Abstract
The objective of this multicentre, randomized, controlled field study was to determine the efficacy of ketanserin gel in preventing exuberant granulation tissue formation (hypergranulation) and infection in equine lower limb wounds. Horses and ponies (n = 481) with naturally occurring wounds were randomized to either topical treatment with ketanserin gel (n = 242) or a positive control (Belgium, Germany: ethacridin lactate solution, n = 120; France, United Kingdom: malic, benzoic, and salicylic acid [MBS] cream, n = 119). Treatment continued until the wound healed (success), formed hypergranulation tissue (failure), or became infected (failure). Treatment was terminated after 6 months in all remaining animals.
Ketanserin was successful in 88% of cases. Wounds treated with ketanserin were 2 and 5 times more likely to heal successfully than were those treated with MBS or ethacridin lactate, respectively. Ketanserin gel is thus more effective than these standard treatments in preventing hypergranulation tissue and infection of equine lower limb wounds.
Abstract
Résumé — Les effets d’un gel de kétanserin sur la guérison de plaies situées à l’aspect distal du membre équin. L’objectif de cette étude de terrain multicentrique, était de déterminer l’efficacité d’un gel de kétanserin dans la prévention de l’hypergranulation et l’infection de plaies appendiculaires équines. Des chevaux et des poneys (n = 481) ayant des plaies traumatiques furent placés de façon aléatoire dans deux groupes. Les animaux du premier groupe reçurent un traitement topique de gel de kétanserin (n = 242) alors que ceux du deuxième groupe reçurent un contrôle positif (Belgique, Allemagne : solution lactate d’éthacridine, n = 120; France, Royaume Uni : onguent d’acides malique, benzoïque et salicylique [MBS], n = 119). Le traitement fut poursuivi jusqu’à guérison (succès), hypergranulation (échec), ou infection (échec) de la plaie.
Le gel de kétanserin fut un succès dans 88 % des cas. Les plaies soignées avec ce traitement topique furent 2 et 5 fois plus aptes à guérir de façon convenable que celles recevant le MBS ou le lactate d’éthacridine. Il est donc apparent que le gel de kétanserin est un traitement efficace pour la prévention d’hypergranulation et d’infection de plaies à l’aspect distal du membre équin.
(Traduit par Docteure Christine Théorêt)
Introduction
Healing of equine lower limb wounds is frequently impaired by formation of excessive granulation tissue (hypergranulation or “proud flesh”; 1). This exuberant tissue may halt healing by impeding epithelialization and wound contraction (2). Hypergranulation may also occur as a secondary event, when epithelialization and wound contraction are arrested by other factors (1,2). Once formed, excessive granulation tissue predisposes the wound to infection and further trauma (2), and surgical resection is often necessary to allow healing to progress (2).
The pathophysiology of delayed healing in equine lower limb wounds is poorly understood, but slow fibroblast growth (3,4) and changes in the expression of transforming growth factor-beta (TGF-β) (5–7) may contribute. Factors that promote hypergranulation tissue include large body size, wound location (lower limb wounds are predisposed), motion, and bandaging (1,2,8). Tissue hypoperfusion, infection, and trauma also predispose to hypergranulation tissue by impairing the speed of healing (1,8).
Numerous treatments have been advocated for promotion of wound healing and prevention of hypergranulation tissue in horses, but the majority of these are of unproven efficacy. Recent controlled studies investigating the efficacy of a variety of topical and systemic treatments (25% propylene glycol hydrogel, recombinant human TGF-β1, and recombinant equine growth hormone) have also failed to demonstrate a beneficial effect on equine wound healing (9–11). This paper presents the results of a multicentre, randomized, controlled field trial that compared the efficacy of topical ketanserin gel and 2 control products in promoting healing of equine lower limb wounds. Ketanserin is a potent serotonin (5-hydroxytryptamine [5-HT2])-receptor antagonist with moderate affinity for histamine H1, α1-adrenergic, and (5-HT1c) receptors (12). Ketanserin’s efficacy as a peripheral vasodilator led to its use as an antihypertensive drug (13). More recently, ketanserin has been used to treat chronic wounds in humans (14–17). Addition of ketanserin ointment to the usual care regimen of patients with decubitus, venous, and ischemic skin ulcers resulted in a 150% increase in the initial velocity of wound closure, more rapid and extensive formation of healthy granulation and epithelial tissues, and faster reductions in wound surface area (14). These beneficial results are attributed to ketanserin’s effects on the microcirculation, wound debridement, and fibroblast function (12). The efficacy of ketanserin gel in promoting equine wound healing was previously evaluated in an open-label field trial involving 91 horses (18). The majority of the wounds (74%) were on the limbs. All infections present at the start of the study resolved during treatment, and no new infections occurred (18). Moderate hypergranulation tissue occurred in 5.5% of wounds.
Materials and methods
Animals
The study involved 481 horses that were presented to 44 practitioners in 26 veterinary practices in Belgium (n = 5 practices), France (n = 9), Germany (n = 6), and the United Kingdom (UK) (n = 6). The study population included 253 (53%) animals classified as “riding horses,” 85 (18%) Thoroughbreds, 45 (9%) trotters, and 38 (8%) ponies. A variety of breeds were represented by the remaining 60 animals. Animals ranged in weight from 50 to 700 kg, and in age from 15 d to 30 y.
Animals were eligible if they had a single full-thickness skin wound on or below the carpus or tarsus. Deeper soft tissue wounds were also eligible. There was no restriction on the age of the wound. All foreign bodies, sequestrae, and hypergranulation tissue were removed surgically prior to enrollment. Animals were excluded if they had 2 or more unconnected wounds on the lower limbs, if the wound involved a joint penetration, if the animal required systemic antibiotics, or if the wound had been treated medically for prevention of hypergranulation tissue within the previous 2 wk. The application of topical antibacterial preparations in conjunction with the test products was not allowed during the trial. All owners gave written informed consent before enrollment. The study was conducted under conditions of Good Clinical Practice (European Union guideline on the Good Clinical Practice for the Conduct of Clinical Trials for Veterinary Medicinal Products).
Test products
Participating animals were randomized within each practice to ketanserin or the control. Since no single product was approved for topical use on equine wounds in all 4 countries, 2 reference products were used. In Belgium and Germany, the control preparation was ethacridin lactate (Rivanol; ASID Veterinär Vertriebs GmbH, D-85716 Unterschleissheim, Germany). This topical antiseptic is dissolved in water (500 mg/L) before application. In France and the UK, the control preparation was a cream containing malic, benzoic, and salicylic acids (MBS) (Dermaflon Crème Elevage; Pfizer Animal Health, Orsay, France; Dermisol cream; Pfizer Animal Health, Sandwich, UK). Ketanserin was supplied as a gel containing 2.5 mg ketanserin tartrate per mL (Vulketan gel; Janssen Animal Health, Toronto, Ontario). Veterinarians were blinded to the treatment allocation of each horse until initial treatment administration.
Treatment
Treatment was performed by the animals’ owners after demonstration of the correct technique by the veterinarian and provision of written instructions to the owner. Wounds that had been treated surgically (foreign body removal) were not treated topically until all postsurgical hemorrhage had stopped. Each product was applied q12h to the wound surface and surrounding skin after it had been washed with clean water from a hosepipe or rinsed with water and a soft cloth or sponge. At no time were the wounds dressed. Treatment continued until 1 of 4 events occurred: i) the wound healed (became completely covered with epithelium); ii) the wound hypergranulated; iii) the wound); became infected; iv) 6 mo elapsed from study enrollment. Any animal that sustained a 2nd wound at the same site as the original wound was withdrawn.
Assessment of efficacy
The first day of treatment was designated day 0 for each animal. The wound was assessed at this time and, thereafter, within ± 1 d of days 3, 7, 14, and 21, and within ± 5 d of days 35, 49, 80, 110, 140, 170, and 180. These assessments were designated visits 1 through 12. Sequential observations on each animal were made by the same veterinarian. At each visit, the wound was measured and assessed for the presence of infection, normal granulation tissue formation, and hypergranulation tissue. Visits were discontinued once the animal was no longer receiving treatment.
Granulation tissue was classified as exuberant (hypergranulation), if it protruded ≥ 3 mm above the skin surface at any point around the edge of the wound. A wound was classified as infected, if it showed clinical signs of inflammation and if a stained impression smear showed abundant bacteria and neutrophils. Wound size was assessed by using 2 linear measurements: “wound length” (maximum length of the wound in any direction) and “wound width” (maximum width of the wound perpendicular to its length). These measurements were multiplied to estimate wound area. The presence or absence of normal granulation tissue was recorded on a 3-point discontinuous scale (absent, wound partially filled, wound completely filled) and the exercise regimen of the horse was recorded as “stabled,” “on pasture,” or “back in exercise.” All adverse events and concomitant treatments were recorded.
The primary efficacy parameter was the presence or absence of hypergranulation tissue or infection. Any animal that developed hypergranulation tissue or a confirmed wound infection requiring antiinfectious treatment was classified as a treatment failure. Animals that failed to complete the study were also classified as failures. A wound was classified as a treatment success a) if it healed completely during the course of the study or b) if it had not healed by the end of the study but showed neither hypergranulation tissue nor an infection requiring antiinfectious treatment at the final visit. The results were thus analyzed on an intention-to-treat basis. Wound size, formation of normal granulation tissue, and the speed with which animals resumed their normal exercise regime were secondary efficacy parameters.
Statistical analyses
All tests were 2-tailed and results were deemed significant if P ≤ 0.05. The analysis was carried out in a blind manner using software (SAS system for information delivery, version 6.12; SAS Institute, Cary, North Carolina, USA).
Assessment of efficacy
Equivalence of the treatment groups at the start of treatment was assessed by comparing wound and patient characteristics with the use of the asymptotic Pearson chi-square test, Fisher’s exact test, the Kruskal-Wallis test, or Student’s t-test, as appropriate.
Univariate analyses were performed to determine whether there was an association between each variable and outcome (success or failure) when the asymptotic Pearson chi-square test, Fisher’s exact test, or the Kruskal-Wallis test were used. All variables whose distribution among treatment groups was associated with a P-value of < 0.20 were included in these tests. The effect of treatment group on outcome was also analyzed.
Any factor associated with a P-value < 0.20 in the univariate analyses was entered as a covariable into a univariate regression model and examined by using Wald’s test. Factors that remained significant were included as main effects in a final multivariate logistic regression analysis that compared the efficacy of ketanserin with that of the reference products in achieving treatment success.
Secondary efficacy parameters were assessed by using a descriptive approach. For each visit, wound size and the number of animals achieving each of the 3 possible outcomes of normal granulation tissue formation and exercise regime were calculated.
Assessment of safety
The frequency of adverse events deemed possibly or probably related to treatment was compared among the 3 groups by using the asymptotic Pearson chi-square test. All horses exposed to treatment were included in this analysis.
Results
Primary efficacy parameter
A successful outcome was achieved in 88.0% of the ketanserin group (n = 213), 80.7% of the MBS group (n = 96), and 58.3% of the ethacridin lactate group (n = 70). Hypergranulation tissue formed in 4% (n = 10) of the ketanserin group, 9% (n = 11) of the MBS group, and 27% (n = 32) of the ethacridin lactate group. By visit 6 (day 35), infection requiring antiinfectious treatment had occurred in 9 (3.7%) ketanserin-treated animals, 3 (2.5%) MBS-treated animals, and 16 (13.3%) ethacridin lactate-treated animals. Between visit 7 and the end din of the study, only 2 additional horses (1 in each of the ketanserin and ethacridin lactate groups) developed infections requiring treatment. The overall infection rate was thus substantially lower for ketanserin (4.1%) and MBS (2.5%) than for ethacridin lactate (14.2%).
By the time of the final visit (day 180), only 2 wounds (ketanserin: n = 1, 0.4%; MBS: n = 1, 0.8%) had not epithelialized. The number of wounds classified as treatment successes that had not fully healed is thus small. Six animals in the ketanserin group, 6 in the MBS group, and 1 in the ethacridin lactate group were withdrawn from the study for reasons unrelated to efficacy. These animals were designated as failures.
A number of wound and patient signalment characteristics showed uneven distribution among the 3 groups at the start of the study (Table 1). Category of animal (P = 0.001), = 0.001), bodyweight (P = 0.02), age of wound (= 0.02), age of wound (P = 0.001), prior = 0.001), prior nonsurgical (P = 0.001) and surgical (= 0.001) and surgical (P = 0.002) treatment, = 0.002) treatment, and limb affected (left versus right, P = 0.008) significantly different among the groups. Univariate analyses showed that, of these 6 factors, bodyweight and prior surgery were of potential prognostic significance (P = 0.15 = 0.15 and 0.013, respectively). Treatment group also significantly affected outcome (P < 0.001). Treatment group, body-weight, and prior surgery were therefore entered as covariables into the univariate regression model. This analysis showed no significant association between bodyweight and outcome. The multivariate logistic regression model thus contained only treatment group and prior surgery as main effects.
Table 1.
Wound and patient signalment characteristics in the 3 treatment groups at study entry
| Treatment | |||||
|---|---|---|---|---|---|
| Variable | Ketanserin | MBS | Ethacridin lactate | P-valuea | |
| Category | 0.001 | ||||
| Riding horse | 53% | 33% | 72% | ||
| Trotter or Throughbred | 27% | 48% | 7% | ||
| Pony | 7% | 12% | 5% | ||
| Other | 13% | 8% | 17% | ||
| Gender | NS | ||||
| Bodyweight (kg) | 418 | 417 | 454 | 0.02 | |
| Age of animal (years) | 5.9 | 6.5 | 6.2 | NS | |
| Age of wound (days) | 8.2 | 8.6 | 6.0 | 0.001 | |
| Wound size (cm2) | 28.9 | 27.5 | 24.6 | NS | |
| Wound had received non-surgical treatment prior to study | 41% | 50% | 24% | 0.001 | |
| Wound had received surgical treatment prior to study | 21% | 7% | 21% | 0.002 | |
| Limb affected (fore versus [vs] hind) | 41% vs 59% | 45% vs 55% | 38% vs 62% | NS | |
| Limb affected (left vs right) | 43% vs 57% | 61% vs 39% | 46% vs 54% | 0.008 | |
| Location of wound | NS | ||||
| Carpur/tarsus | 23% | 26% | 24% | ||
| Metacarpus/metatarsus | 49% | 45% | 47% | ||
| Foot | 28% | 29% | 29% | ||
| Circumferential location of wound | NS | ||||
MBS — malic, benzoic, and salicylic acids; NS — not significant (P > 0.2)
The table shows mean values or percentages, as indicated.
aDifferences among groups were examined using the asymptotic Pearson Chi-square test, the Kruskal-Wallis test, or Fisher’s exact test
Surgical treatment had occurred in 21% of both the ketanserin and ethacridin lactate groups, but in only 7% of the MBS group, prior to enrollment. Since prior surgery approximately halved the chance of a successful outcome (odds ratio for comparison of prior surgery with no surgery = 0.48, 95% confidence interval 0.27 to 0.86, P = 0.013), this imbalance among treatment groups affected the raw success figures. The final regression analysis, which took prior surgical treatment into account, showed that MBS and ethacridin lactate were significantly less effective than ketanserin (Table 2). The probability of success in MBS-treated animals was exactly half that in ketanserin-treated animals (odds ratio for comparison of MBS with ketanserin = 0.50, 95% confidence interval 0.27 to 0.92, P = 0.03). Animals treated with ethacridin lactate also had a significantly lower probability of success than did those treated with ketanserin (odds ratio for comparison with ketanserin = 0.19, 95% confidence interval 0.11 to 0.32, P < 0.001). By visit 8 (day 79), ketanserin was associated with a greater cumulative percentage of treatment successes than either MBS or ethacridin lactate. This advantage was maintained until the end of the trial (Figure 1). Ketanserin was associated with fewer cases of hypergranulation tissue than either MBS or ethacridin lactate (Figure 2A). Infection occurred in a substantially greater percentage of wounds treated with ethacridin lactate than in those treated with ketanserin or MBS (Figure 2B).
Table 2.
Results of the final multivariate logistic regression model
| Covariable | Odds ratio | 95% CI | P-valuea |
|---|---|---|---|
| Treatment | |||
| Ketanserinb | 1.00 | ||
| MBS | 0.50 | 0.27, 0.92 | 0.03 |
| Ethacridin lactate | 0.19 | 0.11, 0.32 | <0.001 |
| Surgery | |||
| Nob | 1.00 | ||
| Yes | 0.48 | 0.27, 0.86 | 0.013 |
MBS — malic, benzoic, and salicylic acids
aStatistical comparisons were performed using Wald’s test
bReference group for calculation of odds ratio, 95% confidence interval (CI), and P-value
Figure 1.

Cumulative treatment successes in wounds treated with ketanserin; malic, benzoic, and salicylic acid (MBS); and ethacridin lactate over the 6-month period of the study. For each visit, the data are presented as a percentage of the total number of animals in the group.
Figure 2.
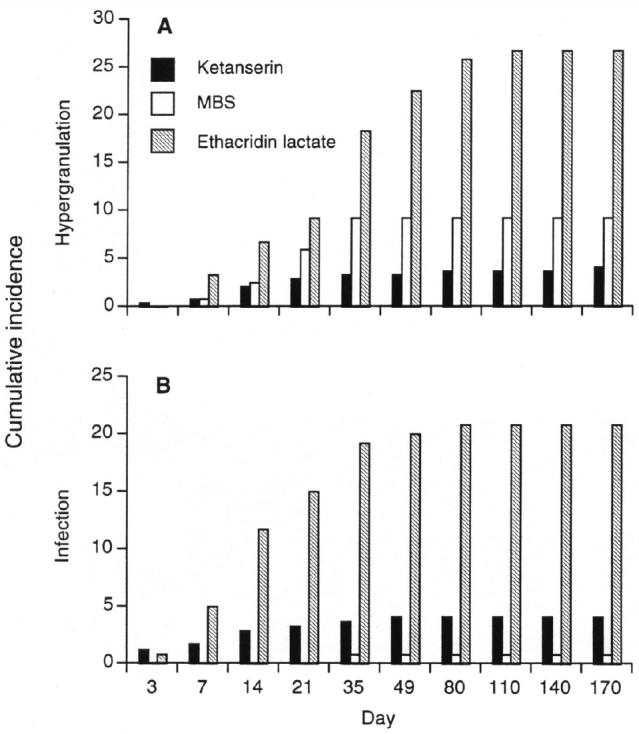
Cumulative occurrence (as a percentage of the total number of horses entering the trial) of hypergranulation tissues (A) and infection (B) in wounds treated with ketanserin; malic, benzoic, and salicylic acid (MBS); and ethacridin lactate during the first 11 visits (170 d) of the study. The data are presented as a percentage of the total number of animals in the group.
Secondary efficacy parameters
The speed with which normal granulation tissue formed did not differ substantially among the groups (data not shown). The percentage of wounds undergoing treatment that were fully filled with normal granulation tissue at visit 2 (day 3) was 21% in all 3 groups. This percentage rose steadily in all groups with increasing time after injury, ranging from 68% to 80% at visit 5 (day 21). The percentage of the animals remaining in the study that had returned to their normal exercise regimen also rose steadily during the study in all 3 treatment groups (data not shown).
There was substantial variation in wound size at each visit in all 3 groups, and the evolution of this parameter did not appear to differ among the groups (data not shown). At visit 4 (day 14), the mean wound sizes in the ketanserin, MBS, and ethacridin lactate groups were 21.2 cm2, 22.2 cm2, and 20.7 cm2, respectively. By visit 8 (day 80), these values had changed to 4.6 cm2 (ketanserin), 5.6 cm2 (MBS), and 11.8 cm2 (ethacridin lactate).
Safety
Six animals sustained adverse events that could be classified as possibly related to treatment (lymphangitis, n = 3; limb swelling, n = 3). Three of the affected animals were in the ketanserin-treated group (1.2%), and 3 in the MBS-treated group (2.5%). No adverse events were classified as probably related to treatment. The frequency of adverse events was not significantly different among the 3 groups.
Discussion
This study has shown that ketanserin gel is significantly more effective than 2 standard positive control treatments in preventing hypergranulation and infection in equine lower limb wounds. Wounds treated with ketanserin were 2 and 5 times more likely to heal successfully than those treated with MBS and ethacridin lactate, respectively. The success rate in ponies did not differ from that of other breed categories.
Ketanserin achieved a successful outcome in 88% (n = 213) of cases, and hypergranulation occurred in only 4% (n = 10). However, 21% of the ketanserin-treated wounds had been treated surgically prior to randomization. Since there was a strong association between prior surgical treatment and poor outcome (odds ratio for success, 0.48), it is likely that ketanserin’s success rate would have been higher had a smaller percentage of the group received surgical treatment. Figure 2 suggests that ketanserin was the most successful of the 3 treatments in reducing the occurrence of hypergranulation tissue, whereas the occurrence of wound infection was lowest in the MBS-treated animals. The low occurrence of infection in the MBS-treated animals may have been influenced by the relatively low occurrence of prior surgical treatment in this group. However, the analysis did not allow this relationship to be examined.
Wounds that have formed exuberant granulation tissue once often do so repeatedly, and multiple resections may be required before healing is achieved (19,20). Since prior surgery is strongly associated with poor outcome, the chance of achieving successful healing will be maximized if hypergranulation tissue never forms. Early institution of treatment with ketanserin may thus substantially reduce the number of equine lower limb wounds that require treatment for infection or hypergranulation tissue before successful healing is achieved.
Previous studies have shown that ketanserin promotes healing in a wide variety of wound types (14,15). When added to the usual care regimen of human patients with venous ulcers, topical application of ketanserin significantly accelerated granulation, thereby facilitating more rapid epithelialization (15). Topical ketanserin also promotes granulation and epithelialization of decubitus and ischemic skin ulcers (14).
Ketanserin’s beneficial effects on healing are attributed to its multiple effects on the microcirculation, wound debridement, and formation of granulation tissue. Wound healing is classically divided into 4 overlapping periods, designated as the phases of inflammation, debridement, repair, and maturation (8). Macrophages are essential for success during the debridement phase (8), but macrophage activation may be suppressed by the levels of 5-HT present in the wound at this time (21). Ketanserin antagonizes this 5-HT–induced suppression of macrophage activation (21). Ketanserin also reverses 5-HT–induced inhibition of endothelial cell migration (22) and stimulates fibroblast and endothelial cell proliferation (23). In addition, ketanserin promotes vasodilation, inhibits microthrombus formation, and reduces local tissue ischemia, all of which improve the local microcirculation (12). Ketanserin thus promotes many of the processes that are important during the repair phase of healing, when migration and activity of fibroblasts and establishment of an adequate blood supply are important. Ketanserin’s effects on macrophage function and the microcirculation may also facilitate the control of infection.
European Union guidelines require that, where possible, new treatments be compared with positive control products that have a licensed claim for the indication under investigation. Topical corticosteroid preparations, which are preferred by some practitioners for the control of hypergranulation tissue in horses, are not universally licensed for this use, and it is debatable whether any steroid-induced reduction in granulation tissue formation compensates for the negative effects that these agents have on wound healing (2). The positive control treatments with which ketanserin was compared in this study have both been shown to encourage wound healing in species other than horses. Ethacridin lactate is an antiseptic (24), whereas MBS is described as a “desloughing” agent with no inherent antibacterial activity (25, Dermisol data sheet). In dogs, naturally occurring wounds treated with ethacridin lactate healed more quickly than those treated with water irrigation (24). Ethacridin lactate has also demonstrated selective antibacterial activity when used in the management of canine wounds (24). Its antibacterial spectrum is not optimal for the treatment of equine wounds (24), however, and the results of the current experiment demonstrate the inability of this agent to prevent infection in this species (Figure 2B). The product MBS has shown efficacy in the treatment of burns and ulcerative skin lesions in humans (25,26). To our knowledge, the efficacy of these agents in promoting healing of equine lower limb wounds has not previously been demonstrated.
The field conditions under which this trial was performed imposed a number of constraints on the study design. Among these were the necessity for “unblinding” of the veterinarians after treatment allocation. The possibility that this biased the results is refuted by the raw data, which show that the rates of infection and hypergranulation tissue were lowest in different treatment groups (Figure 2). The choice of assessment methods was also constrained by the study design. For example, wound infection was assessed by using a clinical score rather than biopsy. Biopsies would have yielded continuous data but would have carried the possibility of modifying the outcome (by increasing the risk of infection), since each biopsy would constitute a fresh insult to the wound. Biopsies would also have been ethically questionable, particularly in cases where multiple repetitions were required. The use of simple measurement methods may also have limited the ability of the study to distinguish among the 3 treatments’ effects on secondary efficacy parameters (wound size, speed of normal granulation tissue formation, and speed of return to normal exercise regime).
When equine lower limb wounds heal by second intention, hypergranulation tissue and delayed closure are common (1). The contamination of many equine wounds, trauma sustained during wounding, and the relatively poor blood supply to the distal extremities further predispose to infection, hypergranulation tissue, and delayed healing (1,2,27). An effective method of preventing this outcome is needed. Ketanserin has previously been shown to promote the formation of healthy granulation tissue and increase the rate of healing in human wounds (14). This study has shown that topical application of ketanserin gel is highly effective in preventing hypergranulation tissue and infection in equine lower limb wounds and that it is superior to 2 current treatments in this regard.
Footnotes
This study was supported by Janssen Animal Health, Beerse, Belgium.
References
- 1.Bertone AL. Principles of wound healing. In: Booth LC, ed. Wound Management. Vet Clin North Am Equine Pract. Philadelphia: WB Saunders Company, 1989;5:449–463. [DOI] [PubMed]
- 2.Bertone AL. Management of exuberant granulation tissue. In: Booth LC, ed. Wound management. Vet Clin North Am Equine Pract. Philadelphia: WB Saunders Company, 1989;5:551–562. [DOI] [PubMed]
- 3.Miller CB, Wilson DA, Keegan KG, Kreeger JM, Adelstein EH, Ganjam VK. Growth characteristics of fibroblasts isolated from the trunk and distal aspect of the limb of horses and ponies. Vet Surg. 2000;29:1–7. doi: 10.1111/j.1532-950x.2000.00001.x. [DOI] [PubMed] [Google Scholar]
- 4.Wilmink JM, Nederbragt H, van Weeren PR, Stolk PW, Barneveld A. Differences in wound contraction between horses and ponies: the in vitro contraction capacity of fibroblasts. Equine Vet J. 2001;33:499–505. doi: 10.2746/042516401776254817. [DOI] [PubMed] [Google Scholar]
- 5.Theoret CL, Barber SM, Moyana TN, Gordon JR. Expression of transforming growth factor beta(1), beta(3), and basic fibroblast growth factor in full-thickness skin wounds of equine limbs and thorax. Vet Surg. 2001;30:269–277. doi: 10.1053/jvet.2001.23341. [DOI] [PubMed] [Google Scholar]
- 6.Theoret CL, Barber SM, Moyana TN, Gordon JR. Preliminary observations on expression of transforming growth factors beta 1 and beta 3 in equine full-thickness skin wounds healing normally or with exuberant granulation tissue. Vet Surg. 2002;31:266–273. doi: 10.1053/jvet.2002.32394. [DOI] [PubMed] [Google Scholar]
- 7.Theoret CL, Barber SM, Gordon JR. Temporal localization of immunoreactive transforming growth factor beta1 in normal equine skin and in full-thickness dermal wounds. Vet Surg. 2002;31:274–280. doi: 10.1053/jvet.2002.32397. [DOI] [PubMed] [Google Scholar]
- 8.Stashak TS. Principles of wound healing. In: Stashak TS, ed. Equine Wound Management. Philadelphia: Lea and Febiger, 1991:1–18.
- 9.Steel CM, Robertson ID, Thomas J, Yovich JV. Effect of topical rh-TGF-beta 1 on second intention wound healing in horses. Aust Vet J. 1999;77:734–737. doi: 10.1111/j.1751-0813.1999.tb12916.x. [DOI] [PubMed] [Google Scholar]
- 10.Dart AJ, Cries L, Jeffcott LB, Hodgson DR, Rose RJ. Effects of 25% propylene glycol hydrogel (Solugel) on second intention wound healing in horses. Vet Surg. 2002;31:309–313. doi: 10.1053/jvet.2002.33585. [DOI] [PubMed] [Google Scholar]
- 11.Dart AJ, Cries L, Jeffcott LB, Hodgson DR, Rose RJ. The effect of equine recombinant growth hormone on second intention wound healing in horses. Vet Surg. 2002;31:314–319. doi: 10.1053/jvet.2002.33589. [DOI] [PubMed] [Google Scholar]
- 12.Rooman RP, Janssen H. Ketanserin promotes wound healing: clinical and preclinical results. Proc 3rd Int Symp Tissue Repair, 1990. 1991:115–128. [PubMed]
- 13.Symoens J. Ketanserin: a novel cardiovascular drug. Blood Coagul Fibrinolysis. 1990;1:219–224. [PubMed] [Google Scholar]
- 14.Janssen PAJ, Janssen H, Cauwenbergh G, et al. Use of topical ketanserin in the treatment of skin ulcers: A double-blind study. J Am Acad Dermatol. 1989;21:85–90. doi: 10.1016/s0190-9622(89)70153-5. [DOI] [PubMed] [Google Scholar]
- 15.Roelens P. Double-blind placebo-controlled study with topical 2% ketanserin ointment in the treatment of venous ulcers. Dermatologica. 1989;178:98–102. doi: 10.1159/000248400. [DOI] [PubMed] [Google Scholar]
- 16.Apelqvist J, Castenfors J, Larsson J, Stenström A, Persson, G Ketanserin in the treatment of diabetic foot ulcer with severe peripheral vascular disease. Int Angiol. 1990;9:120–124. [PubMed] [Google Scholar]
- 17.Martinez-de Jesus FR, Morales-Guzman M, Castañeda M, Perez- Morales A, Garcia-Alonso J, Mendiola-Segura I. Randomized single-blind trial of topical ketanserin for healing acceleration of diabetic foot ulcers. Arch Med Res. 1996;27:95–99. [PubMed] [Google Scholar]
- 18.Ooms LAA, Vlaminck K, Dony J, Desplenter L, Marsboom R. Wound healing in horses: Experience with ketanserin, an S2 antagonist. Proc 4th Congr Europ Assoc Vet Pharmacol Toxicol. 1988:239–243.
- 19.Bertone AL, Sullins KE, Stashak TS, Norrdin RW. Effect of wound location and the use of topical collagen gel on exuberant granulation tissue formation and wound healing in the horse and pony. Am J Vet Res. 1985;46:1438–1444. [PubMed] [Google Scholar]
- 20.Woollen N, DeBowes RM, Leipold HW, Schneider LA. A comparison of four types of therapy for the treatment of full-thickness skin wounds of the horse. Proc Am Assoc Equine Pract. 1987;33:569–576. [Google Scholar]
- 21.Sternberg EM, Trial J, Parker CW. Effect of serotonin on murine macrophages: suppression of Ia expression by serotonin and its reversal by 5-HT2 serotonergic receptor antagonists. J Immunol. 1986;137:276–282. [PubMed] [Google Scholar]
- 22.Bottaro D, Shepro D, Peterson S, Hechtman HB. Serotonin, histamine, and norepinephrine mediation of endothelial and vascular smooth muscle cell movement. Am J Physiol. 1985;248:C252–257. doi: 10.1152/ajpcell.1985.248.3.C252. [DOI] [PubMed] [Google Scholar]
- 23.Beele H, Thierens H, de Ridder L. Direct effects of serotonin and ketanserin on the functional morphology of embryonic chick skin in vitro. In Vitro Cell Dev Biol Anim. 1989;25:923–933. doi: 10.1007/BF02624005. [DOI] [PubMed] [Google Scholar]
- 24.Richter K, Kohn B, Hermanns W, Brunnberg L. Antiseptische Wundbehandlung Ein Vergleich der Wirksamkeit von Lavasept®, Rivanol® und Wasser. Kleintierpraxis. 1998;43:271–287. [Google Scholar]
- 25.Young TW. The place of Aserbine in the healing of ulcerative disorders. Clin Trials J 1966:579–589.
- 26.de Kock M, van der Merwe AE. A study to assess the effects of a new Betadine cream formulation compared to a standard topical treatment regimen for burns. Burns, including thermal injury. 1987;13:69–74. doi: 10.1016/0305-4179(87)90261-0. [DOI] [PubMed] [Google Scholar]
- 27.Stashak TS. Principles of wound management and selection of approaches to wound closure. In: Stashak TS, ed. Equine Wound Management. Philadelphia: Lea and Febiger, 1991:36–51.


