6′), 124.0 (C3), 124.1 (C1), 126.8 (C5), 127.0 (C6), 128.4 (C2′′, C6′′), 129.1 (C3′, C5′), 129.8 (C3′′, C5′′), 132.4 (C4), 135.9 (C1′), 137.2 (C1′′), 137.7 (C4′′), 143.7 (C2), 166.7 (C=O); GC-MS (EI) m/z: 405 [M]+.
3.1.6. General Synthetic Procedures for 11–13
To a suspension of 10% Pd-C (200 mg) in ethanol (50 mL) was added 1N-HCl (1 mL) and nitriles 11a–13a (1.28 mmol) and the reaction mixture was catalytically hydrogenated in parr hydrogenation apparatus (50 psi of hydrogen) at room temperature for 6 h. When the starting material could be no longer detected by TLC, the reaction mixture was filtrated on celite pad. The filtrate was concentrated to give the oily product which was diluted in ethanol and then crystallized with diethyl ether to form the light brown precipitate which was recrystallized with ethanol and diethyl ether mixture to afford 11–13 as a white powder.
N-(3’-Aminomethyl)phenyl-2-(4”-methylphenylsulfonamido)benzamide (11). Yield: 72%; m.p: 163–166 °C; 1H NMR (400 MHz, CD3OD) δ: 2.29 (3H, s, CH3), 4.03 (2H, s, CH2), 7.23 (1H, t, J = 7.2 Hz, H4), 7.27 (2H, d, J = 8.4 Hz, H3′′ + H5′′), 7.32 (1H, d, J = 7.6 Hz, H4′), 7.40–7.45 (2H, m, H5′ + H6′), 7.42 (1H, t, J = 7.6 Hz, H5), 7.55 (1H, d, J = 8.4 Hz, H3), 7.63 (2H, d, J = 8.4 Hz, H2′′ + H6′′), 7.83 (1H, d, J = 7.6 Hz, H6), 7.90 (1H, s, H2′), 8.50 (3H, br s, aminium chloride), 10.52 (1H, s, NHSO2), 10.69 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 20.9 (CH3), 42.3 (CH2), 121.0 (C2′), 121.2 (C6′), 121.5 (C3), 123.6 (C1), 124.8 (C5), 126.8 (C2′′, C6′′), 128.9 (C6), 129.2 (C4′), 129.8 (C3′′, C5′′), 132.5 (C4, C1′), 134.5 (C3′), 137.4 (C1′′), 138.5 (C4′′), 143.7 (C2), 166.8 (C=O); DIP-MS (EI): m/z 395 [M − HCl]+; HRMS (FAB): calcd for C21H22N3O3S [M + H]+ 396.1382, found 396.1396.
N-(4′-Aminomethyl)phenyl-2-(4”-methylphenylsulfonamido)benzamide (12). Yield: 81%; m.p: 92–95 °C; 1H NMR (400 MHz, CD3OD) δ: 2.29 (3H, s, CH3), 4.03 (2H, s, CH2), 7.18–7.24 (3H, m, H4 + H3′′ + H5′′), 7.42 (1H, t, J = 8.0 Hz, H5), 7.45–7.53 (3H, m, H3 + H3′+ H5′), 7.61 (2H, d, J = 8.4 Hz, H2′′ + H6′′), 7.69 (2H, d, J = 8.4 Hz, H2′ + H6′), 7.80 (1H, d, J = 8.0 Hz, H6), 8.36 (3H, br s, aminium chloride), 10.44 (1H, s, NHSO2), 10.61 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 20.9 (CH3), 41.8 (CH2), 120.9 (C2′, C6′), 121.4 (C3), 124.0 (C1), 124.1 (C5), 126.8 (C2′′, C6′′), 129.1 (C6), 129.3 (C3′, C5′), 129.8 (C3′′, C5′′), 132.4 (C4, C1′), 135.9 (C4′), 137.2 (C1′′), 138.4 (C4′′), 143.7 (C2), 166.7 (C=O); DIP-MS (EI): m/z 395 [M − HCl]+; HRMS (FAB): calcd for C21H22N3O3S [M]+ 396.1382, found 396.1399.
N-(4′-Aminoethyl)phenyl-2-(4”-methylphenylsulfonamido)benzamide (13). Yield: 77%; m.p: 253–255 °C; 1H NMR (400 MHz, CD3OD) δ: 2.27 (3H, s, CH3), 2.98 (2H, t, J = 7.8 Hz, CH2), 3.21 (2H, t, J = 7.8 Hz, CH2), 7.14 (2H, m, H3′ + H5′), 7.23 (1H, t, J = 7.6 Hz, H4), 7.30 (2H, d, J = 8.0 Hz, H3′′ + H5′′), 7.46–7.61 (6H, m, H3 + H5 + H2′ + H6′ + H2′′ + H6′′), 7.67 (1H, d, J = 7.6 Hz, H6), 8.33 (3H, br s, aminium chloride), 10.41 (1H, s, NHSO2), 10.63 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 20.2 (CH3), 32.9 (CH2), 40.7 (CH2), 121.8 (C2′, C6′), 123.5 (C3), 124.8 (C1), 125.3 (C5), 127.1 (C2′′, C6′′), 128.2 (C6), 129.0 (C3′, C5′), 129.5 (C3′′, C5′′), 132.2 (C4), 133.2 (C4′), 136.1 (C1′), 137.3 (C1′′), 137.7 (C4′′), 144.1 (C2), 167.7 (C=O); DIP-MS (EI): m/z 409 [M − HCl]+; HRMS (FAB): calcd for C22H24N3O3S [M + H]+ 410.1538, found 410.1520.
3.1.7. General Synthetic Procedures for 14–20
To a solution of 4-(methylphenylsulfonamido)benzoyl chloride (2, 1.50 mmol) was dissolved in anhydrous benzene (30 mL) was added 4-chloroaniline or 4-bromoaniline or 4-methoxyaniline or 4-carboxyaniline or 2-(thiophen-2-yl)ethylamine or 4-morpholinoaniline or 4-(2-oxomorpholino)aniline (1.36 mmol) and triethylamine (0.1 mL). The following procedures were same as procedures for 1a–5a. Compounds 14–20 were recrystallized with dichloromethane and n-hexane mixture to afford a white powder, respectively.
N-(4′-Chlorophenyl)-2-(4"-methylphenylsulfonamido)benzamide (14). Yield: 82%; m.p: 220–223 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.29 (3H, s, CH3), 7.20–7.30 (3H, m, H4 + H3′′ + H5′′), 7.58 (2H, d, J = 7.6 Hz, H3′ + H5′), 7.69 (2H, d, J = 8.0 Hz, H2′′ + H6′′), 7.71 (2H, d, J = 7.6 Hz, H2′ + H6′), 7.98 (1H, t, J = 7.6 Hz, H5), 8.03 (1H, d, J = 8.0 Hz, H3), 8.18 (1H, d, J = 8.0 Hz, H6) 11.95 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.6 (CH3), 121.2 (C2′, C6′), 123.1 (C3), 125.2 (C1), 126.4 (C5), 127.5 (C6), 128.0 (C2′′, C6′′), 129.9 (C4′), 130.4 (C3′′, C5′′), 133.1 (C4, C3′, C5′), 136.8 (C1′′), 137.2 (C4′′), 144.3 (C2), 167.6 (C=O); DIP-MS (EI) m/z: 400 [M]+; HRMS (FAB): calcd for C20H18ClN2O3S [M + H]+ 401.0727, found 401.0741.
N-(4′-Bromophenyl)-2-(4"-methylphenylsulfonamido)benzamide (15). Yield: 80%; m.p: 211–213 °C; 1H NMR (400 MHz, CDCl3) δ: 2.26 (3H, s, CH3), 7.12–7.21 (3H, m, H4 + H3′′ + H5′′), 7.38 (2H, d, J = 8.8 Hz, H3′ + H5′), 7.43–7.51 (4H, m, H3 + H5 + H2′ + H6′), 7.62 (2H, d, J = 8.4 Hz, H2′′ + H6′′), 7.69 (1H, d, J = 7.8 Hz, H6) 10.1 (1H, s, NHSO2), 10.45 (1H, s, CONH); 13C NMR (100 MHz, CDCl3) δ: 21.7 (CH3), 118.2 (C4′), 122.2 (C2′, C6′), 122.7 (C3), 122.9 (C1), 124.3 (C5), 126.9 (C6), 127.5 (C2′′, C6′′), 129.8 (C3′′, C5′′), 132.3 (C3′, C5′), 133.3 (C4), 136.4 (C1′), 136.5 (C1′′), 138.8 (C4′′), 144.0 (C2), 166.7 (C=O); DIP-MS (EI) m/z: 444 [M]+; HRMS (FAB): calcd for C20H18BrN2O3S [M + H]+ 445.0222, found 445.0239.
N-(4′-Methoxyphenyl)-2-(4"-methylphenylsulfonamido)benzamide (16). Yield: 87%; m.p: 157–159 °C; 1H NMR (400 MHz, CDCl3) δ: 2.28 (3H, s, CH3), 3.76 (3H, s, OCH3), 6.95 (2H, d, J = 8.8 Hz, H3′ + H5′), 7.21 (1H, t, J = 6.8 Hz, H4), 7.26 (2H, d, J = 8.0 Hz, H3′′ + H5′′), 7.44–7.50 (2H, m, H3 + H5), 7.54 (2H, d, J = 8.8 Hz, H2′+ H6′), 7.61 (2H, d, J = 8.4 Hz, H2′′ + H6′′), 7.77 (1H, d, J = 7.6 Hz, H6), 10.23 (1H, s, NHSO2), 10.81 (1H, s, CONH); 13C NMR (100 MHz, CDCl3) δ: 21.6 (CH3), 55.9 (OCH3), 114.4 (C3′, C5′), 121.6 (C3), 123.4 (C2′, C6′), 124.0 (C1), 124.6 (C5), 127.5 (C2′′, C6′′), 129.6 (C6), 130.5 (C3′′, C5′′), 131.8 (C1′), 133.0 (C4), 136.5 (C1′′), 138.2 (C4′′), 144.4 (C2), 156.8 (C4′), 167.0 (C=O) DIP-MS (EI) m/z: 396 [M]+; HRMS (FAB): calcd for C21H21N2O4S [M + H]+ 397.1222, found 397.1241.
N-(4′-Carboxyphenyl)-2-(4"-methylphenylsulfonamido)benzamide (17). Yield: 85%; m.p: 210–212 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.23 (3H, s, CH3), 7.18–7.25 (3H, m, H4 + H3′′ + H5′′), 7.36 (1H, d, J = 8.4 Hz, H3), 7.47 (1H, t, J = 7.6 Hz, H5), 7.57 (2H, d, J = 8.0 Hz, H2′′ + H6′′), 7.61 (2H, d, J = 8.8 Hz, H2′ + H6′), 7.86 (2H, d, J = 8.8 Hz, H3′ + H5′), 7.71 (1H, d, J = 8.0 Hz, H6), 10.31 (1H, s, NHSO2), 10.52 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.3 (CH3), 117.5 (C2′, C6′), 122.0 (C3), 124.3 (C1), 124.3 (C5), 126.8 (C4′), 127.1 (C2′′, C6′′), 129.0 (C6), 128.9 (C3′′, C5′′), 130.8 (C3′, C5′), 132.8 (C4), 136.1 (C1′′), 138.5 (C4′′), 144.1 (C2), 167.3 (C=O) DIP-MS (EI) m/z: 410 [M]+; HRMS (FAB): calcd for C21H19N2O5S [M + H]+ 411.1015, found 411.1036.
N-(2-(thiophen-2′-yl)ethyl)-2-(4"-methylphenylsulfonamido)benzamide (18). Yield: 60%; m.p: 119–121 °C; 1H NMR (400 MHz, CDCl3) δ: 2.31 (3H, s, CH3), 3.02 (2H, t, J = 6.8 Hz, CH2), 3.45 (2H, q, J = 6.8 Hz, CH2), 6.91 (1H, d, J = 2.4 Hz, H3′), 6.96 (1H, d, J = 5.2, 3.6 Hz, H4′), 7.13 (1H, t, J = 7.2 Hz, H4), 7.31 (2H, d, J = 8.0 Hz, H3′′ + H5′′), 7.35 (1H, dd, J = 5.2, 1,2 Hz, H5′), 7.42–7.53 (2H, m, H3 + H5), 7.61 (2H, d, J = 8.4 Hz, H2′′ + H6′′), 7.66 (1H, d, J = 8.0 Hz, H6), 8.91 (1H, s, NHSO2), 11.57 (1H, s, CONH); 13C NMR (100 MHz, CDCl3) δ: 21.0 (CH3), 28.7 (CH2), 40.9 (CH2), 119.7 (C3), 120.6 (C1), 123.4 (C5), 124.2 (C5′), 125.3 (C4′), 126.8 (C2′′, C6′′), 127.0 (C3′), 128.3 (C6), 129.9 (C3′′, C5′′), 132.4 (C4), 135.9 (C1′′), 138.4 (C4′′), 141.2 (C2′), 143.8 (C2), 168.0 (C=O); DIP-MS (EI) m/z: 400 [M]+; HRMS (FAB): calcd for C20H21N2O3S2 [M + H]+ 401.0994, found 401.0982.
N-(4′-morpholinophenyl)-2-(4"-methylphenylsulfonamido)benzamide (19). Yield: 55%; m.p: 206–209 °C; 1H NMR (400 MHz, CDCl3) δ: 2.29 (3H, s, CH3), 3.09 (4H, t, J = 4.8 Hz, CH2), 3.75 (4H, t, J = 4.4 Hz, CH2), 6.95 (2H, d, J = 8.8 Hz, H3′ + H5′), 7.20 (1H, t, J = 6.0 Hz, H4), 7.26 (2H, d, J = 8.4 Hz, H3′′ + H5′′), 7.45–7.53 (4H, m H3 + H5 + H2′ + H6′), 7.61 (2H, d, J = 8.4 Hz, H2′′ + H6′′), 7.77 (1H, d, J = 7.6 Hz, H6), 10.18 (1H, s, NHSO2), 10.89 (1H, s, CONH); 13C NMR (100 MHz, CDCl3) δ: 21.7 (CH3), 49.4 (2 x CH2), 66.8 (2 x CH2), 115.8 (C3′, C5′), 122.9 (C3), 123.9 (C1, C2′, C6′), 124.6 (C5), 127.6 (C2′′, C6′′), 129.6 (C6), 130.5 (C3′′, C5′′), 130.7 (C1′), 133.0 (C4), 136.5 (C1′′), 138.2 (C4′′), 144.4 (C2), 148.8 (C4′), 166.9 (C=O); DIP-MS (EI) m/z: 451 [M]+; HRMS (FAB): calcd for C24H26N3O4S [M + H]+ 452.1644, found 452.1627.
N-(4′-(2-oxomorpholino)phenyl)-2-(4"-methylphenylsulfonamido)benzamide (20). Yield: 44%; m.p: 270–273 °C; 1H NMR (400 MHz, CDCl3) δ: 2.28 (3H, s, CH3), 3.74 (2H, t, J = 4.8 Hz, CH2), 3.99 (2H, t, J = 4.8 Hz, CH2), 4.21 (2H, s, CH2), 7.20–7.30 (3H, m, H4 + H3′ + H5′), 7.39 (2H, d, J = 8.8 Hz, H3′′ + H5′′), 7.40–7.52 (2H, m, H3 + H5), 7.61 (2H, d, J = 8.4 Hz, H2′+ H6′), 7.67 (2H, d, J = 8.8 Hz, H2′′ + H6′′), 7.77 (1H, d, J = 7.6 Hz, H6), 10.40 (1H, s, NHSO2), 10.61 (1H, s, CONH); 13C NMR (100 MHz, CDCl3) δ: 21.0 (CH3), 40.1 (CH2), 63.5 (CH2), 67.8 (CH2), 117.8 (C3′, C5′), 121.2 (C3), 124.0 (C1), 124.1 (C5), 125.7 (C2′, C6′), 126.8 (C2′′, C6′′), 129.1 (C6), 129.8 (C3′′, C5′′), 132.4 (C4), 135.9 (C1′), 136.4 (C4′), 137.2 (C1′′), 137.7 (C4′′), 143.8 (C2), 166.0 (C=O), 166.6 (C=O); DIP-MS (EI) m/z: 465 [M]+; HRMS (FAB): calcd for C24H24N3O5S [M + H]+ 466.1437, found 466.1454.
3.1.8. General Synthetic Procedures for 21a–23a
2-Nitrobenzoyl chloride (8.99 mmol) and triethylamine (7.19 mmol) were added to a solution of 3-aminophenylcyanide or 4-aminophenylcyanide or 4-aminobenzyl cyanide (7.19 mmol) in anhydrous dichloromethane (30 mL) and stirred at room temperature for 3 h. The reaction was terminated with water and extracted with dichloromethane (3 × 30 mL), dried with anhydrous magnesium sulfate and filtrated. The filtrate was concentrated to give the crude oily reside, which was recrystallized with ethyl acetate and n-hexane mixture to afford pure white or pale yellow compounds 21a–23a.
N-(3′-Cyanophenyl)-2-nitrobenzamide (21a). Yield: 80%; m.p.: 170–171 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.59–7.92 (6H, m, H4 + H5 + H6 + H4′ + H5′ + H6′), 8.13 (1H, s, H2′), 8.18 (1H, d, J = 8.0 Hz, H3), 11.02 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 111.7 (C3′), 118.6 (C≡N), 122.2 (C6′), 124.2 (C5′), 124.4 (C1), 127.5 (C2′), 129.3 (C3), 130.4 (C4′), 131.3 (C6), 132.0 (C4), 134.3 (C5), 139.6 (C1′), 146.3 (C2), 164.6 (C=O); GC-MS (EI) m/z: 267 [M]+.
N-(4′-Cyanophenyl)-2-nitrobenzamide (22a). Yield: 84%; m.p.: 217–218 °C; ; 1H NMR (400 MHz, DMSO-d6) δ: 7.77–7.84 (6H, m, H4 + H6 + H2′ + H3′ + H5′ + H6′), 7.90 (1H, td, J = 7.6, 1.2 Hz, H5), 8.19 (1H, dd, J = 8.0, 4.0 Hz, H3), 11.08 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 105.7 (C4′), 118.9 (C≡N), 119.6 (C2′, C6′), 124.8 (C1), 129.3 (C3), 131.3 (C6), 132.1 (C4), 133.4 (C3′, C5′), 134.3 (C5), 142.9 (C1′), 146.2 (C2), 164.7 (C=O); GC-MS (EI) m/z: 267 [M]+.
N-(4′-Cyanomethylphenyl)-2-nitrobenzamide (23a). Yield: 76%; m.p.: 174–175 °C; 1H NMR (400 MHz, DMSO-d6) δ: 4.01 (2H, s, CH2), 7.35 (2H, d, J = 8.0 Hz, H3′ + H5′), 7.68 (2H, d, J = 8.0 Hz, H2′ + H6′), 7.75–7.79 (2H, m, H4 + H6), 7.88 (1H, t, J = 8.0 Hz, H5), 8.16 (1H, d, J = 8.0 Hz, H3), 10.71 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.9 (CH2), 119.3 (C≡N), 120.0 (C2′, C6′), 124.3 (C1), 126.6 (C4′), 128.6 (C3′, C5′), 129.3 (C3), 131.0 (C6), 132.5 (C4), 134.1 (C5), 138.2 (C1′), 146.4 (C2), 164.1 (C=O); GC-MS (EI) m/z: 281 [M]+.
3.1.9. General Synthetic Procedures for 21b–23b
To a nitro compounds 21a–23a (1.79 mmol) in methanol (20 mL) was added ammonium chloride (17.9 mmol) and iron powder (3.58 mmol), and then the reaction mixture was refluxed for 7 h. The following procedures were same as synthetic procedures for 1b–5b. Compounds 21b–23b were recrystallized with ethyl acetate and n-hexane mixture to provide pure white or pale-yellow compounds 21b–23b.
2-Amino-N-(3′-cyanophenyl)benzamide (21b). Yield: 82%; m.p.: 158–160 °C, (161–163 °C [40]).
2-Amino-N-(4′-cyanophenyl)benzamide (22b). Yield: 64%; m.p.: 233–234 °C, (231 °C [40]).
2-Amino-N-(4′-cyanomethylphenyl)benzamide (23b). Yield: 61%; m.p.: 144–145 °C; 1H NMR (400 MHz, DMSO-d6) δ: 3.98 (2H, s, CH2), 6.32 (2H, s, NH2), 6.59 (1H, t, J = 6.8 Hz, H5), 6.75 (1H, d, J = 7.6 Hz, H3), 7.18–7.22 (2H, m, H4 + H6), 7.30 (2H, d, J = 8.0 Hz, H3′ + H5′), 7.73 (2H, d, J = 8.0 Hz, H2′ + H6′), 10.03 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.8 (CH2), 114.6 (C1), 115.0 (C3), 116.3 (C5), 119.3 (C≡N), 120.8 (C2′, C6′), 128.2 (C3′, C5′), 128.6 (C4′), 129.3 (C6), 132.1 (C4), 138.6 (C1′), 149.7 (C2), 167.8 (C=O); DIP-MS (EI) m/z: 251 [M]+.
3.1.10. General Synthetic Procedures of 21c–23c
Compounds 21b–23b (1.48 mmol) were dissolved in anhydrous benzene (20 mL) and added with triethylamine (1.48 mmol). To the stirred solution, thiophene-2-carbonyl chloride (1.85 mmol) was added at room temperature and then refluxed for 1 h. The following procedures were same as synthetic procedures for 1–10. Compounds 21c–23c were recrystallized with ethyl acetate and n-hexane mixture to give pure white or pale yellow compounds 21c–23c.
N-(3′-Cyanophenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide (21c). Yield: 98%; m.p.: 208–209 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.25 (1H, dd, J = 5.0, 4.0 Hz, H4′′), 7.32 (1H, td, J = 7.6, 1.2 Hz, H5), 7.56–7.65 (3H, m, H4 + H4′ + H6′), 7.80 (1H, dd, J = 3.8, 0.8 Hz, H5′′), 7.88–7.91 (2H, m, H3 + H6), 7.99–8.02 (1H, m, H5′), 8.19 (1H, s, H2′), 8.24 (1H, dd, J = 8.2, 0.8 Hz, H3′′), 10.80 (1H, s, NHCO), 11.36 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 111.2 (C3′), 118.5 (C≡N), 121.8 (C3), 123.3 (C1), 124.5 (C5), 125.2 (C6′), 127.3 (C2′), 128.2 (C6), 128.7 (C4′′), 128.8 (C5′′), 129.9 (C4′), 132.0 (C4), 132.1 (C3′′), 137.6 (C2′′), 139.2 (C1′), 139.3 (C2), 159.5 (CONH), 167.5 (NHCO); DIP-MS (EI) m/z: 347 [M]+.
N-(4′-Cyanophenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide (22c). Yield: 69%; m.p.: 266–267 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.24 (1H, t, J = 4.0 Hz, H4′′), 7.32 (1H, t, J = 7.6 Hz, H5), 7.62 (1H, t, J = 8.0 Hz, H4), 7.79 (1H, d, J = 3.2 Hz, H5′′), 7.83 (2H, d, J = 8.0 Hz, H3′ + H5′), 7.87–7.89 (2H, m, H3 + H6), 7.94 (2H, d, J = 8.0 Hz, H2′ + H6′), 8.20 (1H, d, J = 8.0 Hz, H3′′), 10.86 (1H, s, NHCO), 11.23 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 105.6 (C4′), 119.0 (C≡N), 120.6 (C3′, C5′), 122.2 (C3), 123.7 (C1), 124.2 (C5), 128.3 (C6), 128.9 (C4′′), 129.1 (C5′′), 132.2 (C4), 132.3 (C3′′), 133.1 (C2′, C6′), 137.7 (C2′′), 139.4 (C2), 143.1 (C1′), 159.5 (CONH), 167.6 (NHCO); DIP-MS (EI) m/z: 347 [M]+.
N-(4′-Cyanomethylphenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide (23c). Yield: 88%; m.p.: 221–222 °C; 1H NMR (400 MHz, DMSO-d6) δ: 4.02 (2H, s, CH2), 7.25 (1H, dd, J = 5.0, 4.0 Hz, H4′′), 7.29 (1H, dt, J = 7.7, 1.1 Hz, H5), 7.36 (2H, d, J = 8.0 Hz, H3′ + H5′), 7.61 (1H, dt, J = 8.0, 1.2 Hz, H4), 7.74 (2H, d, J = 8.0 Hz, H2′ + H6′), 7.75 (1H, d, J = 7.6 Hz, H6), 7.89 (1H, d, J = 5.0 Hz, H5′′), 7.93 (1H, dd, J = 7.8, 1.2 Hz, H3), 8.35 (1H, dd, J = 8.2, 1.2 Hz, H3′′), 10.60 (1H, s, NHCO), 11.66 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.9 (CH2), 119.3 (C≡N), 121.4 (C3), 121.5 (C2′, C6′), 122.8 (C1), 123.4 (C5), 126.9 (C4′), 128.4 (C3′, C5′), 128.8 (C6), 129.0 (C4′′), 129.0 (C5′′), 132.2 (C4), 132.3 (C3′′), 137.9 (C2′′), 138.3 (C1′), 139.5 (C2), 159.4 (CONH), 167.3 (NHCO); DIP-MS (EI) m/z: 361 [M]+.
3.1.11. General Synthetic Procedures for 21d–23d
The nitriles 21c–23c (0.86 mmol) were added in absolute ethanol (25 mL) and 1,4-dioxane (5 mL) mixture and added with triethylamine (2.59 mmol) and then refluxed to dissolve. The reaction mixture was added with hydroxylamine·HCl (3.45 mmol) and refluxed for 4–6 h. The ethanol and trimethylamine were removed under reduced pressure and the residue was poured with water to form the precipitate. The obtained precipitate was filtrated, washed with cold water to afford pure white or pale yellow compounds 21d–23d.
N-(3′-Amidoximephenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide (21d). Yield: 94%; m.p.: 230–232 °C; 1H NMR (400 MHz, DMSO-d6) δ: 5.78 (2H, s, NH2), 7.25 (1H, dd, J = 5.0, 4.0 Hz, H4′′), 7.29 (1H, dt, J = 7.7, 1.1 Hz, H5), 7.38 (1H, t, J = 8.0 Hz, H5′), 7.43 (1H, dt, J = 7.7, 1.4 Hz, H4′), 7.61 (1H, dt, J = 7.6, 2.4 Hz, H4), 7.68–7.71 (2H, m, H6 + H6′), 7.89 (1H, dd, J = 5.0, 1.1 Hz, H5′′), 7.95 (1H, dd, J = 7.6, 1.2 Hz, H3), 8.04 (1H, t, J = 1.7 Hz, H2′), 8.38 (1H, dd, J = 4.0, 0.8 Hz, H3′′), 9.65 (1H, s, =NOH), 10.59 (1H, s, NHCO), 11.71 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 116.6 (C2′), 122.8 (C3), 123.4 (C1), 124.6 (C5), 124.9 (C3′), 129.2 (C4′′), 129.3 (C5′′), 129.8 (C6), 132.1 (C4), 132.8 (C3′′), 134.8 (C5′), 138.1 (C2′′), 138.7 (C1′), 139.3 (C2), 159.1 (CONH), 167.7 (NHCO); FAB-MS m/z: 363 [M + 1 − H2O]+, 380 [M]+, 381 [M + 1]+, 403 [M + Na]+.
N-(4′-Amidoximephenyl)-2-(thiophen-2′′-ylcarbonylamino)benzamide (22d). Yield: 52%; m.p.: 241–243 °C; 1H NMR (400 MHz, DMSO-d6) δ: 5.40 (2H, s, NH2), 7.20–7.29 (4H, m, H5 + H2′ + H6′ + H4′′), 7.56–7.64 (3H, m, H4 + H3′ + H5′), 7.80 (1H, d, J = 7.6 Hz, H6), 7.88 (1H, d, J = 4.0 Hz, H5′′), 7.96 (1H, d, J = 7.6 Hz, H3), 8.30 (1H, d, J = 8.4 Hz, H3′′), 8.80 (1H, s, =NOH), 10.20 (1H, s, NHCO), 11.12 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 120.6 (C2′, C6′), 122.5 (C3), 123.2 (C1), 124.3 (C5), 128.8 (C3′, C5′), 128.7 (C6), 129.0 (C4′′, C5′′), 133.1 (C4), 134.2 (C3′′), 136.6 (C1′), 138.0 (C2′′), 138.7 (C2), 150.2 (C=NOH), 154.3 (CONH), 165.3 (NHCO); FAB-MS m/z: 363 [M + 1 − H2O]+, 380 [M]+, 381 [M + 1]+.
N-(4′-Amidoximemethylphenyl)-2-(thiophen-2′′-ylcarbonylamino)benzamide (23d). Yield: 89%; m.p.: 209–211 °C; 1H NMR (400 MHz, DMSO-d6) δ: 3.26 (2H, s, CH2), 5.43 (2H, s, NH2), 7.19–7.27 (4H, m, H5 + H3′ + H5′ + H4′′), 7.56–7.62 (3H, m, H4 + H2 + H6′), 7.73 (1H, d, J = 2.8 Hz, H6), 7.89 (1H, d, J = 4.0 Hz, H5′′), 7.93 (1H, d, J = 7.6 Hz, H3), 8.39 (1H, d, J = 8.4 Hz, H3′′), 8.92 (1H, s, =NOH), 10.50 (1H, s, NHCO), 11.79 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 36.7 (CH2), 121.1 (C2′, C6′), 122.5 (C3), 123.2 (C1), 128.4 (C4′), 128.8 (C3′, C5′), 128.9 (C6), 128.9 (C4′′, C5′′) 132.3 (C4), 133.9 (C3′′), 136.6 (C1′), 138.4 (C2′′), 139.7 (C2), 152.2 (C=NOH), 159.3 (CONH), 167.3 (NHCO); FAB-MS m/z: 377 [M + 1 − H2O]+, 394 [M]+, 395 [M + 1]+, 417 [M + Na]+.
3.1.12. General Synthetic Procedures for 21–23
Amidoximes 21d–23d (0.80 mmol) were dissolved in anhydrous dichloromethane (15 mL) and added with triethylamine (2.37 mmol) at room temperature and acetyl chloride (0.9 mmol) was added at 0 °C. The mixture was reacted for 1–2 h at room temperature and ice water was added. The aqueous layer was extracted with dichloromethane (3 × 40 mL) and the organic phase was washed with water, saturated sodium bicarbonate and water. The organic layer was dried with anhydrous MgSO4, filtrated, and concentrated in reduced pressure to give the acetyl amidoximes (21e–23e), which were used in next reaction without purification. The acetyl amidoximes (1.0 eq) was added to a suspension of 10% Pd-C in absolute ethanol (10 mL) and c-HCl (1.0 eq), and carried out catalytic hydrogenatin reaction for 2 h at 60 psi, 45 °C. The reaction mixture was filtrated on celite pad and the filtrate was evaporated under reduced pressure to give a crude oily residue, which was purified to yield white compounds 21–23 by medium pressure liquid chromatography.
N-(3′-Amidinophenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide·HCl (21). Yield: 32%; m.p.: 262–264 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.24 (1H, dd, J = 5.0, 4.0 Hz, H4′′), 7.33 (1H, t, J = 8.0 Hz, H5), 7.53 (1H, d, J = 8.0 Hz, H4′), 7.60–7.65 (2H, m, H4 + H5′), 7.79 (1H, dd, J = 3.7, 0.8 Hz, H6), 7.89 (1H, dd, J = 5.0, 0.8 Hz, H5′′), 7.92 (1H, d, J = 8.0 Hz, H6′), 8.00 (1H, d, J = 8.3 Hz, H3), 8.21 (1H, s, H2′), 8.26 (1H, dd, J = 8.0, 0.8 Hz, H3′′), 9.08 (2H, br s, hydrogens of amidinium chloride), 9.36 (2H, br s, hydrogens of amidine·HCl), 10.86 (1H, s, NHCO), 11.45 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 120.0 (C2′), 122.0 (C3), 123.5 (C1), 123.7 (C5), 125.7 (C6′), 128.3 (C6), 128.9 (C4′′), 129.0 (C5′′), 129.5 (C4′), 132.3 (C4), 132.4 (C3′′), 137.9 (C2′′), 139.2 (C1′), 139.5 (C2), 159.4 (carbon of amidine), 166.0 (CONH), 167.6 (NHCO); FAB-MS m/z: 388 [M − Cl + Na]+, 365 [M − Cl]+; HRMS (FAB): calcd for C19H17N4O2S [M + H]+ 365.1072, found 365.1084.
N-(4′-Amidinophenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide·HCl (22). Yield: 51%; m.p.: 211–213 °C; 1H NMR (400 MHz, DMSO-d6) δ: 7.24 (1H, dd, J = 5.0, 4.0 Hz, H4′′), 7.30 (1H, t, J = 8.0 Hz, H5), 7.61 (1H, dt, J = 8.0, 1.2 Hz, H4), 7.82 (1H, dd, J = 4.0, 1.2 Hz, H6), 7.88 (2H, d, J = 8.0 Hz, H3′ + H5′), 7.88 (1H, d, J = 4.0, 1.2 Hz, H5′′), 7.94 (1H, dd, J = 8.0, 1.2 Hz, H3), 7.97 (2H, d, J = 8.0 Hz, H2′ + H6′), 8.18 (1H, dd, J = 8.0, 0.8 Hz, H3′′), 9.36 (4H, br s, hydrogens of amidinium chloride), 10.77 (1H, s, NHCO), 11.53 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 120.2 (C2′, C6′), 122.4 (C3), 122.5 (C4′), 123.6 (C1), 124.4 (C5), 128.3 (C6), 128.9 (C3′, C5′, C4′′), 129.2 (C5′′), 132.1 (C4), 132.2 (C3′′), 137.9 (C2′′), 139.7 (C2), 143.9 (C1′), 159.6 (carbon of amidine), 164.9 (CONH), 167.5 (NHCO); FAB-MS m/z: 365 [M − Cl]+; HRMS (FAB): calcd for C19H17N4O2S [M + H]+ 365.1072, found 365.1089.
N-(4′-Amidinomethylphenyl)-2-((thiophen-2′′-yl)carbonylamino)benzamide·HCl (23). Yield: 42%; m.p.: 172–174 °C; 1H NMR (400 MHz, DMSO-d6) δ: 3.69 (2H, s, CH2), 7.24 (1H, dd, J = 5.0, 4.0 Hz, H4′′), 7.30 (1H, dt, J = 7.7, 1.1 Hz, H5), 7.42 (2H, d, J = 8.0 Hz, H3′ + H5′), 7.61 (1H, dt, J = 8.0, 1.2 Hz, H4), 7.72 (2H, d, J = 8.0 Hz, H2′+ H6′), 7.75 (1H, dd, J = 4.0, 1.2 Hz, H6), 7.89 (1H, dd, J = 4.0, 1.2 Hz, H5′′), 7.92 (1H, dd, J = 8.0, 1.2 Hz, H6), 8.33 (1H, d, J = 8.0 Hz, H3′′), 8.92 (4H, br s, hydrogens of amidinium chloride), 10.61 (1H, s, NHCO), 11.64 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 121.4 (C2′, C6′), 121.5 (C3), 123.0 (C1), 123.4 (C5), 128.4 (C6), 128.8 (C4′′), 129.0 (C5′′), 129.1 (C3′, C5′), 130.7 (C4′), 132.2 (C4), 132.3 (C3′′), 138.0 (C2′′), 138.2 (C1′), 139.6 (C2), 159.4 (carbon of amidine), 167.3 (CONH), 169.1 (NHCO); FAB-MS m/z: 379 [M − Cl]+; HRMS (FAB): calcd for C20H19N4O2S [M + H]+ 379.1229, found 379.1243.
3.1.13. General Synthetic Procedures for 24b–26b
To a solution of 11a–13a (1.54 mmol) in ethanol (40 mL) was added with hydroxylamine hydrochloride (300 mg, 4.60 mmol) and triethylamine (0.5 mL) at room temperature. The reaction mixture was refluxed for 12 h and the following procedures were same as synthetic procedure for 21b–23b. Compounds 24b–26b were recrystallized with dichloromethane and n-hexane mixture to afford as a white powder, respectively.
N-(3′-Amidoximino)-2-(4"-methylphenylsulfonamido)benzamide (24b). Yield: 85%; m.p: 182–184 °C; 1H NMR (400 MHz, CDCl3) δ: 2.23 (3H, s, CH3), 5.05 (2H, s, NH2), 6.61 (1H, s, =NOH), 7.02–7.12 (4H, m, H3 + H4 + H5 + H5′), 7.37 (2H, d, J = 8.0 Hz, H3′′ + H5′′), 7.50–7.60 (3H, m, H6 + H4′ + H6′), 7.61 (2H, d, J = 8.0 Hz, H2′′ + H6′′), 7.85 (1H, s, H2′), 7.91 (1H, s, NHSO2), 8.26 (1H, s, CONH); 13C NMR (100 MHz, CDCl3) δ: 21.4 (CH3), 118.3 (C2′), 121.9 (C6′), 122.2 (C3), 122.5 (C1), 124.0 (C5), 124.1 (C3′), 127.2 (C2′′, C6′′), 129.4 (C3′′, C5′′), 129.7 (C6), 132.9 (C4), 133.1 (C5′), 136.2 (C1′′), 137.5 (C4′′), 138.6 (C1′), 143.9 (C2), 158.8 (C=NOH), 166.8 (C=O); GC-MS (EI) m/z: 409 [M + 1-NH2]+, 392 [M + 1 − NH2OH]+.
N-(4′-Amidoximino)-2-(4"-methylphenylsulfonamido)benzamide (25b). Yield: 74%; m.p: 213–215 °C; 1H NMR (400 MHz, (CD3)2CO) δ: 2.26 (3H, s, CH3), 6.60 (1H, s, =NOH), 7.19 (3H, m, H4 + H3′′ + H5′′), 7.54 (1H, t, J = 7.2 Hz, H5), 7.62 (2H, d, J = 7.6 Hz, H2′′ + H6′′), 7.69 (1H, d, J = 8.4 Hz, H3), 7.75–7.88 (3H, m, H6 + H2′ + H6′), 7.98 (2H, d, J = 8.4 Hz, H3′ + H5′), 9.72 (1H, s, NHSO2), 10.50 (1H, s, CONH); 13C NMR (100 MHz, (CD3)2CO) δ: 21.4 (CH3), 120.9 (C3), 122.9 (C1), 124.2 (C5), 125.0 (C4′), 128.0 (C2′, C6′), 129.2 (C3′, C5′, C2′′, C6′′), 130.6 (C3′′, C5′′), 131.2 (C6), 133.6 (C4), 137.3 (C1′′), 139.5 (C4′′), 142.0 (C1′), 144.8 (C2), 168.2 (C=NOH), 168.3 (C=O); GC-MS (EI) m/z: 409 [M + 1-NH2]+, 392 [M + 1 − NH2OH]+.
N-(4′-Amidoximinobenzyl)-2-(4"-methylphenylsulfonamido)benzamide (26b). Yield: 63%; m.p: 70–74 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.29 (3H, s, CH3), 3.52 (2H, s, CH2), 7.22 (1H, t, J = 7.2 Hz, H4), 7.25 (2H, d, J = 7.6 Hz, H3′ + H5′), 7.26 (2H, d, J = 8.4 Hz, H3′′ + H5′′), 7.42–7.50 (2H, m, H3 + H5), 7.52 (2H, d, J = 7.6 Hz, H2′ + H6′), 7.61 (2H, d, J = 8.4 Hz, H2′′ + H6′′), 7.78 (1H, d, J = 8.0 Hz, H6), 8.49 (2H, br s, NH2), 9.50 (1H, br s, =NOH), 10.35 (1H, s, NHSO2), 10.75 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 21.4 (CH3), 120.9 (C3), 122.9 (C1), 124.2 (C5), 125.0 (C4′), 128.0 (C2′, C6′), 129.2 (C3′, C5′, C2′′, C6′′), 130.6 (C3′′, C5′′), 131.2 (C6), 133.6 (C4), 137.3 (C1′′), 139.5 (C4′′), 142.0 (C1′), 144.8 (C2), 168.2 (C=NOH), 168.3 (C=O); GC-MS (EI) m/z: 423 [M + 1 − NH2]+, 406 [M + 1 − NH2OH]+.
3.1.14. General Experimental Procedures for 24–26
To a solution of amidoximes 24b–26b (1.18 mmol) in anhydrous dichloromethane (10 mL) was added triethylamine (1.18 mmol) and acetyl chloride (1.20 mmol) at 0 °C. The following procedures were same as synthetic procedures for acetylated amidoximes 21c–23c. To a suspention of 10% Pd-C in absolute ethanol (50 mL) was added 21c–23c (1.12 mmol) and c-HCl (1.12 mmol) and the reaction mixture was shaken on Paar hydrogenation apparatus for 12 h at 50 psi, 45 °C and filtrated on celite pad. The filtrate was evaporated under reduced pressure to provide a crude compound, which was purified to give white compounds 24–26 by column chromatography.
N-(3′-Amidinophenyl)-2-(4"-methylphenylsulfonamido)benzamide·HCl (24). Yield: 37% m.p: 216–218 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.29 (3H, s, CH3), 7.20–7.29 (3H, m, H4 + H3′′ + H5′′), 7.39 (1H, d, J = 6.8 Hz, H3), 7.55 (1H, t, J = 8.0 Hz, H5), 7.58–7.68 (4H, m, H3′ + H5′ + H2′′ + H6′′), 7.86 (1H, d, J = 7.6 Hz, H6), 7.92 (1H, d, J = 6.8 Hz, H6′), 8.24 (1H, s, H2′), 9.28 (2H, br s, hydrogens of amidinium chloride), 9.46 (2H, s, hydrogens of amidinium chloride), 10.57 (1H, s, NHSO2), 10.80 (1H, s, CONH); 13C NMR (100 MHz, CD3OD) δ: 20.2 (CH3), 119.4 (C2′), 121.3 (C3), 121.8 (C1), 122.4 (C5), 123.0 (C6′), 124.2 (C4′), 125.8 (C3′), 126.8 (C2′′, C6′′), 126.9 (C3′′, C5′′), 129.1 (C4), 129.2 (C6), 129.8 (C5′), 130.6 (C1′), 132.3 (C1′′), 139.9 (C4′′), 142.9 (C2), 167.3 (C=NH), 167.6 (C=O); DIP-MS (EI) m/z: 392 [M – HCl − NH2]+; HRMS (FAB): calcd for C21H21N4O3S [M + H]+ 409.1334, found 409.1347.
N-(4′-Amidinophenyl)-2-(4"-methylphenylsulfonamido)benzamide·HCl (25). Yield: 43%; m.p: 250–252 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.27 (3H, s, CH3), 7.23–7.32 (3H, m, H4 + H3′′ + H5′′), 7.35 (1H, d, J = 8.0 Hz, H3), 7.50 (1H, t, J = 8.4 Hz, H5), 7.60 (2H, d, J = 8.0 Hz, H2′′ + H6′′), 7.78 (1H, dd, J = 8.0, 1.2 Hz, H6), 9.10 (2H, br s, hydrogens of amidinium chloride), 9.35 (2H, br s, hydrogens of amidinium chloride), 10.34 (1H, s, NHSO2), 10.73 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 20.9 (CH3), 120.1 (C2′, C6′), 122.2 (C3), 122.6 (C1), 124.5 (C5), 125.4 (C4′), 126.8 (C3′, C5′), 128.3 (C2′′, C6′′), 129.3 (C6), 129.5 (C3′′, C5′′), 132.4 (C4), 136.0 (C1′′), 136.7 (C4′′), 143.5 (C1′), 143.6 (C2), 164.8 (C=NH), 166.9 (C=O); DIP-MS (EI) m/z: 392 [M – HCl − NH2]+; HRMS (FAB): calcd for C21H21N4O3S [M + H]+ 409.1334, found 409.1348.
N-(4′-Amidinomethylphenyl)-2-(4"-methylphenylsulfonamido)benzamide·HCl (26). Yield: 33%; m.p.: 172–173 °C; 1H NMR (400 MHz, DMSO-d6) δ: 2.28 (3H, s, CH3), 3.67 (2H, s, CH2), 7.23 (1H, t, J = 8.0 Hz, H4), 7.26 (2H, d, J = 8.0 Hz, H3′′ + H5′′), 7.41–7.53 (4H, m, H3 + H5 + H3′,+ H5′), 7.60 (2H, d, J = 8.4 Hz, H2′ + H6′), 7.66 (2H, d, J = 8.8 Hz, H2′′ + H6′′), 7.81 (1H, dd, J = 8.0, 1,2 Hz, H6), 8.79 (2H, br s, hydrogens of amidinium chloride), 9.28 (2H, br s, hydrogens of amidinium chloride), 10.44 (1H, s, NHCO), 10.65 (1H, s, CONH); 13C NMR (100 MHz, DMSO-d6) δ: 20.9 (CH3), 37.1 (CH2), 121.2 (C2′, C6′), 123.8 (C3), 124.1 (C1), 126.8 (C3′, C5′), 128.3 (C6), 129.2 (C2′′, C6′′), 129.8 (C3′′, C5′′), 129.9 (C4), 132.4 (C4′), 135.9 (C1′), 137.3 (C1′′), 137.7 (C4′′), 143.7 (C2), 166.7 (C=NH), 169.2 (NHCO); DIP-MS (EI) m/z: 406 [M – HCl − NH2]+; HRMS (FAB): calcd for C22H23N4O3S [M + H]+ 423.1491, found 423.1478.
3.2. Biology
3.2.1. Reagents and Instruments
Heparin was purchased from Sigma (St. Louis, MO, USA) and TNF-α was purchased from Abnova (Taipei, Taiwan). The molecular weight of heparin used as positive control in our experiments is 3500. Anti-tissue factor (TF) antibody, rivaroxaban, and argatroban were purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA). Thromboxane A2 (TXA2) analog and U46619 were purchased from Calbiochem-Novabiochem Corp. (San Diego, CA, USA). Prothrombin, thrombin, Factor VIIa, X, and Xa were purchased from Haematologic Technologies (Essex Junction, VT, USA) and APTT-XL and thromboplastin-D were purchased from Fisher Diagnostics (Middletown, VA, USA). S-2222 and S-2238 were purchased as substrate of FXa and thrombin from ChromogenixAB (Mölndal, Sweden). Aggregometer (Chronlog, Havertown, PA, USA), thrombotimer (Behnk Elektronik, Norderstedt, Germany), microplate reader (Tecan Austria GmbH, Grödig, Austria), and spectrophotometer (TECAN, Männedorf, Switzerland) were used.
3.2.2. Preparation of Plasma
Blood samples were collected in the morning from 10 healthy volunteers after fasting overnight (aged between 24 and 28 years, four males and six females) without cardiovascular disorders, allergy and lipid or carbohydrate metabolism disorders, untreated with drugs. All volunteers gave written informed consent before participation. Human blood was collected into sodium citrate (0.32% final concentration) and immediately centrifuged (2000× g, 15 min) to prepare plasma. The study protocol was approved by the Institutional Review Board of Kyungpook National University Hospitals (Daegu, Republic of Korea).
3.2.3. In Vitro Anticoagulant Assay
According to the manufacturer’s instructions, as described previously [41], aPTT and PT of compounds 5, 9, and 21–23 were measured by a Thrombotimer (Behnk Elektronik, Norderstedt, Germany). The aPTT and PT results are expressed in seconds and PT results are also expressed as INR. INR (International Normalized Rations) = (PT sample/PT control) ISI, ISI = international sensitivity index.
3.2.4. In Vivo Bleeding Time
According to procedure reported by Dejana et al. [42], tail bleeding times were measured. Briefly, ICR mice were fasted overnight before experiments. One hour after intravenous administration of 5, 9, and 21–23, tails of mice were transected at 2 mm from their tips. Bleeding time was defined as the time elapsed until bleeding stopped. When the bleeding time exceeded 10 min, in vivo bleeding time was recorded as 10 min. All animals were treated in accordance with the guidelines for the Care and Use of Laboratory Animals issued by Kyungpook National University.
3.2.5. Thrombin Activity Assay
According to procedure previously described [22], compounds 5, 9, and 21–23 were dissolved in 50 nM Tris-HCl buffer (pH 7.4) containing 7.5 mM ethylenediaminetetraacetic acid (EDTA) and added with 150 mM NaCl. After incubation at 37 °C for 2 min, thrombin solution (150 µL; 10 U/ mL) was added and incubated at 37 °C for 1 min. S-2238 as substrate (150 µL; 1.5 mM) solution was added and then absorbance was measured at 405 nm for 2 min using a spectrophotometer (TECAN, Männedorf, Switzerland).
3.2.6. Factor Xa Activity Assay
Factor Xa activity assay was carried out in the same method as the thrombin activity assay, but using FXa (150 µL; 1 U/mL) and S-2222 as FXa substrate.
3.2.7. Cell Culture
Primary HUVECs were purchased from Cambrex Bio Science (Charles City, IA, USA) and were maintained by a previously described method [43,44]. Briefly, cells were cultured until confluent at 37 °C in 5% CO2 in EBM-2 basal media supplemented from Cambrex Bio Science (Charles City, IA, USA).
3.2.8. Thrombin Generation on the Surfaces of Human Umbilical Vein Endothelial Cells (HUVECs)
The thrombin generation of HUVECs was quantitated as previously described [40]. Briefly, HUVECs were pre-incubated with synthesized compounds (300 µL) dissolved in 50 mM Tris-HCl buffer, 100 pM FVa, and 1 nM FXa for 10 min, and then added with prothrombin to a final concentration of 1 µM. After 10 min, Each 10 µL of triplicate samples was transferred to a 96-well plate containing 40 µL of 0.5 M EDTA in Tris-buffered saline per well. Activated prothrombin was determined by measuring the rate of hydrolysis of S2238 at 405 nm. Standard curves were prepared using amounts of purified thrombin.
3.2.9. Factor Xa Generation on the Surfaces of HUVECs
The FXa generation of HUVECs was measured as previously described [41]. The amounts of FXa generated by indicated concentrations of 5, 9, and 21–23 were measured at 405 nm over 2 min by a microplate reader. Initial rates of color development were converted into FXa concentrations using a standard curve prepared with known dilutions of purified human FXa.
3.2.10. Thrombin-Catalyzed Fibrin Polymerization
Thrombin-catalyzed polymerization was monitored turbidity at 360 nm every 6 s for 20 min by using a spectrophotometer (TECAN, Männedorf, Switzerland) at ambient temperature. Both control plasma and plasma incubated with compounds 5, 9, and 21–23 were diluted three times in TBS 50 mM Tris-buffered saline and clotted with thrombin (final concentration-0.5 U/mL). The maximum rate of fibrin polymerization (Vmax, ΔmOD/min) was recorded for each absorbance curve. All experiments were performed in triplicate.
3.2.11. Platelet Aggregation
The platelet aggregation assay was measured following to the previously reported method [44]. Washed plasma was pre-incubated with the indicated concentration of compounds 5, 9, and 21–23 for 3 min, and then stimulated with thrombin (0.1 U/mL, Sigma) and U46619 (2 µM) in 0.9% saline for 5 min. Platelet aggregations were performed in an aggregometer (Chronolog, Havertown, PA, USA).
3.2.12. Cell Viability Assay
The colorimetric MTT was used for counting the number of live cells to determine cell viability. Cells (5 × 103/well) were grown in a 96-well plate at 37 °C and after incubation for 24 h, cells were washed with fresh medium, and treated with compounds 5, 9, and 21–23. After a 48 h incubation period, cells were washed and 100 µL of 1 mg/mL MTT was added, followed by incubation for 4 h. Finally, the resultant formazan salt was dissolved in 150 µL DMSO and the absorbance intensity determinded by a microplate reader (Tecan Austria GmbH, Grödig, Austria) with a reference wavelength of 540 nm using. Data were expressed as means ± SEM at least three independent experiments.
3.2.13. Statistical Analysis
Data are expressed as means ± SEM of at least three independent experiments. Statistical significance (p < 0.05) was determiced using the Student’s t-test.
4. Conclusions
Non-amidino N2-thiophenecarbonyl- and N2-tosylanthranilamides 1–20, and amidino N2- thiophenecarbonyl- and N2-tosylanthranilamides 21–26 synthesized and evaluated against PT and aPTT in vitro. Compounds 5, 9, and 21–23 showed prolongation in aPTT in vitro and ex vivo, and in vivo bleeding time. Compounds 5 and 21–23 exhibited prolongation in PT in vitro and ex vivo. The activities of FXa and thrombin as well as the generation of thrombin and FXa in HUVECs were dose-dependently inhibited but weak. These compounds inhibited thrombin-catalyzed fibrin polymerization and platelet aggregation induced by U46619. Any sulfonamide group containing compounds 11–20, 24, and 26 did not exhibit prolongation of PT and aPTT, but 4-amidino derivative 25 showed high aPTT. The anticoagulant activity was governed to a great extent by an amidine group at the meta or para position of phenyl ring and thiophenecarbonyl linker of 21–23.
Acknowledgments
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (2010-0013516) and by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI15C0001).
Author Contributions
Soo Hyun Lee, Wonhwa Lee, ThiHa Nyugen, and Il Soo Um performed the research and analyzed the data; Jong-Sup Bae and Eunsook Ma wrote the paper. All authors read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Silverstein M.D., Heit J.A., Mohr D.N., Petterson T.M., O’Fallon W.M., Melton L.J. Trends in the incidence of deep vein thrombosis and pulmonary embolism: A 25-year population-based study. Arch. Intern. Med. 1998;158:585–593. doi: 10.1001/archinte.158.6.585. [DOI] [PubMed] [Google Scholar]
- 2.Hirsh J. Heparin. N. Engl. J. Med. 1991;324:1565–1574. doi: 10.1056/NEJM199105303242206. [DOI] [PubMed] [Google Scholar]
- 3.Fareed J., Thethi I., Hoppensteadt D. Old versus new oral anticoagulants: Focus on pharmacology. Annu. Rev. Pharmacol. Toxicol. 2012;52:79–99. doi: 10.1146/annurev-pharmtox-010611-134633. [DOI] [PubMed] [Google Scholar]
- 4.Mackman N. Triggers, targets and treatments for thrombosis. Nature. 2008;451:914–918. doi: 10.1038/nature06797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nar H., Bauer M., Schmid A., Stassen J.M., Wienen W., Priepke H.W.M., Kauffmann I.K., Ries U.J., Hauel N.H. Structural basis for inhibition promiscuity of dual specific thrombin and factor Xa blood coagulation inhibitors. Structures. 2001;9:29–37. doi: 10.1016/S0969-2126(00)00551-7. [DOI] [PubMed] [Google Scholar]
- 6.Walenga J.M., Jeske W.P., Samama M.-M., Frapaise F.X., Bick R.L., Fareed J. Fondaparinux: A synthetic heparin pentasaccharide as a new antithrombotic agent. Expert. Opin. Investig. Drugs. 2002;11:397–407. doi: 10.1517/13543784.11.3.397. [DOI] [PubMed] [Google Scholar]
- 7.Samama M.-M., Gerotziafas G.T. Evaluation of the pharmacological properties and clinical results of the synthetic pentasaccharide (Fondaparinux) Thromb. Res. 2003;109:1–11. doi: 10.1016/S0049-3848(03)00030-6. [DOI] [PubMed] [Google Scholar]
- 8.Blizzard T.A., Singh S., Patil B., Chidurala N., Komanduri V., Debnath S., Belyakov S., Crespo A., Struck A., Kurtz M., et al. Heterocyclic core analogs of direct thrombin inhibitor. Bioorg. Med. Chem. Lett. 2014;25:1111–1115. doi: 10.1016/j.bmcl.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Kranjc A., Kekelj D. Dual inhibitors of the blood coagulation enzymes. Curr. Med. Chem. 2004;11:2535–2547. doi: 10.2174/0929867043364432. [DOI] [PubMed] [Google Scholar]
- 10.Herbert J.-M., Herault J.-P., Bernat A., Savi P., Schaeffer P., Driguez P.-A., Duchaussoy P., Petitou M. SR123781A, a synthetic heparin mimetic. Thromb. Haemost. 2001;85:852–860. [PubMed] [Google Scholar]
- 11.Petitou M., Hérault J.-P., Bernat A., Driguez P.-A., Duchaussoy P., Lormeau J.-C., Herbert J.-M. Synthesis of thrombin-inhibiting heparin mimetics without side effects. Nature. 1999;398:417–422. doi: 10.1038/18877. [DOI] [PubMed] [Google Scholar]
- 12.Bauer K.A., Hawkins D.W., Peters P.C., Petitou M., Herbert J.-M., Constant A.A., van Boeckel C.A.A., Meuleman D.G. Fondaparinux, a synthetic pentasaccharide: The first in a new class of antithrombotic agents—The selective factor Xa inhibitors. Cardiovasc. Drug Rev. 2002;20:37–52. doi: 10.1111/j.1527-3466.2002.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 13.De Kort M., Buijsmain R.C., van Boeckel A.A. Synthetic heparin derivatives as new anticoagulant drugs. Drug Discov. Today. 2005;10:769–779. doi: 10.1016/S1359-6446(05)03457-4. [DOI] [PubMed] [Google Scholar]
- 14.Petitou M., Nancy-Porteboi V., Dubreucq G., Motte V., Meuleman D., de Kort M., van Boeckel C.A.A., Vogel G.M.T., Wisse J.A.J. The long way to a new pentasaccharidebased neutralisable anticoagulant with an unprecedented pharmacological profile. Thromb. Haemost. 2009;102:804–810. doi: 10.1160/TH09-01-0063. [DOI] [PubMed] [Google Scholar]
- 15.Olson S.T., Swanson R., Petitou M. Specificity and selectivity profile of EP217609: A new neutralizable dual-action anticoagulant targets thrombin and factor Xa. Blood. 2012;119:2187–2195. doi: 10.1182/blood-2011-09-381764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng J.Z., Daniel R., McMasters D.R., Rabbat P.M.A., Williams P.D., Coburn C.A., Yan Y., Kuo L.C., Lewis S.D., Lucas B.J., et al. Development of an oxazolopyridine series of dual thrombin/factor Xa inhibitors via structure-guided lead optimization. Bioorg. Med. Chem. Lett. 2005;15:4411–4416. doi: 10.1016/j.bmcl.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 17.Trstenjak U., Ilas J., Kikelj D. Transformation of a selective factor Xa inhibitor rivaroxaban into a dual factor Xa/thrombin inhibitor by modification of the morpholin-3-one moiety. Med. Chem. Commun. 2014;5:197–202. doi: 10.1039/c3md00250k. [DOI] [Google Scholar]
- 18.Meneyrol J., Follmann M., Lassalle G., Wehner V., Barre G., Rousseaux T., Altenburger J.-M., Petit F., Bocskei Z., Schreuder H., et al. 5-Chlorothiophene-2-carboxylic acid [(S)-2-[2-methyl-3-(2-oxopyrrolidin-1-yl)benzenesulfonylamino]-3-(4-methylpiperazin-1-yl)-3-oxopropyl]amide (SAR107375), a selective and potent orally activated thrombin and Factor Xa Inhibitor. J. Med. Chem. 2013;56:9441–9456. doi: 10.1021/jm4005835. [DOI] [PubMed] [Google Scholar]
- 19.Young R.J., Brown D., Burns-Kurtis C.L., Chan C., Convery M.A., Hubbard J.A., Kelly H.A., Pateman A.J., Patikis A., Senger S., et al. Selective and dual action orally active inhibitors of thrombin and factor Xa. Bioorg. Med. Chem. Lett. 2007;17:2927–2930. doi: 10.1016/j.bmcl.2007.03.080. [DOI] [PubMed] [Google Scholar]
- 20.Schonig A., Sarafoff N., Seyfarth M. Triple antithrombotic management after stent implantation: When and how? Heart. 2009;95:1280–1285. doi: 10.1136/hrt.2008.160457. [DOI] [PubMed] [Google Scholar]
- 21.Lee S.H., Lee W., Bae J.-S., Ma E. Synthesis and in vitro and in vivo anticoagulant and antiplatelet activities of amidino- and non-amdino benzamides. Molecules. 2016;21:676–697. doi: 10.3390/molecules21050676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mendel D., Marquart A.L., Joseph S., Waid P., Yee Y.K., Tebbe A.L., Ratz A.M., Herron D.K., Goodson T., Masters J.J., et al. Anthranilamide inhibitors of factor Xa. Bioorg. Med. Chem. Lett. 2007;17:4832–4836. doi: 10.1016/j.bmcl.2007.06.051. [DOI] [PubMed] [Google Scholar]
- 23.Kochanny M.J., Adler M., Ewing J., Griedel B.D., Ho E., Karanjawala R., Lee W., Lentz D., Liang A.M., Morrissey M.M., et al. Substituted thiophene-anthranilamides as potent inhibitors of human factor Xa. Bioorg. Med. Chem. 2007;15:2127–2145. doi: 10.1016/j.bmc.2006.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Zhang P., Bao L., Zuckett J.F., Goldman E.A., Jia J.J., Arfsten A., Edwards S., Sinha U., Hutchaleelaha A., Park G., et al. Design, synthesis and SAR of anthranilamide-based factor Xa inhibitors incorporating substituted biphenyl P4 motifs. Bioorg. Med. Chem. Lett. 2004;14:983–987. doi: 10.1016/j.bmcl.2003.11.079. [DOI] [PubMed] [Google Scholar]
- 25.Zhang P., Bao L., Zuckett J.F., Jia Z.J., Woolfrey J., Arfsten A., Edwards S., Sinha U., Hutchaleelaha A., Lambing J.L., et al. Design, synthesis, and SAR of anthranilamide-based factor Xa inhibitors with improved functional activity. Bioorg. Med. Chem. Lett. 2004;14:989–993. doi: 10.1016/j.bmcl.2003.11.080. [DOI] [PubMed] [Google Scholar]
- 26.Haginoya N., Kobayashi S., Komoriya S., Yoshino T., Suzuki M., Shimada T., Watanabe K., Hirokawa Y., Furugori T., Nagahara T. Synthesis and conformational analysis of a non-amidine factor Xa inhibitor that incorporates 5-methyl-4,5,6,7-tetrahydrothiazolo[5,4-c]pyridine as S4 binding element. J. Med. Chem. 2004;47:5167–5182. doi: 10.1021/jm049884d. [DOI] [PubMed] [Google Scholar]
- 27.Diehl K.H., Hull R., Morton D., Pfister R., Rabemampianina Y., Smith D. A good practice guide to the aministration of substances and removal of blood, including routes and volumes. J. Appl. Toxicol. 2001;21:15–23. doi: 10.1002/jat.727. [DOI] [PubMed] [Google Scholar]
- 28.Sugo T., Nakamikawa C., Tanabe S. Activation of prothrombin by factor Xa bound to the membrane surface of human umbilical vein endothelial cells: Its catalytic efficiency is similar to that of prothrombinase complex on platelets. J. Biochem. 1995;117:244–250. doi: 10.1093/jb/117.2.244. [DOI] [PubMed] [Google Scholar]
- 29.Rao L.V., Rapaport S.I., Lorenzi M. Enhancement by human umbilical vein endothelial cells of factor Xa- catalyzed activation of factor VII. Blood. 1988;71:791–796. [PubMed] [Google Scholar]
- 30.Waris G., Siddigi H.M., Florke U., Butt M.S., Hussain R. N-(4′-Chlorophenyl)-2-nitrobenzamide. Acta Cryst. Sec. E. 2012;E68:o2768. doi: 10.1107/S1600536812036082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bae J.-S. The Result of Reptilase-Catalyzed Polymerization Assay Showed Significantly Decreased by the Indicated Concentration of Compounds 5 or 9 or 21–23. Kyungpook National University; Daegu, Korea: 2016. [Google Scholar]
- 32.Sareed A., Hussain S., Bolte M. N-(4-Chlorophenyl)-2-nitrobenzamide. Acta Cryst. 2007;E63:o4615. [Google Scholar]
- 33.Bold G., Furet P., Monley P.W. Anthranilic acid amides and pharmaceutical Use thereof. 7,067,543. US Patent. 2006 Jun 27;
- 34.Gao J., Wang G.-W. Direct oxidation of aldehyde with anilines under mechanical milling contions. J. Org. Chem. 2008;73:2955–2958. doi: 10.1021/jo800075t. [DOI] [PubMed] [Google Scholar]
- 35.Beight D.W., Craft T.J., Franciskovich J.B., Goodson T., Hall S.E., Herran D.K., Klimkowski V.J., Shuman R.T., Smith G.F., Tebber A.L., et al. Antithrombotic agents. 6,605,626. US Patent. 2003 Aug 12;
- 36.Kang S.K., Kim D.-H., Park J.-N. The copper-catalyzed N-arylation of aryl iodides with benzamides or nitrogen heterocycles in the presence of ethylenediamines. Synlett. 2002;3:427–430. doi: 10.1055/s-2002-20457. [DOI] [Google Scholar]
- 37.Mabkhot Y.N., Al-Majid A.M., Barakat A., Al-Showiman S.S., Al-Har M.S., Radi S., Naseer M.M., Hadda T.B. Synthesis and biological evaluation of 2-aminobenzamide derivatives as antimicrobial agents: Opening/closing pharmacophore site. Int. J. Mol. Sci. 2014;15:5115–5127. doi: 10.3390/ijms15035115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cabrera-Rivera F.A., Oxtiz-Nava C., Roman-Bravo P., Leyva M.A., Escalante J. Direct halogenation reactions in 2,3-dihydro-4(1H)-quinazolinones. Heterocycles. 2012;85:2173–2195. [Google Scholar]
- 39.Altmann K.-H., Bold G., Furet P., Manley P., Wood J., Ferran S., Hofmann F., Mestan J., Huth A., Kruger M., et al. N-(Aryl)thioanthranilic acid amide derivatives their preparation and their use as VEGF receptor tyrosine kinas inhibitors. 200,200,194 A1. US Patent. 2002 Feb 14;
- 40.Kim T.H., Gu S.K., Bae J.S. Antithrombotic and profibrinolytic activities of eckol and dieckol. J. Cell Biochem. 2012;113:2877–2883. doi: 10.1002/jcb.24163. [DOI] [PubMed] [Google Scholar]
- 41.Dejana E., Callioni A., Quintana A., de Gaetano G. Bleeding time in laboratory animals II. A comparison of different assay conditions in rats. . Thromb. Res. 1979;15:191–197. doi: 10.1016/0049-3848(79)90064-1. [DOI] [PubMed] [Google Scholar]
- 42.Bae J.S., Rezaie A.R. Protease activated receptor 1 (PAR-1) activation by thrombin is protective in human pulmonary artery endothelial cells if endothelial protein C receptor is occupied by its natural ligand. Thromb. Haemost. 2008;100:101–109. doi: 10.1160/TH08-02-0127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bae J.S. Antithrombotic and profibrinolytic activities of phloroglucinol. Food Chem. Toxicol. 2011;49:1572–1577. doi: 10.1016/j.fct.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 44.Kim S.Y., Kim S., Kim J.M., Jho E.H., Park S., Oh D., Yun-Choi H.S. PKC inhibitors RO 31-8220 and Go 6983 enhance epinephrine-induced platelet aggregation in catecholamine hypo-responsive platelets by enhancing Akt phosphorylation. BMB Rep. 2011;44:140–145. doi: 10.5483/BMBRep.2011.44.2.140. [DOI] [PubMed] [Google Scholar]