Abstract
A recent death projection has placed pancreatic ductal adenocarcinoma as the second cause of death by cancer in 2030. The prognosis for pancreatic cancer is very poor and there is a great need for new treatments that can change this poor outcome. Developments of therapeutic innovations in combination with conventional chemotherapy are needed urgently. Among innovative treatments the gene therapy offers a promising avenue. The present review gives an overview of the general strategy of gene therapy as well as the limitations and stakes of the different experimental in vivo models, expression vectors (synthetic and viral), molecular tools (interference RNA, genome editing) and therapeutic genes (tumor suppressor genes, antiangiogenic and pro-apoptotic genes, suicide genes). The latest developments in pancreatic carcinoma gene therapy are described including gene-based tumor cell sensitization to chemotherapy, vaccination and adoptive immunotherapy (chimeric antigen receptor T-cells strategy). Nowadays, there is a specific development of oncolytic virus therapies including oncolytic adenoviruses, herpes virus, parvovirus or reovirus. A summary of all published and on-going phase-1 trials is given. Most of them associate gene therapy and chemotherapy or radiochemotherapy. The first results are encouraging for most of the trials but remain to be confirmed in phase 2 trials.
Keywords: gene therapy, pancreatic cancer, expression vectors, clinical trial, oncovirus, vaccination, endoscopic ultrasound
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is the fifth leading cause of cancer-related death in Western countries, and its incidence has increased over the last 40 years [1]. Unfortunately the prognostic remains poor and a recent death projection of the “top cancer killers” due to demographic changes has placed PDAC as the second cause of death by cancer in 2030 behind the pulmonary carcinoma [2]. Curative surgery to manage PDAC is possible in only a fraction of patients. Indeed, a vast majority (85%) of patients are diagnosed with locally advanced tumors and/or metastases because of lack specific symptoms and early markers for this dismal disease. For these patients, palliative armamentarium consists of conventional chemotherapeutic agents such as gemcitabine and, more recently, FOLFIRINOX (protocol 5-FU, Folinic Acid, Irinotecan, Oxaliplatin) and nab-paclitaxel, which offer marginal survival benefits [3,4,5,6]. Even if promising innovative treatments based on patient genetics (PARP inhibitor), or targeting the tumor microenvironment (hyaluronidase, sonic hedgehog inhibitors), are currently being evaluated in early phase clinical trials, the prognosis for PDAC is still very poor and there is great need for new treatments that can change this poor outcome [7,8,9].
Unlike other digestive-cancer entities, such as colon cancer or gastrointestinal stromal tumors, molecular-targeted therapies have, so far, largely failed to improve patient survival in PDAC [1]. Indeed, large-scale genetic analyses have recently revealed that PDAC is defined by numerous exomic alterations in diverse signalling pathways, which vary considerably between patients. In addition, primary tumors are intrinsically heterogeneous and specific cellular subclones can be identified in metastasis. Such heterogeneity hampers the development of targeted therapies for the intervention in PDAC [9,10,11].
In this context, and in the past few years, several teams and we have developed innovative approaches based on therapeutic gene transfer namely gene therapy. In this review, we will comment on the latest developments in PDAC gene therapy, including gene-based tumor cell sensitization to chemotherapy, tumor targeting, and the development of oncolytic virus therapies.
2. Specificity and Tools of the Gene Therapy Applied to Pancreatic Cancer
2.1. General Strategy and Experimental Models
There are several specificities of pancreatic cancer gene therapy. First, it requires not only efficient vectors but also capable of transferring large amounts of genes that do not necessarily have prolonged expression. It may also be noted that the inflammatory reactions associated with the use of certain vectors are of lesser importance compared to other indications insofar as the drug genes are not intended to be administered on a regular and continuous basis. The anatomic localization of pancreas is of importance due to a profoundly localized organ with a close relationship with abundant vascularization and surrounding organs. For a clinical point of view, imaging techniques have also of importance to diagnose and perform staging PDAC. Among them not only computed tomodensitometry but especially endoscopic ultrasound (EUS) have a crucial role. EUS combined endoscopy and high-resolution echography by means of a high frequency ultrasonic device placed at the tip of this endoscope. Fine needle aspiration of pancreatic lesions can be performed under ultrasound imaging and nowedays EUS is the major technique to obtain a cytopathological confirmation of PDAC. By means of the same needle intratumor injection of therapeutic compounds can be easily and safely done such gene therapy products [12].
It is well known that pancreatic cancer cells are aggressive with a high rate of proliferation and a powerful local invasive and metastatic properties (peritoneum, lymph nodes, liver, etc.). An over-expression of growth factors and/or their receptors (EGF, nerve growth factor, gastrin) as well as of pro-angiogenic factors (VEGF, FGF, Platelet Derived Growth Factor), and increased invasive factors (metalloproteinases, tissue plasminogen activators) participate to these proliferative and aggressive properties. In addition, this aggressiveness occurs whatever the size of the tumor with a special role of the tumor microenvironment. Indeed, this microenvironment plays also a key role in the invasive and metastatic process of pancreatic carcinoma, with a strong relationship between cancerous cells and pancreatic stellate cells, as well as the extracellular matrix. It strongly participates in tumor fibrosis, hypoxia, and hypovascularization, resulting in inaccessibility of drugs [13,14]. The genetic background of PDAC is well known and INK4a/ARF (p16), TP53, DPC4/Smad4 tumor suppressor pathways are genetically inactivated in the majority of pancreatic carcinomas (associated with losses of heterozygosity of, respectively, 9p21, 17p, and 18q), whereas oncogenic Kras is activated [15,16,17]. At a late stage of tumor development, there is an increase in telomerase activity. However, the activating point-mutation of the Kras oncogene on codon 12 (Exon 2) remains the major event (70–95% of PDAC cases: 71% of pancreatic cancer specimens in the COSMICS database harbour Kras mutations) [17,18,19].
Most of models used for pre-clinical approaches of gene therapy are well-established human pancreatic cancer cell lines that can be implanted in athymic mice heterotopically or orthotopically. One limitation of these models is the lack of immunity despite a stromal reaction arising from mouse itself. Other in vivo models are transgenic ones including the mouse model displaying a G12D Kras mutation (Kras LSL-G12D/+ i.e., KPC model) [20]. In addition, crossed models are also available with animals bearing a knockout of TP53 or other tumor suppressor genes [21,22]. However, transgenic models take often time to obtain primary PDAC and its environment and the immunity remains a mouse context [20,21,22]. Finally, humanized models such as PDX (patient-derived xenograft) could be theoretically applied but require preliminary characterization in term of differentiation, somatic mutations, chemosensitivity or resistance. In our team, we have largely used an immune model with phenotypic and genotypic properties close related with the human model, i.e., orthotopic allotransplantation or portal injection of pancreatic cancer cells in hamster. It is a rapid and reproductive model but the molecular tools for studying hamster are quite poor [23,24,25].
On the other hand, we demonstrated many years ago that human pancreatic cancer cells are difficult to transfect using various synthetic vectors and also adenovirus [23,24]. The use of viral vectors offers generally a better yield of DNA transfection and expression but they cannot be used optimally in all models as specific receptors are sometimes required and human viruses do not necessarily work in rodent cells and organs. Last, two points are peculiarly important when one can consider a gene therapy strategy for PDAC: (i) the evolution of 80% of tumor is rapid with a median survival reaching 8 to 11 months [1,3,4,5] requiring to take into account the fact that chemotherapy should be often included in the therapeutic strategy (before as a first line or in combination with gene therapy) and that the time to set up any second (and hopefully) lines treatment is very short with an otherwise aggressive and often metastatic disease; (ii) the choice of optimal gene, vector and route (frequency) of future administration at the clinical stage should be minded and investigated as soon as possible and thus integrated in the different stage of the clinical development “proof of concept, pre-clinical experiments (up-scaling of gene therapy production, biodistribution, toxicology, etc.) and phase 1.
2.2. Methods of Gene Delivery
The three main methods are ex vivo, in vivo and in situ gene therapy. Ex vivo gene therapy involves sampling cells from the patient, genetically modifying them with a vector carrying the therapeutic gene and then reintroducing them in the same patient [26]. This method applies very well to blood stem cells as it is clinically used in the gene therapy protocols for combined immunodeficiency syndroms or hemoglobinopathy [27,28]. This strategy requires an ex vivo culture as currently practice in the context of cell therapy with adult stem cell sampling and more commonly in heterologous bone marrow transplantation. This took times and several controls within at least four to eight weeks procedures. In the context of a tumor like PDAC with a rapid growth and invasive process the ex vivo strategy is not adapted and a gene therapy product “ready to use” is theoretically better. The second type of gene therapy is the in vivo approach. It involves injecting the vector carrying the therapeutic gene directly into the bloodstream. In this case, the injection is easy (it is an intravenous or intra-arterial injection) but several additional barriers are raised like the circulating blood and the vessels. Indeed, in the circulating blood, several actors will try to eliminate the foreign DNA and its vector such as macrophages and endonucleases. Finally, if the transgene must reach an organ, it will have to cross the barrier of capillaries and Endothelial cells. The third type of gene therapy is in situ, delivering the therapeutic gene directly within the target tissue (or tumor). This technique is tested in particular in cases of cystic fibrosis (transfer of vectors in the trachea and bronchial network with aerosols), myopathy (for example injection into the muscle of a vector carrying the dystrophin gene in the myopathy of Duchenne) and cancers in order to inject into the tumor a vector carrying a therapeutic gene. This approach requires two things: a good targeting of the organ and an efficient vectorisation system. In terms of targeting, these are mainly “physical” methods that can be carried out by the hand of doctors or surgeons by means of a simple needle or catheters. In case of PDAC, therapeutic gene injection is guided by surgeon during laparotomy, by the radiologist under ultrasound or CT-scan and last (and better) by a gastroenterologist under EUS which is the most precise technique to target primary PDAC whatever its localization within the pancreatic gland.
This route seems optimal for PDAC gene therapy but require also a specific and elective tropism of the gene therapy product for PDAC cells. This suppose specific promoter as a “molecular targeting” or a specific viral tropism. Molecular targeting is theoretically possible, so far we use specific promoters of PDAC cells. Unfortunately, there is no highly specific promoter even if mesothelin and MUC-1 appear the best candidates to obtain an elective transgene expression in PDAC cells. Finally, the choice of in situ gene therapy appears the best one for PDAC.
2.3. Vectors
Besides naked DNA, that do not offer a significant advantage for cancer gene therapy, there are synthetic vectors which have the advantage of being easy to produce by the chemical industry. They are relatively innocuous (although some cellular toxicity in vitro is often found) and interesting properties for gene therapy. Indeed, all the nucleic acids can be complexed (such as plasmid DNA, siRNAs, shRNAs and even some viral vectors). There are two main types of synthetic vectors: lipid vectors and polycationic vectors [29].
The pure lipid vectors have the power of enveloping the DNA and facilitating its passage through the lipid bilayer constituted by the cell membrane. These vectors did not appeared efficacious to produce significant gene transfer into human PDAC cells. They rapidly evolved towards mixed vectors associating a lipid part and a cationic part. The principle of the cationic part is very simple: it is a succession of positively charged molecules which envelop and complex with DNA which is negatively charged. The vector envelops the DNA in order to facilitate the membrane passage by the lipid part and to be protected during its “travel” in the cytoplasm towards the nucleus thanks to the cationic part of the vector. Finally, if mixed vectors displayed higher performances than pure lipid vectors to induce a significant DNA transfection in PDAC cells. A second type of synthetic vector appeared more interesting. Indeed, polycations or cationic polymers are vectors derived from polylysine and polyethylenimine. The polylysine vector is therefore a basic amino acid polymer, lysine, which has the power to condense DNA and protect it from digestion by intracellular lysosomes. Certain chemical groups such as polyethylene glycol (PEG) have been added to improve stability in the blood and the internal environment, or even to decrease cell toxicity.
Among the polycationic vectors, we also find that of the polyethylenimine (PEI) type which is a pure polycation, linear in its structure and which facilitates the cellular and nuclear penetration of the DNA by conferring it a good resistance to lysosomal digestion. Several vectors are derived from the PEI and have parts added by chemists such as PEG, cholesterol, mannose and dextrose and many other chemical compounds [30,31,32,33,34,35]. A number of these DNA-complexed vectors have been tested in patients and have not had any major adverse effects at any site of administration (lung cancer, subcutaneous vaccination, ovarian cancer, lymphocytes or bladder cancer). Clinical studies have been conducted in PDAC patients (including our experience) using PEI and with safety and possible efficacy pending results of ongoing phase 2 trials. Finally, it should be noted that the DNA-polycation complexes take a final structure and especially a size close to the manometer. As a result, they are also called “nanocomplexes” or “DNA nanoparticles”.
Besides synthetic vectors, viral vectors have been developed for their natural property to infect human cells [29,36,37] (Table 1). Adenoviruses are responsible in children and adults of head and neck, pulmonary and digestive infections. They infect many cells and, as a result, vectors derived from adenoviruses have long been used in preclinical and clinical gene therapy strategies for PDAC. They also induce transient expression and of provoke strong inflammatory and immune reactions. However, several clinical trials have been conducted with adenoviral vectors, without major incident. In order to overcome some problems of toxicity, third generation adenoviral vectors lacking viral sequence have been developed (so called “gutless” or “helper dependent”) [36,37,38]. Adeno-Associated Viruses (AAV) are non-pathogenic, naturally defective viruses that are widespread in humans. AAVs are derived from viruses of the parvovirus family. They are used as single-stranded DNA vectors, are very small in size, unwrapped and transduce quiescent cells and dividing cells. They only rarely and randomly integrate and therefore present a low mutagenic risk. They are termed “associated adenoviral vectors” because the parental viral strain, the adeno-associated virus, depends, for replication, on the presence of adenovirus, herpes simplex virus or papillomavirus. They can effectively transduce cells from the brain, liver and some blood cells but have been rarely tested in human PDAC cells. Retroviral (most derived from MuLV for Murine Leukemia Virus) vectors and lentiviral (derived from HIV-1 for Human Immunodeficiency Virus Type 1) belong to retroviridae family. These are small enveloped RNA vectors which have the advantage of efficiently transducing a lot of cells of interest, being not very immunogenic (and without any pre-existing immunity because they are very rare in nature) and which are easy to produce. Nevertheless, the weak point of this type of vectors is their integration in open areas of the chromatin of the nucleus of the target cell, which presents a risk of mutagenic insertion (they can be considered as onco-retroviruses). They are mainly used in ex vivo strategies but their ability of the transduction of proliferating cells such cancer cells authorize also an in situ administration [27,28,37,38]. A new generation retroviral and lentiviral vectors have been modified to avoid the transactivation of oncogenes when they are integrated into the host cells: these are the so-called SIN (self-activating vectors, avoiding transactivation of oncogene such as LMO oncogene). Another type of modified lentivirus has been also successfully tested by our team in PDAC models using a integrase-defective lentiviral vectors [39].
Table 1.
Main viral vector and their characteristics used in pre-clinical and clinical strategies of gene therapy for pancreatic cancer.
| Virus | Insertion Capacity | Target Cells | Delivery | Transgene Expression | Level of Expression | Pre-existant Immunity | Bio-Safety |
|---|---|---|---|---|---|---|---|
| AdV | 35 kb | Dividing or non dividing | Ex vivo or In situ | Transient | High | Yes | Immunogenic, inflammation No integration |
| AAV | 4.8 kb | Dividing or non dividing | Ex vivo or In situ | Stable | Moderate | Yes | Mutational integration |
| Retrov | 8 kb | Dividing | Ex vivo or In situ | Stable | Moderate | No | Mutational integration |
| LentiV | 10 kb | Dividing or non dividing | Ex vivo or In situ | Stable | High | No | Mutational integration, recombination with WT HIV |
| HSV | 30 kb | Dividing or non dividing | Ex vivo or In situ | Transient | High | Yes | Mutational integration, neurotoxicity |
| Pox | 25 kb | Dividing or non dividing | In vivo or In situ | Stable | High | No | Immunogenic, Adjuvant to vaccination |
| SV40 | 5 kb | Dividing or non dividing | Ex vivo or In situ | Stable | Moderate | No | Mutational integration |
AdV: adenovirus derived vector; AAV: Adeno-associated derived vector; RetroV: retroviral derived vector; LentiV: lentiviral derived vector; HSV: herpes simplex viros derived vector; Pox: Pox and vaccinia virus derived vector; SV40: simian virus 40 (papova virus). AdV, HSV and Pox are double stranded DNA virus; AAV is a single stranded DNA virus; RetroV and LentiV are single stranded RNA virus. WT: wild type.
Some researchers have studied herpes viruses as gene therapy tools, such as HSV-1. It is the opposite of the AAV virus from the point of view of its advantages and disadvantages. Indeed, HSV-1 virus can carry large amounts of DNA but it is highly immunogenic (and human HSV-1 antibodies frequently). On the other hand, this virus has a strong tropism for the nerve cells in contrast to most other viral vectors. However, its genome is complex and the passage from a highly pathogenic virus to a clinically effective harmless vector is far from simple. Other viruses have been used for in vitro and in vivo gene transfer and are derived from other pathogenic viruses such as measles, vaccinia, pox or Sendai virus. Their application in gene therapy remains marginal but a development is currently observed in strategies of vaccination (pox virus) or in vivo administration (vaccinia virus). Main viral vectors and their characteristics are detailed in Table 1. In addition some authors add to this list the concept of “biological vectors” taking into account that attenuated strain of bacteria can be used for gene therapy (and vaccination protocols) by introducing therapeutic transgene [38,40]. Finally, it is noteworthy that the field of gene transfer has been considerably opened since the nanotechnologies and nanomaterials have been adapted to DNA transport [41].
The route of administration depends on the type of vectors. Generally adenovirus, AAV and synthetic vectors are used for in situ administration, retroviral and lentiviral vectors are applied for ex-vivo approaches. Oncolytic viruses can be injected by IV route while subcutaneous injections can be performed with plasmidic DNA as well as transfected cells or bacteria. Whatever the system of gene delivery and in case of solid tumors such as PDAC it is difficult to reach all tumors cells as doing chemotherapy. The in situ approach can benefit from a radiological targeting but on cannot really know how many cancer cells are transfected. In the vivo approach gene transfer can be tone down by natural barriers such as blood stream, nucleases, capillaries or tumor stroma. Once again the best way would be a “robust” molecular targeting (promoter driving the gene of interest or molecular motif within the vector itself) specific of pancreatic cancer cells.
3. Therapeutic Genes and Pre-Clinical Strategies
The general strategy and tools for gene therapy of cancers will vary depending on the final goal: either to reintroduce a deficient gene (such as transfer of a tumor suppressor gene) or to introduce a missing or under-expressed therapeutic gene (such as a cytokine or a suicide gene) or to inhibit the expression of a gene (such as an oncogene). On the other hand, whatever the strategy of administration (in vivo or in situ), one should not expect to reach a large part of the tumor in terms of gene transfer and expression. Indeed, many successive obstacles are to be overcome such as access to tumor cells, intracellular and intranuclear penetration, expression of mature mRNA and protein. In our experiment by applying a plasmid vector complexed to PEI or a recombinant adenovirus, we obtained a maximum of only 15% of the tumor cells of the primary tumor after administration in situ [23,24,25]. This percentage implies to obtain a bystander effect in order to hopefully induce a significant antitumor effect. The bystander effect realizes a diffusion of the therapeutic effect to the neighboring cells following the transfer of the therapeutic gene. This prerequisite must be observed in any proof of concept for gene therapy program of epithelial cancers. This bystander effect has been described initially after in vitro and in vivo transfer of suicide genes. It generally involves the diffusion of pro-apoptotic signals, activated prodrugs and even anti-angiogenic paracrine signals. The various pre-clinical approaches of gene therapy for the PDAC are detailed in Table 2 and developed in the sub-chapters below.
Table 2.
Main experiments and strategies tested for gene therapy of pancreatic cancer.
| Strategy | Detailed Genes |
|---|---|
| Gene transfer | Tumor suppressor genes (P16, p21, pRb, p53, Smad4/DPC4…) Ani-angiogenic genes (endostatin, thrombospondin-1, angiostatin, Matrix metalloproteinase inhibitors, somatostatin receptor sst2 subtype, arrestin…) Apoptosis related genes (TRAIL, TNF) Suicide genes |
| Gene invalidation | antisens therapy (Kras, HER-2/ErbB-2, MDR-1) MicroRNA (MIR-21) |
| Active immunotherapy | Interleukin expression Cytokine expression |
| Vaccination | Pulsed dendritic cells DNA, peptide, engineered cells and bacteria |
| Adoptive immunotherapy | CAR-T cells (targeting mesothelin or MUC-1) |
3.1. Tumor Suppressor Genes
This strategy aims to obtain the transduction of an antioncogene, the expression of which has been lost during carcinogenesis. The most well-known tumor suppressor gene is that encoding TP53, a protein that controls the entry of apoptosis cells that are mutated in 50% of human tumors. The direct in vitro inhibitory effect of the restoration of TP53 expression on tumor growth is complemented by an in vivo inhibitory action on neoangiogenesis. This TP53 gene transfer strategy has been applied to the treatment of tumors of the liver, stomach, colon, lung, ovary and head and neck cancers. The results obtained are poor and often reflect a poor efficiency of gene transfer in vivo, but also differences in the sensitivity of cell types or even the inconstant mutation of TP53 in tumors in general. However, it should be noted that the first gene therapy medication authorized for the treatment of cancers involves the transfer of the p53 gene (see below in chapter on oncoviruses). Beside TP53, others tumor suppressor genes has been tested in vitro and or in vivo in PDAC models such as p21WAF1, p16INK4A, or DPC [38,42,43,44,45,46,47,48].
3.2. Antiangiogenic and Proapototic Genes
Always in vitro and in vivo models, several strategies has been tested by transfer of gene encoding for anti-angiogenic molecules such as endostatin, angiostatin, thrombospondin-1, vasostatin, soluble fibroblast growth-factor receptors as well as soluble vascular endothelial growth factor receptor [38,49,50,51,52,53,54,55]. As a proof of concept of a future gene therapy protocol for PDAC (see chapter clinical trials) we demonstrated that by re-introducing the somatostatin receptor subtype 2 (SSTR2) gene (the expression of which is lost in PDAC), we obtained in a mouse and hamster models a local and distant antitumor bystander effect. This bystander effect is mediated by apoptosis and antiangiogenic effect through a negative autocrine loop induced by gene transfer with secretion of the natural ligand somatostatin [23,24].
3.3. Suicide Genes
An important issue is that a gene transfer of a suicide gene with a strong neighboring antitumor effect (so called the “bystander effect”) may compensate for the weakness of gene expression within the tumor. The most classic example of this suicide gene strategy is that of the herpes virus thymidine kinase gene (the HSV-TK gene). This gene encodes the thymidine kinase enzyme which is able to metabolize ganciclovir, an antiviral drug that is normally devoid of antitumor effect, into a toxic compound that interferes with DNA replication and results in cell apoptosis. This system provides conditional toxicity based on the presence of the sensitizing gene and its substrate. In other words, the gene is theoretically harmless to the cells in which it will be expressed but becomes highly toxic when its substrate, ganciclovir, is added. The latter is also harmless in its initial form (before administration) but will become toxic in the cell after its phosphorylation by thymidine kinase. Using this enzyme for therapeutic purposes, it was quickly apparent in vitro that its expression in only 10% of the cell population was sufficient to cause the death of all cells in culture after treatment with ganciclovir. Several studies have demonstrated that this remote toxicity was due to the phosphorylated release of ganciclovir from cells expressing the HSV-TK enzyme to those that do not express it, via intercellular communications. Pro-apoptotic messages and enzymes would also be diffused from cells expressing the HSV-TK gene. In vivo, this system also induces a strong immune response directed against tumor cells. Indeed, it is suspected that the fact of destroying the tumor cells releases tumor antigens that will lead to a response of the body against other tumor cells. This is called a distant bystander effect [56,57,58]. The other examples of suicide/prodrug gene systems that has been also successfully tested in PDAC models are as follow: the cytosine deaminase gene (also coupled to the uracil phosphoribosyltransferase gene) that transforms 5-fluorocytosine (with antifungal properties) into 5-fluorouracil [38,59,60]; the nitroreductase gene transforms CB1954 (for [5-(aziridin-1-yl)-2,4-dinitrobenzamide]) into a toxic compound, the 4-hydroxylamine [61,62]; the cytochrome P450 gene that transforms ifosfamide to acrolein (nitrogen mustard);. At a clinical point of view, the cytochrome P450/isofosfamide system has been developed crossing in vitro and in vivo proof of concept in order to subsequently conduct a phase 1 trial in PDAC patients (see also chapter clinical trials) [63,64].
3.4. Small Non-Coding mRNA, Interferent mRNA and Antisense Therapy
Besides the conventional gene transfer using vectorized DNA (synthetic or viral vector), there is another strategy, acting directly on the RNA and thus shunting the DNA and its transcription process. Small non-coding RNAs have been discovered since the late 1990s and two-classes of small RNA, the microRNAs (miRNAs) and interfering RNAs such as siRNAs (for “Small Interfering RNA”) and shRNAs (for “Short Hairpin RNA”). These non-coding RNAs have many functions, in particular the post-transcriptional inhibition of gene expression.
MicroRNAs (or miRNAs) are single-stranded RNAs of 21–24 ribonucleotides specific. They are natural molecules endowed with a post-transcriptional regulatory power and therefore capable of extinction of the expression of a gene. Indeed, their pairing with a sequence complementary to the target messenger RNA can lead to the inhibition of the translation of the mRNA or its degradation. They were notably involved in the modulation of processes such as apoptosis, cell cycle or differentiation. Loss of their expression (by chromosomal impairment, mutation, polymorphism, epigenetic modifications) can lead to major dysfunctions, especially during carcinogenesis. The study of the expression on a large scale (called the microRNome) made it possible to determine the role of some microRNA in the development of many cancers as well as the mechanisms that lead to these dysfunctions. These miRNAs so-called oncoMIR can be down regulated through a gene therapy approach. We successfully demonstrated that targeting the oncogenic miRNA-21 (down regulation by an antisense expressed with a lentiviral vector) strongly inhibit pancreatic cancer tumor growth both in vitro and in vivo [65].
An interfering RNA is a single or double-stranded RNA (composed of 21 ribonucleotides) whose interference with a specific mRNA leads to its degradation and to the reduction of its translation into protein. Like micro-RNA, the interfering RNA makes it possible to block translation by making “silent” this or that gene. To this arsenal must be added the small hairpin or shRNAs (shRNAs for short hairpin RNAs), the structure of which is in the form of a stem and a loop, which are also involved in the phenomenon of Interference with RNA. The expression of the shRNAs in cells is carried out mostly by means of plasmids or viral vectors. Finally, using the RNA tool avoids the nuclear compartment of the cell (unlike naked or vectorized DNA) and therefore all transcriptional and post-transcriptional machinery. Some successes in vitro and in vivo have been recorded and several pre-clinical and clinical trials currently apply the strategy of interfering RNA. In the same class of tools that directly address gene expression, there is the antisense strategy. It is indeed possible to synthesize a nucleic acid strand (DNA, RNA or a chemical analogue) intended to bind to the mRNA of the target gene when it is expressed. This has the effect of inactivating the gene (sometimes destroying it). It is also possible to affect the splicing of the pre-RNA, thereby changing the exon content of the mRNA. The synthetic nucleic acid sequence used is called “antisense” because it is complementary to that of the messenger RNA of the gene, which is called the “sense sequence”. It is, therefore, possible to synthesize oligonucleotides to block the expression of a deleterious gene by its expression or overexpression (such as an oncogene, a growth factor, pro-inflammatory molecules, mediators, etc.) [66].
This strategy has been applied to PDAC both at the experimental and clinical levels targeting Kras oncogene expression with antisense, siRNA and ribozymes molecular tools. Unfortunately clinical results did not reflect those positively obtained in vitro [19,38,67,68,69,70]. A compound is now evaluated at the clinical stage, the LODER® (Silenseed Ltd., Modi’in, Israel) that is a miniature biodegradable intratumoral implant containing and releasing within 4 months siRNA against Kras.
A recent most sophisticated approach, is genome editing that encompasses a set of techniques for manipulating the genome via “rewriting genetic material” that can be applied to plants, animals, fungi and bacteria. Some laboratories also propose to apply it to the human genome and use it as a gene therapy tool. Three new systems have potential in biomedical research and perhaps in personalized medicine. The first two use endonucleases as Zinc-Finger Nucleases (ZFN) and Transcription Activator-Like Effector Nucleases (TALEN). The third system is considered to be a real technological revolution, the system CRISPR-Cas9. Known since 2012, it comes from a system of bacterial defense. Indeed, bacteria integrate into their genes, at the level of the CRISPR (“Clustered Regulatory Interspaced Short Palindromic Repeats”) sequences, foreign DNA fragments, as a “souvenir” of past infections. These latter are transcribed into RNA, which can then guide the enzyme Cas9 (for “CRISPR associated protein 9” which is also an endonuclease) to the foreign DNA when it presents itself in order to eliminate it. On this principle, it is possible to obtain a CRISPR-Cas9 artificial complex specific for a given DNA sequence (generally between 10 and 24 nucleotides) in order to cut and modify it. Of course, this is an extraordinary tool for molecular biology but also for gene therapy: to remove a specific deleterious sequence, to replace it but also to add a sequence of crucial interest, in short, to modify the genome at will [71,72,73]. This strategy is already applied in gene therapy approach of HIV infection, paludism or pulmonary cancer [74]. An in vivo gene transfer of this system is possible in various models of cancer including PDAC [75]. One question remains to resolve, which crucial gene should be targeted to reduce cell proliferation and/or invasion? Besides the specificity of the gene (and a lot of candidates may be chosen in the case of PDAC) some milestones must be passed such as accuracy, efficiency and safety of CRISPR-Cas9 technology applied for a gene therapy strategy.
3.5. Immunotherapy and Vaccination
The lack of recognition and elimination of tumor cells by the immune system is strongly implicated in the development and progression of tumors. This is one of the explanations for the aggressiveness of certain cancers. Indeed, PDAC is characterized by the accumulation of a desmoplastic stroma rich in inflammatory cell infiltrates [76]. While immune cells are abundant within the stroma, they mostly belong to immunosuppressive subsets, such as regulatory T cells (Tregs), T-helper (Th)17 cells, tumor-associated macrophages (TAMs) and multiple subsets of immature myeloid cells/myeloid-derived suppressor cells (MDSCs) [77,78,79]. Intratumoral effector T-cells are rare, in contrast to many other solid tumors, and when present express high levels of immune checkpoint receptors such as PD-1, indicating an exhausted status. As observed in many cancers, PDAC is subjected to a microenvironment remodeling to survive. PDAC specifically promotes an immunosuppressive microenvironment and a pro-tumoral inflammation. One can underline the specific role of PDAC stellate cells with a paracrine signaling mediated by cytokines such as GM-CSF (Granulocyte-macrophage colony-stimulating factor) and IL-6 [80]. In addition, cancer cells themselves expressed checkpoint molecules (such as programmed death ligand-1/PDL-1) [81]. A limited number of studies targeting theses immune checkpoint inhibitors (ICI) have been completed in PDAC. Both Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4) and Programmed death-ligand 1 (PD-L1) inhibitors were investigated in patients with locally advanced or metastatic PDAC in two clinical trials. Unfortunately, PDAC is not melanoma and the clinical outcomes were disappointing [77,82]. On the whole PDAC microenvironment is comprised of an intricate network of signals between immune cells, stellate cells and PDAC cells resulting in an immunosuppressive environment resistant to single-agent immunotherapy including gene therapy [77,81].
There are many strategies of immunotherapy applied to the treatment of cancers. The more classical is the non-specific immunotherapy aiming to administrate cytokines with antitumor effect (interferons, interleukins, etc.). To overcome some side effects provided by systemic administration gene therapy strategies has been developed to induce local interleukin or cytokine either by the tumor cells themselves or via ex-vivo approach (example of human fibroblast transduced with interleukin-12) [38,83,84,85,86,87,88]. Pre-clinical experiments have been conducted in vitro and in vivo aiming to produced interleukins (IL-2, IL-4, IL-6, IL-27, IL-1β, IL-24) and cytokines (GM-CSF, IFNα and β, TNFα). Most of experiments were positive with antitumor effect as proof of concept of future clinical trials.
In the field of specific immunotherapy, there is several routes. The first one is the administration of monoclonal antibodies to receptors or molecules involved in the growth, immunity or vascularization of tumors. The second one is the adoptive immunotherapy by introducing a tumor antigen into the lymphocytes. It consists in stimulating the patient's immune system against his own tumor so as to facilitate the recognition of cancer cells and their elimination. The antigen (peptide or gene transfer) is introduced in the T lymphocytes or antigen-presenting cells (so called “dendritic cells”) of patients. The gene therapy approach consists in introducing a gene encoding a protein involved in tumor cell recognition, in their destruction (tumor antigens, cytokines, tumor suppressor gene) and to reinsert them into the organism of the patients. Antigens commonly expressed for PDAC are MUC-1 (Mucin-1) and mesothelin. Antigen-pulsed dendritic cells vaccine experiments have been conducted in PDAC models. In term of adoptive active immunotherapy of cancers, a new approach has been developed. It is based on the principle of the Chimeric Antigen Receptors. Autologous lymphocytes of the CD8+ T cell type are removed from the patient and are modified to recognize and kill the tumor cells carrying tumor antigen. By means of a lentiviral (or retroviral) vector, a monoclonal antibody fragment (in fact several fragments, hence the term “chimeric”) is transduced into the patient's lymphocytes and then reinjected. Some initial tests for malignant hemopathies such as acute B cell lymphocytic leukemia, lymphocytic B cell lymphoma and chronic lymphocytic leukemia are promising and first trials are in progress for PDAC [89,90,91,92,93].
The last approach of immunotherapy is vaccination by systemic administration (i.e., intravenous infusion), subcutaneous or local administration of tumor antigens in the form of a peptide, a protein, a tumor cell lysate, a DNA plasmid or a recombinant viral vector. Several programs have been initiated and reached the clinical application during early phases. Beside antigen-pulses dendritic cells, various strategies are developed such as: DNA vaccine with the VEGF-receptor DNA, peptide vaccination (Gastrine17-DT vaccination, mutated peptides of Kras), CRS-207 (attenuated form of Listeria monocytogenes that has been genetically-modified to express mesothelin), TELOVAC (vaccination trial using a 16-amino acid telomerase peptide - GV1001) and GVAX (mix of allogenic human pancreatic cancer cells expressing and secretin GM-CSF) [38,94,95,96,97,98,99,100]. Most of the strategies of vaccination are associated with GM-CSF administration and/or chemotherapy.
4. Oncolytic Virotheray
Development of PDAC is associated with a series of changes that provide a selective growth advantage to the cancer cells. However, these very changes (among them, lack of interferon response, elevated metabolic activity, and disengagement of cell cycle control) render cancer cells highly sensitive to viral infection. Consequently, selectively replicating viruses (oncolytic viruses) represent a promising new therapeutic strategy. These viruses can be naturally “cancer selective” for their replication or may require genetic modifications. A recent clinical-trial-watch report has summarized the use of oncolytic virus for cancer therapy [101]. Oncolytic viruses induce their anticancer activity through several mechanisms such as a preferential replication in dividing tumor cells until apoptosis and cell death, tumor cell specificity, the targeting of dysfunction pathway (such as TP53), the induction of specific and non-specific anti-tumor immunity and last suppression of cancer-stem cells [102]. Oncolytic viruses are natural pathogens that have been selected or specifically designed to infect and destroy cancer cells. These oncovirus are under development in academic and pharmaceutical laboratories around the world for more than twenty years. They can be engineered to produce cytokines, antigens or suicide genes.
The first oncovirus that has been approved for clinical indication is an adenovirus, Oncorine H101 (developed by Shangai Sunway biotech) that is engineered and modified selectively to replicate in and kill tumor cells that harbor p53 mutations. It is close related to the adenovirus Onyx-15. The clinical indication is head and neck cancers but Onyx-15 has been evaluated in PDAC. The second one is an herpes virus (HSV-1), OncoVex GM-CSF (T-VEC, Talimogen laherparepvec, Imlygic® developed by Amgen, Thousand Oaks, CA, USA) was approved for the treatment for advanced melanoma by FDA (US Food and Drug Administration) and EMA (European Medicine Agency). Others oncoviruses reached the stage of clinical evaluation such as JX-594 (developed by Jenerex, a vaccinia virus expressing GM-CSF) tested in hepatocellular carcinoma, Reolysin® (Oncolytics Biothech Inc., Calgary, Canada) (a reovirus for “respiratory enteric orphan virus”) tested in brain tumors and PDAC, Toca 511 (a retroviral replicating vector encoding a modified yeast cytosine desaminase in combination with 5-fluorocytosine developed by Tocagen) tested brain tumors but also in PDAC, the TG4023 (vaccinia virus encoding for expressing cytosine deaminase and uracil phosphoribosyltransferase in combination with 5-fluorocytosine developed by Trangene) tested in hepatocellular carcinoma and hepatic metastasis [103].
In the case of PDAC, several viruses have been explored for their ability to replicate and to inhibit tumor growth in experimental model [37,104,105]. Preclinical studies have demonstrated that conditionally replicative adenoviruses can be exploited for PDAC therapy, when combined with gemcitabine [106]. Pioneering studies demonstrated that intratumorally injected Onyx-15 combined with gemcitabine was well tolerated in a phase I/II clinical trial for PDAC patients [107]. Current refinements in conditionally replicative adenoviruses as a result of a German–French collaboration comprise modifications of the hexon to improve viral replication in both tumor and stromal cells [108], as PDAC is characterized by a dismal stromal reaction that impedes the intratumoral dissemination of therapeutic drugs. Interestingly, growing body of evidences indicate that PDAC tumor microenvironment is not a watertight barrier, but rather facilitates oncolytic viruses replication and therapeutic activity [109].
We recently demonstrated that engineered herpes simplex type 1 viruses (HSV-1) are efficient in blocking experimental tumor growth, used alone or in combination with gemcitabine [110]. HSV-1-based viruses infect various tumor cell types, do not integrate into the genome of infected cells, are safe in patients, and there are several anti-HSV-1-specific drugs available in case of adverse events. In addition, cell killing of entire cancer cell populations can be achieved rapidly with a relatively low dose of virus. The most widely used strategy to restrict HSV-1 replication to cancer cells is to delete genes that are important for viral replication as well as the evasion of innate immune responses. First-generation oncolytic HSV-1 viruses, such as Oncovex and HF10, which have reached clinical applications, were ablated for the γ34.5 viral protein and resulted in reduced therapeutic efficacy [105]. In our work performed by the HSV-1-based virus, Myb34.5, in which the expression of the γ34.5 viral protein is controlled by a tumor-specific B-Myb promoter, efficiently replicates and kills human PDAC cells. When injected in orthotopic human tumors developed in nude mice, Myb34.5 successfully replicates, strongly inhibits tumor progression and blocks metastatic dissemination. The Myb34.5 antitumor effect is potentiated by gemcitabine administration, both in vitro and in vivo [110].
The oncolytic parvovirus, H-1 (H-1PV), belongs to the Parvoviridae family and is composed of a small, non-enveloped, icosahedral capsid containing a linear, single-stranded DNA around 5 kb in size. The mechanism underlying the natural selective replication as well as the specific toxicity of the virus for rat and human cancer cells is complex and multifactorial. If H-1PV binds and infects equivalently normal and transformed permissive cells, it is thought that the molecular abnormalities encountered in cancer cells favor nuclear envelope breakdown, viral gene expression, viral DNA amplification, capsid assembly, virion maturation and cytopathic effect. Variable levels of constitutive activation of the specific metabolic/signalling pathways leading to these cellular events may explain the difference in potency of H-1PV, as permissive, semi permissive and a few resistant cancer-cell types have been described [111].
H-1PV efficacy and safety was tested in a rat orthotopic model of PDAC, involving either rat cells in a syngeneic rat or human carcinoma cells in an immuno-incompetent model [112,113,114]. Other experiments led to similar conclusions on human PDAC tumors that were implanted immuno-incompetent mice, that are non-permissive to H-1PV. The efficacy of H-1PV was also tested in combination therapies, in the context of PDAC. Considering that H-1PV induces cancer-cell killing using a different mechanism (cathepsin dependent), oncolytic H-1PV could potentially kill cells resistant to gemcitabine. Considering the clinical application of H-1PV in patients, a French study, in which the skin metastases of twelve patients with melanoma, breast adenocarcinoma, lung large cell carcinoma, pancreatic carcinoma, or kidney leiomyosarcoma were injected with escalading doses of H-1PV, reported only mild signs of toxicity. The presence of viral DNA and proteins was detected in the injected tumor lesions, as well as at distant tumor sites, suggesting a systemic spread of the virus. In light of the lack of significant toxicity encountered in this early clinical trial, an oncolytic H-1PV trial (ParvOryx01) proposing escalating doses of H-1PV was initiated in patients with malignant brain tumors [113], following injection into the primary site, or intravenous administration, and was found to be safe. Altogether, the clinical trials reported so far involving administration of H-1PV in cancer patients have demonstrated that this virus is safe and devoid of clinically relevant side effects. In addition, the ParvOryx01 trial, which has just been completed and is under current clinical assessment, provided intriguing evidence of viral intracellular replication, induction of viral and cellular cytotoxic effectors, and activation of specific T-cell responses [114], which could be translated in the future to the therapy of pancreatic cancer. A new therapeutic concept is emerging by combining viral oncolysis with immunotherapy. In fact, at the same time, virus mediated tumor lysis leads to the liberation of tumor associated antigens and/or mutant proteins that have arisen during tumor evolution [115]. Imlygic® has provided the first convincing human data supporting the idea that direct tumor lysis by a replicating virus can locally stimulate sufficient anti-tumor immune responses to provide systemic, long-lasting cancer killing immune responses in advanced cancer patients [116]. This oncolytic virus was found to increase T cell infiltration into tumors and generate a systemic immune response against tumor associated antigens [117]. Thus, combining oncolytic virus with immune checkpoint inhibitors to override immune tolerance has emerged as one of the most promising anticancer modality to date. It is tempting to speculate that heating up immunologically cold pancreatic tumors with oncolytic virus will overcome resistance to ICI treatment for best patient benefit.
Additionally, data from preclinical models and patients suggests that the natural induction of anti-tumor immunity is “hit and miss” with a pure oncolytic approach. Rather than hoping for an immune response to be induced with this strategy, oncolytic virus offers the opportunity to encode foreign transgenes [118] and to create an improved oncolytic virus vaccine for PDAC. In this strategy, a “supercharged” immune response is directed against a specific antigen expressed on the patient’s cells, to create the perfect immunological storm within the tumor leading to profound therapeutic effect both on primary site and metastatic deposits. To explore this hypothesis, oncolytic virus could be generated to express diverse PDAC antigen (mesothelin, MUC-1, etc) that were recently found to elicit a potent antitumoral immune response when combined with GVAX [119] or used in chimeric antigen receptor-T cells strategies [115], respectively. Supercharged oncolytic virus could be assessed for tumor destruction and immune response against the tumors, including in combination with immune checkpoint inhibitors.
5. Clinical Trials
Main clinical trials published and for which we have sufficient data are detailed in Table 3. Main indication is PDAC at an advanced stage (locally advanced and/or metastatic) with phases 1 and 2 trials and one phase 3 [101,120,121,122,123,124,125,126,127,128,129,130,131,132,133,134,135,136,137,138,139,140,141,142,143,144,145,146]. Three types of strategies have been conducted: in situ gene therapy product injection (by means of EUS), vaccination (intradermal or subcutaneous route) and oncolytic virus administration. Gene transfer has been performed either with adenovirus or with synthethic vectors. All treatments have been well tolerated (i.e. no serious adverse event). As observed for pre-clinical studies the transgene provided active immunotherapy with interleukin production but also after suicide genes expression. Oncoviruses were adenovirus Onyx-15, adenovirus H101, herpes virus plus GM-CSF (Imlygic® Amgen, Thousand Oaks, CA, USA) and reovirus Reolysin® Oncolytics Biothech Inc., Calgary, AB, Canada). In most of the programs, patients frequently received chemotherapy or radio-chemotherapy. Gene therapy in these cases can be considered as an “add-on strategy”. All these strategies are schematized in Figure 1. We also developed a gene therapy program, the Thergap Trials, which are based on chemosensitization to gemcitabine. In collaboration with InvivoGen Company, a gene therapy product GMP was produced and tested during pre-clinical phases. We thus devised a novel approach based on intratumoral therapeutic gene transfer to inhibit PDAC tumor growth. We identified SSTR2 and DCK::UMK as complementary therapeutic genes to sensitize cancer cells to chemotherapy, to induce tumor regression and to block metastatic dissemination in experimental models [23,24,25]. We previously demonstrated that in situ gene transfer of SSTR2 (somatostatin receptor subtype 2 gene) induced a significant antitumor effect (with a bystander effect) together with antiangiogenic properties and chemosensitization to gemcitabine. The DCK::UMK (deoxycitine kinase::uridyl monophosphate kinase) fusion gene, by phosphorylating twice intracellular gemcitabine, also induced a significant tumor regression and bystander effect in our relevant models of PDAC. We thus generated a clinical-grade gene therapy product, namely CYL-02, encoding for the above-mentioned genes delivered by non-viral vectors, that was first validated in experimental models. We next administrated CYL-02 to patients with advanced PDAC and demonstrate that gene therapy is feasible and well tolerated without serious adverse events. In selected patients, tumor volume and cancer biomarker levels decreased, metastatic spread was blocked while median overall and progression-free survivals were significantly improved when compared to historical series of patients. We identified circulating Alpha-2-macroglobulin as predictive of treatment response [144]. These promising results warrant further evaluation in selected PDAC patients during an ongoing phase 2 gene therapy trials (Thergap-2 trial, supported in part by the InvivoGen Company (Toulouse, France)—Table 4).
Table 3.
Main published clinical trials of gene therapy for pancreatic cancer.
| Author, Year [Reference] | Phase, (Patient Number-StAge III–IV) | Route | Vector/Strategy | Results |
|---|---|---|---|---|
| Gilly et al., 1999 [120] | I/II (7) | IT, during surgery | AdV/Interleukin 2 | Well tolerated, 1 tumor regression |
| Mulvihill et al., 2001 [121] | I (3) | IT (CT, surgery) | AdV/ONYX-015 | No objective response |
| Löhr et al., 2001 Salmons et al.,2003 [122,123] | I/II (14) | angiography | Lipofectamine/Cyto. P450 (*) | Well tolerated, 2 PR, 12 SD, OS survival: 10 months |
| Pecher et al., 2002 [124] | I/II (10) | SC | Cationic liposome/dendritic cells transfected with MUC1 cDNA | No side effects, 9 PD, 1 SD |
| Hecht et al., 2003 [107] | I/II (21) | IT, EUS-guided | AdV/ONYX-015 + gemcitabine | 2 duodenal perforations, 4 PR, 6 SD, 11 PD |
| Gordon et al., 2003 [125] | I (3) | Intravenous | Rv/Rexin-G | Well tolerated 2 SD (4, 5 months) 1 PD (21 months) |
| Sangro et al., 2004 [126] | I (7) | IT, EUS-guided | AdV/Interleukin 12 | Well tolerated, SD |
| Senzer et al., 2004 [127] | I (30) | IT EUS-and CT-guided | TNFerade | 22% fever, 19% chills 5 complete responses and 16 PR |
| Mazzolini et al., 2005 [128] | I (11) | IT, EUS-guided | Autologous dendritic cells transfected with an Adv encoding interleukin-12 gene | Well tolerated, 1 PR, 2 SD, 8 PD |
| Kaufman et al., 2007 [129] | 1 (10) | ID | Vaccination with Vaccinia and Pox virus expressing CEA MUC-1) and co-stimulatory molecules | 10/10 Antibody responses against vaccinia virus 6, 3 months of OS |
| Galanis et al., 2008 [130] | I (12) | IV | Rv/Rexin-G | Well tolerated, no evidence of anti-tumor activity |
| Laheru et al., 2008 [131] | I (50) | ID | Plasmid/GVAX Arm A: GVAX alone Arm B: GVAX with cyclophosphamide |
Well tolerated, Median survival : A: 2.3 months B: 4.3 months |
| Chawla et al., 2010 [132] | I/II (9) | IV | Rv/Rexin-G at two dosages | Well tolerated, 8 SD, 1 PR, Median overall survival between 4.3 and 9.2 months |
| Nakao et al., 2011 [133] | I (6) | IT during surgery | HF10 oncolytic herpes virus | Well tolerated, 3 SD, 1 PR, 2 PD |
| Lutz et al., 2011 [134] | II (60) | ID | Plasmid/GVAX with chemoradiation | Well tolerated Median disease free survival 17.3 months (resected tumors) |
| Kubusho et al., 2012 [135] | I (7 with mutated KRAS) | SC | EBV/Peripheral blood lymphocytes genetically modified with an episomal EBV expressing Ras mutant | No acute EBV infection 4 SD, 6 T cell response |
| Hanna et al., 2012 [136] | I/Iia (9) | IT EUS- and CT-guided | Plasmid / expression of diphtheria-toxin gene | Well tolerated, 3 PR |
| Hecht et al., 2012 [137] | I (50) | IT EUS- and CT-guided | AdV/TNFerade with chemoradiation (5 FU) | 21 mild adverse events 1 complete response, 7 PR, 12 SD, 19 PD Median OS: 10 months |
| Le et al., 2012 [138] | I (26) | IV (GI metastatic cancers included) | Attenuated listeria vaccine expressing (17) or not (9) mesotheline | Immune response, OS > 15 months in 10 patients |
| Le et al., 2013 [139] | SC | A: Ipilimumab alone B: GVAX + Ipilimumab | 20% Grade 3/4 immune-relate adverse events A and B : SD OS: 3.2 vs 5.7 (NS) 1 year survival 7 Vas 27% | |
| Hardacre et al., 2013 [140] | II (70 after tumor resection) | SC | Algenpantucel-L + gemcitabine + 5FU | 12% induration at the injection site one year OS: 86% |
| Herman et al., 2013 [141] | III (304) | IT EUS- and CT-guided | AdV/TNFerade + Chemoradiation Vs chemoradiation | More grade 1 to 2 AE in the TNFerade + chemoradiation arm OS: 10 months, both arms |
| Löhr et al., 2014 [142] | II (13) | angiography | Lipofectamine/Cyto. P450 (*) | Well tolerated 4 PR, 4 PD, 5 SD OS: 9.5 months |
| Aguilar et al., 2015 [143] | I (24) | IT EUS- and CT-guided | AdV/HSV thymidine kinase Arm A borderline: HSK −TK + valacyclovir Arm B non resectable: HSV − TK + valacyclovir + chemoradiation | Well tolerated Median OS: 10 months in Arm A and 12 months in arm B with 25% of RECIST response. |
| Buscail et al., 2015 [144] | I (22) | IT EUS- guided | Complexed plasmid/CYL-02 + gemcitabine | Well tolerated, 12 SD, OS in non metastatic patients 12.6 months |
| Golan et al., 2015 [145] | I/IIa (15) | IT EUS- and CT-guided | IT placement of SiG12-LODER® + gemcitabine | 90% minimal AE 2 PR, 10 SD, 2 PR OS: 15 months |
| Le et al., 2015 [119] | II (90) | SC | GVAX + CRS 2017 (Arm A) vs GVAX alone (Arm B) | Local reactions 77%, general minor AE 53–62% OS: 6.1 months in arm A Vs 3.9 months in arm B |
| Noonan et al., 2016 [146] | II (73) | IV | Arm A: Reolysin + paclitaxel + carboplatin Arm B: paclitaxel + carboplatin | Well tolerated No difference I term of PFS and OS between the two arms |
IT: intratumoral route; ID: intradermal route; IV: intravenous route; SC: subcutaneous route; CT: CT scan; AdV: adenoviral vector; Rv: retroviral vector; PR: partial response; SD: stable disease; PD: progressive disease; PFS: progression free survival; OS: overall survival; AE: adverse event ; RECIST: Response Evaluation Criteria In Solid Tumors; ONYX-1: E1B-55 kDa region- deleted adenovirus that selectively replicates in and lyses tumor cells with abnormalities in p53 function; *: Encapsuled genetically modified allogenic fiboblastic cells (in cellulose sulfate) that expressed cytochrome P450 enzyme transforming the Isofosfamide IV; TNFerade is an adenovector encoding human human tumor necrosis alfa with a radiation-inducible promoter. Administration was combined with chemoradiation therapy; Rexin-G: cytocidal retrovirus gene construct encoding mutant cyclin G1 gene; GVAX: allogenic pancreatic cancer cells expressing and secreting GM-CSF (plasmidic transfection); Algenpantucel-L: composed of two human pancreatic cancer cell lines genetically engineered to express αGal using a retroviral transfer; HSV-TK: herpes virus thymidine kinase that phosphorylate an antiherpetic prodrug valacyclovir becoming toxic for pancreatic cancer cells expressing the suicide gene HSV-TK; CYL-02: gene therapy product combining SSTR2 (human somatostatin receptor subtype 2), DCK (deoxycytine kinase) and UMK (uridyl moniphosphate kinase) genes and polycationic polyethylenimine vector with antioncogenic and chemosensitizing (gemcitabine) activities; LODER®: miniature biodegradable implant (intratumoral insertion) containing and releasing within 4 months a siRNA drug against Kras (mutation G12D); CRS 2017: live-attenuated listeria monocytogene genetically ingeneered to express mesothelin; Reolysin®: pelareorep, Reovirus Serotype3-Dearing Strain.
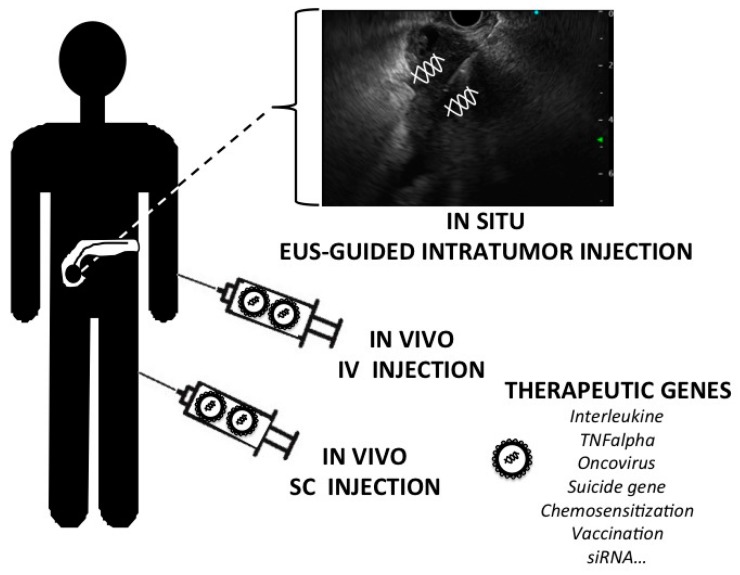
Figure 1.
Clinical approaches of gene therapy for pancreatic cancer: type of gene therapy and routes of administration, main therapeutic genes. (EUS: endoscopic ultrasound; IV: intravenous; SC: subcutaneous).
Table 4.
Main on-going clinical trials of gene therapy for pancreatic cancer (source ClinicalTrials.gov, available online: https://clinicaltrials.gov/).
| Identification Number Date of Start Trial Stage | Phase, (Patient Number) | Route | Vector/Strategy |
|---|---|---|---|
| NCT00711997 August 2009 Completed | I/II (9) | IT CT- or US- or EUS-guided | jetPEI/DTA-H19 |
| NCT00669734 February 2010 Active, not recruiting | I (18) | IT or SC | Lowlpox and Vaccinia virus/PANVAC-V+ PANVAC-F + GM-CSF) |
| NCT01088789 April 2010 Recruiting | II (72) | ID | PANC 10.05 pcDNA-1/GM-Neo and PANC 6.03 pcDNA-1 neo vaccine (allogenic pancreatic tumor cell vaccine transfected with the GM-CSF gene) +/− cyclophosphamide |
| NCT01191684 October 2011 Completed | I (2) | SC | Vaccinia virus/Vaccinia virus ankara vaccine expressing p53 |
| NCT01583686 April 2012 Recruiting | I/II (136) | IV | Rv/Anti-mesothelin CAR-T cell |
| NCT01836432 May 2013 Active, not recruiting | III (302) | ID | Rv/Algenpantucel-L +/− chemotherapy (Folfirinox or Gemcitabine + Nab-paclitaxel) |
| NCT02239861 September 2014 Recruiting | I (18) | IV | Tumor-associated antigen (TAA)-specific cytotoxic T lymphocytes 5 common TAAs: NY-ESO-1, MAGEA4, PRAME, Survivin and SSX. |
| NCT02340117 January 2015 Recruiting | II (28) | IV | Cationic liposome/SGT-53 + Gemcitabine + Nab-Paclitaxel |
| NCT02416466 April 2015 Active, not recruiting | I (8) | Percutan-eous hepatic artery infusion | SIR-Spheres microspheres/Anti-CEA CAR-T cells |
| NCT02465983 May 2015 Active, not recruiting | I (12) | IV | CART-meso-19 T cells + Cyclophosphamide |
| NCT02432963 November 2015 Recruiting | I (12) | ID | Vaccinia Ankara/Modified Vaccinia Ankara vector expressing full length wild type human p53 + Pembrolizumab |
| NCT02806687 June 2016 Recruiting | II (100) | IT EUS-guided | JetPEI/CYL-02 + Gemcitabine |
| NCT02576665 July 2016 Recruiting | I (26) | IV or IT | Rv/Toca 511 + Toca FC |
| NCT02894944 August 2016 Recruiting | I (9) | AdV/Theragene + Chemotherapy | |
| NCT02705196 November 2016 Not yet recruiting | I/II (26) | IT EUS- or US-guided | AdV/LOAd703 + Gemcitabine + Nab-paclitaxel |
IT: intratumoral route; ID: intradermal route; IV: intravenous route ; SC: subcutaneous route ; CT: CT scan; AdV: adenoviral vector; Rv: retroviral vector; CART-meso-19 T cells: patients′ own T cells modified to express a receptor specific to the mesothelin protein and receptor specific to CD19; DTA-H19: a doubled stranded DNA plasmid that carries the gene for the diphtheria toxin A (DT-A) chain under the regulation of the H19 promoter sequence; GVAX: allogenic pancreatic cancer cells expressing and secreting GM-CSF (plasmidic transfection); Algenpantucel-L: composed of two human pancreatic cancer cell lines genetically engineered to express αGal using a retroviral transfer. HSV-TK: herpes virus thymidine kinase that phosphorylate an antiherpetic prodrug valacyclovir becoming toxic for pancreatic cancer cells expressing the suicide gene HSV-TK; ADV-TK: Adenovirus-mediated herpes simplex virus thymidine kinase gene therapy; CYL-02: gene therapy product combining SSTR2 (human somatostatin receptor subtype 2), DCK (deoxycytine kinase) and UMK (uridyl moniphosphate kinase) genes and polycationic polyethylenimine vector with antioncogenic and chemosensitizing (gemcitabine) activities; LOAd703 : oncolytic adenovirus modified to include additional immune system stimulators; PANVAC-F: Intratumoral Recombinant Fowlpox PANVAC. PANVAC-V: Subcutaneous Recombinant Vaccinia PANVAC; SGT-53: complex of cationic liposome encapsulating a normal human wild type p53 DNA sequence in a plasmid backbone; Theragene: Replication-competent Adenovirus-mediated Double Suicide Gene Therapy (Theragene®, Ad5-yCD/mutTKSR39rep-ADP – Theragen Pharamceuticals Inc. San Diego, CA, USA); Toca 511: a retroviral replicating vector encoding a modified yeast cytosine deaminase (CD) gene; The CD gene converts the antifungal 5-flurocytosine (5FC) to the anticancer drug 5-FU in cells that have been infected by the Toca 511 vector; Toca FC: an extended-release formulation of flucytosine (Tocagen Company, San Diego, CA, USA).
We can conclude from all these trials that the first results are encouraging for most of phase 1 but remain “fragile” in phase 2, in which the difference between standard treatment and gene therapy did not reach high significance. However, that requires trials with larger groups and selected patients such as first line treatment. In Table 4 the main protocol inserted by investigators in the clinical trial web is detailed, recruiting or not, but not published. Besides classical approaches (in situ gene transfer, vaccination and oncoviral therapy) new concepts of adoptive immunotherapy through CAR-T technology reached the clinical stage.
6. Conclusions
We have come a long way since the first gene transfer tests in vitro and in vivo. We are now at the stage of clinical trials for which a long and difficult process has to be overcome as with drug development: proof of concept, preclinical safety tests, early phases and phase 2. In addition these are unconventional and genetically modified medication adding a degree of complexity. The severity of the disease requires urgently therapeutic innovations in combination with conventional chemotherapy, the contribution of which is modest. From these therapeutic combinations will surely come the solution. Gene therapy also opens up the field of oncovirotherapy and adoptive immunotherapy that diversifies and strengthens this approach. Open question: Will the future give us reason?
Abbreviations
| PDAC | Pancreatic Ductal Adenocarcinoma |
| EUS | Endoscopic Ultrasound |
| CAR | Chimeric Antigen Receptors |
| VEGF | Vascular endothetial Growth Factor |
| EGF | Epidermal Growth Factor |
| MUC-1 | Mucin 1 |
| HSV-TK | Herpes Virus Thymidine Kinase |
| TNF | Tumor Necrosis Factor |
| CAR | Chimeric Antigen Receptor |
| GM-CSF | Granulocyte-Macrophage Colony-Stimulating Factor |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Ryan D.P., Hong T.S., Bardeesy N. Pancreatic adenocarcinoma. N. Engl. J. Med. 2014;371:2140–2141. doi: 10.1056/NEJMra1404198. [DOI] [PubMed] [Google Scholar]
- 2.Rahib L., Smith B.D., Aizenberg R., Rosenzweig A.B., Fleshman J.M., Matrisian L.M. Projecting cancer incidence and deaths to 2030: The unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–2921. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 3.Burris H.A., Moore M.J., Andersen J., Green M.R., Rothenberg M.L., Modiano M.R., Cripps M.C., Portenoy R.K., Storniolo A.M., Tarassoff P., et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: A randomized trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 1997;15:2403–2413. doi: 10.1200/JCO.1997.15.6.2403. [DOI] [PubMed] [Google Scholar]
- 4.Conroy T., Desseigne F., Ychou M., Bouché O., Guimbaud R., Bécouarn Y., Adenis A., Raoul J.L., Gourgou-Bourgade S., de la Fouchardière C., et al. Groupe Tumeurs Digestives of Unicancer; PRODIGE Intergroup FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 2011;364:1817–1825. doi: 10.1056/NEJMoa1011923. [DOI] [PubMed] [Google Scholar]
- 5.Von Hoff D.D., Ervin T., Arena F.P., Chiorean E.G., Infante J., Moore M., Seay T., Tjulandin S.A., Ma W.W., Saleh M.N., et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ducreux M., Boige V., Malka D. Treatment of advanced pancreatic cancer. Semin. Oncol. 2007;34:S25–S30. doi: 10.1053/j.seminoncol.2007.01.006. [DOI] [PubMed] [Google Scholar]
- 7.Drew Y., Mulligan E.A., Vong W.T., Thomas H.D., Kahn S., Kyle S., Mukhopadhyay A., Los G., Hostomsky Z., Plummer E.R., et al. Therapeutic potential of poly(ADP-ribose) polymerase inhibitor AG014699 in human cancers with mutated or methylated BRCA1 or BRCA2. J. Natl. Cancer Inst. 2011;103:334–346. doi: 10.1093/jnci/djq509. [DOI] [PubMed] [Google Scholar]
- 8.Provenzano P.P., Cuevas C., Chang A.E., Goel V.K., von Hoff D.D., Hingorani S.R. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rhim A.D., Oberstein P.E., Thomas D.H., Mirek E.T., Palermo C.F., Sastra S.A., Dekleva E.N., Saunders T., Becerra C.P., Tattersall I.W., et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J., Jiao Y., dal Molin M., Maitra A., de Wilde R.F., Wood L.D., Eshleman J.R., Goggins M.G., Wolfgang C.L., Canto M.I., et al. Whole-exome sequencing of neoplastic cysts of the pancreas reveals recurrent mutations in components of ubiquitin-dependent pathways. Proc. Natl. Acad. Sci. USA. 2011;108:21188–21193. doi: 10.1073/pnas.1118046108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waddell N., Pajic M., Patch A.M., Chang D.K., Kassahn K.S., Bailey P., Johns A.L., Miller D., Nones K., Quek K., et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buscail L., Faure P., Bournet B., Selves J., Escourrou J. Interventional endoscopic ultrasound in pancreatic diseases. Pancreatology. 2006;6:7–16. doi: 10.1159/000090022. [DOI] [PubMed] [Google Scholar]
- 13.Koorstra J.B.M., Hustinx S.R., Offerhaus G.J.A., Maitra A. Pancreatic carcinogenesis. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al. 2008;8:110–125. doi: 10.1159/000123838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klemm F., Joyce J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015;25:198–213. doi: 10.1016/j.tcb.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hahn S.A., Schmiegel W.H. Recent discoveries in cancer genetics of exocrine pancreatic neoplasia. Digestion. 1998;59:493–501. doi: 10.1159/000007526. [DOI] [PubMed] [Google Scholar]
- 16.Delpu Y., Hanoun N., Lulka H., Sicard F., Selves J., Buscail L., Torrisani J., Cordelier P. Genetic and epigenetic alterations in pancreatic carcinogenesis. Curr. Genom. 2011;12:15–24. doi: 10.2174/138920211794520132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Forbes S.A., Bindal N., Bamford S., Cole C., Kok C.Y., Beare D., Jia M., Shepherd R., Leung K., Menzies A., et al. COSMIC: Mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39:D945–D950. doi: 10.1093/nar/gkq929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Magliano M.P., Logsdon C.D. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bournet B., Buscail C., Muscari F., Cordelier P., Buscail L. Targeting KRAS for diagnosis, prognosis, and treatment of pancreatic cancer: Hopes and realities. Eur. J. Cancer Oxf. Engl. 1990. 2015;54:75–83. doi: 10.1016/j.ejca.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 20.Hingorani S.R., Petricoin E.F., Maitra A., Rajapakse V., King C., Jacobetz M.A., Ross S., Conrads T.P., Veenstra T.D., Hitt B.A., et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/S1535-6108(03)00309-X. [DOI] [PubMed] [Google Scholar]
- 21.Aguirre A.J., Bardeesy N., Sinha M., Lopez L., Tuveson D.A., Horner J., Redston M.S., DePinho R.A. Activated Kras and Ink4a/Arf deficiency cooperate to produce metastatic pancreatic ductal adenocarcinoma. Genes Dev. 2003;17:3112–3126. doi: 10.1101/gad.1158703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bardeesy N., Aguirre A.J., Chu G.C., Cheng K.H., Lopez L.V., Hezel A.F., Feng B., Brennan C., Weissleder R., Mahmood U., et al. Both p16(Ink4a) and the p19(Arf)-p53 pathway constrain progression of pancreatic adenocarcinoma in the mouse. Proc. Natl. Acad. Sci. USA. 2006;103:5947–5952. doi: 10.1073/pnas.0601273103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rochaix P., Delesque N., Estève J.P., Saint-Laurent N., Voight J.J., Vaysse N., Susini C., Buscail L. Gene therapy for pancreatic carcinoma: Local and distant antitumor effects after somatostatin receptor sst2 gene transfer. Hum. Gene Ther. 1999;10:995–1008. doi: 10.1089/10430349950018391. [DOI] [PubMed] [Google Scholar]
- 24.Vernejoul F., Faure P., Benali N., Calise D., Tiraby G., Pradayrol L., Susini C., Buscail L. Antitumor Effect of in Vivo Somatostatin Receptor Subtype 2 Gene Transfer in Primary and Metastatic Pancreatic Cancer Models. Cancer Res. 2002;62:6124–6131. [PubMed] [Google Scholar]
- 25.Benali N., Cordelier P., Calise D., Pages P., Rochaix P., Nagy A., Esteve J.P., Pour P.M., Schally A.V., Vaysse N., et al. Inhibition of growth and metastatic progression of pancreatic carcinoma in hamster after somatostatin receptor subtype 2 (SST2) gene expression and administration of cytotoxic somatostatin analog AN-238. Proc. Natl. Acad. Sci. USA. 2000;97:9180–9185. doi: 10.1073/pnas.130196697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Thorne B., Takeya R., Vitelli F., Swanson X. Gene Therapy. Adv. Biochem. Eng. Biotechnol. 2017 doi: 10.1007/10_2016_53. [DOI] [PubMed] [Google Scholar]
- 27.Hacein-Bey-Abina S., Hauer J., Lim A., Picard C., Wang G.P., Berry C.C., Martinache C., Rieux-Laucat F., Latour S., Belohradsky B.H., et al. Efficacy of gene therapy for X-linked severe combined immunodeficiency. N. Engl. J. Med. 2010;363:355–364. doi: 10.1056/NEJMoa1000164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavazzana-Calvo M., Payen E., Negre O., Wang G., Hehir K., Fusil F., Down J., Denaro M., Brady T., Westerman K., et al. Transfusion independence and HMGA2 activation after gene therapy of human β-thalassaemia. Nature. 2010;467:318–322. doi: 10.1038/nature09328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yin H., Kanasty R.L., Eltoukhy A.A., Vegas A.J., Dorkin J.R., Anderson D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014;15:541–555. doi: 10.1038/nrg3763. [DOI] [PubMed] [Google Scholar]
- 30.Doody A.M., Korley J.N., Dang K.P., Zawaneh P.N., Putnam D. Characterizing the structure/function parameter space of hydrocarbon-conjugated branched polyethylenimine for DNA delivery in vitro. J. Control. Release. 2006;116:227–237. doi: 10.1016/j.jconrel.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 31.Forrest M.L., Meister G.E., Koerber J.T., Pack D.W. Partial acetylation of polyethylenimine enhances in vitro gene delivery. Pharm. Res. 2004;21:365–371. doi: 10.1023/B:PHAM.0000016251.42392.1e. [DOI] [PubMed] [Google Scholar]
- 32.Thomas M., Klibanov A.M. Enhancing polyethylenimine’s delivery of plasmid DNA into mammalian cells. Proc. Natl. Acad. Sci. USA. 2002;99:14640–14645. doi: 10.1073/pnas.192581499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oskuee R.K., Dehshahri A., Shier W.T., Ramezani M. Alkylcarboxylate grafting to polyethylenimine: A simple approach to producing a DNA nanocarrier with low toxicity. J. Gene Med. 2009;11:921–932. doi: 10.1002/jgm.1374. [DOI] [PubMed] [Google Scholar]
- 34.Midoux P., Pichon C., Yaouanc J.J., Jaffrès P.A. Chemical vectors for gene delivery: A current review on polymers, peptides and lipids containing histidine or imidazole as nucleic acids carriers. Br. J. Pharmacol. 2009;157:166–178. doi: 10.1111/j.1476-5381.2009.00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bertrand E., Gonçalves C., Billiet L., Gomez J.P., Pichon C., Cheradame H., Midoux P., Guégan P. Histidinylated linear PEI: A new efficient non-toxic polymer for gene transfer. Chem. Commun. Camb. Engl. 2011;47:12547–12549. doi: 10.1039/c1cc15716g. [DOI] [PubMed] [Google Scholar]
- 36.Nayerossadat N., Maedeh T., Ali P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012;1:27. doi: 10.4103/2277-9175.98152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Touchefeu Y., Harrington K.J., Galmiche J.P., Vassaux G. Review article: Gene therapy, recent developments and future prospects in gastrointestinal oncology. Aliment. Pharmacol. Ther. 2010;32:953–968. doi: 10.1111/j.1365-2036.2010.04424.x. [DOI] [PubMed] [Google Scholar]
- 38.Liu S.X., Xia Z.S., Zhong Y.Q. Gene therapy in pancreatic cancer. World J. Gastroenterol. 2014;20:13343–13368. doi: 10.3748/wjg.v20.i37.13343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hanoun N., Gayral M., Pointreau A., Buscail L., Cordelier P. Initial Characterization of Integrase-Defective Lentiviral Vectors for Pancreatic Cancer Gene Therapy. Hum. Gene Ther. 2016;27:184–192. doi: 10.1089/hum.2015.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vassaux G., Nitcheu J., Jezzard S., Lemoine N.R. Bacterial gene therapy strategies. J. Pathol. 2006;208:290–298. doi: 10.1002/path.1865. [DOI] [PubMed] [Google Scholar]
- 41.Riley M.K., Vermerris W. Recent Advances in Nanomaterials for Gene Delivery-A Review. Nanomater. Basel Switz. 2017;7:94. doi: 10.3390/nano7050094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kobayashi S., Shirasawa H., Sashiyama H., Kawahira H., Kaneko K., Asano T., Ochiai T. P16INK4a expression adenovirus vector to suppress pancreas cancer cell proliferation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1999;5:4182–4185. [PubMed] [Google Scholar]
- 43.Joshi U.S., Dergham S.T., Chen Y.Q., Dugan M.C., Crissman J.D., Vaitkevicius V.K., Sarkar F.H. Inhibition of pancreatic tumor cell growth in culture by p21WAF1 recombinant adenovirus. Pancreas. 1998;16:107–113. doi: 10.1097/00006676-199803000-00001. [DOI] [PubMed] [Google Scholar]
- 44.Craig C., Kim M., Ohri E., Wersto R., Katayose D., Li Z., Choi Y.H., Mudahar B., Srivastava S., Seth P., et al. Effects of adenovirus-mediated p16INK4A expression on cell cycle arrest are determined by endogenous p16 and Rb status in human cancer cells. Oncogene. 1998;16:265–272. doi: 10.1038/sj.onc.1201493. [DOI] [PubMed] [Google Scholar]
- 45.Hill R., Rabb M., Madureira P.A., Clements D., Gujar S.A., Waisman D.M., Giacomantonio C.A., Lee P.W.K. Gemcitabine-mediated tumour regression and p53-dependent gene expression: Implications for colon and pancreatic cancer therapy. Cell Death Dis. 2013;4:e791. doi: 10.1038/cddis.2013.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gupta S., Sathishkumar S., Ahmed M.M. Influence of cell cycle checkpoints and p53 function on the toxicity of temozolomide in human pancreatic cancer cells. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al. 2010;10:565–579. doi: 10.1159/000317254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Azmi A.S., Philip P.A., Aboukameel A., Wang Z., Banerjee S., Zafar S.F., Goustin A.S., Almhanna K., Yang D., Sarkar F.H., et al. Reactivation of p53 by novel MDM2 inhibitors: Implications for pancreatic cancer therapy. Curr. Cancer Drug Targets. 2010;10:319–331. doi: 10.2174/156800910791190229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Shen W., Tao G.Q., Li D.C., Zhu X.G., Bai X., Cai B. Inhibition of pancreatic carcinoma cell growth in vitro by DPC4 gene transfection. World J. Gastroenterol. 2008;14:6254–6260. doi: 10.3748/wjg.14.6254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kuo C.J., Farnebo F., Yu E.Y., Christofferson R., Swearingen R.A., Carter R., von Recum H.A., Yuan J., Kamihara J., Flynn E., et al. Comparative evaluation of the antitumor activity of antiangiogenic proteins delivered by gene transfer. Proc. Natl. Acad. Sci. USA. 2001;98:4605–4610. doi: 10.1073/pnas.081615298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ogawa T., Takayama K., Takakura N., Kitano S., Ueno H. Anti-tumor angiogenesis therapy using soluble receptors: Enhanced inhibition of tumor growth when soluble fibroblast growth factor receptor-1 is used with soluble vascular endothelial growth factor receptor. Cancer Gene Ther. 2002;9:633–640. doi: 10.1038/sj.cgt.7700478. [DOI] [PubMed] [Google Scholar]
- 51.Zhang X., Xu J., Lawler J., Terwilliger E., Parangi S. Adeno-associated virus-mediated antiangiogenic gene therapy with thrombospondin-1 type 1 repeats and endostatin. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2007;13:3968–3976. doi: 10.1158/1078-0432.CCR-07-0245. [DOI] [PubMed] [Google Scholar]
- 52.Shan Y., Fang Y., Wang X., Jin R., Zhang Q., Andersson R. Experimental studies on treatment of pancreatic cancer with double-regulated duplicative adenovirus AdTPHre-hEndo carrying human endostatin gene. Pancreatol. Off. J. Int. Assoc. Pancreatol. IAP Al. 2013;13:393–400. doi: 10.1016/j.pan.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 53.Tysome J.R., Briat A., Alusi G., Cao F., Gao D., Yu J., Wang P., Yang S., Dong Z., Wang S., et al. Lister strain of vaccinia virus armed with endostatin-angiostatin fusion gene as a novel therapeutic agent for human pancreatic cancer. Gene Ther. 2009;16:1223–1233. doi: 10.1038/gt.2009.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rigg A.S., Lemoine N.R. Adenoviral delivery of TIMP1 or TIMP2 can modify the invasive behavior of pancreatic cancer and can have a significant antitumor effect in vivo. Cancer Gene Ther. 2001;8:869–878. doi: 10.1038/sj.cgt.7700387. [DOI] [PubMed] [Google Scholar]
- 55.Katz M.H., Spivack D.E., Takimoto S., Fang B., Burton D.W., Moossa A.R., Hoffman R.M., Bouvet M. Gene therapy of pancreatic cancer with green fluorescent protein and tumor necrosis factor-related apoptosis-inducing ligand fusion gene expression driven by a human telomerase reverse transcriptase promoter. Ann. Surg. Oncol. 2003;10:762–772. doi: 10.1245/ASO.2003.01.021. [DOI] [PubMed] [Google Scholar]
- 56.Wang J., Lu X.X., Chen D.Z., Li S.F., Zhang L.S. Herpes simplex virus thymidine kinase and ganciclovir suicide gene therapy for human pancreatic cancer. World J. Gastroenterol. 2004;10:400–403. doi: 10.3748/wjg.v10.i3.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fogar P., Greco E., Basso D., Habeler W., Navaglia F., Zambon C.F., Tormen D., Gallo N., Cecchetto A., Plebani M., et al. Suicide gene therapy with HSV-TK in pancreatic cancer has no effect in vivo in a mouse model. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2003;29:721–730. doi: 10.1016/j.ejso.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 58.Greco E., Fogar P., Basso D., Stefani A.L., Navaglia F., Zambon C.F., Mazza S., Gallo N., Piva M.G., Scarpa A., Pedrazzoli S., et al. Retrovirus-mediated herpes simplex virus thymidine kinase gene transfer in pancreatic cancer cell lines: An incomplete antitumor effect. Pancreas. 2002;25:e21–e29. doi: 10.1097/00006676-200208000-00020. [DOI] [PubMed] [Google Scholar]
- 59.Erbs P., Regulier E., Kintz J., Leroy P., Poitevin Y., Exinger F., Jund R., Mehtali M. In vivo cancer gene therapy by adenovirus-mediated transfer of a bifunctional yeast cytosine deaminase/uracil phosphoribosyltransferase fusion gene. Cancer Res. 2000;60:3813–3822. [PubMed] [Google Scholar]
- 60.Fogar P., Navaglia F., Basso D., Greco E., Zambon C.F., Fadi E., Falda A., Stranges A., Vannozzi F., Danesi R., et al. Suicide gene therapy with the yeast fusion gene cytosine deaminase/uracil phosphoribosyltransferase is not enough for pancreatic cancer. Pancreas. 2007;35:224–231. doi: 10.1097/mpa.0b013e3180622519. [DOI] [PubMed] [Google Scholar]
- 61.Grove J.I., Searle P.F., Weedon S.J., Green N.K., McNeish I.A., Kerr D.J. Virus-directed enzyme prodrug therapy using CB1954. Anticancer. Drug Des. 1999;14:461–472. [PubMed] [Google Scholar]
- 62.Green N.K., Youngs D.J., Neoptolemos J.P., Friedlos F., Knox R.J., Springer C.J., Anlezark G.M., Michael N.P., Melton R.G., Ford M.J., et al. Sensitization of colorectal and pancreatic cancer cell lines to the prodrug 5-(aziridin-1-yl)-2,4-dinitrobenzamide (CB1954) by retroviral transduction and expression of the E. coli nitroreductase gene. Cancer Gene Ther. 1997;4:229–238. [PubMed] [Google Scholar]
- 63.Carrió M., Visa J., Cascante A., Estivill X., Fillat C. Intratumoral activation of cyclophosphamide by retroviral transfer of the cytochrome P450 2B1 in a pancreatic tumor model. Combination with the HSVtk/GCV system. J. Gene Med. 2002;4:141–149. doi: 10.1002/jgm.247. [DOI] [PubMed] [Google Scholar]
- 64.Löhr M., Müller P., Karle P., Stange J., Mitzner S., Jesnowski R., Nizze H., Nebe B., Liebe S., Salmons B., et al. Targeted chemotherapy by intratumour injection of encapsulated cells engineered to produce CYP2B1, an ifosfamide activating cytochrome P450. Gene Ther. 1998;5:1070–1078. doi: 10.1038/sj.gt.3300671. [DOI] [PubMed] [Google Scholar]
- 65.Sicard F., Gayral M., Lulka H., Buscail L., Cordelier P. Targeting miR-21 for the therapy of pancreatic cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2013;21:986–994. doi: 10.1038/mt.2013.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brummelkamp T.R., Bernards R., Agami R. Stable suppression of tumorigenicity by virus-mediated RNA interference. Cancer Cell. 2002;2:243–247. doi: 10.1016/S1535-6108(02)00122-8. [DOI] [PubMed] [Google Scholar]
- 67.Réjiba S., Wack S., Aprahamian M., Hajri A. K-ras oncogene silencing strategy reduces tumor growth and enhances gemcitabine chemotherapy efficacy for pancreatic cancer treatment. Cancer Sci. 2007;98:1128–1136. doi: 10.1111/j.1349-7006.2007.00506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Morioka C.Y., Machado M.C.C., Saito S., Nakada Y., Matheus A.S., Jukemura J., Bacchella T., Takahara T., Watanabe A. Suppression of invasion of a hamster pancreatic cancer cell line by antisense oligonucleotides mutation-matched to K-ras gene. Vivo Athens Greece. 2005;19:535–538. [PubMed] [Google Scholar]
- 69.Shen Y.M., Yang X.C., Yang C., Shen J.K. Enhanced therapeutic effects for human pancreatic cancer by application K-ras and IGF-IR antisense oligodeoxynucleotides. World J. Gastroenterol. 2008;14:5176–5185. doi: 10.3748/wjg.14.5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Alberts S.R., Schroeder M., Erlichman C., Steen P.D., Foster N.R., Moore D.F., Rowland K.M., Nair S., Tschetter L.K., Fitch T.R. Gemcitabine and ISIS-2503 for patients with locally advanced or metastatic pancreatic adenocarcinoma: A North Central Cancer Treatment Group phase II trial. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004;22:4944–4950. doi: 10.1200/JCO.2004.05.034. [DOI] [PubMed] [Google Scholar]
- 71.Chew W.L., Tabebordbar M., Cheng J.K.W., Mali P., Wu E.Y., Ng A.H.M., Zhu K., Wagers A.J., Church G.M. A multifunctional AAV-CRISPR-Cas9 and its host response. Nat. Methods. 2016;13:868–874. doi: 10.1038/nmeth.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Song M. The CRISPR/Cas9 system: Their delivery, in vivo and ex vivo applications and clinical development by startups. Biotechnol. Prog. 2017 doi: 10.1002/btpr.2484. [DOI] [PubMed] [Google Scholar]
- 73.Mout R., Ray M., Lee Y.W., Scaletti F., Rotello V.M. In Vivo Delivery of CRISPR/Cas9 for Therapeutic Gene Editing: Progress and Challenges. Bioconjug. Chem. 2017;28:880–884. doi: 10.1021/acs.bioconjchem.7b00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang Z., Tomitaka A., Raymond A., Nair M. Current application of CRISPR/Cas9 gene-editing technique to eradication of HIV/AIDS. Gene Ther. 2017 doi: 10.1038/gt.2017.35. [DOI] [PubMed] [Google Scholar]
- 75.Chiou S.H., Winters I.P., Wang J., Naranjo S., Dudgeon C., Tamburini F.B., Brady J.J., Yang D., Grüner B.M., Chuang C.H., et al. Pancreatic cancer modeling using retrograde viral vector delivery and in vivo CRISPR/Cas9-mediated somatic genome editing. Genes Dev. 2015;29:1576–1585. doi: 10.1101/gad.264861.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Clark C.E., Hingorani S.R., Mick R., Combs C., Tuveson D.A., Vonderheide R.H. Dynamics of the Immune Reaction to Pancreatic Cancer from Inception to Invasion. Cancer Res. 2007;67:9518–9527. doi: 10.1158/0008-5472.CAN-07-0175. [DOI] [PubMed] [Google Scholar]
- 77.Sideras K., Braat H., Kwekkeboom J., van Eijck C.H., Peppelenbosch M.P., Sleijfer S., Bruno M. Role of the immune system in pancreatic cancer progression and immune modulating treatment strategies. Cancer Treat Rev. 2014;40:513–522. doi: 10.1016/j.ctrv.2013.11.005. [DOI] [PubMed] [Google Scholar]
- 78.Wang W.Q., Liu L., Xu H.X., Wu C.T., Xiang J.F., Xu J., Liu C., Long J., Ni Q.X., Yu X.J. Infiltrating immune cells and gene mutations in pancreatic ductal adenocarcinoma. Br. J. Surg. 2016;103:1189–1199. doi: 10.1002/bjs.10187. [DOI] [PubMed] [Google Scholar]
- 79.Seo Y.D., Pillarisetty V.G. T-cell programming in pancreatic adenocarcinoma: A review. Cancer Gene Ther. 2017;24:106–113. doi: 10.1038/cgt.2016.66. [DOI] [PubMed] [Google Scholar]
- 80.Von Ahrens D., Bhagat T.D., Nagrath D., Maitra A., Verma A. The role of stromal cancer-associated fibroblasts in pancreatic cancer. J. Hematol. Oncol. 2017;10:76. doi: 10.1186/s13045-017-0448-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Johnson B.A., III, Yarchoan M., Lee V., Laheru D.A., Jaffee E.M. Strategies for Increasing Pancreatic Tumor Immunogenicity. Clin. Cancer Res. 2017;23:1656–1669. doi: 10.1158/1078-0432.CCR-16-2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johansson H., Andersson R., Bauden M., Hammes S., Holdenrieder S., Ansari D. Immune checkpoint therapy for pancreatic cancer. World J. Gastroenterol. 2016;22:9457. doi: 10.3748/wjg.v22.i43.9457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kimura M., Tagawa M., Takenaga K., Kondo F., Yamaguchi T., Saisho H., Nakagawara A., Sakiyama S. Loss of tumorigenicity of human pancreatic carcinoma cells engineered to produce interleukin-2 or interleukin-4 in nude mice: A potentiality for cancer gene therapy. Cancer Lett. 1998;128:47–53. doi: 10.1016/S0304-3835(98)00050-0. [DOI] [PubMed] [Google Scholar]
- 84.Liu L., Meng J., Zhang C., Duan Y., Zhao L., Wang S., Shan B. Effects on apoptosis and cell cycle arrest contribute to the antitumor responses of interleukin-27 mediated by retrovirus in human pancreatic carcinoma cells. Oncol. Rep. 2012;27:1497–1503. doi: 10.3892/or.2012.1663. [DOI] [PubMed] [Google Scholar]
- 85.Ravet E., Lulka H., Gross F., Casteilla L., Buscail L., Cordelier P. Using lentiviral vectors for efficient pancreatic cancer gene therapy. Cancer Gene Ther. 2010;17:315–324. doi: 10.1038/cgt.2009.79. [DOI] [PubMed] [Google Scholar]
- 86.Armstrong L., Davydova J., Brown E., Han J., Yamamoto M., Vickers S.M. Delivery of interferon alpha using a novel Cox2-controlled adenovirus for pancreatic cancer therapy. Surgery. 2012;152:114–122. doi: 10.1016/j.surg.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Murugesan S.R., King C.R., Osborn R., Fairweather W.R., O’Reilly E.M., Thornton M.O., Wei L.L. Combination of human tumor necrosis factor-α (hTNF-α) gene delivery with gemcitabine is effective in models of pancreatic cancer. Cancer Gene Ther. 2009;16:841–847. doi: 10.1038/cgt.2009.32. [DOI] [PubMed] [Google Scholar]
- 88.Lortal B., Gross F., Peron J.M., Pénary M., Berg D., Hennebelle I., Favre G., Couderc B. Preclinical study of an ex vivo gene therapy protocol for hepatocarcinoma. Cancer Gene Ther. 2009;16:329–337. doi: 10.1038/cgt.2008.88. [DOI] [PubMed] [Google Scholar]
- 89.Mohammed S., Sukumaran S., Bajgain P., Watanabe N., Heslop H.E., Rooney C.M., Brenner M.K., Fisher W.E., Leen A.M., Vera J.F. Improving Chimeric Antigen Receptor-Modified T Cell Function by Reversing the Immunosuppressive Tumor Microenvironment of Pancreatic Cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2017;25:249–258. doi: 10.1016/j.ymthe.2016.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Posey A.D., Schwab R.D., Boesteanu A.C., Steentoft C., Mandel U., Engels B., Stone J.D., Madsen T.D., Schreiber K., Haines K.M., et al. Engineered CAR T Cells Targeting the Cancer-Associated Tn-Glycoform of the Membrane Mucin MUC1 Control Adenocarcinoma. Immunity. 2016;44:1444–1454. doi: 10.1016/j.immuni.2016.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.O’Hara M., Stashwick C., Haas A.R., Tanyi J.L. Mesothelin as a target for chimeric antigen receptor-modified T cells as anticancer therapy. Immunotherapy. 2016;8:449–460. doi: 10.2217/imt.16.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.DeSelm C.J., Tano Z.E., Varghese A.M., Adusumilli P.S. CAR T-cell therapy for pancreatic cancer. J. Surg. Oncol. 2017 doi: 10.1002/jso.24627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ren J., Liu X., Fang C., Jiang S., June C.H., Zhao Y. Multiplex Genome Editing to Generate Universal CAR T Cells Resistant to PD1 Inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017;23:2255–2266. doi: 10.1158/1078-0432.CCR-16-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kudrin A. Overview of cancer vaccines: Considerations for development. Hum. Vaccines Immunother. 2012;8:1335–1353. doi: 10.4161/hv.20518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Plate J.M.D. Advances in therapeutic vaccines for pancreatic cancer. Discov. Med. 2012;14:89–94. [PubMed] [Google Scholar]
- 96.Maki R.G., Livingston P.O., Lewis J.J., Janetzki S., Klimstra D., Desantis D., Srivastava P.K., Brennan M.F. A phase I pilot study of autologous heat shock protein vaccine HSPPC-96 in patients with resected pancreatic adenocarcinoma. Dig. Dis. Sci. 2007;52:1964–1972. doi: 10.1007/s10620-006-9205-2. [DOI] [PubMed] [Google Scholar]
- 97.Rong Y., Qin X., Jin D., Lou W., Wu L., Wang D., Wu W., Ni X., Mao Z., Kuang T., et al. A phase I pilot trial of MUC1-peptide-pulsed dendritic cells in the treatment of advanced pancreatic cancer. Clin. Exp. Med. 2012;12:173–180. doi: 10.1007/s10238-011-0159-0. [DOI] [PubMed] [Google Scholar]
- 98.Gjertsen M.K., Buanes T., Rosseland A.R., Bakka A., Gladhaug I., Søreide O., Eriksen J.A., Møller M., Baksaas I., Lothe R.A., et al. Intradermal ras peptide vaccination with granulocyte-macrophage colony-stimulating factor as adjuvant: Clinical and immunological responses in patients with pancreatic adenocarcinoma. Int. J. Cancer. 2001;92:441–450. doi: 10.1002/ijc.1205. [DOI] [PubMed] [Google Scholar]
- 99.Bernhardt S.L., Gjertsen M.K., Trachsel S., Møller M., Eriksen J.A., Meo M., Buanes T., Gaudernack G. Telomerase peptide vaccination of patients with non-resectable pancreatic cancer: A dose escalating phase I/II study. Br. J. Cancer. 2006;95:1474–1482. doi: 10.1038/sj.bjc.6603437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pol J., Bloy N., Obrist F., Eggermont A., Galon J., Hervé Fridman W., Cremer I., Zitvogel L., Kroemer G., Galluzzi L. Trial Watch: DNA vaccines for cancer therapy. Oncoimmunology. 2014;3:e28185. doi: 10.4161/onci.28185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pol J., Bloy N., Obrist F., Eggermont A., Galon J., Cremer I., Erbs P., Limacher J.M., Preville X., Zitvogel L., et al. Trial Watch: Oncolytic viruses for cancer therapy. Oncoimmunology. 2014;3:e28694. doi: 10.4161/onci.28694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Huang F., Wang B.R., Wu Y.Q., Wang F.C., Zhang J., Wang Y.G. Oncolytic viruses against cancer stem cells: A promising approach for gastrointestinal cancer. World J. Gastroenterol. 2016;22:7999–8009. doi: 10.3748/wjg.v22.i35.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Husseini F., Delord J.P., Fournel-Federico C., Guitton J., Erbs P., Homerin M., Halluard C., Jemming C., Orange C., Limacher J.M., et al. Vectorized gene therapy of liver tumors: Proof-of-concept of TG4023 (MVA-FCU1) in combination with flucytosine. Ann. Oncol. 2017;28:169–174. doi: 10.1093/annonc/mdw440. [DOI] [PubMed] [Google Scholar]
- 104.Wennier S., Li S., McFadden G. Oncolytic virotherapy for pancreatic cancer. Expert Rev. Mol. Med. 2011;13:e18. doi: 10.1017/S1462399411001876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Xu C., Li H., Su C., Li Z. Viral therapy for pancreatic cancer: Tackle the bad guys with poison. Cancer Lett. 2013;333:1–8. doi: 10.1016/j.canlet.2013.01.035. [DOI] [PubMed] [Google Scholar]
- 106.Bhattacharyya M., Francis J., Eddouadi A., Lemoine N.R., Halldén G. An oncolytic adenovirus defective in pRb-binding (dl922-947) can efficiently eliminate pancreatic cancer cells and tumors in vivo in combination with 5-FU or gemcitabine. Cancer Gene Ther. 2011;18:734–743. doi: 10.1038/cgt.2011.45. [DOI] [PubMed] [Google Scholar]
- 107.Hecht J.R., Bedford R., Abbruzzese J.L., Lahoti S., Reid T.R., Soetikno R.M., Kirn D.H., Freeman S.M. A phase I/II trial of intratumoral endoscopic ultrasound injection of ONYX-015 with intravenous gemcitabine in unresectable pancreatic carcinoma. Clin. Cancer Res. 2003;9:555–561. [PubMed] [Google Scholar]
- 108.Lucas T., Benihoud K., Vigant F., Schmidt C.Q., Schmidt C.Q.A., Wortmann A., Bachem M.G., Simmet T., Kochanek S. Hexon modification to improve the activity of oncolytic adenovirus vectors against neoplastic and stromal cells in pancreatic cancer. PLoS ONE. 2015;10:e0117254. doi: 10.1371/journal.pone.0117254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ilkow C.S., Marguerie M., Batenchuk C., Mayer J., Ben Neriah D., Cousineau S., Falls T., Jennings V.A., Boileau M., Bellamy D., et al. Reciprocal cellular cross-talk within the tumor microenvironment promotes oncolytic virus activity. Nat. Med. 2015;21:530–536. doi: 10.1038/nm.3848. [DOI] [PubMed] [Google Scholar]
- 110.Gayral M., Lulka H., Hanoun N., Biollay C., Sèlves J., Vignolle-Vidoni A., Berthommé H., Trempat P., Epstein A.L., Buscail L., et al. Targeted oncolytic herpes simplex virus type 1 eradicates experimental pancreatic tumors. Hum. Gene Ther. 2015;26:104–113. doi: 10.1089/hum.2014.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Angelova A.L., Aprahamian M., Grekova S.P., Hajri A., Leuchs B., Giese N.A., Dinsart C., Herrmann A., Balboni G., Rommelaere J., et al. Improvement of gemcitabine-based therapy of pancreatic carcinoma by means of oncolytic parvovirus H-1PV. Clin. Cancer Res. 2009;15:511–519. doi: 10.1158/1078-0432.CCR-08-1088. [DOI] [PubMed] [Google Scholar]
- 112.Réjiba S., Bigand C., Parmentier C., Masmoudi A., Hajri A. Oncosuppressive suicide gene virotherapy “PVH1-yCD/5-FC” for pancreatic peritoneal carcinomatosis treatment: NFκB and Akt/PI3K involvement. PLoS ONE. 2013;8:e70594. doi: 10.1371/journal.pone.0070594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Geletneky K., Huesing J., Rommelaere J., Schlehofer J.R., Leuchs B., Dahm M., Krebs O., von Knebel Doeberitz M., Huber B., Hajda J. Phase I/IIa study of intratumoral/intracerebral or intravenous/intracerebral administration of Parvovirus H-1 (ParvOryx) in patients with progressive primary or recurrent glioblastoma multiforme: ParvOryx01 protocol. BMC Cancer. 2012;12:99. doi: 10.1186/1471-2407-12-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Geletneky K., Leoni A.L., Pohlmeyer-Esch G., Loebhard S., Leuchs B., Hoefer C., Jochims K., Dahm M., Huber B., Rommelaere J., et al. Bioavailability, biodistribution, and CNS toxicity of clinical-grade parvovirus H1 after intravenous and intracerebral injection in rats. Comp. Med. 2015;65:36–45. [PMC free article] [PubMed] [Google Scholar]
- 115.Breitbach C.J., Lichty B.D., Bell J.C. Oncolytic Viruses: Therapeutics with an Identity Crisis. EBioMedicine. 2016;9:31–36. doi: 10.1016/j.ebiom.2016.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kaufman H.L., Kim D.W., DeRaffele G., Mitcham J., Coffin R.S., Kim-Schulze S. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 2010;17:718–730. doi: 10.1245/s10434-009-0809-6. [DOI] [PubMed] [Google Scholar]
- 117.Andtbacka R.H.I., Kaufman H.L., Collichio F., Amatruda T., Senzer N., Chesney J., Delman K.A., Spitler L.E., Puzanov I., Agarwala S.S., et al. Talimogene Laherparepvec Improves Durable Response Rate in Patients With Advanced Melanoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015;33:2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 118.Vassaux G., Angelova A., Baril P., Midoux P., Rommelaere J., Cordelier P. The Promise of Gene Therapy for Pancreatic Cancer. Hum. Gene Ther. 2016;27:127–133. doi: 10.1089/hum.2015.141. [DOI] [PubMed] [Google Scholar]
- 119.Le D.T., Wang-Gillam A., Picozzi V., Greten T.F., Crocenzi T., Springett G., Morse M., Zeh H., Cohen D., Fine R.L., et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J. Clin. Oncol. 2015;33:1325–1333. doi: 10.1200/JCO.2014.57.4244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Gilly F.N., Beaujard A., Bienvenu J., Trillet Lenoir V., Glehen O., Thouvenot D., Malcus C., Favrot M., Dumontet C., Lombard-Bohas C., et al. Gene therapy with Adv-IL-2 in unresectable digestive cancer: Phase I-II study, intermediate report. Hepatogastroenterology. 1999;46:1268–1273. [PubMed] [Google Scholar]
- 121.Mulvihill S., Warren R., Venook A., Adler A., Randlev B., Heise C., Kirn D. Safety and feasibility of injection with an E1B-55 kDa gene-deleted, replication-selective adenovirus (ONYX-015) into primary carcinomas of the pancreas: A phase I trial. Gene Ther. 2001;8:308–315. doi: 10.1038/sj.gt.3301398. [DOI] [PubMed] [Google Scholar]
- 122.Löhr M., Hoffmeyer A., Kröger J., Freund M., Hain J., Holle A., Karle P., Knöfel W.T., Liebe S., Müller P., et al. Microencapsulated cell-mediated treatment of inoperable pancreatic carcinoma. Lancet Lond. Engl. 2001;357:1591–1592. doi: 10.1016/S0140-6736(00)04749-8. [DOI] [PubMed] [Google Scholar]
- 123.Salmons B., Löhr M., Günzburg W.H. Treatment of inoperable pancreatic carcinoma using a cell-based local chemotherapy: Results of a phase I/II clinical trial. J. Gastroenterol. 2003;38(Suppl. S15):78–84. [PubMed] [Google Scholar]
- 124.Pecher G., Häring A., Kaiser L., Thiel E. Mucin gene (MUC1) transfected dendritic cells as vaccine: Results of a phase I/II clinical trial. Cancer Immunol. Immunother. CII. 2002;51:669–673. doi: 10.1007/s00262-002-0317-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gordon E.M., Cornelio G.H., Lorenzo C.C., Levy J.P., Reed R.A., Liu L., Hall F.L. First clinical experience using a “pathotropic” injectable retroviral vector (Rexin-G) as intervention for stage IV pancreatic cancer. Int. J. Oncol. 2004;24:177–185. doi: 10.3892/ijo.24.1.177. [DOI] [PubMed] [Google Scholar]
- 126.Sangro B., Mazzolini G., Ruiz J., Herraiz M., Quiroga J., Herrero I., Benito A., Larrache J., Pueyo J., Subtil J.C., et al. Phase I trial of intratumoral injection of an adenovirus encoding interleukin-12 for advanced digestive tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004;22:1389–1397. doi: 10.1200/JCO.2004.04.059. [DOI] [PubMed] [Google Scholar]
- 127.Senzer N., Mani S., Rosemurgy A., Nemunaitis J., Cunningham C., Guha C., Bayol N., Gillen M., Chu K., Rasmussen C., et al. TNFerade biologic, an adenovector with a radiation-inducible promoter, carrying the human tumor necrosis factor alpha gene: A phase I study in patients with solid tumors. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004;22:592–601. doi: 10.1200/JCO.2004.01.227. [DOI] [PubMed] [Google Scholar]
- 128.Mazzolini G., Alfaro C., Sangro B., Feijoó E., Ruiz J., Benito A., Tirapu I., Arina A., Sola J., Herraiz M., et al. Intratumoral injection of dendritic cells engineered to secrete interleukin-12 by recombinant adenovirus in patients with metastatic gastrointestinal carcinomas. J. Clin. Oncol. 2005;23:999–1010. doi: 10.1200/JCO.2005.00.463. [DOI] [PubMed] [Google Scholar]
- 129.Kaufman H.L., Kim-Schulze S., Manson K., DeRaffele G., Mitcham J., Seo K.S., Kim D.W., Marshall J. Poxvirus-based vaccine therapy for patients with advanced pancreatic cancer. J. Transl. Med. 2007;5:60. doi: 10.1186/1479-5876-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Galanis E., Carlson S.K., Foster N.R., Lowe V., Quevedo F., McWilliams R.R., Grothey A., Jatoi A., Alberts S.R., Rubin J. Phase I trial of a pathotropic retroviral vector expressing a cytocidal cyclin G1 construct (Rexin-G) in patients with advanced pancreatic cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2008;16:979–984. doi: 10.1038/mt.2008.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Laheru D., Lutz E., Burke J., Biedrzycki B., Solt S., Onners B., Tartakovsky I., Nemunaitis J., Le D., Sugar E., et al. Allogeneic granulocyte macrophage colony-stimulating factor-secreting tumor immunotherapy alone or in sequence with cyclophosphamide for metastatic pancreatic cancer: A pilot study of safety, feasibility, and immune activation. Clin. Cancer Res. 2008;14:1455–1463. doi: 10.1158/1078-0432.CCR-07-0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Chawla S.P., Chua V.S., Fernandez L., Quon D., Blackwelder W.C., Gordon E.M., Hall F.L. Advanced phase I/II studies of targeted gene delivery in vivo: Intravenous Rexin-G for gemcitabine-resistant metastatic pancreatic cancer. Mol. Ther. 2010;18:435–441. doi: 10.1038/mt.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nakao A., Kasuya H., Sahin T.T., Nomura N., Kanzaki A., Misawa M., Shirota T., Yamada S., Fujii T., Sugimoto H., et al. A phase I dose-escalation clinical trial of intraoperative direct intratumoral injection of HF10 oncolytic virus in non-resectable patients with advanced pancreatic cancer. Cancer Gene Ther. 2011;18:167–175. doi: 10.1038/cgt.2010.65. [DOI] [PubMed] [Google Scholar]
- 134.Lutz E., Yeo C.J., Lillemoe K.D., Biedrzycki B., Kobrin B., Herman J., Sugar E., Piantadosi S., Cameron J.L., Solt S., et al. A lethally irradiated allogeneic granulocyte-macrophage colony stimulating factor-secreting tumor vaccine for pancreatic adenocarcinoma. A Phase II trial of safety, efficacy, and immune activation. Ann. Surg. 2011;253:328–335. doi: 10.1097/SLA.0b013e3181fd271c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Kubuschok B., Pfreundschuh M., Breit R., Hartmann F., Sester M., Gärtner B., König J., Murawski N., Held G., Zwick C., et al. Mutated Ras-transfected, EBV-transformed lymphoblastoid cell lines as a model tumor vaccine for boosting T-cell responses against pancreatic cancer: A pilot trial. Hum. Gene Ther. 2012;23:1224–1236. doi: 10.1089/hum.2011.153. [DOI] [PubMed] [Google Scholar]
- 136.Hanna N., Ohana P., Konikoff F.M., Leichtmann G., Hubert A., Appelbaum L., Kopelman Y., Czerniak A., Hochberg A. Phase 1/2a, dose-escalation, safety, pharmacokinetic and preliminary efficacy study of intratumoral administration of BC-819 in patients with unresectable pancreatic cancer. Cancer Gene Ther. 2012;19:374–381. doi: 10.1038/cgt.2012.10. [DOI] [PubMed] [Google Scholar]
- 137.Hecht J.R., Farrell J.J., Senzer N., Nemunaitis J., Rosemurgy A., Chung T., Hanna N., Chang K.J., Javle M., Posner M., et al. EUS or percutaneously guided intratumoral TNFerade biologic with 5-fluorouracil and radiotherapy for first-line treatment of locally advanced pancreatic cancer: A phase I/II study. Gastrointest. Endosc. 2012;75:332. doi: 10.1016/j.gie.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Le D.T., Brockstedt D.G., Nir-Paz R., Hampl J., Mathur S., Nemunaitis J., Sterman D.H., Hassan R., Lutz E., Moyer B., et al. A Live-attenuated Listeria Vaccine (ANZ-100) and a Live-attenuated Listeria Vaccine Expressing Mesothelin (CRS-207) for Advanced Cancers: Phase 1 Studies of Safety and Immune Induction. Clin. Cancer Res. 2012;18:858. doi: 10.1158/1078-0432.CCR-11-2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Le D.T., Lutz E., Uram J.N., Sugar E.A., Onners B., Solt S., Zheng L., Diaz L.A., Donehower R.C., Jaffee E.M., et al. Evaluation of Ipilimumab in combination with allogeneic pancreatic tumor cells transfected with a GM-CSF gene in previously treated pancreatic cancer. J. Immunother. Hagerstown Md 1997. 2013;36:382–389. doi: 10.1097/CJI.0b013e31829fb7a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Hardacre J.M., Mulcahy M., Small W., Talamonti M., Obel J., Krishnamurthi S., Rocha-Lima C.S., Safran H., Lenz H.J., Chiorean E.G. Addition of algenpantucel-L immunotherapy to standard adjuvant therapy for pancreatic cancer: A phase 2 study. J. Gastrointest. Surg. 2013;17:94–101. doi: 10.1007/s11605-012-2064-6. [DOI] [PubMed] [Google Scholar]
- 141.Herman J.M., Wild A.T., Wang H., Tran P.T., Chang K.J., Taylor G.E., Donehower R.C., Pawlik T.M., Ziegler M.A., Cai H., et al. Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: Final results. J. Clin. Oncol. 2013;31:886–894. doi: 10.1200/JCO.2012.44.7516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Löhr J.M., Haas S.L., Kröger J.C., Friess H.M., Höft R., Goretzki P.E., Peschel C., Schweigert M., Salmons B., Gunzburg W.H. Encapsulated cells expressing a chemotherapeutic activating enzyme allow the targeting of subtoxic chemotherapy and are safe and efficacious: Data from two clinical trials in pancreatic cancer. Pharmaceutics. 2014;6:447–466. doi: 10.3390/pharmaceutics6030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Aguilar L.K., Shirley L.A., Chung V.M., Marsh C.L., Walker J., Coyle W., Marx H., Bekaii-Saab T., Lesinski G.B., Swanson B., et al. Gene-mediated cytotoxic immunotherapy as adjuvant to surgery or chemoradiation for pancreatic adenocarcinoma. Cancer Immunol. Immunother. CII. 2015;64:727–736. doi: 10.1007/s00262-015-1679-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Buscail L., Bournet B., Vernejoul F., Cambois G., Lulka H., Hanoun N., Dufresne M., Meulle A., Vignolle-Vidoni A., Ligat L., et al. First-in-man Phase 1 Clinical Trial of Gene Therapy for Advanced Pancreatic Cancer: Safety, Biodistribution, and Preliminary Clinical Findings. Mol. Ther. 2015;23:779–789. doi: 10.1038/mt.2015.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Golan T., Khvalevsky E.Z., Hubert A., Gabai R.M., Hen N., Segal A., Domb A., Harari G., David E.B., Raskin S., et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560–24570. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Noonan A.M., Farren M.R., Geyer S.M., Huang Y., Tahiri S., Ahn D., Mikhail S., Ciombor K.K., Pant S., Aparo S., et al. Randomized Phase 2 Trial of the Oncolytic Virus Pelareorep (Reolysin) in Upfront Treatment of Metastatic Pancreatic Adenocarcinoma. Mol. Ther. 2016;24:1150–1158. doi: 10.1038/mt.2016.66. [DOI] [PMC free article] [PubMed] [Google Scholar]