Abstract
Postmortem examination of a Boer buck kid that died peracutely revealed diffusely congested, edematous bowel. Clostridium perfringens Type A was isolated. Some isolates carried the gene for β2 toxin, suggesting a role for β2 toxin in caprine enterotoxemia, a common cause of death in growing kids.
Abstract
Résumé — Clostridium perfringens type A et sa toxine β2 associés à l’entérotoxémie chez un chevreau. Suite à une mort suraiguë, l’examen d’un chevreau mâle de race Boer a révélé de la congestion diffuse et de l’œdème au niveau de l’intestin. Du Clostridium perfringens de type A a été isolé. Certains isolats portaient le gène de la toxine β2, laissant présager un rôle pour la toxine β2 dans l’entérotoxémie caprine, maladie causant de nombreux décès chez les chevreaux.
(Traduit par Docteur André Blouin)
A 5-week-old, Boer buck in a 500-head commercial goat herd was found in acute distress, intensely vocalizing and recumbent. The kid had appeared normal the night before and had not experienced any previous health problems. The owners noted hypothermia (rectal temperature 36.2°C) and an absence of feces. Concerned about possible enterotoxemia, the owner treated the kid with Clostridium perfringens antitoxin, types C and D (Boehringer Ingelheim Vetmedica, Burlington, Ontario), 20 mL, SC, and 10 mL, PO; florfenicol (Nuflor; Schering-Plough Animal Health, Pointe Claire, Quebec), 10 mg/kg bodyweight (BW), IM; isoflupredone acetate (Predef; Pharmacia and Upjohn, Orangeville, Ontario), 0.015 mg/kg BW, IM; and penicillin G (PENPRO; APA, Cambridge, Ontario), 10 000 IU/kg BW, PO. The buck’s condition continued to deteriorate and he died en route to the veterinary clinic.
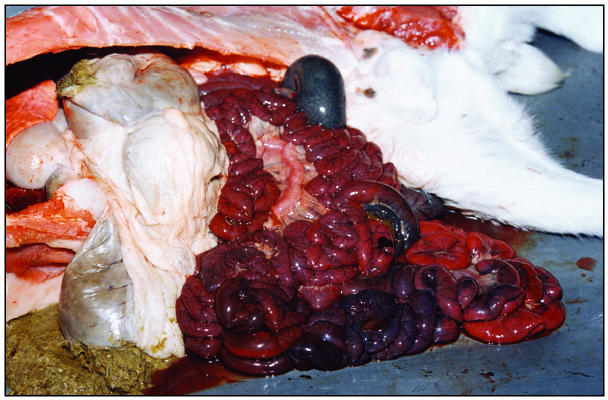
A postmortem examination revealed good body condition, no obvious lesions externally, and well-formed fecal pellets in the rectum. There was approximately 500 mL of serosanguinous fluid in the peritoneal cavity. The small and large intestines were diffusely congested, edematous, and filled with hemorrhagic fluid (Figure 1). The proximal part of the intestines appeared to be less affected than the distal portions. There were no signs of obstruction or torsion of the mesenteric root. The mesenteric lymph nodes were enlarged and congested. Body fat stores were adequate and there were no lesions indicative of white muscle disease. A presumptive diagnosis of enterotoxemia was made.
Figure 1.
Gross pathological changes in a 5-week-old Boer buck kid that died suddenly. The bowel is congested and edematous, and mesenteric lymph nodes are enlarged and congested.
Samples of heart, lung, liver, spleen, kidney, skeletal muscle, small and large intestine, mesenteric lymph node, thymus, thyroid, and brain were collected into 10% buffered formalin and submitted to the Animal Health Laboratories (AHL; University of Guelph, Guelph, Ontario) for histopathological analysis. Bowel content and tissue samples (at 4ºC) from the duodenum, jejunum, ileum, and large intestine were also submitted to AHL for bacteriological culture and polymerase chain reaction (PCR) testing.
A fecal sample was collected and analyzed in clinic by the standard vial gravitational flotation technique. An occasional coccidial oocyst was noted at 40× magnification. Because of the intensive nature of the commercial operation, subclinical infection with an Eimeria sp. was not unexpected. The small number of oocysts and absence of diarrhea made coccidiosis an unlikely contributor to the pathogenesis in this case. Coccidia control on the farm consisted of monensin in the feed at a concentration of 26.5 mg/kg (Rumensin; Elanco Animal Health, Guelph, Ontario).
The histopathological report noted mild acute myocardial necrosis and intestinal mucosal congestion. The muscle lesions might have been indicative of agonal changes or a myopathy, such as white muscle disease. However, the peracute and painful clinical presentation, lack of obvious muscle lesions at necropsy, and history of prophylactic administration of vitamin E-selenium at birth (E-Sel; Citadel Animal Health, Cambridge, Ontario), 0.25 mL, made white muscle disease an unlikely diagnosis. Congestion of the intestinal mucosal and lack of other remarkable findings was considered compatible with a diagnosis of enterotoxemia.
A large number of C. perfringens isolates were cultured from the bowel content. Polymerase chain reaction analysis of the isolates identified 2 different genotypes. Both were type A, with one encoding only the α toxin and the other encoding both α and β2 toxins. The clinical signs, postmortem lesions, and bacteriological results suggested a diagnosis of C. perfringens type A variant enterotoxemia.
Three forms of caprine enterotoxemia have been described. The peracute form typically affects young growing kids and, as in this case, causes death within hours. The rapid clinical course may or may not be associated with clinical signs, which may include severe pain, vocalization, recumbency, fever, fibrinohemorrhagic diarrhea, and convulsions (1). Acute enterotoxemia has a less rapid clinical course, and signs are less severe than those observed with the peracute form; however, if left untreated, acute enterotoxemia usually culminates in death. This form affects mature animals more commonly than kids (1). The chronic form has been recognized in adult goats and is associated with chronic intermittent diarrhea, anorexia, weight loss, and decreased milk production (1).
Several types of C. perfringens have been categorized on the basis of toxin production. Caprine enterotoxemia is caused primarily by C. perfringens type D, which produces both α and ɛ toxins, with the ɛ toxin being the main virulence factor (1). Clostridium perfringens type C, producing α and β toxins, has occasionally been associated with enterotoxemia, particularly in young kids (1). Clostridium perfringens type B, producing α, β, and ɛ toxins, rarely contributes to disease in goats and is more commonly associated with porcine enteritis (2). Clostridium perfringens type A, producing α toxin, is a common isolate from cases of caprine enterotoxemia (2), but its pathologic role is equivocal, as it is a normal commensal of the gastrointestinal tract (1).
As C. perfringens types C and D are the principal causes of enterotoxemia in goats, vaccination with ovine type C and D toxoids is a routine procedure on most goat farms. The case herd was vaccinated 3 to 4 times annually with C. perfringens type D, C. tetani, and Corynebacterium pseudotuberculosis (ovis) bacterin-toxoid (Glanvac-3; Vetrepharm, Lindon, Ontario). In addition, C. perfringens types C and D and C. tetani toxoid (Bar-Vac CD/T; Boerhinger Ingelheim Vetmedica) was administered to all kids at birth and at 3 to 4 wk of age.
The efficacy of vaccination against caprine enterotoxemia has been questioned. Most reports agree that response to vaccination is variable, although disagreement exists as to whether vaccine-induced antibody levels are sufficiently protective (3,4). It has been reported that to produce protective titers, 3 to 4 doses of vaccine annually were required to maintain a protective antibody level (3). Further controversy exists as to whether systemic immunity, mucosal immunity, or both are required to protect against caprine enterotoxemia, which is thought to be a predominantly enteric disease with systemic effects (1,5). One challenge study found that parenteral vaccination was protective against both systemic and enteric effects (5). However, poor efficacy is more commonly reported (1). Some researchers have suggested that inadequate protection in goats, despite apparently adequate antibody levels, may be attributed to the mainly enteric expression of enterotoxemia in goats compared with the systemic effects seen in sheep (6). The possible involvement of other clostridial toxins in caprine enterotoxemia has not been ruled out.
A novel clostridial toxin, β2, has been isolated from cases of necrotic enteritis in piglets (7,8), enterocolitis in horses (7), enterotoxemia in calves (8), and clostridial dysentery in lambs (9). This toxin may be associated with any of the clostridial biotypes, although current literature suggests that, predominantly, type A, and occasionally types C and D, are involved. The β2 toxin is encoded by the cpb2 gene (7). Sequence characterization of the gene and its product have revealed that β2 toxin is distinct from β1, with no significant amino acid sequence homology (7). The 2 toxins are of different sizes and are serologically only weakly cross-reactive (5). However, cytopathological and biological similarities do exist, as both toxins cause cytotoxic effects in cell culture, lethality in mice, and intestinal hemorrhage and necrosis in vivo in cattle and sheep (8,10).
While the cpb2 gene has been identified in a number of enteritis cases from a variety of species, the role of β2 toxin in pathogenesis of enterotoxemia is not straight-forward. As with other forms of clostridial enterotoxemia, the connection between the presence of a toxin and its role in disease is complicated by the fact that apparently healthy individuals may be carriers of the organisms that produce the toxin, and that enteritis may be caused by a variety of bacterial pathogens, including other clostridial biotypes. From studies that investigated foal diarrhea, lamb diarrhea, and calf enterotoxemia, β2 toxin has been reported at similar frequencies in both diseased and unaffected control populations (7,8). This finding is consistent with previous studies in which ɛ toxin, which is associated with C. perfringens type D enterotoxemia, was identified in healthy animals (9). These researchers postulated that the proportion of C. perfringens organisms in the intestinal tract that carry the gene encoding for the toxin determines disease occurrence. The absolute number of toxigenic C. perfringens in the intestinal tract is another important factor, as more isolates were reported in diseased animals than in the control population in a study of type A clostridial enterotoxemia in calves (8). Recently, increased intestinal lesions in a calf ligated-intestinal-loop assay were attributed to synergism between clostridial α and β2 toxins (8). In general, clostridial enterotoxemia appears to result from many factors occurring concurrently, which may include the immune status of the individual, the number of clostridial organisms in the gastrointestinal tract, and the toxigenic potential of these organisms.
Management plays a large role in the prevention and control of enterotoxemia. Sudden access to carbohydrate-rich feed, such as lush pasture or grain, stimulates C. perfringens population growth and subsequent pathogenesis, particularly the peracute form of the disease (1). The buck kid in this case was being weaned and was beginning to access creep feed to a significant extent. This may have placed him at greater risk for clostridial enterotoxemia. Failure of passive immunity is another important consideration in diseases that affect young animals. Five other kids in the herd had died with similar signs in the 5 mo prior to this case. All but one of these had been deprived of sufficient colostrum because of triplet/quadruplet births or poor colostral production. Vaccination of pregnant does with a clostridial toxoid may help to prevent classical type D enterotoxemia in the offspring. In this case, however, herd vaccination for C. perfringens types C and D would not have helped, as β2 toxin has no homology with other clostridial toxins, and α and β2 toxoids are not commercially available.
This case demonstrates a possible role for a Clostridium perfringens type A β2-variant in caprine enterotoxemia. Definitive diagnosis is difficult as healthy individuals may be carriers (7,8). However, the clinical signs, necropsy findings, culture results, isolation of α and β2 toxins, and the absence of other enterotoxigenic pathogens make this the most likely diagnosis.
Acknowledgments
The author thanks Dr. Peter Brouwers, Dr. Bian De Salvo, Dr. Tamara Lightbody, and the staff at Peterborough Veterinary Services for their assistance and guidance. Photo credit goes to Roger Dray. The author also thanks Pat and Bernie Steger, owners of Bethany Hill’s Boers, for their valuable input. CVJ
Footnotes
Dr. Dray’s current address is Strathroy Animal Clinic, 425 Frances Street, PO Box 158, Strathroy, Ontario N7G 3J2.
Dr. Dray will receive 50 free reprints of her article, courtesy of The Canadian Veterinary Journal.
References
- 1.Smith MC, Sherman DM. Goat Medicine. Baltimore: Lippincott, Williams and Wilkins, 1994:298–302.
- 2.Daube G, Simon P, Limbourg B, Manteca C, Mainil J, Kaeckenbeeck A. Hybridization of 2659 Clostridium perfringens isolates with gene probes for seven toxins (α, β, ɛ, τ, θ, μ and enterotoxin) and for sialidase. Am J Vet Res. 1996;57:496–501. [PubMed] [Google Scholar]
- 3.Blackwell TE, Butler DG, Bell JA. Enterotoxemia in the goat: The humoral response and local tissue reaction following vaccination with two different bacterin-toxoids. Can J Comp Med. 1983;47:127–132. [PMC free article] [PubMed] [Google Scholar]
- 4.Uzal FA, Bodero DA, Kelly WR, Nielson K. Variability of serum antibody responses of goat kids to a commercial Clostridium perfringens epsilon toxoid vaccine. Vet Rec. 1998;143:472–474. doi: 10.1136/vr.143.17.472. [DOI] [PubMed] [Google Scholar]
- 5.Uzal FA, Kelly WR. Protection of goats against experimental enterotoxaemia by vaccination with Clostridium perfringens type D epsilon toxoid. Vet Rec. 1998;142:722–725. doi: 10.1136/vr.142.26.722. [DOI] [PubMed] [Google Scholar]
- 6.Blackwell TE, Butler DG, Prescott JF, Wilcock BP. Differences in signs and lesions in sheep and goats with enterotoxemia induced by intraduodenal infusion of Clostridium perfringens type D. Am J Vet Res. 1991;52:1147–1152. [PubMed] [Google Scholar]
- 7.Garmony HS, Chanter N, French NP, Bueschel D, Songer JG, Titball RW. Occurrence of Clostridium perfringens β2 toxin amongst animals determined using genotyping and subtyping PCR assays. Epidemiol Infect. 2000;124:61–67. doi: 10.1017/s0950268899003295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manteca C, Daube G, Jauniaux T, et al. A role for the Clostridium perfringens β2 toxin in bovine enterotoxemia? Vet Microbiol. 2002;86:191–202. doi: 10.1016/S0378-1135(02)00008-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miserez R, Frey J, Buogo C, et al. Detection of α- and ɛ- toxigenic - Clostridium perfringens Type D in sheep and goats using a DNA amplification technique (PCR) Lett Appl Microbiol. 1998;26:382–386. doi: 10.1046/j.1472-765x.1998.00356.x. [DOI] [PubMed] [Google Scholar]
- 10.Gkiourtzidis K, Frey J, Bourtzi-Hatzopoulou E, Iliadis N, Sarris K. PCR detection and prevalence of α-, β-, β2-, ɛ-, τ- and enterotoxin genes in Clostridium perfringens isolated from lambs with clostridial dysentery. Vet Microbiol. 2001;82:39–43. doi: 10.1016/s0378-1135(01)00327-3. [DOI] [PubMed] [Google Scholar]