Abstract
Thyroid cancers are common endocrine malignancies that comprise tumors with different clinical and histological features. Indeed, papillary and follicular thyroid cancers are slow-growing, well-differentiated tumors, whereas anaplastic thyroid cancers are undifferentiated neoplasias that behave much more aggressively. Well-differentiated thyroid carcinomas are efficiently cured by surgery and radioiodine, unlike undifferentiated tumors that fail to uptake radioactive iodine and are usually resistant to chemotherapy. Therefore, novel and more effective therapies for these aggressive neoplasias are urgently needed. Whereas most genetic events underlying the pathogenesis of well-differentiated thyroid cancers have been identified, the molecular mechanisms that generate undifferentiated thyroid carcinomas are still unclear. To date, one of the best-characterized genetic alterations leading to the development of poorly differentiated thyroid tumors is the loss of the p53 tumor suppressor gene. In addition, the existence of a complex network among p53 family members (p63 and p73) and their interactions with other factors that promote thyroid cancer progression has been well documented. In this review, we provide an update on the current knowledge of the role of p53 family proteins in thyroid cancer and their possible use as a therapeutic target for the treatment of the most aggressive variants of this disease.
Keywords: thyroid cancer, p53, p63, p73, genetic alterations, p53 inhibition mechanisms, target therapies
1. Introduction
Thyroid cancer is the most common endocrine neoplasm, accounting for about 1.7% of total cancer diagnoses each year in the USA [1]. Worldwide, disease incidence has increased three-fold over the past 30 years because of both a higher prevalence in thyroid gland screening and environmental and life-style changes [2]. The risk of developing a thyroid tumor depends on genetic factors, age, gender, histological type, radiation exposure, and geographical region [3]. Neoplastic transformation of the follicular epithelium generates well-differentiated thyroid cancers (WDTC), including papillary (PTC) and follicular (FTC) thyroid carcinomas, and undifferentiated tumors represented by poorly differentiated (PDTC) and anaplastic thyroid carcinomas (ATC). PTCs and FTCs account for 85% and 10% of all thyroid carcinomas, respectively, with the remaining tumors unevenly distributed between PDTCs and ATCs [4].
The treatment for WDTC is based on surgery, radioactive iodine, and thyroid hormone therapy [5]. On the other hand, PDTCs or ATCs are refractory to hormone therapy and lack expression of the Sodium/Iodide Symporter (NIS) required for radioactive iodine uptake. Moreover, although metastases are observed in 10 to 20% of patients with ATC, most of them succumb to locally invasive, inoperable disease [6]. Furthermore, ATC is usually refractory to conventional chemotherapy [6] with more than 50% of affected individuals dying within one year from diagnosis [7].
Multiple studies have improved our understanding of the mechanisms underlying thyroid carcinogenesis. Presently, we know that several forms of thyroid cancer are characterized by mutually exclusive somatic mutations and/or gene rearrangements, which trigger increased cell proliferation and survival [8,9,10]. PTCs are often characterized by activating mutations of the BRAF (V600E substitution) that have been reported in up to 70% of cases. Rat Sarcoma (RAS) point mutations in codons 12, 13, and 61 and rearrangements of the Rearrangement During Transfection (RET) gene (generating RET/PTC chimeric oncogenes) represent other frequent genetic alterations (Figure 1). These events share a common downstream signaling pathway as they lead to the improper activation of the mitogen-activated protein kinase (MAPK) [11].
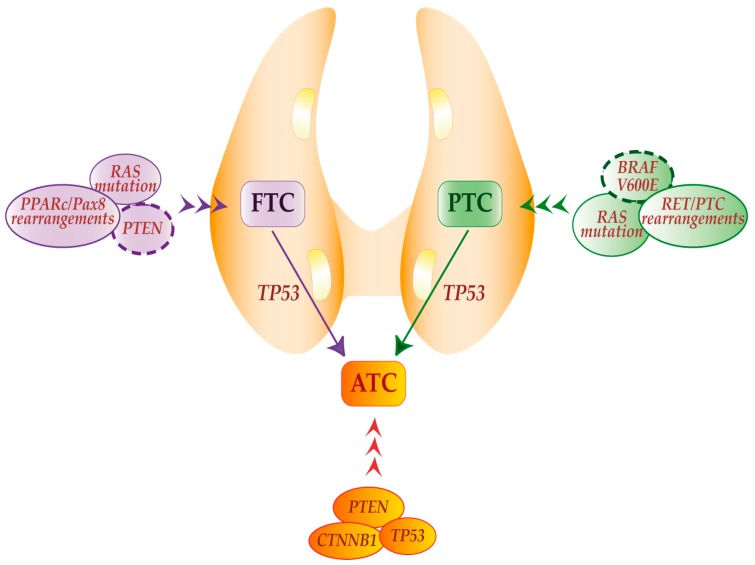
Figure 1.
Schematic representation of thyroid cancer hystotypes and their causative genetic events. Papillary (PTC), Follicular (FTC) and Anaplastic Thyroid carcinomas (ATC) originate from thyroid follicular cells. PTCs display BRAF (V600E substitution) and/or Rat Sarcoma (RAS) mutations as well as Rearrangement During Transfection (RET)/PTC rearrangements. FTCs present PPARc/Pax8 rearrangements, RAS, mutations and PTEN inactivating mutations or deletions. ATCs are characterized by PTEN and CTNNB1 mutations and p53 inactivation. Furthermore, ATCs may arise from FTCs and PTCs as a result of p53 loss of function, dysregulation of the PTEN/PI3K/AKT pathway or additional genetic alterations. Dashed lines indicate genes and mechanisms involved in progression to ATC.
FTCs display a different genetic profile (Figure 1) characterized by the presence of peroxisome proliferator-activated receptor c/paired box 8 (PPARc/Pax8) genetic rearrangements (30%), RAS activating point mutations (20–40%) and PTEN inactivation by point mutations, deletions, or promoter methylation (<10%) [12].
On the other hand, ATCs can be initiated by the coexistence of activating mutations in CTNNB1 (β-catenin encoding gene) and inactivation of both p53 and PTEN [13]. In addition, ATCs may arise from WDTC when PTEN inactivation (in the case of FTC) or BRAF activation (in the case of PTC) are associated with p53 loss of function (Figure 1). Finally, ATC may display mutations in the catalytic subunit alpha of the phosphatidyl-inositol-3-kinase (PIK3CA) that, in addition to PTEN inactivation, cause constitutive activation of the AKT pathway resulting in a more aggressive phenotype [14].
These alterations represent prognostic markers associated with tumor progression and potential targets that could become therapeutically actionable in the near future. In this review we intend to clarify the role of the p53 protein family in thyroid tumorigenesis. Furthermore we provide an update on the new therapeutic approaches involving p53 for the treatment of the most aggressive variants of the disease.
2. The p53 Protein Family
p53, p63 and p73 are tumor suppressor genes encoding for proteins with high homology. The common protein domains consist of an amino-terminal transactivation domain (TAD), a central DNA binding domain (DBD), a carboxy-terminal oligomerization domain (OD), and a proline-rich sequence recognition domain (PRD) [15] (Figure 2A).
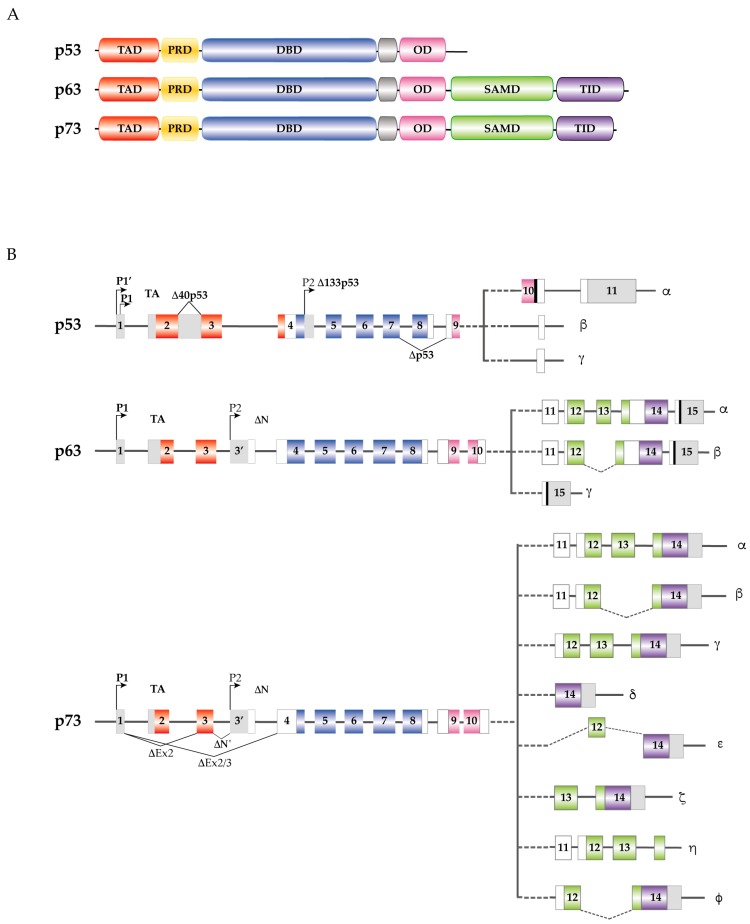
Figure 2.
p53 family proteins and isoforms. (A) Functional domains of the p53 family members. Red, Transactivation Domain (TAD); yellow, Proline-Rich sequence Domain (PRD); blue, DNA Binding Domain (DBD); pink, Oligomerization Domain (OD); green, Sterile α Motif Domain (SAMD); purple, Transactivation Inhibitory Domain (TID); (B) p53, p63 and p73 intron/exon structure. Introns are depicted in gray, while exon coloring reflects the corresponding functional domains. All three genes express multiple splice variants and contain different internal promoters. p53 includes TAp53, Δ40p53 (generated by an alternative splicing of intron 2), Δp53 (produced by alternative splicing of exons 7/9), and Δ133p53 (generated using an internal promoter in intron 4). The alternative splicing of intron 9 gives rise to α, β, and γ isoforms. In p63, the proximal P1 promoter yields the TA isoforms, while the distal P2 promoter in intron 3′, gives rise to ΔNp63 truncated variants. In addition, the COOH-terminal splicing leads to p63 α, β and γ isoforms for both the TA and ΔN variants. As for p73 the P1 promoter generates the TA isoforms, while the P2 distal promoter in intron 3′, gives rise to ΔNp73 truncated variants. Moreover, p73 can use an additional NH2-terminal splicing site, within exon 2, that produces ΔN like proteins Ex2p73, Ex2/3p73 and ΔN’p73. The COOH-terminal splicing leads to p73 α, β, γ, δ, ε, ζ, η, and ϕ isoforms for both TA and ΔN variants.
The TAD is the binding site for several transcription regulators [16]. The DBD mediates binding to target DNA sequences [17] while the OD induces oligomers formation that influence DNA-binding and transcriptional activity [18]. In addition to the aforementioned domains, p63 and p73 present a sterile α motif domain (SAMD) that modulates protein-protein interactions [19,20] and a transactivation inhibitory domain (TID) that regulates their transcription activity [21] (Figure 2A).
p53, p63 and p73 express different internal promoters located at the amino-terminus of each gene. The alternative activation of each promoter leads to the expression of several isoforms containing a complete N-terminal transactivation domain (TA-isoform) and/or an N-terminally truncated isoform lacking the transactivation domain (Figure 2B). In addition, several COOH-terminus transcripts, originated by alternative splicing, have been identified in p53 family members: α, β, and γ in the case of p53 and p63; α, β, γ, δ, ε, ζ, η, and φ in the case of p73 [15] (Figure 2B).
Since p53, p63, and p73 play a pivotal role in DNA damage response, cell differentiation, proliferation, and death, they currently represent very appealing targets in anticancer drug development [22,23]. Early evidence suggested significant redundancy in the biologic role of the p53 family members. However, additional findings have also indicated non p53-redundant functions for TAp63 and TAp73 as they regulate genes that are not p53 transcriptional targets [24]. Furthermore, while p53 is a tumor suppressor inactivated in half of human cancers, p63 and p73 rarely display mutations in their sequence. It was later demonstrated that TAp63 and TAp73 transactivate distinct but functionally overlapping subsets of known p53-regulated genes involved in cell-cycle arrest and apoptosis [25,26]. It should also be noted that although TAp63 and TAp73 show tumor suppressor properties in different tumors [27,28], several reports suggest an oncogenic function for these proteins as they may inhibit p53 DNA binding activity [29,30,31]. Likewise, the p63 and p73 ΔN variants were considered dominant-negative inhibitors of their respective TA isoforms and of the p53 tumor-suppressor. Indeed, their overexpression in a wide range of tumors is usually associated with an inferior prognosis [17]. However, later experiments also revealed a physiologic role for ΔN isoforms as expression modulators for different p53 family members. For example, p53 and TAp73 are both transcriptional inducers of ΔNp73, thus defining a negative regulatory loop in which p53 and TAp73 activation leads to their functional inhibition by ΔNp73 [26].
In summary, the need to account for a plethora of biological parameters—such as the TA/ΔN p63/p73 ratios, p63/p73 protein-protein interactions, and p63/p73 binding to the promoter of different p53-target genes—has yet to define an unequivocal role for p63 and p73 in human tumorigenesis [16].
2.1. p53 and Thyroid Cancer
It is well documented that thyroid carcinoma initiation and progression occurs through the gradual accumulation of multiple genetic alterations. One of the pivotal molecular alterations discriminating ATCs from WDTCs is the inactivation of the p53 tumor suppressor gene. p53 mutations are common in undifferentiated thyroid tumors (50–80% in ATCs) [32,33]. However, several findings indicate that alterations in the p53 sequence may also play a role in the early stages of thyroid cancerogenesis. Indeed, p53 mutations were recently found in up to 40% of PTCs and in 22% of oncocytic FTCs [34,35]. Usually, p53 point mutations are located in the region between exons 5 and 8 [36].
DNA damaging agents activate p53, leading to its binding of specific DNA responsive elements that transcriptionally regulate selected target genes. In thyroid cancer cells, p53 tumor suppressor activity is inhibited by three different mechanisms hindering p53 transcriptional activity, protein stability, and downstream signaling.
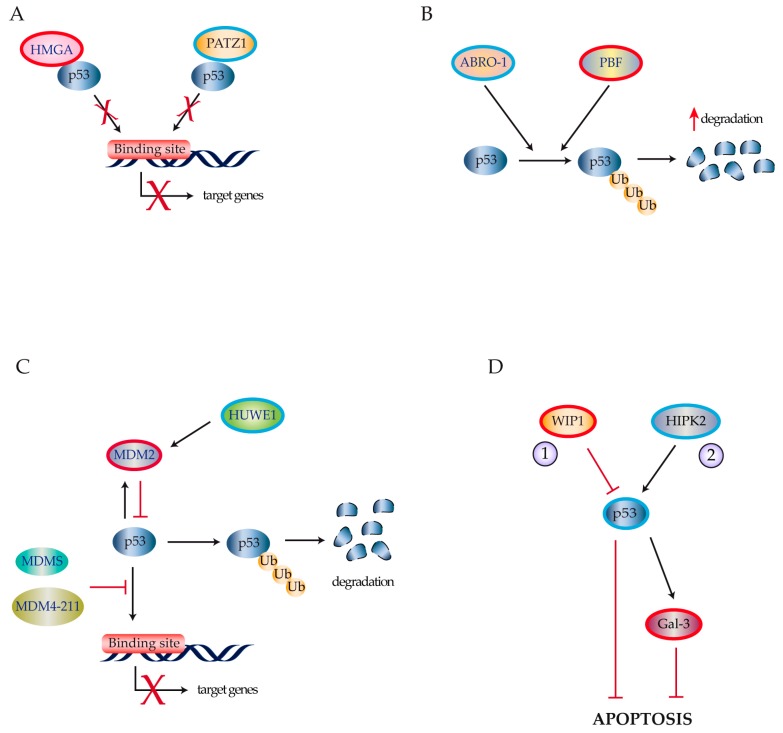
Frasca and colleagues reported that overexpression of high mobility group A factors (HMGA1a, HMGA1b, and HMGA2) functionally disables all p53 family members [15,37] possibly by reducing their DNA-binding activity [37,38,39] (Figure 3A). The POZ/BTB and AT hook containing zinc finger protein (PATZ1) is down-regulated in PDTCs and ATCs. As PATZ1 facilitates p53 binding to its responsive elements. Its down-regulation in thyroid cancer cells reduces p53 biological activity favoring both epithelial-mesenchymal transition and cell migration [40] (Figure 3A).
Figure 3.
p53 inactivating mechanisms in thyroid cancer. (A) HGMA1 over-expression (red) and PATZ1 down-regulation (blue) inhibit p53 ability to bind to its DNA responsive elements; (B) ABRO-1 down-regulation (blue) and PBF over-expression (red) decrease p53 stability through an increase in ubiquitin-mediated p53 degradation; (C) MDM-S, MDM4-211, and MDM-2 over-expression (red), following ubiquitin E3 ligase HUWE1 down-regulation (blue), inhibit p53 transactivation activity through p53 poly-ubiquitination and by blocking p53 interaction with the DNA binding sites of its target genes; (D) Mechanisms leading to reduced apoptotic sensibility in thyroid cancer cells include (1) WIP1 over-expression (red) and (2) decreased HIPK2 expression (blue) causing a reduction of p53 levels (blue) and subsequent increases of Gal-3 (red).
p53 stability is regulated through a process of ubiquitin-dependent protein degradation. In thyroid cancer cells, the over-expression or down-regulation of different p53 regulatory proteins heavily influence this mechanism.
Zhang et al. reported that Abraxas brother 1 (ABRO1), a component of the BRISC multiprotein complex that specifically cleaves “Lys-63”-linked ubiquitin, is frequently downregulated in thyroid, breast, liver, and kidney tumors [41]. The authors showed that ABRO1 stabilizes p53 by facilitating its interaction with deubiquitinase USP7. Depletion of ABRO1 in thyroid cancer reduces this interaction causing p53 poly-ubiquitination and enhancing cellular transformation of thyroid neoplastic clones [41] (Figure 3B). Likewise, the proto-oncogene PTTG1-Binding Factor (PBF) is highly expressed in WDTC [42], where it interacts with p53 thereby enhancing its poly-ubiquitination, which depends on the E3 ligase activity of MDM2 [43] (Figure 3B).
Murine double minute (MDM) family members are key regulators of p53 expression and function. Both MDM2 and MDM4 negatively regulate p53 [44,45] (Figure 3C). Furthermore, Prodosmo et al. found that MDM-S and MDM4-211—shorter MDM4 spliced variants expressed in thyroid tumors—were strong in vitro p53 inhibitors [46] (Figure 3C). The HECT, UBA, and WWE domain-containing protein 1 (HUWE1) is a ubiquitin E3 ligase for MDM2 that shows deregulated expression in several human cancers. Recently, Ma and colleagues demonstrated that HUWE1 is down-regulated in human thyroid carcinomas thus increasing MDM2 expression and reducing p53 protein stability [47] (Figure 3C).
Alterations in p53 signaling can also contribute to thyroid carcinogenesis by determining checkpoint defects, genomic instability, and inhibition of apoptosis.
Wild type p53 induced phosphatase 1 (WIP1) is a member of the PP2C family of evolutionarily conserved protein phosphatases and is considered a novel proto-oncogene [48,49]. Originally described as a p53-regulated gene, it is overexpressed in several tumors including PTCs where it inhibits p53 as well as p38MAPK and p16 [50] (Figure 3D). Galectin-3 (Gal-3) is an anti-apoptotic molecule that is down-regulated by wild-type p53 [51]. Thyroid tumors usually display low levels of transcription factor Homeodomain Interacting Protein Kinase 2 (HIPK2) that reduces p53 activation resulting in Gal-3 overexpression thus promoting neoplastic transformation [52] (Figure 3D). Zou et al. have recently described an additional mechanism contributing to p53 inactivation in a murine thyroid cancer model. They demonstrated that mice with thyroid-specific expression of the BRAFV600E mutation rapidly developed thyroid carcinomas. In this model, high levels of thyroid-stimulating hormone upregulated the PI3K/AKT pathway thus reducing p53 expression and suppressing BRAFV600E-induced senescence [53]. A further mechanism disregulating p53 downstream signaling involves FOXE1, a single-exon coding gene belonging to the forkhead/winged helix-domain protein family. FOXE1 is essential for thyroid gland development and has also been implicated in thyroid cancerogenesis following the discovery of inactivating mutations detected in neoplastic thyrocytes [54,55,56]. Recent evidence suggests that myosin-9 (MYH9) is a binding partner of the papillary thyroid cancer susceptibility candidate 2 (PTCSC2) long noncoding RNA. The interaction between MYH9 and PTCSC2 inhibits the FOXE1 promoter. In turn, lack of FOXE1 results in the transcriptional repression of THBS1 and IGFBP3, pivotal members of the p53 signaling pathway [57].
2.2. p63 and Thyroid Cancer
The p63 gene is infrequently mutated in human cancer. p63 overexpression has been reported in basal and squamous cell carcinomas of the head and neck, in thymomas, basal-like breast cancer, adenocarcinoma of the prostate, and poorly differentiated cervical tumors. p63 may also be aberrantly expressed in thyroid cancer [27,58,59,60].
Several evidences support the tumor suppressor role of TAp63 after interaction with ΔNp63 or other p53 family members. Furthermore, TAp63 induces cell death and suppress metastasis formation by decreasing mobility and invasion [27].
Different studies reported a possible role for p63 in thyrocyte neoplastic transformation [15,30]. Malaguarnera et al. demonstrated that, unlike normal thyroid cells and benign adenomas, most thyroid neoplasias express TAp63α. They also showed that endogenous TAp63α does not play a p53-like role as it fails to induce p21, BAX, and MDM2 and therefore does not cause cell growth arrest and apoptosis. Furthermore, they reported that TAp63α antagonizes p53 by interfering with the binding of more transcriptionally active p63 homologues (TAp63β and TAp63γ) and that of TAp73 [30]. As both TAp63α and ΔNp63α successfully inhibit p53-dependent suppression of colony formation, these findings imply that in thyroid cancer cells both TAp63α and ΔNp63α display a tumor-promoting role [15]. In this respect, Lazzari and colleagues have reported that HIPK2 induces ΔNp63α phosphorylation causing its proteasomal degradation. This mechanism removes the dominant negative effect of ΔNp63α on p53 restoring its pro-apoptotic activity [61].
2.3. p73 and Thyroid Cancer
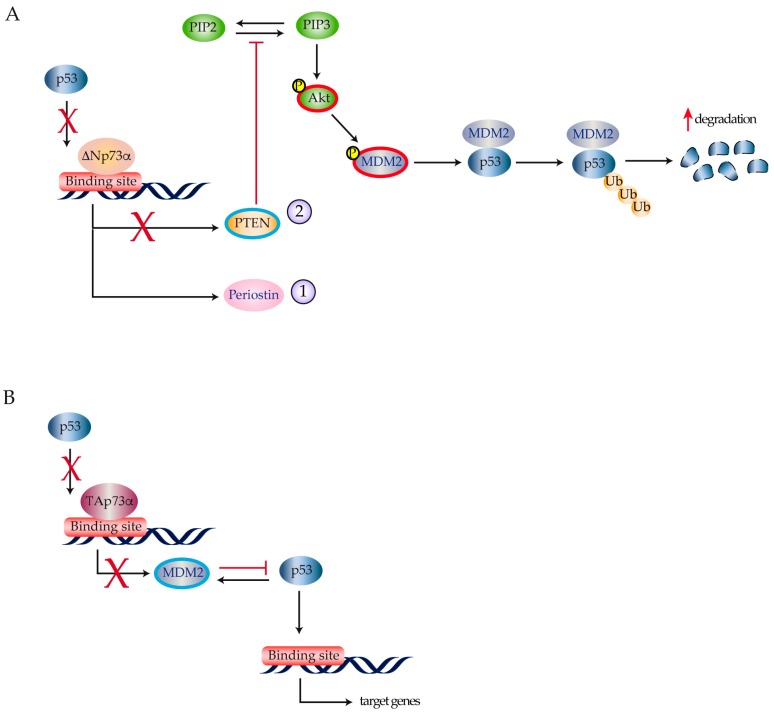
Different studies have reported p73 expression in human thyroid tumors, even if the role of p73 in thyroid cancer progression is still controversial [15,62,63]. Both TAp73α and ΔNp73 were detected by RQ-PCR and immunoblots in the large majority of thyroid cancer cell lines isolated from different histotypes (papillary, follicular, and anaplastic), but not in normal cultured thyrocytes [15,64]. Likewise, TAp73 and ΔNp73 transcripts were found in a consistent number of human thyroid carcinomas, although no correlation was found with their clinical and pathological characteristics [65]. Puppin and colleagues published evidence suggesting that ΔNp73α transcriptionally stimulates periostin gene expression in papillary, follicular, and undifferentiated thyroid cancer cells [66] (Figure 4A).
Figure 4.
p73 pathway activation in thyroid cancer. (A) ΔNp73α binding on the p53 promoter causes the activation of periostin (1) and a reduction in PTEN expression (blue) (2). Transcriptional repression of the PTEN promoter determines an activation of the PI3K-Akt pathway resulting in MDM2 phosphorylation that enhances MDM2-dependent degradation of p53; (B) TAp73α blocks p53-dependent transcription on the MDM2 promoter. In turn, this leads to reduced MDM2 expression and increased p53 stability.
As periostin is associated with accelerated tumor growth, increased neoangiogenesis and higher metastatic potential [67], ΔNp73α expression results in a more aggressive cancer phenotype [68,69]. Vella et al. reported that ΔNp73α represses the PTEN promoter [63]. PTEN down-regulation increases PIP3 half-life reducing its conversion in PIP2. High levels of PIP3 increase AKT phosphorylation causing MDM2-mediated ubiquitination and p53 degradation [70,71,72] (Figure 4A). PTEN repression by ΔNp73α requires binding to a DNA region outside of the canonical p53 site, confirming that p53 and its family members may recognize different sequences on the same promoter. In this scenario, the functional interactions among p53 family members may be either synergistic or antagonistic with respect to tumor suppression. This is in line with previous reports showing that, in different thyroid cancer models, co-expression of p53 and TAp73α may alternatively result in TAp73α down-regulation [62] or stronger p53 tumor suppressor activity [73]. In the latter case, TAp73α inhibits MDM2-mediated p53 protein degradation by reducing MDM2 binding to p53 and by directly antagonizing p53 activity on the MDM2 promoter (Figure 4B).
3. Novel Therapeutic Approaches for Undifferentiated Thyroid Cancer
The aforementioned advances in understanding the contribution of p53 family members to the pathogenesis of thyroid cancer have provided several opportunities for the use of molecular targeted therapies.
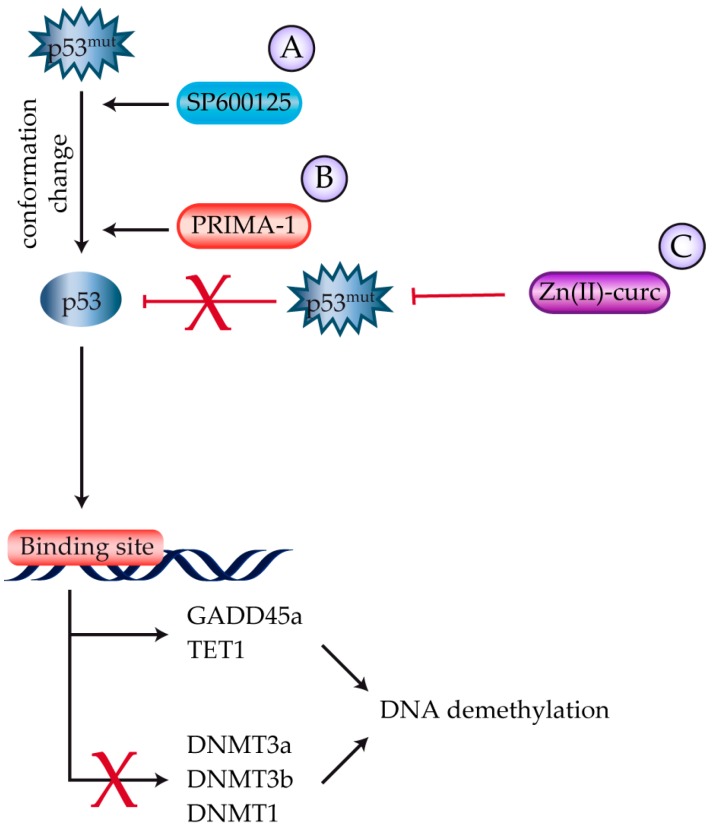
Multiple clinical trials using various multi-kinase inhibitors (MKIs) have led to the FDA and EMA approval of Sorafenib [74] and Lenvatinib [75] for undifferentiated thyroid cancer. Grassi and colleagues have also investigated SP600125, a reversible ATP-competitive MKI with anticancer properties against undifferentiated thyroid cancer. The compound reduces cell migration while killing human thyrocytes through the activation of mutant p53 and the concomitant inhibition of the ROCK/HDAC6 pathway [76] (Figure 5).
Figure 5.
Strategies to reactivate p53 in thyroid cancer. (A) SP600125 and (B) PRIMA-1 reactivate mutant p53 via conformational changes. In thyroid cancer, modifications caused by PRIMA-1 induce global DNA demethylation through the up-regulation of GADD45a and TET1 and down-regulation of DNMT1, 3a, and 3b; (C) Zn(II)-curc reduces mutant p53 expression by restoring wild-type p53-DNA binding activity to target gene promoters.
Besides MKIs, additional strategies for thyroid cancer treatment are currently being investigated that employ strategies that modulate epigenetic changes in thyroid cancer DNA, restore the transcriptional activity of mutant p53, and block signal transduction downstream of different p53 family members.
Studies on histone post-translational modifications that support tumor development and progression have focused on DNA methyltransferases (DNMT) inhibitors such as 5′-azadeoxycytidine with the aim of enabling thyroid cancer differentiation, thus restoring its sensitivity to radioactive iodine treatment [77]. This effect has also been investigated with the MEK1/MEK2 inhibitor Selumetinib [78]. Thailandepsin A (TDP-A) is a novel class I histone deacetylase inhibitor that induces a dose- and time-dependent anti-proliferative effect on human thyroid cancer cells mainly attributed to activation of the extrinsic apoptotic pathway coupled with cell cycle arrest [79]. Proteasome inhibitors such as Bortezomib have also been tested on thyroid cancer cells leading to significant inhibition of neoplastic thyrocyte proliferation [80,81].
p53 reactivation and induction of massive apoptosis (PRIMA-1) is a compound that reactivates the DNA binding ability of mutant p53 by restoring its conformation through two different mechanisms: (i) it induces Hsp90 over-expression thus enhancing its binding to mutant p53 and (ii) it covalently binds to mutant p53 [82]. Qiang and colleagues have recently defined the mechanism responsible for PRIMA-1-dependent rescue of mutant p53 function in thyroid cancer cells [83]. They showed that PRIMA-1 causes global DNA demethylation in cancer cells expressing mutant p53 mainly through inhibition of DNMT 1, 3a, and 3b, and upregulation of GADD45a. PRIMA-1 also increased the expression of the ten-eleven translocation (TET) family of 5mC-hydroxylases, particularly TET1, further contributing to DNA demethylation. Messina et al. studied the effect of PRIMA-1 in thyroid cancer cell lines with both wild-type and D259Y and K286E p53 mutants. They found that PRIMA-1 successfully killed undifferentiated thyroid carcinoma cells carrying mutant p53 that were refractory to chemotherapy [84] (Figure 5).
A curcumin-based zinc compound [Zn(II)-curc] also reduced mutant p53 expression in thyroid cancer cells, reactivating p53 tumor suppressor activity and resulting in increased response to anti-cancer therapies [85] (Figure 5).
Finally, the mammalian target of rapamycin (mTOR) pathway inhibitor Everolimus has been studied in a phase II trial of 40 patients with locally advanced or metastatic thyroid cancer [86] producing stable disease in 29 (76%) patients and a median Progression Free Survival of 47 weeks (95% CI 14.9–78.5).
4. Conclusions
The concept of precision medicine postulates that effective treatment strategies should be tailored to the individual variability of both the patient and her/his disease. While alterations in p53 sequence, stability, and downstream signaling are heavily involved in thyroid carcinogenesis, the role of the remaining p53 family members in the development and progression of thyroid cancer has yet to be fully elucidated. Nevertheless, increasing evidence indicates that p53 family members contribute to the development of multiple thyroid cancer variants and an ever-increasing number of therapeutic molecules targeting these proteins may soon be available in the clinical setting.
Abbreviations
| WDTC | Well Differentiated Thyroid Cancer |
| PDTC | Poorly Differentiated Thyroid Cancer |
| ATC | Anaplastic Thyroid Cancer |
| RET | Rearranged During Transfection |
| PTC | Papillary Thyroid Cancer |
| FTC | Follicular Thyroid cancer |
| MAPK | Mitogen-Activated Protein Kinase |
| PPARc | Peroxisome Proliferator-Activated Receptor C |
| PAX8 | Paired Box 8 |
| RAS | Rat Sarcoma |
| PI3K | Phosphatidyl-Inositol-3-Kinase |
| PTEN | Phosphatase and Tensin Homolog |
| CTNNB1 | Catenin beta 1 |
| TAD | Transactivation Domain |
| DBD | DNA Binding Domain |
| OD | Oligomerization Domain |
| PRD | Prolin-Rich Sequence recognition Domain |
| SAMD | Steril Alpha Motif Domain |
| TID | Transactivation Inhibitory Domain |
| HMGA | High Mobility Group A |
| PATZ1 | POZ7BTB and AT Hook containing Zinc-finger Protein |
| ABRO1 | Abraxas Brother 1 |
| PBF | PTTG1-Binding Factor |
| MDM | Murine Double Minute |
| HUWE1 | HECT, UBA, and WWE Domain-Containing Protein 1 |
| WIP1 | Wild Type p53 Induced Phosphatases 1 |
| Gal-3 | Galectin-3 |
| HIPK2 | Homeodomain Interacting Protein Kinase 2 |
| FOXE1 | Forkhead7Winged Helix-Domain Protein |
| MYH9 | Myosin-9 |
| PTCSC2 | Papillary Thyroid Cancer Susceptibility Candidate 2 |
| THBS1 | Thrombospondin 1 |
| IGFBP3 | Insulin-Like Growth Factor Binding Protein 3 |
| MKIs | Multi-Kinase Inhibitors |
| HDAC | Histone Deacetylase |
| ROCK | Rho Kinase |
| DNMT | DNA Methyltransferase |
| MEK | Mitogen-Activated protein kinase kinase |
| TDP-A | Thailandepsin A |
| PRIMA-1 | p53 Reactivation and Induction of Massive Apoptosis |
| GADD45A | Growth Arrest and DNA Damage Inducible Alpha |
| TET | Ten-Eleven Translocation |
| mTOR | Mammalian Target of Rapamycine |
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Siegel R., Ma J., Zou Z., Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 2.Chen A.Y., Jemal A., Ward E.M. Increasing incidence of differentiated thyroid cancer in the United States, 1988–2005. Cancer. 2009;115:3801–3807. doi: 10.1002/cncr.24416. [DOI] [PubMed] [Google Scholar]
- 3.Pellegriti G., Frasca F., Regalbuto C., Squatrito S., Vigneri R. Worldwide increasing incidence of thyroid cancer: Update on epidemiology and risk factors. J. Cancer Epidemiol. 2013;2013:965212. doi: 10.1155/2013/965212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikiforov Y.E., Steward D.L., Robinson-Smith T.M., Haugen B.R., Klopper J.P., Zhu Z., Fagin J.A., Falciglia M., Weber K., Nikiforova M.N. Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J. Clin. Endocrinol. Metab. 2009;94:2092–2098. doi: 10.1210/jc.2009-0247. [DOI] [PubMed] [Google Scholar]
- 5.Sherman S.I. Thyroid carcinoma. Lancet. 2003;361:501–511. doi: 10.1016/S0140-6736(03)12488-9. [DOI] [PubMed] [Google Scholar]
- 6.McIver B., Hay I.D., Giuffrida D.F., Dvorak C.E., Grant C.S., Thompson G.B., van Heerden J.A., Goellner J.R. Anaplastic thyroid carcinoma: A 50-year experience at a single institution. Surgery. 2001;130:1028–1034. doi: 10.1067/msy.2001.118266. [DOI] [PubMed] [Google Scholar]
- 7.Hadar T., Mor C., Shvero J., Levy R., Segal K. Anaplastic carcinoma of the thyroid. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 1993;19:511–516. [PubMed] [Google Scholar]
- 8.Massimino M., Vigneri P., Fallica M., Fidilio A., Aloisi A., Frasca F., Manzella L. IRF5 promotes the proliferation of human thyroid cancer cells. Mol. Cancer. 2012;11:21. doi: 10.1186/1476-4598-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Soares P., Trovisco V., Rocha A.S., Lima J., Castro P., Preto A., Maximo V., Botelho T., Seruca R., Sobrinho-Simoes M. BRAF mutations and RET/PTC rearrangements are alternative events in the etiopathogenesis of PTC. Oncogene. 2003;22:4578–4580. doi: 10.1038/sj.onc.1206706. [DOI] [PubMed] [Google Scholar]
- 10.Xing M. Molecular pathogenesis and mechanisms of thyroid cancer. Nat. Rev. Cancer. 2013;13:184–199. doi: 10.1038/nrc3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kimura E.T., Nikiforova M.N., Zhu Z., Knauf J.A., Nikiforov Y.E., Fagin J.A. High prevalence of BRAF mutations in thyroid cancer: Genetic evidence for constitutive activation of the RET/PTC-RAS-BRAF signaling pathway in papillary thyroid carcinoma. Cancer Res. 2003;63:1454–1457. [PubMed] [Google Scholar]
- 12.Kroll T.G., Sarraf P., Pecciarini L., Chen C.J., Mueller E., Spiegelman B.M., Fletcher J.A. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma [corrected] Science. 2000;289:1357–1360. doi: 10.1126/science.289.5483.1357. [DOI] [PubMed] [Google Scholar]
- 13.Omur O., Baran Y. An update on molecular biology of thyroid cancers. Crit. Rev. Oncol. Hematol. 2014;90:233–252. doi: 10.1016/j.critrevonc.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Xing M. Genetic alterations in the phosphatidylinositol-3 kinase/Akt pathway in thyroid cancer. Thyroid Off. J. Am. Thyroid Assoc. 2010;20:697–706. doi: 10.1089/thy.2010.1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malaguarnera R., Vella V., Vigneri R., Frasca F. p53 family proteins in thyroid cancer. Endocr. Relat. Cancer. 2007;14:43–60. doi: 10.1677/erc.1.01223. [DOI] [PubMed] [Google Scholar]
- 16.Ferraiuolo M., Di Agostino S., Blandino G., Strano S. Oncogenic Intra-p53 Family Member Interactions in Human Cancers. Front. Oncol. 2016;6:77. doi: 10.3389/fonc.2016.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Collavin L., Lunardi A., Del Sal G. p53-family proteins and their regulators: Hubs and spokes in tumor suppression. Cell Death Differ. 2010;17:901–911. doi: 10.1038/cdd.2010.35. [DOI] [PubMed] [Google Scholar]
- 18.Sauer M., Bretz A.C., Beinoraviciute-Kellner R., Beitzinger M., Burek C., Rosenwald A., Harms G.S., Stiewe T. C-terminal diversity within the p53 family accounts for differences in DNA binding and transcriptional activity. Nucleic Acids Res. 2008;36:1900–1912. doi: 10.1093/nar/gkn044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bernard H., Garmy-Susini B., Ainaoui N., Van Den Berghe L., Peurichard A., Javerzat S., Bikfalvi A., Lane D.P., Bourdon J.C., Prats A.C. The p53 isoform, Δ133p53α, stimulates angiogenesis and tumour progression. Oncogene. 2013;32:2150–2160. doi: 10.1038/onc.2012.242. [DOI] [PubMed] [Google Scholar]
- 20.Melino G., Lu X., Gasco M., Crook T., Knight R.A. Functional regulation of p73 and p63: Development and cancer. Trends Biochem. Sci. 2003;28:663–670. doi: 10.1016/j.tibs.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Ghioni P., Bolognese F., Duijf P.H., Van Bokhoven H., Mantovani R., Guerrini L. Complex transcriptional effects of p63 isoforms: Identification of novel activation and repression domains. Mol. Cell. Biol. 2002;22:8659–8668. doi: 10.1128/MCB.22.24.8659-8668.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Candi E., Agostini M., Melino G., Bernassola F. How the TP53 family proteins TP63 and TP73 contribute to tumorigenesis: Regulators and effectors. Hum. Mutat. 2014;35:702–714. doi: 10.1002/humu.22523. [DOI] [PubMed] [Google Scholar]
- 23.Gomes S., Leao M., Raimundo L., Ramos H., Soares J., Saraiva L. p53 family interactions and yeast: Together in anticancer therapy. Drug Discov. Today. 2016;21:616–624. doi: 10.1016/j.drudis.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 24.Barbieri C.E., Pietenpol J.A. p63 and epithelial biology. Exp. Cell Res. 2006;312:695–706. doi: 10.1016/j.yexcr.2005.11.028. [DOI] [PubMed] [Google Scholar]
- 25.Deyoung M.P., Ellisen L.W. p63 and p73 in human cancer: Defining the network. Oncogene. 2007;26:5169–5183. doi: 10.1038/sj.onc.1210337. [DOI] [PubMed] [Google Scholar]
- 26.Melino G., De Laurenzi V., Vousden K.H. p73: Friend or foe in tumorigenesis. Nat. Rev. Cancer. 2002;2:605–615. doi: 10.1038/nrc861. [DOI] [PubMed] [Google Scholar]
- 27.Melino G. p63 is a suppressor of tumorigenesis and metastasis interacting with mutant p53. Cell Death Differ. 2011;18:1487–1499. doi: 10.1038/cdd.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Su X., Chakravarti D., Flores E.R. p63 steps into the limelight: Crucial roles in the suppression of tumorigenesis and metastasis. Nat. Rev. Cancer. 2013;13:136–143. doi: 10.1038/nrc3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freebern W.J., Smith J.L., Chaudhry S.S., Haggerty C.M., Gardner K. Novel cell-specific and dominant negative anti-apoptotic roles of p73 in transformed leukemia cells. J. Biol. Chem. 2003;278:2249–2255. doi: 10.1074/jbc.M208517200. [DOI] [PubMed] [Google Scholar]
- 30.Malaguarnera R., Mandarino A., Mazzon E., Vella V., Gangemi P., Vancheri C., Vigneri P., Aloisi A., Vigneri R., Frasca F. The p53-homologue p63 may promote thyroid cancer progression. Endocr. Relat. Cancer. 2005;12:953–971. doi: 10.1677/erc.1.00968. [DOI] [PubMed] [Google Scholar]
- 31.Vikhanskaya F., D’Incalci M., Broggini M. p73 competes with p53 and attenuates its response in a human ovarian cancer cell line. Nucleic Acids Res. 2000;28:513–519. doi: 10.1093/nar/28.2.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quiros R.M., Ding H.G., Gattuso P., Prinz R.A., Xu X. Evidence that one subset of anaplastic thyroid carcinomas are derived from papillary carcinomas due to BRAF and p53 mutations. Cancer. 2005;103:2261–2268. doi: 10.1002/cncr.21073. [DOI] [PubMed] [Google Scholar]
- 33.Salvatore D., Celetti A., Fabien N., Paulin C., Martelli M.L., Battaglia C., Califano D., Monaco C., Viglietto G., Santoro M., et al. Low frequency of p53 mutations in human thyroid tumours; p53 and Ras mutation in two out of fifty-six thyroid tumours. Eur. J. Endocrinol. 1996;134:177–183. doi: 10.1530/eje.0.1340177. [DOI] [PubMed] [Google Scholar]
- 34.Cancer Genome Atlas Research Network Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159:676–690. doi: 10.1016/j.cell.2014.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dralle H., Machens A., Basa J., Fatourechi V., Franceschi S., Hay I.D., Nikiforov Y.E., Pacini F., Pasieka J.L., Sherman S.I. Follicular cell-derived thyroid cancer. Nat. Rev. Dis. Primers. 2015;1:15077. doi: 10.1038/nrdp.2015.77. [DOI] [PubMed] [Google Scholar]
- 36.Farid N.R. P53 mutations in thyroid carcinoma: Tidings from an old foe. J. Endocrinol. Investig. 2001;24:536–545. doi: 10.1007/BF03343889. [DOI] [PubMed] [Google Scholar]
- 37.Frasca F., Rustighi A., Malaguarnera R., Altamura S., Vigneri P., Del Sal G., Giancotti V., Pezzino V., Vigneri R., Manfioletti G. HMGA1 inhibits the function of p53 family members in thyroid cancer cells. Cancer Res. 2006;66:2980–2989. doi: 10.1158/0008-5472.CAN-05-2637. [DOI] [PubMed] [Google Scholar]
- 38.Reeves R. Molecular biology of HMGA proteins: Hubs of nuclear function. Gene. 2001;277:63–81. doi: 10.1016/S0378-1119(01)00689-8. [DOI] [PubMed] [Google Scholar]
- 39.Sgarra R., Rustighi A., Tessari M.A., Di Bernardo J., Altamura S., Fusco A., Manfioletti G., Giancotti V. Nuclear phosphoproteins HMGA and their relationship with chromatin structure and cancer. FEBS Lett. 2004;574:1–8. doi: 10.1016/j.febslet.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Chiappetta G., Valentino T., Vitiello M., Pasquinelli R., Monaco M., Palma G., Sepe R., Luciano A., Pallante P., Palmieri D., et al. PATZ1 acts as a tumor suppressor in thyroid cancer via targeting p53-dependent genes involved in EMT and cell migration. Oncotarget. 2015;6:5310–5323. doi: 10.18632/oncotarget.2776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J., Cao M., Dong J., Li C., Xu W., Zhan Y., Wang X., Yu M., Ge C., Ge Z., et al. ABRO1 suppresses tumourigenesis and regulates the DNA damage response by stabilizing p53. Nat. Commun. 2014;5:5059. doi: 10.1038/ncomms6059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stratford A.L., Boelaert K., Tannahill L.A., Kim D.S., Warfield A., Eggo M.C., Gittoes N.J., Young L.S., Franklyn J.A., McCabe C.J. Pituitary tumor transforming gene binding factor: A novel transforming gene in thyroid tumorigenesis. J. Clin. Endocrinol. Metab. 2005;90:4341–4349. doi: 10.1210/jc.2005-0523. [DOI] [PubMed] [Google Scholar]
- 43.Read M.L., Seed R.I., Fong J.C., Modasia B., Ryan G.A., Watkins R.J., Gagliano T., Smith V.E., Stratford A.L., Kwan P.K., et al. The PTTG1-binding factor (PBF/PTTG1IP) regulates p53 activity in thyroid cells. Endocrinology. 2014;155:1222–1234. doi: 10.1210/en.2013-1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marine J.C., Dyer M.A., Jochemsen A.G. MDMX: From bench to bedside. J. Cell Sci. 2007;120:371–378. doi: 10.1242/jcs.03362. [DOI] [PubMed] [Google Scholar]
- 45.Marine J.C., Francoz S., Maetens M., Wahl G., Toledo F., Lozano G. Keeping p53 in check: Essential and synergistic functions of MDM2 and MDM4. Cell Death Differ. 2006;13:927–934. doi: 10.1038/sj.cdd.4401912. [DOI] [PubMed] [Google Scholar]
- 46.Prodosmo A., Giglio S., Moretti S., Mancini F., Barbi F., Avenia N., Di Conza G., Schunemann H.J., Pistola L., Ludovini V., et al. Analysis of human MDM4 variants in papillary thyroid carcinomas reveals new potential markers of cancer properties. J. Mol. Med. 2008;86:585–596. doi: 10.1007/s00109-008-0322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ma W., Zhao P., Zang L., Zhang K., Liao H., Hu Z. Tumour suppressive function of HUWE1 in thyroid cancer. J. Biosci. 2016;41:395–405. doi: 10.1007/s12038-016-9623-z. [DOI] [PubMed] [Google Scholar]
- 48.Bulavin D.V., Demidov O.N., Saito S., Kauraniemi P., Phillips C., Amundson S.A., Ambrosino C., Sauter G., Nebreda A.R., Anderson C.W., et al. Amplification of PPM1D in human tumors abrogates p53 tumor-suppressor activity. Nat. Genet. 2002;31:210–215. doi: 10.1038/ng894. [DOI] [PubMed] [Google Scholar]
- 49.Li J., Yang Y., Peng Y., Austin R.J., van Eyndhoven W.G., Nguyen K.C., Gabriele T., McCurrach M.E., Marks J.R., Hoey T., et al. Oncogenic properties of PPM1D located within a breast cancer amplification epicenter at 17q23. Nat. Genet. 2002;31:133–134. doi: 10.1038/ng888. [DOI] [PubMed] [Google Scholar]
- 50.Yang D., Zhang H., Hu X., Xin S., Duan Z. Abnormality of pl6/p38MAPK/p53/Wipl pathway in papillary thyroid cancer. Gland Surg. 2012;1:33–38. doi: 10.3978/j.issn.2227-684X.2012.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lavra L., Ulivieri A., Rinaldo C., Dominici R., Volante M., Luciani E., Bartolazzi A., Frasca F., Soddu S., Sciacchitano S. Gal-3 is stimulated by gain-of-function p53 mutations and modulates chemoresistance in anaplastic thyroid carcinomas. J. Pathol. 2009;218:66–75. doi: 10.1002/path.2510. [DOI] [PubMed] [Google Scholar]
- 52.Lavra L., Rinaldo C., Ulivieri A., Luciani E., Fidanza P., Giacomelli L., Bellotti C., Ricci A., Trovato M., Soddu S., et al. The loss of the p53 activator HIPK2 is responsible for galectin-3 overexpression in well differentiated thyroid carcinomas. PLoS ONE. 2011;6:e20665. doi: 10.1371/journal.pone.0020665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zou M., Baitei E.Y., Al-Rijjal R.A., Parhar R.S., Al-Mohanna F.A., Kimura S., Pritchard C., Binessa H.A., Alzahrani A.S., Al-Khalaf H.H., et al. TSH overcomes Braf(V600E)-induced senescence to promote tumor progression via downregulation of p53 expression in papillary thyroid cancer. Oncogene. 2016;35:1909–1918. doi: 10.1038/onc.2015.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.He H., Li W., Liyanarachchi S., Jendrzejewski J., Srinivas M., Davuluri R.V., Nagy R., de la Chapelle A. Genetic predisposition to papillary thyroid carcinoma: Involvement of FOXE1, TSHR, and a novel lincRNA gene, PTCSC2. J. Clin. Endocrinol. Metab. 2015;100:E164–E172. doi: 10.1210/jc.2014-2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landa I., Ruiz-Llorente S., Montero-Conde C., Inglada-Perez L., Schiavi F., Leskela S., Pita G., Milne R., Maravall J., Ramos I., et al. The variant rs1867277 in FOXE1 gene confers thyroid cancer susceptibility through the recruitment of USF1/USF2 transcription factors. PLoS Genet. 2009;5:e1000637. doi: 10.1371/journal.pgen.1000637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mond M., Bullock M., Yao Y., Clifton-Bligh R.J., Gilfillan C., Fuller P.J. Somatic Mutations of FOXE1 in Papillary Thyroid Cancer. Thyroid Off. J. Am. Thyroid Assoc. 2015;25:904–910. doi: 10.1089/thy.2015.0030. [DOI] [PubMed] [Google Scholar]
- 57.Wang Y., He H., Li W., Phay J., Shen R., Yu L., Hancioglu B., de la Chapelle A. MYH9 binds to lncRNA gene PTCSC2 and regulates FOXE1 in the 9q22 thyroid cancer risk locus. Proc. Natl. Acad. Sci. USA. 2017;114:474–479. doi: 10.1073/pnas.1619917114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bonzanini M., Amadori P.L., Sagramoso C., Dalla Palma P. Expression of cytokeratin 19 and protein p63 in fine needle aspiration biopsy of papillary thyroid carcinoma. Acta Cytol. 2008;52:541–548. doi: 10.1159/000325595. [DOI] [PubMed] [Google Scholar]
- 59.Candi E., Dinsdale D., Rufini A., Salomoni P., Knight R.A., Mueller M., Krammer P.H., Melino G. TAp63 and DeltaNp63 in cancer and epidermal development. Cell Cycle. 2007;6:274–285. doi: 10.4161/cc.6.3.3797. [DOI] [PubMed] [Google Scholar]
- 60.Candi E., Rufini A., Terrinoni A., Giamboi-Miraglia A., Lena A.M., Mantovani R., Knight R., Melino G. DeltaNp63 regulates thymic development through enhanced expression of FgfR2 and Jag2. Proc. Natl. Acad. Sci. USA. 2007;104:11999–12004. doi: 10.1073/pnas.0703458104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lazzari C., Prodosmo A., Siepi F., Rinaldo C., Galli F., Gentileschi M., Bartolazzi A., Costanzo A., Sacchi A., Guerrini L., et al. HIPK2 phosphorylates DeltaNp63alpha and promotes its degradation in response to DNA damage. Oncogene. 2011;30:4802–4813. doi: 10.1038/onc.2011.182. [DOI] [PubMed] [Google Scholar]
- 62.Frasca F., Vella V., Aloisi A., Mandarino A., Mazzon E., Vigneri R., Vigneri P. p73 tumor-suppressor activity is impaired in human thyroid cancer. Cancer Res. 2003;63:5829–5837. [PubMed] [Google Scholar]
- 63.Vella V., Puppin C., Damante G., Vigneri R., Sanfilippo M., Vigneri P., Tell G., Frasca F. DeltaNp73alpha inhibits PTEN expression in thyroid cancer cells. Int. J. Cancer. 2009;124:2539–2548. doi: 10.1002/ijc.24221. [DOI] [PubMed] [Google Scholar]
- 64.Dominguez G., Garcia J.M., Pena C., Silva J., Garcia V., Martinez L., Maximiano C., Gomez M.E., Rivera J.A., Garcia-Andrade C., et al. DeltaTAp73 upregulation correlates with poor prognosis in human tumors: Putative in vivo network involving p73 isoforms, p53, and E2F-1. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2006;24:805–815. doi: 10.1200/JCO.2005.02.2350. [DOI] [PubMed] [Google Scholar]
- 65.Ferru A., Denis S., Guilhot J., Gibelin H., Tourani J.M., Kraimps J.L., Larsen C.J., Karayan-Tapon L. Expression of TAp73 and DeltaNp73 isoform transcripts in thyroid tumours. Eur. J. Surg. Oncol. J. Eur. Soc. Surg. Oncol. Br. Assoc. Surg. Oncol. 2006;32:228–230. doi: 10.1016/j.ejso.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 66.Puppin C., Passon N., Frasca F., Vigneri R., Tomay F., Tomaciello S., Damante G. In thyroid cancer cell lines expression of periostin gene is controlled by p73 and is not related to epigenetic marks of active transcription. Cell. Oncol. 2011;34:131–140. doi: 10.1007/s13402-011-0009-9. [DOI] [PubMed] [Google Scholar]
- 67.Ruan K., Bao S., Ouyang G. The multifaceted role of periostin in tumorigenesis. Cell. Mol. Life Sci. CMLS. 2009;66:2219–2230. doi: 10.1007/s00018-009-0013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fluge O., Bruland O., Akslen L.A., Lillehaug J.R., Varhaug J.E. Gene expression in poorly differentiated papillary thyroid carcinomas. Thyroid Off. J. Am. Thyroid Assoc. 2006;16:161–175. doi: 10.1089/thy.2006.16.161. [DOI] [PubMed] [Google Scholar]
- 69.Puppin C., Fabbro D., Dima M., Di Loreto C., Puxeddu E., Filetti S., Russo D., Damante G. High periostin expression correlates with aggressiveness in papillary thyroid carcinomas. J. Endocrinol. 2008;197:401–408. doi: 10.1677/JOE-07-0618. [DOI] [PubMed] [Google Scholar]
- 70.Mayo L.D., Dixon J.E., Durden D.L., Tonks N.K., Donner D.B. PTEN protects p53 from MDM2 and sensitizes cancer cells to chemotherapy. J. Biol. Chem. 2002;277:5484–5489. doi: 10.1074/jbc.M108302200. [DOI] [PubMed] [Google Scholar]
- 71.Mayo L.D., Donner D.B. A phosphatidylinositol 3-kinase/Akt pathway promotes translocation of MDM2 from the cytoplasm to the nucleus. Proc. Natl. Acad. Sci. USA. 2001;98:11598–11603. doi: 10.1073/pnas.181181198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ogawara Y., Kishishita S., Obata T., Isazawa Y., Suzuki T., Tanaka K., Masuyama N., Gotoh Y. Akt enhances MDM2-mediated ubiquitination and degradation of p53. J. Biol. Chem. 2002;277:21843–21850. doi: 10.1074/jbc.M109745200. [DOI] [PubMed] [Google Scholar]
- 73.Malaguarnera R., Vella V., Pandini G., Sanfilippo M., Pezzino V., Vigneri R., Frasca F. TAp73 alpha increases p53 tumor suppressor activity in thyroid cancer cells via the inhibition of MDM2-mediated degradation. Mol. Cancer Res. MCR. 2008;6:64–77. doi: 10.1158/1541-7786.MCR-07-0005. [DOI] [PubMed] [Google Scholar]
- 74.Brose M.S., Nutting C.M., Jarzab B., Elisei R., Siena S., Bastholt L., de la Fouchardiere C., Pacini F., Paschke R., Shong Y.K., et al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: A randomised, double-blind, phase 3 trial. Lancet. 2014;384:319–328. doi: 10.1016/S0140-6736(14)60421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schlumberger M., Tahara M., Wirth L.J., Robinson B., Brose M.S., Elisei R., Habra M.A., Newbold K., Shah M.H., Hoff A.O., et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N. Engl. J. Med. 2015;372:621–630. doi: 10.1056/NEJMoa1406470. [DOI] [PubMed] [Google Scholar]
- 76.Grassi E.S., Vezzoli V., Negri I., Labadi A., Fugazzola L., Vitale G., Persani L. SP600125 has a remarkable anticancer potential against undifferentiated thyroid cancer through selective action on ROCK and p53 pathways. Oncotarget. 2015;6:36383–36399. doi: 10.18632/oncotarget.5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Riesco-Eizaguirre G., Santisteban P. New insights in thyroid follicular cell biology and its impact in thyroid cancer therapy. Endocr. Relat. Cancer. 2007;14:957–977. doi: 10.1677/ERC-07-0085. [DOI] [PubMed] [Google Scholar]
- 78.Ho A.L., Grewal R.K., Leboeuf R., Sherman E.J., Pfister D.G., Deandreis D., Pentlow K.S., Zanzonico P.B., Haque S., Gavane S., et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N. Engl. J. Med. 2013;368:623–632. doi: 10.1056/NEJMoa1209288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Weinlander E., Somnay Y., Harrison A.D., Wang C., Cheng Y.Q., Jaskula-Sztul R., Yu X.M., Chen H. The novel histone deacetylase inhibitor thailandepsin A inhibits anaplastic thyroid cancer growth. J. Surg. Res. 2014;190:191–197. doi: 10.1016/j.jss.2014.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Altmann A., Markert A., Askoxylakis V., Schoning T., Jesenofsky R., Eisenhut M., Haberkorn U. Antitumor effects of proteasome inhibition in anaplastic thyroid carcinoma. J. Nucl. Med. 2012;53:1764–1771. doi: 10.2967/jnumed.111.101295. [DOI] [PubMed] [Google Scholar]
- 81.Putzer D., Gabriel M., Kroiss A., Madleitner R., Eisterer W., Kendler D., Uprimny C., Bale R.J., Gastl G., Virgolini I.J. First experience with proteasome inhibitor treatment of radioiodine nonavid thyroid cancer using bortezomib. Clin. Nucl. Med. 2012;37:539–544. doi: 10.1097/RLU.0b013e31824c5f24. [DOI] [PubMed] [Google Scholar]
- 82.Rehman A., Chahal M.S., Tang X., Bruce J.E., Pommier Y., Daoud S.S. Proteomic identification of heat shock protein 90 as a candidate target for p53 mutation reactivation by PRIMA-1 in breast cancer cells. Breast Cancer Res. 2005;7:R765–R774. doi: 10.1186/bcr1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qiang W., Jin T., Yang Q., Liu W., Liu S., Ji M., He N., Chen C., Shi B., Hou P. PRIMA-1 selectively induces global DNA demethylation in p53 mutant-type thyroid cancer cells. J. Biomed. Nanotechnol. 2014;10:1249–1258. doi: 10.1166/jbn.2014.1862. [DOI] [PubMed] [Google Scholar]
- 84.Messina R.L., Sanfilippo M., Vella V., Pandini G., Vigneri P., Nicolosi M.L., Giani F., Vigneri R., Frasca F. Reactivation of p53 mutants by prima-1 [corrected] in thyroid cancer cells. Int. J. Cancer. 2012;130:2259–2270. doi: 10.1002/ijc.26228. [DOI] [PubMed] [Google Scholar]
- 85.Garufi A., D’Orazi V., Crispini A., D’Orazi G. Zn(II)-curc targets p53 in thyroid cancer cells. Int. J. Oncol. 2015;47:1241–1248. doi: 10.3892/ijo.2015.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lim S.M., Chang H., Yoon M.J., Hong Y.K., Kim H., Chung W.Y., Park C.S., Nam K.H., Kang S.W., Kim M.K., et al. A multicenter, phase II trial of everolimus in locally advanced or metastatic thyroid cancer of all histologic subtypes. Ann. Oncol. 2013;24:3089–3094. doi: 10.1093/annonc/mdt379. [DOI] [PubMed] [Google Scholar]