Abstract
In a continuing effort to develop safe and efficacious vaccine and immunotherapeutic vectors, we constructed recombinant vaccinia virus (rVV) vaccines lacking either the B13R (SPI-2) or the B22R (SPI-1) immune-modulating gene and coexpressing IFN-γ. B13R and B22R are nonessential VV immune-modulating genes that have antiapoptotic and antiinflammatory properties with sequence homology to serine protease inhibitors (serpins). IFN-γ is a cytokine with potent immunoregulatory, antineoplastic, and antiviral properties. We observed that these rVVs with a deletion in a serpin gene and expressing IFN-γ replicated to high titers in tissue culture yet were avirulent in both immunocompromised and immunocompetent mice with no detectable viral replication in these animals. A single immunization elicited potent humoral, T helper, and cytotoxic T cell immune responses in mice despite the absence of any detectable virus replication in vivo. IFN-γ coexpression and the inactivation of one or more VV immune-modulating genes provide an optimized method for increasing the safety while maintaining the efficacy of rVV vaccines. This strategy provides a method for developing highly safe and efficacious vaccines for smallpox and other diseases and immunotherapeutic vectors.
Keywords: vaccines, safety, efficacy, immune-modulating genes, smallpox
Vaccinia virus (VV), the orthopoxvirus used in the global eradication of smallpox, also has served as an effective vector for eukaryotic protein expression, vaccine development, and immunotherapeutic treatments for cancer (1). The emerging threat of smallpox bioterrorism has once again brought VV vaccines to the forefront. Currently, vaccination of military and emergency response personnel with VV is recommended, and vaccination is being considered for the general public (2). Although VV has not been directly associated with any specific disease, complications have been observed in immunocompromised and immunosuppressed individuals (3). In light of the current threat of bioterrorism and the ongoing AIDS epidemic, the complications of VV infection in immunocompetent and especially in immunocompromised populations must be thoroughly addressed to circumvent any possible pathogenic effects of mass vaccination. Therefore, increasing safety while maintaining proven efficacy is one of the foremost considerations for the widespread use of VV vaccines.
VV has been used extensively as a vector for the development of recombinant live vaccines (1). There are currently two well established effective recombinant VV (rVV) vaccines, one for rabies (4) and the other for rinderpest (5–7, 8).
We and others have demonstrated that the cytokine IFN-γ can be used as both an adjuvant and an attenuating agent for the development of safe and efficacious live VV vaccines (9–11). IFN-γ is a cytokine that plays an essential role in the regulation of the immune system and host defense against pathogens (12). The effects of IFN-γ on the immune system are profound. IFN-γ modulates macrophage tumoricidal and microbicidal activity, natural killer cell cytolysis, and both B and T cell responses to antigens (13). Moreover, IFN-γ is involved in the protection against viral pathogenesis, especially the long-term control of viral infections (14). Experiments also have demonstrated that the expression of murine IFN-γ by rVVs (9–11), as well as alphaviruses (15), expedites viral clearance. In addition, we and others have shown IFN-γ to be an effective adjuvant and a potent enhancer of anamnestic responses (16, 17). The immune-enhancing properties of IFN-γ have been related to the primary effect of increasing the expression of MHC antigens on macrophages and the subsequent recruitment of T helper lymphocytes.
Previously, we showed that the deletion of VV serine protease inhibitor (serpin) homolog genes attenuates VV without compromising the effectiveness of the immune response (18). We conjectured that the expression of IFN-γ, in addition to inactivating either the B13R or B22R serpin homolog gene, might further increase the safety and efficacy of rVVs. B13R (SPI-2) and B22R (SPI-1) are two VV serpins that interfere with host inflammatory responses and have potent antiapoptotic properties. In particular, B13R has 92% amino acid homology to the cowpox virus cytokine response modifier A (crmA) protein, which inhibits the proteolytic activity of IL-1β converting enzyme (ICE), also known as caspase-1, as well as granzyme B (19). Similarly, the VV serpin homolog B22R plays a role in reducing the host's immune responses to the virus. The rabbitpox equivalent of the VV B22R gene has been shown to inhibit apoptosis in a caspase independent manner and decrease host range in human A549 lung cells (20). We tested our hypothesis in mice by evaluating the attenuation of the rVVs in normal and immunodeficient mice and by assessing the immune response to both homologous and heterologous antigens, because VV is used both as a smallpox vaccine and as a vaccine vector.
Materials and Methods
Cells and Viruses. African green monkey kidney cells (BS-C-1 and BS-C-40), murine L929 cells, hamster BHK-21 cells, and human A549 and HeLa S3 cells were grown in DMEM supplemented with 10% FBS in 5% CO2. The rVVs v50ΔB13R and v50ΔB22R (18), as well as the newly developed v50ΔB13RMγ and v50ΔB22RMγ rVVs, were propagated in HeLa S3 cells and titered in BS-C-1 cells. All rVVs were derived from v50 (21), which has the thymidine kinase (TK) locus insertionally inactivated with vesicular stomatitis virus glycoprotein (VSV-G). The New Jersey serotype of vesicular stomatitis virus (VSV) (22) and encephalomyocarditis virus were propagated and titered in BHK-21 cells.
Animals. Athymic nude BALB/cBy (nu/nu) mice were purchased from The Jackson Laboratory, and female (BALB/c × C57BL/6)F1 normal hybrid mice (CB6F1) were purchased from Harlan–Sprague–Dawley. All animals were maintained according to National Institutes of Health guidelines and animal care protocols approved by the Animal Use and Care Administrative Advisory Committee at the University of California, Davis.
Construction of the VV Transfer Vectors. Standard PCR techniques using Vent DNA polymerase (New England Biolabs) were used in the construction of p2B22RgptMγ and p2B13RgptMγ VV transfer vectors. The murine IFN-γ gene was PCR-amplified from the plasmid pMC14 (courtesy of W. Wood, Genentech) with the primers 5′-CTCCTGCGGCCGAGCTCTGA-3′ and 5′-TAGTGCCGGCCGAATTATTCTTAT-3′. The p2B22RgptMγ transfer vector was generated by digesting the 518-bp murine IFN-γ PCR fragment (flanked by EagI sites, underlined in the primers) with EagI and inserting it into the unique NotI site of p2B22Rgpt (18). The cloned IFN-γ PCR fragment was sequenced with primers 5′GCCTGCAGAGAATTCGTTTA-3′ and 5′AATATGAAAGTGGTGATTGTGA-3′. p2B13RgptMγ was generated by ligating the 500-bp IFN-γ fragment isolated from the plasmid p2B22RgptMγ into the unique NotI site of p2B13Rgpt (18).
Generation of rVVs. The rVVs v50ΔB13RMγ and v50ΔB22RMγ were generated by standard homologous recombination by using cationic liposome-mediated transfection of BS-C-1 cells with the plasmids p2B13RgptMγ and p2B22RgptMγ, respectively. Monolayers were infected at 0.05 plaque-forming units (pfu) per cell with the parental virus (v50), which expresses the glycoprotein of VSV at the TK site (21). Recombinant gpt-positive VVs were plaque purified on BS-C-40 cells from transfection supernatants, by using gpt-selection medium (25 μg/ml mycophenolic acid/250 μg/ml xanthine/15 μg/ml hypoxanthine) (23). Blue plaques were visualized with X-Gal to detect the lacZ marker gene (24). The expression of the lacZ gene by the final plaque-purified rVVs was tested by cytochemical staining of infected cell monolayers as described in ref. 25. The overall genomic structure of each respective rVV was determined by restriction analysis of rVV DNA, which was purified by a small-scale method employing micrococcal nuclease (26).
Viral Growth Curves. Briefly, triplicate monolayers of BS-C-1 and L929 cells were infected at a multiplicity of infection (moi) of 0.01 for 1 h in 12-well plates, and in vitro virus replication was determined as described in refs. 18 and 27.
IFN-γ Bioassays. The ability of the rVVs to express IFN-γ was determined by the prevention of cytopathic effects of encephalomyocarditis virus on murine L929 cells (28). Briefly, nearly confluent BS-C-40 cell monolayers in 24-well plates were infected with the rVVs at a moi of 10 and incubated for 24 h in a final volume of 0.5 ml. Supernatants then were harvested, filtered through 0.2-μm filters, and serially diluted in DMEM with 5% FBS. Subsequently, 50-μl aliquots were placed in 96-well plates, which were seeded 4–6 h previously with 2 × 104 L929 cells per well in 100 μl of DMEM with 5% FBS. After 24 h of incubation, cells were challenged with the minimum dose of encephalomyocarditis virus that gave 100% cytopathic effects and were stained with crystal violet 24 h later. IFN-γ titers (in units per milliliter) were expressed as the reciprocal of the dilution of sample that gave 50% protection against the challenge virus.
Virulence Studies in Immunodeficient Mice. Survival was measured in groups of immunodeficient BALB/cBy nude mice (7- to 8-week-old males) challenged i.p. with 107 pfu of each respective rVV in a final volume of 250 μl of sterile PBS. Animals were examined twice daily.
Clearance Studies in Immunodeficient and Immunocompetent Mice. Groups of BALB/cBy nude mice and CB6F1 normal mice (7- to 8-week-old females) were inoculated i.p. with 107 pfu of each respective rVV in a final volume of 250 μl of sterile PBS. Tissues were removed, weighed, homogenized, and resuspended in DMEM at 10% wt/vol. Next, tissues were lysed by freeze-thawing and trypsinization. Viral titers were determined by plaque assay on BS-C-1 cell monolayers.
Humoral Studies. Groups of 11 CB6F1 mice (6- to 7-week-old females), under light anesthesia, were immunized intramuscularly with 105 pfu of each respective rVV in a final volume of 50 μl of sterile PBS. Animals were boosted i.p. 4 weeks after vaccination with 2 × 105 pfu of VSV (in 100 μl) recovered from VSV-inoculated mouse brains (18). Animals were monitored for 14 days after booster immunization and euthanized at the end of the observation period. Mice were bled at 0, 2, 4, and 6 weeks after infection. Serum samples were pooled for each group. Antibody titers to VSV and VV were determined by ELISA.
VSV and VV ELISAs. To detect VSV- or VV-specific antibodies by using ELISA, 96-well microtiter plates (Nunc) were coated overnight with 100 μl per well of pelleted VSV (1:25,600 dilution) or cytosolic extracts from WR-infected HeLa S3 cells (1:500 dilution) suspended in PBS. These dilutions were determined to give the highest readings with positive control samples and the lowest background readings with naïve serum samples. The HeLa S3 cytosolic extracts (comprised mainly of intracellular mature virion particles) were clarified by low-speed centrifugation to remove cell debris. Plates coated with mock-infected HeLa S3 cytosolic extracts were used as an additional control to rule out immune responses to HeLa S3 cell proteins in sera of vaccinated animals. After overnight incubation at 4°C, plates were washed twice with 50 mM Tris and 0.2% Tween 20 and blocked for 1 h at room temperature with 5% nonfat dry milk in PBS. The plates then were washed twice and incubated for 1 h at room temperature with twofold serial dilutions of pooled sera from each respective rVV-inoculated group. Next, plates were washed twice, and antibody binding was detected with horseradish peroxidase-conjugated goat anti-mouse IgG (heavy and light chain) antiserum (1:3,000 dilution; Bio-Rad). After 1 h, plates were washed three times and reacted with tetramethyl benzidine peroxidase substrate solution. After a 10-min incubation, the reaction was stopped by the addition of 1 M H2SO4, and absorbance was read at 450 nm. Sample dilutions were considered positive if the optical density (OD) recorded for that dilution was at least 2-fold higher than the OD recorded for a control serum sample (naïve CB6F1 mice) at the same dilution. Titers were expressed as the reciprocal of the highest dilution of samples scoring positive.
T Helper Proliferation Studies. Groups of six CB6F1 (H-2b/d) mice (7-week-old females) were immunized i.p. with 107 pfu of each respective rVV in a final volume of 250 μl of sterile PBS. Splenocytes were harvested 10 days after vaccination. Baculovirus-expressed VSV-G (0.5 μg/ml) in complete RPMI medium 1640 supplemented with 50 mM 2-mercaptoethanol was added to 105 splenocytes in flat-bottom 96-well plates. The mitogen Con A at 2 μg/ml served as a positive control, whereas uninfected naïve mouse splenocytes served as a negative control. Splenocytes were incubated for 4 days at 37°C. On the 4th day, [3H]thymidine was added [0.5 μCi/well (1 Ci = 37 GBq)], and cells were incubated for 18 h at 37°C. Cells then were harvested to determine incorporation of radioactivity. Data analysis was based on cpm in triplicates and expressed as a stimulation index. The stimulation index was calculated as (cpm in the presence of the antigen)/(cpm in the control culture).
Cytotoxic T Lymphocyte (CTL) Studies. Splenocytes harvested as described for the proliferation studies mentioned above also were used for our CTL studies. For primary immune responses, effector splenocytes (5 × 104 to 5 × 105 cells) in complete RPMI medium 1640 supplemented with 50 mM 2-mercaptoethanol were stimulated with UV-treated VSV-infected target splenocytes (104) for 4 h in U-bottom 96-well plates. For secondary CTL responses, effector splenocytes (5 × 104 to 5 × 105 cells) were stimulated with UV-treated/γ-irradiated (3,000 Gy), VSV-infected target splenocytes (104 cells) as well as a suboptimal level of IL-2 (100 ng/ml) for 5 days at 37°C. Cytotoxicity was measured with the Cytotox 96 Nonradioactive Cytotoxicity Assay (Promega). For both primary and secondary immune responses, infected L929 cells (H-2k) served as a negative target control. Percent specific cytolysis was calculated as (OD Experimental – Effector Spontaneous – Target Spontaneous)/(OD Target Maximum – Target Spontaneous) × 100.
Data Analysis. Statistical analyses were performed with the statistical software programs sas (Release 8.2, SAS Institute, Cary, NC) and prism (Version 4.0, GraphPad, San Diego).
Results
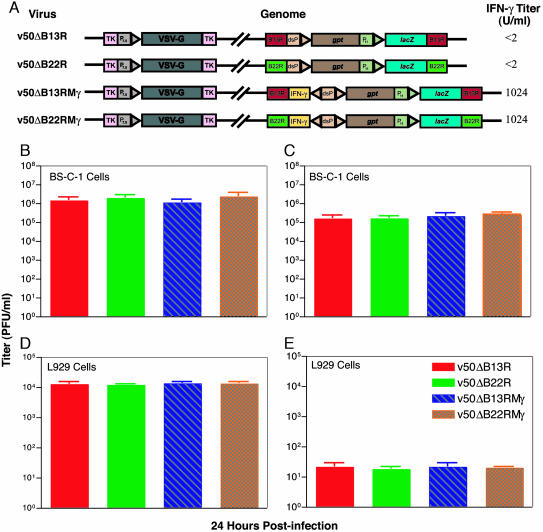
Generation and Characterization of rVVs. We constructed two rVVs (v50ΔB13RMγ and v50ΔB22RMγ) by using the WR strain of the virus expressing the VSV-G within the TK gene of VV and coexpressing the murine IFN-γ gene within the VV B13R or B22R gene (Fig. 1A). The control rVVs, v50ΔB13R and v50ΔB22R, express only VSV-G at the TK locus and have a corresponding deletion in the B13R or B22R gene (18). β-galactosidase cytochemical staining of cell monolayers infected with the rVVs indicated that all of the recombinants were stable and expressed lacZ, thus indicating the absence of v50, the parental strain (data not shown). HindIII restriction analysis of rVV-purified DNA samples confirmed the insertional inactivation of the B13R, B22R, and TK regions with the marker and selection genes (Escherichia coli lacZ and gpt genes), as well as VSV-G and IFN-γ genes (data not shown).
Fig. 1.
rVVs expressing IFN-γ replicate to a high titer in vitro.(A) Schematic representation of rVVs used in this study, including the insertion sites (TK, B13R, and B22R genes), heterologous genes expressed (VSV-G, lacZ, gpt, and murine IFN-γ), and VV promoters used (P7.5, P11, and dsP, the synthetic VV promoters). Numbers to the right refer to average levels of IFN-γ activity in supernatants of BS-C-40 cells 24 h after infection at a moi of 10. (B–E) Monolayers of BS-C-1 (B and C) and L929 (D and E) cells were infected at a moi of 0.01, and, 24 h after infection, both intracellular (B and D) and extracellular (C and E) virus fractions were collected and titered on BS-C-1 cell monolayers. The data shown represent the mean values from triplicate samples assayed in duplicate; error bars indicate SEM.
We constructed the rVVs with the IFN-γ gene cloned specifically under one of the two back-to-back synthetic VV promoters (dsP). This synthetic promoter was chosen because it is active both early and late in infection and provides a heightened, as well as continuous, production of the proteins under its control (29). The expression of murine IFN-γ by rVVs (1,024 units/ml; Fig. 1A) was determined by the prevention of cytopathic effects of encephalomyocarditis virus on murine L929 cells (11). Gene expression studies indicated that IFN-γ production was greatly augmented over time by rVVs under the dsP promoter as compared with rVVs expressing IFN-γ under the natural P7.5 early/late promoter (data not shown).
IFN-γ Coexpression Does Not Affect VV Replication in Vitro. Previously, we have demonstrated that the deletion of the B13R or B22R gene has no effect on virus replication in vitro, although these rVVs are attenuated in vivo (18). In the current study, IFN-γ expression was shown to have no additional effect on the in vitro viral replication kinetics of either B13R- or B22R-deleted rVVs (Fig. 1 B–E). Viral replication of VVs expressing IFN-γ at low moi (0.01) was essentially identical to that of the control viruses in African green monkey BS-C-1 (not responsive to murine IFN-γ) and murine L929 (responsive to murine IFN-γ) cells, for both intracellular and extracellular virus fractions (Fig. 1 B–E), revealing no defects in intracellular mature virion and extracellular enveloped virion formation. Moreover, the plaque phenotypes of the rVVs and their parental strains (v50) were indistinguishable in cell culture (data not shown). In contrast, other highly attenuated VVs, such as modified VV Ankara (MVA) or NYVAC, have reduced or no replication in mammalian cells such as Vero cells, limiting large-scale production for use in mass vaccination.
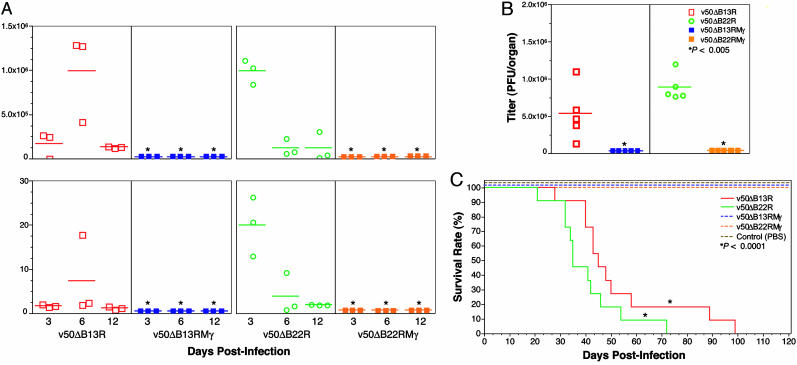
IFN-γ Coexpression Greatly Attenuates VV Virulence in Nude and Normal Mice. The role of IFN-γ expression in reducing VV virulence first was determined by measuring viral clearance. Expression of IFN-γ greatly increased VV clearance, completely blocking VV dissemination in both immunodeficient and immunocompetent mice (Fig. 2 A and B). Female immunodeficient BALB/cBy nude mice were inoculated i.p. with 107 pfu of v50ΔB13R, v50ΔB22R, v50ΔB13RMγ, or v50ΔB22RMγ. The levels of viral replication in ovaries and spleen were quantified by plaque assay (Fig. 2 A); these organs are the major sites of virus replication in mice after i.p. inoculation (10, 18). We previously have shown that B13R or B22R serpin-gene inactivation reduces viral replication levels in vivo (18). Here, we observed that IFN-γ expression by v50ΔB13RMγ and v50ΔB22RMγ appears to cause complete clearance of rVVs (Fig. 2 A) in both ovaries and spleen at 3, 6, and 12 days after infection, because no viral titers could be detected by a standard plaque assay. In contrast, viral titers in tissues of mice inoculated with control viruses v50ΔB13R or v50ΔB22R were as high as 1.3 × 106 pfu per organ (Mann–Whitney test, P < 0.05). We have observed that rVVs expressing murine IFN-γ within the TK site and under the weaker P7.5 promoter could be readily detected in spleens of nude mice 3 days after i.p. inoculation (L.A.J. and T.D.Y., unpublished data). Thus, it appears that the additional deletion of a serpin gene along with the expression of higher levels of IFN-γ has led to this remarkable attenuation, in which no infectious virus was detected in vivo and yet there were undiminished humoral and cellular immune responses.
Fig. 2.
rVVs expressing IFN-γ are attenuated for immunodeficient and immunocompetent mice. (A) Clearance studies in VV-infected immunodeficient mice. No viral replication was observed in ovaries (Upper) and spleen (Lower) of mice inoculated with the rVVs expressing IFN-γ, unlike control viruses (*, P < 0.05, Mann–Whitney test). (B) Pathogenicity studies of VV-infected immunocompetent mice. Viral titers were undetectable in the ovaries of mice inoculated with rVVs v50ΔB13RMγ and v50ΔB22RMγ (*, P < 0.005, Mann–Whitney test). For A and B, viral titers are given as pfu per organ. Bars indicate mean titer per group. (C) Survival studies in BALB/cBy nude mice. Statistical analysis of survival time (log-rank test) yielded P < 0.0001 (*) for v50ΔB13R or v50ΔB22R vs. v50ΔB13RMγ or v50ΔB22RMγ.
We have shown previously that VV replicates to the highest titers in ovaries of CB6F1 mice 3 days after i.p. inoculation (18). We inoculated groups of CB6F1 hybrid mice i.p. with 107 pfu of each respective rVV. Three days after inoculation, mice were killed, and ovaries were removed for virus titration. Again, quite remarkably, VV could not be detected in the ovaries of mice inoculated with v50ΔB13RMγ or v50ΔB22RMγ (Fig. 2B), whereas VV replication could be readily detected (titers as high as 1.2 × 106 pfu per organ) in the ovaries of mice inoculated with the control viruses v50ΔB13R or v50ΔB22R (Mann–Whitney test, P < 0.005). In contrast, a previously developed rVV expressing murine IFN-γ within the TK site and under the weaker P7.5 promoter could be readily detected in ovaries of immunocompetent mice 3 days after i.v. inoculation (10).
Attenuation was further assessed by determining the rate of survival of immunodeficient mice (Fig. 2C). BALB/cBy nude mice were inoculated i.p. with 107 pfu of v50ΔB13R, v50ΔB22R, v50ΔB13RMγ, or v50ΔB22RMγ. All nude mice inoculated with the control viruses with a deletion in either the B13R or B22R gene developed typical pock lesions, showed severe weight loss (data not shown), and died between 21 and 99 days after inoculation (median survival times of 45 and 35 days, respectively); however, these mice survived significantly longer than nude mice inoculated with the parental v50 rVV (18). In contrast, nude mice inoculated with the rVVs expressing IFN-γ (v50ΔB13RMγ or v50ΔB22RMγ) did not exhibit any weight loss or evidence of pock lesion formation and remained healthy throughout the observation period of 120 days, similar to control mice inoculated with sterile PBS (n = 4). Statistical survival analysis between animals inoculated with v50ΔB13RMγ or v50ΔB22RMγ and their respective control groups was highly significant (P < 0.0001, log-rank test).
The remarkable attenuation of v50ΔB13RMγ and v50ΔB22RMγ, observed in vivo (Fig. 2) but not in vitro (L929 cells infected with rVVs at low moi, Fig. 1 D and E), suggests that in vivo IFN-γ expression limits VV replication by a mechanism other than direct induction of an antiviral state in neighboring cells, perhaps by activating cells involved in innate and adaptive immunity such as macrophages, natural killer cells, and T lymphocytes. In any event, it is important to note that this level of attenuation has never been obtained with previous rVVs expressing cytokines, because there was always evidence of virus replication in ovaries and spleens 3 days after inoculation (10, 30, 31).
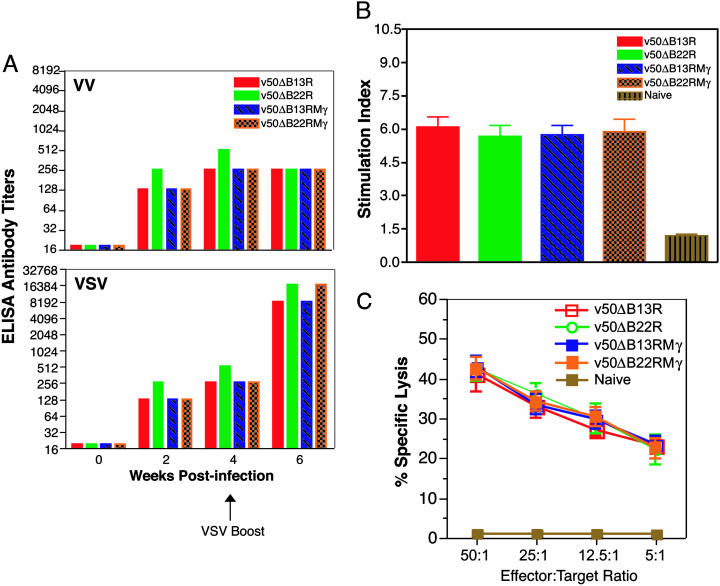
rVVs Coexpressing IFN-γ Induce Potent Immune Responses. We next evaluated the immunogenicity of these avirulent rVVs expressing IFN-γ. Humoral immune responses were determined in groups of normal mice vaccinated intramuscularly, as in previous studies, with 105 pfu of v50ΔB13R, v50ΔB22R, v50ΔB13RMγ, or v50ΔB22RMγ (18). Serum was collected 0, 2, 4, and 6 weeks after infection from each animal, and antibody titers to VV and VSV were determined by ELISA. Although there was a remarkable increase in attenuation as a result of IFN-γ expression and inactivation of a serpin gene, no statistical differences in the humoral immune responses to either homologous (VV) or heterologous (VSV-G) antigens were observed in all vaccinated mice (Fig. 3A). Animals were boosted with VSV 4 weeks after vaccination, and equivalent levels of anamnestic antibody responses to VSV were observed in all vaccinated groups.
Fig. 3.
Potent immune responses are induced by rVVs expressing IFN-γ. (A) IFN-γ expression does not alter humoral responses to rVV. Antibody titers to VV (Upper) and VSV (Lower) were determined by ELISA from pooled samples assayed in duplicate. (B) rVVs coexpressing IFN-γ elicit strong T-helper responses. Splenocytes were harvested 10 days after vaccination and stimulated with 0.5 μg/ml of VSV-G. Data analysis was based on cpm in triplicates and expressed as a stimulation index. Error bars represent SD. (C) rVVs expressing IFN-γ induce potent cytotoxic T cell responses. CTL immune responses were measured 10 days after vaccination in CB6F1 mice inoculated as in B. Effector splenocytes were stimulated with haplotype matched (H-2b/d) γ-irradiated/UV-treated VSV-infected target splenocytes. Specific cytolysis values at the indicated effector/target cell ratios represent the means of triplicate experiments assayed in duplicate. Error bars represent SD.
Cell-mediated immune responses were assessed in groups of normal mice inoculated i.p. with 107 pfu of v50ΔB13R, v50ΔB22R, v50ΔB13RMγ, or v50ΔB22RMγ. The i.p. route is routinely used to measure VV-mediated cellular immune responses (18). Relative T-helper cell proliferative responses to soluble VSV-G protein were measured by the incorporation of [3H]thymidine upon antigenic stimulation with VSV-G and calculated as stimulation index (Fig. 3B). Strong T-helper responses, approximately six times higher than that of naïve controls, were observed for all vaccinated groups. These responses did not differ among the inoculated groups. Finally, specific CTL responses were determined by using the Cytotox 96 Nonradioactive Cytotoxicity Assay (Promega). All rVVs induced potent primary and secondary CTL responses. Primary CTL responses were similar for mice inoculated with B13R and B22R-inactivated recombinants, as well as those expressing IFN-γ, reaching as high as 39% (data not shown). Secondary CTL responses were also strong and similar, nearing 46% (Fig. 3C). Infected haplotype-mismatched L929 cells (H-2k) served as negative target controls, with lysis values of <5% (data not shown).
Discussion
Cytokines have emerged as useful tools for the modulation of immune responses. Specifically, we and others have shown that IFN-γ acts as an effective adjuvant, inducing potent humoral and cell-mediated immune responses in various vaccination regimens (16, 17, 32). IFN-γ plays an integral role in both innate and adaptive immunity (12, 13). These IFN-γ-mediated immune responses to pathogens include the activation of macrophages and the stimulation of specific cytotoxic T cell immunity, as well as the increase in expression of MHC class I and II proteins. We hypothesized that the expression of IFN-γ in an already attenuated serpin-deleted rVV would further augment the safety and immunogenicity of the virus both as a smallpox vaccine and as a vector. To our surprise, we observed an increase in attenuation as a result of IFN-γ expression to the point where no virus could be detected in vivo even though we used a virulent VV strain (WR) to generate these recombinants (Fig. 2). Despite this level of attenuation, humoral and cell-mediated immune responses remained strong (Fig. 3). Despite the undetectable replication of rVVs expressing IFN-γ in vivo, there is apparently a high level of transcription and translation of viral proteins in the infected cells. This amount appears to be sufficient for induction of a strong immune response. Similar events have been observed in the MVA system where there is transcription and translation of viral genes in the absence of production of infectious viruses. Additionally, the strong adjuvant activity of IFN-γ has been shown to enhance antibody responses to viral antigens.
Numerous approaches have been used to enhance the safety of poxviruses. These include the generation of replication-deficient VVs such as MVA (33, 34), nonreplicating defective VV (35), host cell-restricted vectors (avipoxviruses) (1, 36), and poxvirus vectors with deletions in nonessential or host range genes, such as the NYVAC VV with deletions in 18 genes (37). Although these VV vectors have been shown to be safe, they do not replicate efficiently in established mammalian cell culture used for vaccine production, and their limited antigen expression may compromise vaccine efficacy. In fact, MVA frequently requires more than one vaccination to induce optimal levels of immunity (34).
In conclusion, we demonstrated that the deletion of immunomodulating serpin (B13R or B22R) and TK genes, combined with IFN-γ expression, greatly attenuates rVVs to the point that infectious virus was undetectable even in organs where the virus normally replicates to high titers. Despite this level of attenuation, immune responses to a primary immunization were undiminished, thus enhancing safety without compromising efficacy. In addition, unlike other highly attenuated poxviruses such as MVA, ALVAC, and NYVAC, the rVVs described here replicate to high titers in mammalian cell cultures and induce potent humoral and cell-mediated immune responses with a single vaccination. Therefore, our current studies, together with our previous findings (18, 27), indicate that the inactivation of immunomodulating genes, along with the expression of a cytokine such as IFN-γ, can significantly improve the utility of VV as a smallpox vaccine and as a vector for the expression of heterologous antigens. Such rVVs could be administered safely even to immunocompromised individuals, without fear of complications from vaccination.
Acknowledgments
We thank members of the International Laboratory of Molecular Biology for Tropical Disease Agents, especially F. Lin, J. Collins, S. Leung, L. Brown, and C. Tang, for their assistance. We also thank Dr. Bernard Moss from the National Institute of Allergy and Infectious Diseases for providing the back-to-back synthetic VV promoters (dsP). This work was supported by National Institutes of Health Grants AI54951 and AI53811 (to T.D.Y.) and AI59185 (to P.H.V.). F.A.L. was supported by the Floyd and Mary Schwall Fellowship, the Jastro Shields Scholarship, the Peter J. Shields Block Grant, and the University of California Davis Biotechnology Program Fellowship.
Abbreviations: VV, vaccinia virus; rVV, recombinant vaccinia virus; VSV, vesicular stomatitis virus; VSV-G, VSV glycoprotein; TK, thymidine kinase; pfu, plaque-forming units; moi, multiplicity of infection; CTL, cytotoxic T lymphocyte; MVA, modified VV Ankara.
References
- 1.Moss, B. (1996) Proc. Natl. Acad. Sci. USA 93, 11341–11348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Enserink, M. (2003) Science 299, 486–487. [DOI] [PubMed] [Google Scholar]
- 3.Fulginiti, V. A., Papier, A., Lane, J. M., Neff, J. M. & Henderson, D. A. (2003) Clin. Infect. Dis. 37, 251–271. [DOI] [PubMed] [Google Scholar]
- 4.Pastoret, P. P., Brochier, B., Languet, B., Thomas, I., Paquot, A., Bauduin, B., Kieny, M. P., Lecocq, J. P., De Bruyn, J., Costy, F., et al. (1988) Vet. Rec. 123, 481–483. [DOI] [PubMed] [Google Scholar]
- 5.Yilma, T., Hsu, D., Jones, L., Owens, S., Grubman, M., Mebus, C., Yamanaka, M. & Dale, B. (1988) Science 242, 1058–1061. [DOI] [PubMed] [Google Scholar]
- 6.Giavedoni, L., Jones, L., Mebus, C. & Yilma, T. (1991) Proc. Natl. Acad. Sci. USA 88, 8011–8015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Verardi, P. H., Aziz, F. H., Ahmad, S., Jones, L. A., Beyene, B., Ngotho, R. N., Wamwayi, H. M., Yesus, M. G., Egziabher, B. G. & Yilma, T. D. (2002) J. Virol. 76, 484–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pastoret, P. P. & Vanderplasschen, A. (2003) Comp. Immunol. Microbiol. Infect. Dis. 26, 343–355. [DOI] [PubMed] [Google Scholar]
- 9.Yilma, T., Anderson, K., Brechling, K. & Moss, B. (1987) in Vaccines 87: Modern Approaches to New Vaccines including Prevention of AIDS, eds. Chanock, R. M., Ginsberg, H., Lerner, R. & Brown, F. (Cold Spring Harbor Lab. Press, Woodbury, NY), pp. 393–396.
- 10.Kohonen-Corish, M. R., King, N. J., Woodhams, C. E. & Ramshaw, I. A. (1990) Eur. J. Immunol. 20, 157–161. [DOI] [PubMed] [Google Scholar]
- 11.Giavedoni, L. D., Jones, L., Gardner, M. B., Gibson, H. L., Ng, C. T., Barr, P. J. & Yilma, T. (1992) Proc. Natl. Acad. Sci. USA 89, 3409–3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shtrichman, R. & Samuel, C. E. (2001) Curr. Opin. Microbiol. 4, 251–259. [DOI] [PubMed] [Google Scholar]
- 13.Schroder, K., Hertzog, P. J., Ravasi, T. & Hume, D. A. (2004) J. Leukocyte Biol. 75, 163–189. [DOI] [PubMed] [Google Scholar]
- 14.Muller, U., Steinhoff, U., Reis, L. F., Hemmi, S., Pavlovic, J., Zinkernagel, R. M. & Aguet, M. (1994) Science 264, 1918–1921. [DOI] [PubMed] [Google Scholar]
- 15.Binder, G. K. & Griffin, D. E. (2001) Science 293, 303–306. [DOI] [PubMed] [Google Scholar]
- 16.Anderson, K. P., Fennie, E. H. & Yilma, T. (1988) J. Immunol. 140, 3599–3604. [PubMed] [Google Scholar]
- 17.Nakamura, M., Manser, T., Pearson, G. D., Daley, M. J. & Gefter, M. L. (1984) Nature 307, 381–382. [DOI] [PubMed] [Google Scholar]
- 18.Legrand, F. A., Verardi, P. H., Jones, L. A., Chan, K. S., Peng, Y. & Yilma, T. D. (2004) J. Virol. 78, 2770–2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Macen, J. L., Garner, R. S., Musy, P. Y., Brooks, M. A., Turner, P. C., Moyer, R. W., McFadden, G. & Bleackley, R. C. (1996) Proc. Natl. Acad. Sci. USA 93, 9108–9113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moon, K. B., Turner, P. C. & Moyer, R. W. (1999) J. Virol. 73, 8999–9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mackett, M., Yilma, T., Rose, J. K. & Moss, B. (1985) Science 227, 433–435. [DOI] [PubMed] [Google Scholar]
- 22.Yilma, T., Breeze, R. G., Ristow, S., Gorham, J. R. & Leib, S. R. (1985) Adv. Exp. Med. Biol. 185, 101–115. [DOI] [PubMed] [Google Scholar]
- 23.Falkner, F. G. & Moss, B. (1988) J. Virol. 62, 1849–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Earl, P. L. & Moss, B. (1994) in Current Protocols in Molecular Biology, eds. Ausubel, F. M., Brent, R., Kingston, R. E., Moore, D. D., Seidman, J. G., Smith, J. A. & Struhl, K. (Wiley, New York), Vol. 2, pp. 16.17.1–16.17.16. [Google Scholar]
- 25.MacGregor, G. R., Nolan, G. P., Fiering, S., Roederer, M. & Herzenberg, L. A. (1991) in Gene Transfer and Expression Protocols, ed. Murray, E. J. (Humana, Clifton, NJ), Vol. 7, pp. 217–35. [DOI] [PubMed] [Google Scholar]
- 26.Lai, A. C. & Chu, Y. (1991) BioTechniques 10, 564–565. [PubMed] [Google Scholar]
- 27.Verardi, P. H., Jones, L. A., Aziz, F. H., Ahmad, S. & Yilma, T. D. (2001) J. Virol. 75, 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubinstein, S., Familletti, P. C. & Pestka, S. (1981) J. Virol. 37, 755–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chakrabarti, S., Sisler, J. R. & Moss, B. (1997) BioTechniques 23, 1094–1097. [DOI] [PubMed] [Google Scholar]
- 30.Flexner, C., Hugin, A. & Moss, B. (1987) Nature 330, 259–262. [DOI] [PubMed] [Google Scholar]
- 31.Ramshaw, I. A., Andrew, M. E., Phillips, S. M., Boyle, D. B. & Coupar, B. E. (1987) Nature 329, 545–546. [DOI] [PubMed] [Google Scholar]
- 32.Schijns, V. E., Claassen, I. J., Vermeulen, A. A., Horzinek, M. C. & Osterhaus, A. D. (1994) J. Gen. Virol. 75, 55–63. [DOI] [PubMed] [Google Scholar]
- 33.Wyatt, L. S., Earl, P. L., Eller, L. A. & Moss, B. (2004) Proc. Natl. Acad. Sci. USA 101, 4590–4595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Earl, P. L., Americo, J. L., Wyatt, L. S., Eller, L. A., Whitbeck, J. C., Cohen, G. H., Eisenberg, R. J., Hartmann, C. J., Jackson, D. L., Kulesh, D. A., et al. (2004) Nature 428, 182–185. [DOI] [PubMed] [Google Scholar]
- 35.Ober, B. T., Bruhl, P., Schmidt, M., Wieser, V., Gritschenberger, W., Coulibaly, S., Savidis-Dacho, H., Gerencer, M. & Falkner, F. G. (2002) J. Virol. 76, 7713–7723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Taylor, J., Weinberg, R., Tartaglia, J., Richardson, C., Alkhatib, G., Briedis, D., Appel, M., Norton, E. & Paoletti, E. (1992) Virology 187, 321–328. [DOI] [PubMed] [Google Scholar]
- 37.Tartaglia, J., Perkus, M. E., Taylor, J., Norton, E. K., Audonnet, J. C., Cox, W. I., Davis, S. W., van der Hoeven, J., Meignier, B., Riviere, M., et al. (1992) Virology 188, 217–232. [DOI] [PubMed] [Google Scholar]