Abstract
Changes in concentrations of cortisol and progesterone in serial blood samples were used to quantify a stress response to different methods of electroejaculation in 10 Hereford bulls. Treatments included restraint (control), and electroejaculation using rectal probes with segmented electrodes or conventional nonsegmented electrodes, with or without lidocaine caudal epidural anesthesia. A subjective scoring system was used to assess behavioral responses to the different methods of electroejaculation. The increases in concentrations of serum cortisol and progesterone after electroejaculation were higher for all electroejaculation treatments than for restraint alone. The increases in serum progesterone concentrations were significantly lower at 5 and 20 minutes after electro ejaculation with epidural anesthesia than with no anesthesia. However, the change in cortisol or progesterone concentrations did not differ after electroejaculation when comparing the conventional probe or a segmented probe at any time during the study. Subjective scoring showed no differences among electroejaculation methods. Use of epidural anesthesia was beneficial in reducing progesterone, one indicator of an endocrine stress response to electroejaculation.
Abstract
Résumé — Évaluation d’une sonde rectale segmentée et d’une anesthésie épidurale caudale lors d’électroéjaculation chez le taureau. Les variations de concentrations de cortisol et de progestérone provenant d’échantillons sanguins sériés ont été utilisées pour quantifier la réponse au stress de 10 taureaux Hereford soumis à différentes méthodes d’éjaculation. Les manipulations comprenaient l’immobilisation (témoin) et l’électroéjaculation par utilisation de sondes rectales à électrodes segmentées ou non segmentées conventionnelles, avec ou sans anesthésie épidurale caudale à la lidocaïne. Un système subjectif de cotation a été utilisé pour évaluer la réponse comportementale aux différentes méthodes d’électroéjaculation. L’augmentation des concentrations sériques de cortisol et de progestérone après l’électroéjaculation était plus élevée pour toutes les méthodes d’électroéjaculation que pour l’immobilisation seule. L’augmentation des concentrations sériques de progestérone était significativement plus faible 5 et 20 minutes après l’électroéjaculation sous anesthésie épidurale que sans anesthésie. Cependant, les modifications dans les concentrations de cortisol ou de progestérone ne présentaient pas de différence après électroéjaculation avec sonde conventionnelle ou segmentée. La cotation subjective n’a montré aucune différence entre les méthodes d’électroéjaculation. L’utilisation de l’anesthésie épidurale s’est avérée efficace pour réduire le taux de progestérone, un des indicateurs endocriniens de stress lors de l’électroéjaculation.
(Traduit par Docteur André Blouin)
Introduction
Semen collection by electroejaculation (EE) is widely used for evaluation of breeding soundness in bulls, for the majority of bulls it is accomplished easily and does not seem to cause much pain. Bulls that were electroejaculated 2 or 3 times a week for 6 wk did not show aversion to handlers or the restraint facilities over the course of the study (1). However, when higher levels of electrical stimulation are used, some bulls, especially yearling bulls, vocalize, struggle, or lie down. Therefore, EE in animals without the use of any anesthesia has become a welfare concern.
Electroejaculation may be performed in human males who are anejaculatory due to spinal cord injury or disease. Men with normal sensation in the pelvic area are given general or epidural anesthesia before EE, because the procedure is painful (2). Thus, caudal epidural anesthesia may be useful to decrease pain due to EE in bulls. In one study, elevation in heart rate tended to be less when lidocaine or xylazine epidural anesthesia was given to bulls prior to EE (3). There is some anecdotal evidence that finger electrodes, by virtue of a small electrode area, could be used to reduce nerve stimulation and pain during EE in bulls. Recently, rectal probes for bulls have been designed with segmented electrodes to allow operators to reduce the intrapelvic area stimulated during EE. Rectal probes with 3 short caudal segments, 3 short middle segments, and 2 short cranial segments are commercially available. The caudal segments are activated to induce penile protrusion and the middle segments are activated to induce semen emission. The cranial segments are activated only when semen emission cannot be achieved by activation of the caudal and middle segments.
Studies to assess pain in animals during EE are compromised by the difficulty in objectively assessing pain. Stress or pain caused by various veterinary procedures, including transrectal palpation and intramuscular injection, was sufficient to elevate blood cortisol levels in cattle (4). Blood cortisol, progesterone, luteinizing hormone, testosterone, beta-endorphin, and beta-lipoprotein levels have also been used to measure pain due to dehorning in calves (5), castration in calves (6), electro-immobilization in sheep (7), and EE in bulls (8), but with some conflicting results. However, changes in blood cortisol and progesterone concentrations have been shown to be useful indicators of stress or pain suffered by bulls during EE (9). Adverse responses to EE in bulls have also been assessed by scoring behavioral responses (9).
The objective of this study was to determine whether rectal probes with segmented electrodes induced less pain than did probes with long nonsegmented electrodes during EE in bulls and whether the addition of caudal epidural anesthesia with lidocaine further reduced pain due to EE in bulls.
Materials and methods
This study was approved by the university committee on animal care and supply. Ten 16-month-old Hereford bulls were habituated to the chute system by bringing them in daily from outdoor pens for 30 min for 1 wk and offering them alfalfa hay while holding them. Five bulls at a time were held in tandem in individual stalls within the chute system, which allowed them to take a step forward or backward, or lie down. They were then used in an experiment designed to measure blood cortisol and progesterone changes in response to 5 treatments.
The jugular vein was catheterized approximately 6 h before treatments. Most of the catheters remained functional throughout the experimental treatments; however, in 2 bulls catheters became blocked. The catheters were replaced immediately and treatments were resumed 6 h later. On the 1st treatment day, 5 bulls were allowed to enter the chute in random order and these bulls were used for all treatments in the 1st week. All treatments were repeated on the remaining bulls in the 2nd week. One treatment per day was administered to all 5 bulls in the following order: day 1: restraint only control (Cont); day 2: lidocaine caudal epidural anesthesia, followed by EE with a segmented probe (LidoSeg); day 3: lidocaine caudal epidural anesthesia, followed by EE with a conventional probe (LidoConv); day 4: EE with the segmented probe and no epidural anesthesia (Seg); and day 5: EE with a conventional probe and no epidural anesthesia (Conv). In all treatment groups, except the control group, manual transrectal massage of the urethralis muscle, the ampullae of the ductus deferens, the prostate, and the vesicular glands was applied for 2 min prior to electroejaculation. The conventional probe was 75 mm in diameter with 3 longitudinal electrodes ventrally (Lane Manufacturing, Denver, Colorado, USA). The segmented probe was 75 mm in diameter with 3 short caudal electrodes, 3 short middle electrodes, and 2 short cranial electrodes ventrally (Kane Veterinary Supplies, Saskatoon, Saskatchewan). An identical sequence of electrical pulses of increasing intensity was administered for each treatment with an electroejaculator (Masterjac; Kane Veterinary Supplies) until 27 stimuli had been administered. The voltage across the electrodes was measured and electroejaculator settings were determined to provide a similar electrical output between probes. The frequency and duration of electrical stimuli and the voltage level of electrical stimuli were similar for all bulls. When the segmented probe was being used, the caudal 3 segments were active for the first 15 stimuli and the middle 3 segments for the last 12 stimuli. When epidural anesthesia was included in the treatment, electrical stimuli began 15 min after the injection of 6 mL of 2% lidocaine HCl (MTC Pharmaceuticals, Cambridge, Ontario) into the 1st intercoccygeal epidural space. The success of the caudal epidural anesthesia was determined by assessing tail muscle tone.
Following restraint in the chute stalls, blood samples were taken prior to treatments, 5 min after treatments, and at 20-min intervals after treatments until 120 min had elapsed. Blood samples were centrifuged and serum was frozen at −40°C for cortisol and progesterone assays. Cortisol concentrations were determined with an Immulite Cortisol kit and progesterone concentrations were determined by radioimmunoassay (Coat-A-Count Progesterone; Diagnostic Products Corporation, Los Angeles, California, USA). The sensitivity of the assays was 5.5 nmol/L for cortisol and 0.02 ng/mL for progesterone.
Bull vocalization, struggling, or lying down in response to EE were scored subjectively, as described previously (9), to evaluate behavioral responses to treatments. Penile protrusion was scored as none, partial, or full, and semen emission was scored as positive or negative.
The distribution of cortisol and progesterone concentrations were compared graphically by using box and whisker plots by sample time for each treatment type. All data were analyzed using a statistical computer software program (SAS version 8.2 for Windows [PROC MIXED]; SAS Institute, Cary, North Carolina, USA). To measure any movement of the baseline over time because of the potential for either habituation or fatigue, the pretreatment cortisol and progesterone levels were compared across study groups by using a random statement to recognize the repeated use of bulls across treatments. To examine the effects of restraint alone on the untreated bulls, the serum cortisol and progesterone concentrations measured during the study were compared with baseline samples collected immediately after restraint with a random effect to recognize the repeated measures within bull over time. Model specifications also included repeated measures within the same day, with subject equal to the interaction of bull identification number with treatment, by using an autoregressive (AR) (1) covariance structure to approximate the sequential nature of the treatments.
The initial null hypothesis was that the change in levels of serum cortisol and serum progesterone from those at pretreatment to those at 5 min, 20 min, and 40 min posttreatment was not associated with EE, regardless of treatment type (or the mean change in all treatment groups) or time following EE. If there was evidence to refute this initial null hypothesis, the main effect of epidural anesthesia and the main effect of probe type were then examined in a model including time following EE. If any 2 main effects (time, probe type, or anesthesia) were found to be significant (P < 0.05), then the appropriate first-order interaction term was also introduced into the model. Model specifications included repeated measures within the same day, with subject equal to the interaction of bull identification number with treatment, by using an AR(1) covariance structure to approximate the sequential nature of the treatments. Random effects for both bull identification and bull and treatment interaction were also included in the model to account for repeated measures on a bull within a treatment and the repeated use of bulls across treatments. Differences between treatment groups, where present, were reported with 95% confidence intervals (95% CI). The residuals from the final models were graphed to evaluate whether each model met the required assumptions.
Where the individual main effects of time following EE and either epidural anesthesia or probe type were included in the final model (P < 0.05), an interaction between treatment and time was also examined for statistical significance. Where this interaction between treatment and time was significant, posthoc time specific differences between treatments were computed by using PROC MIXED and asking for time specific treatment comparisons with the use of the “estimate” statement, and the resulting P values for the time specific differences values for the time specific differences were adjusted with Bonferonni’s correction to adjust for any perception of increased risk of type I error.
Generalized estimating equations were used to examine the differences in penile protrusion and semen emission across treatment groups, while accounting for repeated observations on the same bull because of the cross-over design.
Results
The distribution of serum concentrations of cortisol and progesterone before and after treatment are shown in Figures 1 and 2.
Figure 1.
A box and whisker plot showing the distribution of serum cortisol concentrations (nmol/L) before and after treatment. The center line reflects the median, the box the interquartile range, and the whiskers extend from the highest to lowest values excluding outliers. The circles indicate out liers between 1.5 and 3 box lengths from the upper or lower edge of the box. An asterisk represents an extreme outlier with a value greater than 3 box lengths from the upper or lower edge of the box.
Figure 2.
A box and whisker plot showing the distribution of serum progesterone concentrations (ng/mL) before and after treatment. The center line reflects the median, the box the interquartile range, and the whiskers extend from the highest to lowest values excluding outliers. The circles indicate out liers between 1.5 and 3 box lengths from the upper or lower edge of the box. An asterisk represents an extreme outlier with a value greater than 3 box lengths from the upper or lower edge of the box.
Cortisol concentrations in pretreatment serum samples for individual bulls did not change during the course of the study (P = 0.80). In addition, there were no significant differences between pre- and posttreatment serum cortisol levels in the first 40 min for the control group (P = 0.25). However, mean serum cortisol concentrations in serial samples from the control group were significantly higher from 60 to 120 min after the start of the sampling period than they were in the pretreatment sample (P < 0.001).
Progesterone concentrations in pretreatment serum samples were also similar for all EE treatments, but they were significantly lower than in the pretreatment samples of the control group (difference, 0.11; 95% CI, 0.5 to 0.18). However, progesterone concentrations in serial serum samples in the control (restraint only) group were not significantly elevated above pretreatment levels at any time during the study (P = 0.18).
Differences from pretreatment hormone levels were examined to account for any potential shift in baseline within bulls over the course of the study. The differences in serum cortisol concentrations between pretreatment levels and levels at 5, 20, and 40 min after EE are shown in Figure 3. The mean increase in serum cortisol concentration from pretreatment baseline for the combined effect of all treatments was significantly greater than the increase in the control (restraint only) series for the period between 5 min and 40 min posttreatment (difference, 14.5 nmol/L; 95% CI, 4.3, 24.8). There was a significant interaction between time and treatment; serum cortisol was 22.0 nmol/L (95% CI, 11.0 to 32.0) higher at 5 min following any treatment and 22.7 nmol/L (95% CI, 6.4 to 39.0) higher at 20 min following any treatment than it was at the comparable time period in the control series. There was no difference in cortisol concentrations among treatment groups at 40 min following treatment.
Figure 3.
A box and whisker plot showing the difference between serum cortisol concentrations (nmol/L) immediately prior to treatment and at 5 min, 20 min, and 40 min posttreatment. The center line reflects the median, the box the interquartile range, and the whiskers extend from the highest to lowest values excluding outliers. The circles indicate out liers between 1.5 and 3 box lengths from the upper or lower edge of the box. An asterisk represents an extreme outlier with a value greater than 3 box lengths from the upper or lower edge of the box.
Given that an overall difference in the serum concentration of cortisol existed between treatment and control periods, further analyses were conducted to determine whether individual treatments differed from each other by examining the main effects of each treatment type, including the control series, in a model containing time. There were no differences in serum cortisol concentrations following the use of a segmented versus conventional probe lowing (difference, 1.7 nmol/L; 95% CI, −7.6, 11.0) or following the use of caudal epidural anesthesia versus no anesthesia (difference, −7.4 nmol/L; 95% CI, −16.4, 1.6).
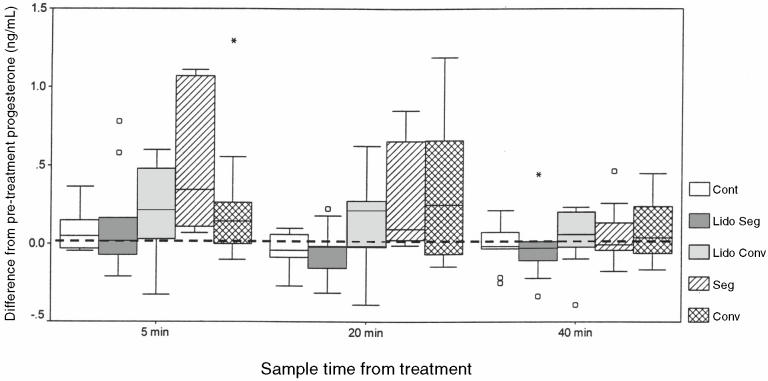
The differences between serum progesterone concentrations at pretreatment levels and at 5, 20, and 40 min after treatments are shown in Figure 4. The mean increase in serum progesterone concentration from pretreatment baseline levels was significantly greater for the combined effect of all treatments than the mean increase in the control series for the period between 5 min and 40 min posttreatment (difference, 0.15 ng/mL; 95% CI, 0.00, 0.31). The amount of increase in serum progesterone following the use of EE did not vary significantly up to 40 min post-EE.
Figure 4.
A box and whisker plot showing the difference between serum progesterone concentrations (ng/mL) immediately prior to treatment and at 5 min, 20 min, and 40 min posttreatment. The center line reflects the median, the box the interquartile range, and the whiskers extend from the highest to lowest values excluding outliers. The circles indicate out liers between 1.5 and 3 box lengths from the upper or lower edge of the box. An asterisk represents an extreme outlier with a value greater than 3 box lengths from the upper or lower edge of the box.
There was no association between probe type and the concentration of progesterone following EE. The mean difference in serum progesterone between the conventional and the segmented probe for the first 3 time periods up to 40 min posttreatment was 0.04 ng/mL (95% CI, −0.11 to 0.18). However, EE after caudal epidural anesthesia compared with EE after no anesthesia resulted in a mean reduction of 0.18 ng/mL (95% CI, 0.05 to 0.31) in serum progesterone levels across this period. There was a significant interaction between time and treatment; therefore, further analyses were done to determine the extent of the differences at different points in time. The progesterone concentrations of the 5-min samples collected following EE with caudal epidural anesthesia were 0.22 ng/mL (95% CI, 0.06 to 0.37) lower than the concentrations of samples following EE without anesthesia. The difference was 0.25 ng/mL (95% CI, 0.10 to 0.41) at 20 min and there was no difference at 40 min.
Complete loss of tail muscle tone occurred in all cases with epidural anesthesia, and none of the bulls showed caudal ataxia due to the epidural anesthesia. None of the bulls vocalized during any treatments, except for 1 bull that vocalized mildly following treatment with the segmented probe. None of the bulls attempted to lie down during any treatment. Only 1 of the bulls struggled during EE and this appeared to be due to the rectal probe being temporarily positioned in contact with the anal sphincter.
Results from the scoring of penile protrusion and semen emission are summarized in Table 1. The degree of penile protrusion was significantly greater when a conventional probe was used than when a segmented probe was used, with or without epidural anesthesia (P < 0.001). The conventional probe and the segmented probe appeared to be equally efficacious for achieving semen emission.
Table 1.
Penile protrusion and semen emission in 10 bulls during electroejaculation with a conventional rectal probe (Conv), a segmented rectal probe (Seg), a conventional rectal probe after lidocaine caudal epidural anesthesia (LidoConv), and a segmented probe after lidocaine caudal epidural anesthesia (LidoSeg)
| Conv | Seg | LidoConv | LidoSeg | ||
|---|---|---|---|---|---|
| Penile protrusion | Full | 9 | 3 | 8 | 2 |
| Partial | 1 | 6 | 2 | 6 | |
| None | 0 | 1 | 0 | 2 | |
| Semen emission | Yes | 10 | 9 | 8 | 8 |
| No | 0 | 1 | 2 | 2 |
Discussion
The absence of a substantial carry-over effect or exhaustion from repeated application of different treatments to individual bulls during the study was substantiated by the observation that cortisol concentrations in pretreatment serum samples for individual bulls across all groups did not change during the course of the study. Furthermore, there were no differences between pre- and posttreatment serum cortisol levels for the first 40 min of the treatment sampling period in the control group and progesterone concentrations did not change in the control group during the course of the study. Mean serum cortisol concentrations were significantly elevated above pretreatment concentrations after 60 min in the control group; however, this elevation may have been due to excessive time spent in the handling facilities. The higher pretreatment progesterone concentrations in the control group compared with those in EE groups may have resulted from the lack of complete acclimatization at the start of the study.
The greatest changes in serum cortisol concentrations were seen at 5 and 20 min after EE. Elevation of serum cortisol and progesterone immediately following treatment indicated that EE using a conventional or segmented probe, with or without caudal epidural anesthesia, was more stressful than restraint alone (control). The differences in elevation in serum cortisol and progesterone after EE may reflect the level of stress to be expected with and without the use of caudal epidural anesthesia (lower stress with anesthesia and higher stress without anesthesia). These findings support those of a similar previous study in which serum cortisol and progesterone tended to be less elevated after EE with the use of caudal epidural anesthesia (9). In another study in which change in heart rate was used as a measure of stress due to EE, elevation in heart rate after EE tended to be lower in bulls that received caudal epidural anesthesia than in those that did not (3). However, in both of those studies, caudal epidural anesthesia did not significantly reduce the stress response to EE.
Caudal epidural anesthesia had no adverse effect on penile protrusion and did not interfere with semen emission. On the other hand, there was little observable difference in behavioral response to the treatments, suggesting that caudal epidural anesthesia offered only a slight reduction in the EE-induced stress. Therefore, the added cost and inconvenience of employing caudal epidural anesthesia on a routine clinical basis may not be warranted. Other caudal epidural treatments, such as combinations of narcotics and lidocaine, may be useful in reducing pain and stress due to EE and these treatments need to be investigated.
Changes in serum progesterone concentrations might be a more sensitive indicator of stress than changes in serum cortisol concentrations since the increase in progesterone was significantly less at 5 and 20 min after EE in bulls with caudal epidural anesthesia than in those without; whereas, the change in cortisol concentrations was not different in bulls with caudal epidural anesthesia versus those without. This finding is in agreement with a previous study that compared changes in cortisol and progesterone concentrations in response to EE procedures (9).
The methods used for EE in this experiment attempted to ensure that quantitatively-similar electrical stimuli were delivered by both types of rectal probe. Since there was no observable difference in changes of either cortisol or progesterone between different probe types, segmented probes likely do not offer a method of pain reduction during EE. Both probe types were equally efficacious for semen emission; however, the degree of penile protrusion was significantly greater with the use of the conventional rectal probe than with the segmented probe. Therefore, segmented probes do not appear to offer any advantages over conventional probes. Many different sequences and durations of activation of the different segments in segmented probes could be employed for EE. Thus, it is possible that different methods than used in this experiment may afford some advantage of segmented versus conventional rectal probes. This may be a fruitful area of study for future experiments. CVJ
Footnotes
Reprints will not be available from the authors.
The authors thank the Interprovincial Summer Student Research Fund and the Saskatchewan Agricultural Development Fund for supporting this project.
References
- 1.Barth AD, Bowman PA. The sequential appearance of sperm abnormalities after scrotal insulation or dexamethasone treatment in bulls. Can Vet J. 1994;35:93–102. [PMC free article] [PubMed] [Google Scholar]
- 2.Ohl DA. Electroejaculation. Urologic Clinics of North America. 1993;20:181–188. [PubMed] [Google Scholar]
- 3.Mosure WL, Meyer RA, Gudmundson J, Barth AD. Evaluation of possible methods to reduce pain associated with electroejaculation in bulls. Can Vet J. 1998;39:504–506. [PMC free article] [PubMed] [Google Scholar]
- 4.Alam MGS, Dobson H. Effect of various veterinary procedures on plasma concentrations of cortisol, luteinizing hormone and prostaglandin F2-alpha metabolite in the cow. Vet Rec. 1986;118:7–10. doi: 10.1136/vr.118.1.7. [DOI] [PubMed] [Google Scholar]
- 5.Cooper C, Evans ACO, Cook S, Rawlings NC. Cortisol, progesterone and beta-endorphin response to stress in calves. Can J Anim terone Sci. 1995;95:197–201. [Google Scholar]
- 6.Robertson IS. Effect of different methods of castration on behavior and plasma cortisol in calves of three ages. Res Vet Anim Sci. 1994;56:8–17. doi: 10.1016/0034-5288(94)90189-9. [DOI] [PubMed] [Google Scholar]
- 7.Jephcott EH, McMillen IC. A comparison of the effects of electroimmobilisation and/or shearing procedures on ovine plasma concentration of beta-endorphin/beta-lipoprotein and cortisol. Res Vet Anim Sci. 1987;43:97–100. [PubMed] [Google Scholar]
- 8.Welsh TH, Jr, Johnson BH. Stress-induced alterations in secretion of corticosteroids, progesterone, luteinizing hormone, and testosterone in bulls. Can Vet J. 1998;39:1–3. doi: 10.1210/endo-109-1-185. [DOI] [PubMed] [Google Scholar]
- 9.Falk AJ, Waldner CL, Cotter BS, Gudmundson J, Barth AD. Effects of epidural lidocaine anesthesia on bulls during electroejaculation. Can Vet J. 2001;42:116–120. [PMC free article] [PubMed] [Google Scholar]
- 10.SAS Institute Inc. SAS/STAT Software: Changes and enhancements through release 6.12. Cary, NC: SAS Institute, 1997.