ABSTRACT
Background: Interest is growing on immune cells involvement in central nervous system tumors such as glioblastoma. Even if a few reports highlighted that immune classifications could have a prognostic value, no paradigm has been clearly yet established on large and homogeneous cohorts. The aim of our study was to analyze the prognostic role of the in situ immune response of cytotoxic T cells (i.e., CD8+), Foxp3 cells, Th17 and tumor-associated macrophages in glioblastoma on two independent large and homogeneous cohorts.
Methods: We worked on two large homogenous cohorts of patients having glioblastoma who underwent standard radiochemotherapy. The first cohort of 186 patients was analyzed using IHC procedures (CD8+, IL-17A, FoxP3 and CD163) of surgery pieces. We next worked with transcriptomic data available online and used metagene strategy analysis for the second cohort of 525 patients.
Results: Cytotoxic CD8+ lymphocytes and Foxp3 cells were associated with a good prognosis, while Th17 were associated with a poor clinical outcome. These data were confirmed with transcriptomic analysis. Moreover, we showed for the first time a strong link between angiogenesis and Th17 metagenes expressions in glioblastoma.
Conclusions: Our study shows that glioblastoma bearing patients can be classified on the immune infiltrate aspects. Beyond this prognostic role of immune biomarkers, subsequent classifications could definitely help clinicians to handle targeted therapy administration and immunotherapeutic interventions.
KEYWORDS: Angiogenesis, CD8+ T cells, glioblastoma, immune infiltrate, Th17 T cells
Introduction
Glioblastomas (GBM) are the most frequent type of glioma, representing 60% of gliomas and have the worst prognosis with less than 3% of survival at 5 y.1 Standard care for newly diagnosed GBM is surgical resection followed by radiotherapy with concurrent and adjuvant temozolomide. Despite such treatment all patients experience recurrence and median survival is only around 15 mo.2 Molecular classification of glioblastoma separates the different transcriptomic type of GBM in three categories (i.e., pro-neural, proliferative and mesenchymal).3,4Although the knowledge of this cancer's biology has improved, few targeted therapies are in development. Due to high vascularization, anti-angiogenic therapy (as single agent or combined with cytotoxic drugs) is the standard for recurrent GBM.5 Although it also improves progression free survival in newly diagnosed GBM, no effect on overall survival (OS) is observed.6 So, GBM is clearly an orphan cancer and finding effective treatments is a must.
Adaptive immune response can control tumor progression in most cancer types.7 As a consequence, in situ immune infiltrates have been clearly associated with human cancer prognosis in various types of cancers. Thus, a balance between the presence of an antitumoral immune response and immunosuppressive actors participate in tumor growth control.8 For brain tumors, a dogma of immune privilege of the central nervous system (CNS) has gradually eroded, with growing data supporting a dynamic interaction between the CNS and the systemic immune system.9 Recent reports showed that GBM are infiltrated with immune cells and specifically with an increase in effector T cells and cytotoxic cells compared with normal brain suggesting that an in situ immune response is generated to control tumor growth.10 In addition to effector cells, immunosuppressive cells like Foxp3+ regulatory T cells (Treg) and myeloid suppressive cells like myeloid-derived suppressor cells (MDSC) or type 2 tumor-associated macrophages (TAM2) are frequently found in GBM and could be involved in tumor progression.11 A recent report demonstrated the efficacy of CAR-T cell immunotherapy against GBM, thus supporting the rational to stratify GBM in a immunological point of view to better define patients more prone to respond to immunotherapy.12
In this work, the objective was to analyze the prognostic role of the in situ immune response in large and homogenous cohorts of GBM patients. We fostered on T cells (CD8+, Th17 and Treg) and myeloid response (TAM2). Interestingly, this study reveals that CD8+ T cell infiltrates are associated with a better OS, while Th17 and angiogenesis are linked with each other and are associated with a poorer prognosis.
Material and methods
Patients
We retrospectively studied formalin-fixed and paraffin-embedded (FFPE) cancer surgical specimens from 186 consecutive glioblastoma cancer patients, who underwent brain surgical resection between January 2006 and December 2013 in the department of neurosurgery of Dijon teaching hospital. Patients were excluded from this study if they received anticancer therapy before surgery or if they did not receive radiochemotherapy after surgery.
Patients' clinical and tumor pathological characteristics are shown in Table S1. The median follow-up at the time of analysis was 32.9 mo. The study was approved by the ethical local committee, and all patients gave written informed consent at the time of the diagnosis for the use of tumor samples for research purposes.
Immunohistochemistry procedures
For single staining, the following antibodies were used: mouse anti-CD8+ (M7103 Dako-Agilent), mouse anti-Foxp3 (ab20034, Abcam), goat anti-IL-17 (AF317- R&D Systems) and mouse anti-CD163 (NCL163, Leica, France). Antigen retrieval was performed by heating slides for 15 min at 95 °C in 1 mmol /L EDTA. The stained arrays were counterstained with haematoxylin, and mounted in Aquamount (Dako). Positive and negative controls were performed with paraffin tonsil sections using each monoclonal antibody and an isotype-matched negative control antibody.
For double staining procedures, the following antibodies were used: rabbit anti-Ki67 (ab16667, Abcam) and mouse anti-CD31 (M0823, Dako-Agilent). Ki67 was detected using a donkey anti-rabbit HRP conjugated and signal was revealed with DAB (SK4103, Vector Laboratories). CD31 was detected using donkey anti-mouse biotin-conjugated. The third step amplification used conjugated streptavidin alkaline phosphatase and the signal was revealed with Vector Red (SK5100, Vector Laboratories).
Quantification of lymphocyte infiltrates and blood vessels
The presence or absence of lymphocyte infiltration and blood vessels, and their quantification in different areas on each tumor sample, was evaluated by two independent physicians (FG and CGK) on tissue section. All samples were previously anonymized and blinded for clinical data. For CD8+, CD163, Foxp3 and IL-17 staining, the number of positive cells was counted in three consecutive high power fields (40×) in the tumor core. The mean count of three fields was used for statistical analysis. The results of the analyses conducted by each independent physician were subsequently compared. Major discrepancies between the two clinicians were reviewed jointly to reach a consensus when the mean count differ more than 10%.
For blood vessels numeration, physician first selected areas with or without IL-17A infiltrate. Then, the same area was chosen on the double stained Ki67/CD31 slide. Blood vessels were then counted at medium magnification (10×). CD8+ quantification was then evaluated in the same area.
Generation of primary GBM cell lines and cell culturing
The procedures described by Azari and colleagues were followed.13 Briefly, fresh surgically removed GBM tumor tissues were minced with scalpels in DMEM/Ham's F12 medium within a sterile Petri dish. Dissociation of tissue was then performed using a trypsin solution during 15 min at 37 °C. Trypsin inhibitor was used after this step to stop tissue digestion. The cell suspension was then passed through a cell strainer (40 µm; Becton-Dickinson-Falcon). GBM primary cell lines were afterward cultured in neurospheres in DMEM/F12 supplemented with EGF, β-FGF (both 15 ng/mL) and heparin 0.2% (1 µL/mL). We also used the two brain cell lines SNB19 and U87 from ATCC. Mycoplasma absence was routinely checked (once every 2 weeks).
VEGF-A assay
Six primary cultures of GBM cells and GBM cell line (SNB19 and U87) were treated with 100 ng/mL of recombinant IL-17A (R&D Systems, France) during 48h. VEGF-A was quantified and analyzed by ELISA (eBioscience, Rennes, France) using manufacturer's protocol. Samples were analyzed in triplicate.
Statistical analysis
All patients were followed up until death or the date of the cut-off for study analysis. OS was calculated from the date when therapy started to the date of death. Median follow-up was calculated using the reverse Kaplan–Meier method. OS probabilities were estimated using the Kaplan–Meier method and were compared by the log-rank test. The optimal cut-off was determined for each histological variable using Cutoff Finder software.14 The optimal cut-off was defined as the point with the most significant (log-rank test) split. A multivariate Cox proportional hazard regression model was applied to assess independent prognostic effects for OS. The hazard ratios (HR) were given with their 95% confidence intervals (CI). All variables with a univariate Cox p value ≤ 0.20 were eligible for multivariate analyses. The multivariate model was adjusted for sex, age, preoperative Karnofsky score (assessed the day before surgery), surgery (resection vs. biopsy). Correlations between co-variables were first tested for eligible variables. To prevent collinearity, when two variables were significantly correlated, one variable was retained according to its clinical relevance or to the value of the likelihood ratio. All reported p values are two-sided. The statistical significance level was set to be p < 0.05. Analyses were performed using SAS 9.3 (Statistical Analysis System).
Transcriptomic analysis
Data sets
We used the TCGA GBM data set downloaded from the Broad Institute GDAC Firebrowse website (http://doi.org/10.7908/C11G0KM9).This data set was obtained for 525 patients using Affymetrix Human Genome U133A array and is provided normalized using the Robust Multi-array Average (RMA) method.15 This data set also displayed age, sex and OS. A total of 217 patients benefited from radiochemotherapy as adjuvant therapy, 205 had radiotherapy and 3 had undergone chemotherapy.
We isolated from the transcriptomic data set different combinations of genes corresponding to specific immune cell metagenes. We focused on two subpopulations of TILS, namely Th17 and CD8+ activated, whose expression was estimated through immune metagenes previously identified by Angelova et al.16 Metagenes of CD8+ activated were CETN3, GEMIN6, GAL, PTRH2, MPZL1, CSE1L, C1GALT1C1, MRPS16, SPC25, TUBB, EIF2S1, CCT6B, RFC5, AHSA1, TIMM13, KIAA0101, PCNA, ADRM1, TK1, TIMM8B and CCT5, and for Th17 were IL23A, MOCOS, TRAF3IP2, YBX2, IL17A, IL17RC, LTK, FURIN, LONRF3, CA2, TNIP2, F12, CEACAM3, GPR25 and ABP1. Note that a few metagenes previously identified were not present in our data set. In addition, we added in the analysis a neoangiogenesis metagene due to the sharp important of angiogenesis in brain tumors (ANGPT1, ANGPT2, ANPEP, EREG, FGF1, FGF2, FIGF, FLT1, JAG1, KDR, LAMA5, NRP1, NRP2, PGF, PLXDC1, STAB1, VEGFA and VEGFC). We also included an endothelial metagene recently reported by Becht and colleagues.17 The expression of all of the metagenes was averaged for each subpopulation as commonly done. Hereafter, we will call these averaged representations of the TILS subpopulation “metagenes.” Correlations between covariates were evaluated using Spearman's rank correlation coefficients and two-tailed p values were estimated using “cor.test” function in R.
Metagenes and prognosis
The survival prognostic value of clinical variables and genes/metagenes expression was evaluated using Kaplan–Meier estimator and log-rank testing. Continuous variables (clinical parameters as well as gene signature expression) were previously dichotomized using the best cut off estimation provided by Budczies.14
Multivariate Cox proportional hazards models were used to evaluate the prognostic value of individual genes or metagenes when combined with clinical parameters. Only tests with p < 0.05 were considered significant. All analysis were performed using the open-source R software (https://cran.r-project.org/).
Results
Patients
A total of 186 patients with GBM consecutively treated with the radiochemotherapy for GBM since 2007 were included in this cohort. The clinical characteristics are summarized in Table S1. There were 105 male and 81 female patients. A total of 99 underwent a complete surgery and 87 were sampled only by biopsy before radiochemotherapy. The collection of blood sample is authorized by the French authorization No AC2014-2460.
Association between immune infiltrates and patients' survival
We analyzed the myeloid marker CD163, lymphoid markers CD8+ and FoxP3 and the cytokine IL-17A with IHC procedures. We performed a manual counting for each cell subset. CD163 was often highly and homogenously stained in the tumor bed (Table S2 and Fig. 1A). In contrast, lymphoid infiltrates were less important. We observed a similar level of CD8+ and Foxp3 infiltrates (i.e., cells per field 130 ± 118 and 128 ± 81, respectively); however, the infiltration was very heterogeneous spanning from the absence of lymphoid cells to high infiltration with more than 500 cells per field (Fig. 1A and Table S2). IL-17A-positive cells were also observed in the tumor bed but at a lower rate (i.e., cells per filed 15 ± 20). These cells seemed to be preferentially located in perivascular area and thus could be testament to a recent diapedesis (Fig. 1A).
Figure 1.
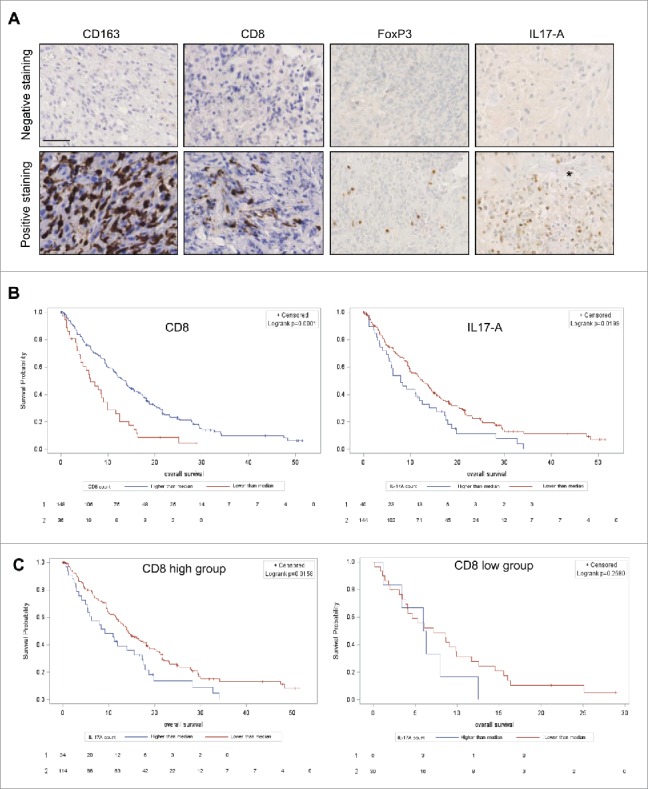
Representative pictures of negative (upper panel) and positive (lower panel) staining for CD163, CD8+, FoxP3 and IL-17A at 40× magnification. The star identifies a blood vessel lumen. Scale bar is 40 µm (A). Kaplan–Meier curves for CD8+ staining (left) and IL-17A (right) (B) (log rank test respectively p = 0.0001 and p = 0.0199). Kaplan–Meier curves of subgroup analysis with CD8+ high group (left) and CD8+ low group (right) (log rank test respectively p = 0.0158 and 0.2580 not significant) (C).
In this cohort, univariate Cox analysis for classical prognostic factors determined that an older age, a lower Karnofsky performance status and the absence of bevacizumab administration at recurrence were associated with a poor survival (Table 1). For immune infiltrates, we observed that while a high number of IL-17-A-positive cells were associated with a poorer OS (p = 0.02; HR: 1.56 [95% CI: 1.02–2.78]), a high number of CD8+ infiltrate (p = 0.0001; HR: 0.47 [95% CI: 0.32–0.7]) (Table 1 and Fig. 1B) were associated with a better OS. Foxp3-positive cells were also associated with a better OS with less significance (p = 0.04; HR: 0.62 [95% CI: 0.4–0.98]) and CD163 infiltrate was finally not associated with prognosis (Fig. S1). Next, we tested the correlation between immune parameters and we noted a strong correlation between Foxp3, CD8+ and CD163 (Table S3). Among those variables, we chose to only retain CD8+ for the multivariate model as it was the most significant. The multivariate Cox regression model showed that age, Karnofsky performance status, CD8+ and IL-17A were independent factors associated with OS (Table 1). Upon subgroup analysis, we observed that IL-17A remained prognostic for patients with a high CD8+ infiltrate (p = 0.01; median OS of 9.2 mo [95% CI: 5.3–15.5] and 14.2 mo [95% CI: 11.6–18.2] for high vs. low IL-17A infiltrate, respectively). In patients with a low CD8+ infiltrate, IL-17A staining was no more significantly associated with a poorer prognosis probably due to the low number of patients in this subgroup (p = 0.25; median OS of 6.1 mo [95% CI: 1.2–12.5] and 7.2 mo [95% CI: 3.9–9.9] for high vs. low IL-17A infiltrate) (Fig. 1C).
Table 1.
Univariate and multivariate analyses (Cox regression) for factors associated with OS in the 186 patients cohort.
| Univariate HR | 95% CI | p | Multivariate HR | 95% CI | p | |
|---|---|---|---|---|---|---|
| Age | ||||||
| <64 | 1 | <0.0001 | 1 | 0.005 | ||
| ≥ 64 | 2.01 | 1.48–2.86 | 1.65 | 1.16–2.34 | ||
| Sex | ||||||
| Female | 1 | 0.94 | ||||
| Male | 0.99 | 0.72–1.37 | ||||
| Diagnosis | ||||||
| Biopsy | 1 | 0.11 | ||||
| Surgery | 0.8 | 0.7–1.1 | ||||
| Karnofsky status | ||||||
| <70 | 1 | <0.0001 | 1 | <0.0001 | ||
| ≥ 70 | 0.23 | 0.16–0.33 | 0.25 | 0.17–0.37 | ||
| Bevacizumab use | ||||||
| No | 1 | 0.003 | 1 | 0.19 | ||
| Yes | 0.61 | 0.44–1.85 | 0.78 | 0.54–1.13 | ||
| CD8+ | ||||||
| <median | 1 | 0.0002 | 1 | 0.01 | ||
| >median | 0.47 | 0.32–0.72 | 0.59 | 0.39–0.91 | ||
| IL-17 | ||||||
| <6,000 /mm3 | 1 | 0.02 | 1 | 0.01 | ||
| >6,000 /mm3 | 1.56 | 1.07–2.78 | 1.65 | 1.11–2.46 | ||
| Foxp3 | ||||||
| <median | 1 | 0.038 | ||||
| >median | 0.62 | 1.05–2.5 | ||||
| CD163 | ||||||
| <median | 1 | 0.09 | ||||
| >median | 1.33 | 0.95–1.81 |
Together these data suggest that CD8+ infiltrates is associated with a better OS, while IL-17A-positive cells infiltrates are associated with a poorer OS independently.
External validation using transcriptomic data
To confirm our observations on another set of data, we used a public large scale transcriptomic study namely the TCGA GBM data set, available on the Broad Institute GDAC Firebrowse. This data set was obtained for 525 patients bearing GBM using Affymetrix Human Genome U133A array. This cohort included 320 males and 205 females, mean age was 57.7 ± 14.6 y. A total of 435 patients received at least radiotherapy as treatment. To analyze the prognostic role of Th17 and CD8+ T cells, we decided to use the metagene strategy described previously to analyze immune infiltrates in colorectal cancer.16 In this cohort, using the univariate Cox model, a highly activated CD8+ metagene expression was associated with a better prognosis (p = 0.0025; HR: 0.25 [95% CI: 0.09–0.66]) (Fig. 2A), while a high Th17 metagene expression was associated with a poorer prognosis (p = 0.0074; HR: 1.58 [95% CI: 1.13–2.22]) (Fig. 2B and Table 2). Similar results were obtained upon multivariate model including age and treatment (Table 2). Upon the subgroup analysis, patients could be separated in four groups. First, a group of patients expressing a high CD8+ activated metagene combined with a low Th17 metagene had the better prognosis. A group of patients with both low CD8+ activated and Th17 metagenes expression had an intermediate prognosis. The third group combining the expression of a low CD8+ activated metagene and a high Th17 metagene had the poorer prognosis (Fig. 2C). A fourth group combining both high CD8+ activated and Th17 metagenes share similar prognostic that the high CD8+ activated metagene combined with a low Th17 metagene. However, due to the few number (only nine patients), results from this final group required careful interpretations.
Figure 2.
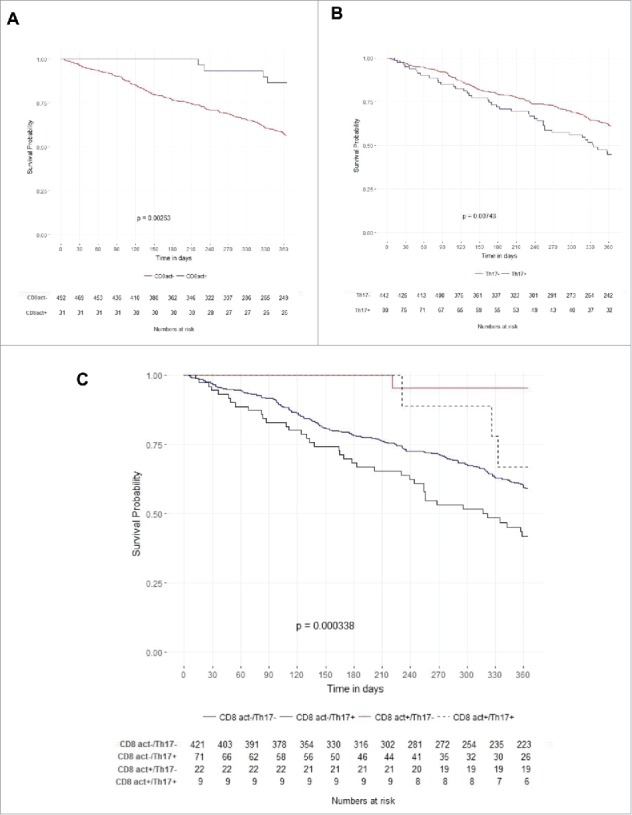
Kaplan–Meier curves for activated CD8+ (CD8act) metagene expression (log rank test p = 0.00253) (A) and Th17 metagene expression (log rank test p = 0.00743) (B). Kaplan–Meier curves for subgroup analysis comparing CD8act+/Th17− (red line), CD8act−/Th17− (blue line), CD8−/Th17+ (black line) and CD8act+/Th17+ (dotted black line) (C).
Table 2.
Univariate and multivariate analysis (Cox regression) for factors associated with OS in the 525 patients cohort.
| Covariates | Threshold | Univariate HR | 95% CI | p-value | Multivariate HR | 95% CI | p-value |
|---|---|---|---|---|---|---|---|
| Age | < 60 y | 0.47 | [0.36–0.63] | 1.84e−07 | 0.70 | [0.52–0.95] | 0.021 |
| ≥ 60 y | 1 | 1 | |||||
| Radiotherapy (22 NA) | Yes | 0.12 | [0.09–0.17] | < 2e−16 | 0.13 | [0.09–0.18] | < 2e−16 |
| No | 1 | 1 | |||||
| Karnofsky score* (129 NA) | ≥ 70 | 1 | [1.99–3.97] | 4.34e−09 | – | — | — |
| < 70 | 2.8 | ||||||
| Th17 metagene | < 5.46 | 1 | [1.13–2.22] | 0.0074 | 1 | [1.17–2.44] | 0.048 |
| ≥ 5.46 | 1.52 | 1.70 | |||||
| CD8+ activated metagene | < 8.48 | 1 | [0.09–0.66] | 0.0025 | 1 | [0.11–0.67] | 0.0048 |
| ≥ 8.48 | 0.30 | 0.28 | |||||
| Angiogenesis metagene | < 5.90 | 1.241 | [0.96–1.67] | 0.092 | 1.21 | [0.89–1.63] | 0.23 |
| ≥ 5.90 | 1 | ||||||
| VEGF-A | < 8.50 | 1 | [1.23–2.14] | 0.00046 | 1 | [1.27–2.32] | 0.00045 |
| ≥ 8.50 | 1.61 | 1.72 |
Not included in the multivariate due to missing data.
All in all, transcriptomic data confirmed the prognostic role of CD8+ and Th17 in another large cohort of GBM.
IL-17A and Th17 cells promote neoangiogenesis
As IL-17A is the main cytokine secreted by Th17 cells and is known to promote VEGF-A production and neoangiogenesis in different tumor types,18,19we investigated its role in GBM related neoangiogenesis. Thus, we treated six GBM primary cultures and two GBM cells lines with recombinant IL-17A. Such stimulation resulted in VEGF-A release from GBM primary cultures and GBM cell lines (Fig. 3A). To address the link between IL-17A and angiogenesis in vivo, we performed a classic IL-17A staining combined with a multiparametric immunohistological staining for CD31 and Ki-67 in an independent cohort of 20 GBM tumors. For patients with a strong IL-17A-positive cells infiltrate within the tumor core, IL-17A-positive areas were systematically surrounded by well-developed blood vessels or neoangiogenic vessels positive for the CD31 endothelial cells marker (Fig. 3B). In contrast, in areas without IL-17A-positive cells, fewer blood vessels were observed (Fig. 3C). Such interaction between IL-17A and CD31 cells was not observed with CD8+ cells, suggesting the specific association between Th17 and endothelial cells. To validate this association between IL-17A and angiogenesis, we used the previous transcriptomic data on 525 patients having GBM. First, using univariate Cox model, we found that VEGF-A expression was associated with a poorer clinical outcome (p = 0.0046, HR: 1.51 [95% CI: 1.13–2.00]) (Table 2). We also observed strong correlations between Th17 metagene and angiogenesis metagene (p < 2.2e−16; R: 0.36) (Fig. 3D) and between Th17 metagene and endothelial cells metagene (p < 3.60e−7; R: 0.22) and between Th17 metagene and VEGF-A (p = 0.00043; R: 0.15) (Table S4). The subgroup analysis showed that patients combining both high Th17 metagene and high VEGF-A expressions had a poorer OS compared with double negative patients (Kaplan–Meier, p < 0.0001) (Fig. 3E and Table 2).
Figure 3.
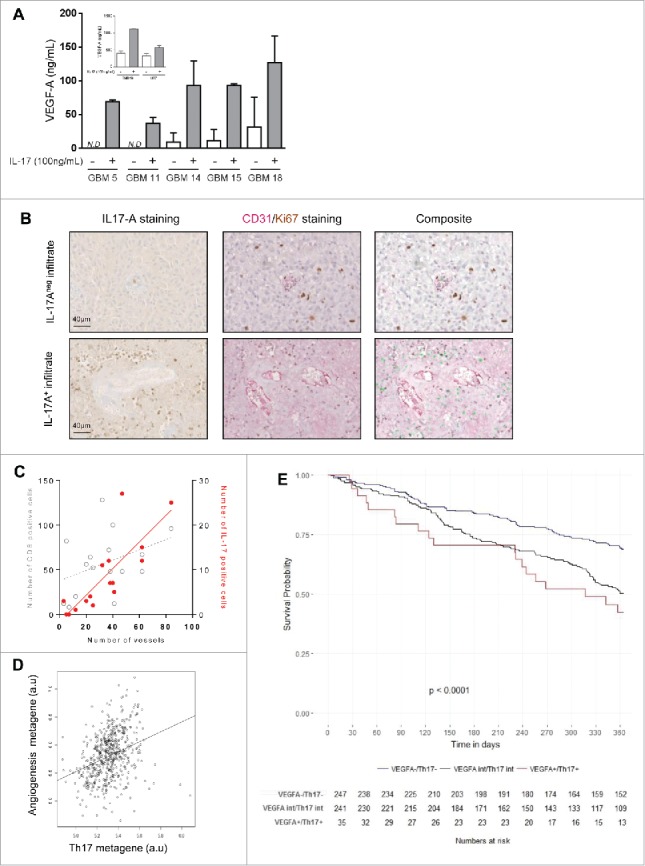
VEGF-A secretion by GBM primary cultures and cell lines (inset) upon IL-17A stimulation (100 ng/mL during 48 h) (N.D. = not determined) (A). Representative pictures of IL17-A staining (left), Ki67/CD31 double staining (middle) and composite (right, IL-17 is merged and appeared green). Scale bar is 40 µm (B). Correlation between blood vessels quantification and Th17 or CD8+ infiltrates in GBM (IL-17-positive cells in red, R2 = 0.67; CD8+ in gray, R2 = 0.15) (C). Correlation between angiogenesis and Th17 metagenes expressions (Spearman's rank correlation coefficient R = 0.36) (D). Kaplan–Meier curves of subgroup analysis comparing VEGF-A−/Th17− (blue line), VEGF-Aint/Th17int (black line) and VEGF-A+/ Th17+ (red line) (E).
All these data suggest that Th17 cells exert protumoral effects notably by their abilities to induce VEGF-A secretion by GBM cells, which in turn promote neoangiogenesis.
Discussion
In this study, we first observed that CD8+ and IL-17A are strong prognosis factors in patients having GBM and treated with the standard radiochemotherapy after surgery. While a high CD8+ infiltrate is associated with a better outcome, a high IL-17A infiltrate is associated with a poorer prognosis. These data were confirmed using metagene strategy on transcriptomic data analysis of a larger cohort. We found that CD8+ activated metagene expression was associated with a better OS, whereas Th17 metagene expression was associated with angiogenesis and a poorer clinical outcome. Importantly, our data highlighted that Th17 immune response and angiogenesis are linked with each other in GBM.
The prognosis role of CD4+ and CD8+ infiltrates in GBM was previously reported in a few small retrospective studies. Recently, Han and colleagues20 underlined that GBM had higher levels of CD4+ TILs than low-grade gliomas, which in combination with a decrease in level of CD8+ TILs induced a decrease in the CD8+/CD4+ ratio in high-grade glioma patients. Consistent with these results, Waziri reported that most of the CD4+ TILs in GBM suppressed the cellular immune response.21 Therefore, the immune function of GBM patients may be compromised due to the relative low level of CD8+ cells compared with immunosuppressive cells. Nevertheless, transcriptomic data from TCGA suggested the presence of a cytotoxic molecular signature in GBM.22 While a first report showed that a high CD8+ T cells infiltrate is more frequently observed in longer survivors compared with shorter survivors,23 further studies suggested that CD8+ alone cannot be associated with clinical outcome.20 Our series using a larger number and probably less heterogeneous patients treated with a standard radiochemotherapy after surgery clearly underlined the prognosis role of CD8+ T cells infiltrate in GBM in two distinct cohorts.
Foxp3 infiltrate is associated with a better outcome in our univariate analysis. However, its prognosis role in GBM is still conflicting in literature. While some reports suggested that a high Foxp3 infiltrate is associated with tumor recurrence and poor survival,10,24some other reports failed to demonstrate a prognostic role of Foxp3 infiltrate.25 The most valuable reason to explain these discrepancies is that Foxp3 is probably not a specific marker of regulatory T as most activated CD4+ T cells in human could express Foxp3 at different levels.26 Efforts for a better characterization of these cells in human tumors are still strongly required for flow cytometry and immunochemistry applications.
Th17 cells are a subpopulation of effector CD4+ helper T cells which produce IL-17A and express the master controller transcriptional factor RORγt. These cells are essential elements of the inflammatory response not only in the context of inflammatory disease but also in the setting of infection by extracellular pathogens. In cancer, the implication of Th17 cells is conflicting, but most reports propose that Th17 cells and notably IL-17A are associated with a poor outcome.27 The deleterious effect of Th17 cells is explained by their ability to exert direct immunosuppressive functions via IL-10 secretion or active ectonucleotidases expression.28,29In GBM, the impact of Th17 is not clearly understood yet. A report on 41 patients suggested that IL-17A was associated with a better outcome.30 Conversely, we observed in our study that IL-17A staining with IHC procedures was a biomarker associated with a poor prognosis. The better specificity of the IL-17A antibody we used for staining procedures may explain the discrepancy between the two studies. It is important to mention that transcriptomic data corroborated our finding using Th17 metagenes strategy in another series of GBM patients. It is also important to point that due to the lack of data, we could not include MGMT promoter methylation status in the multivariate model. Addition works need to be performed to determine whether immune infiltrates are associated with different outcome in function of MGMT status.
Recent reports demonstrated that IL-17A is a proangiogenic factor that could induce production of VEGF-A18 but the capacity of IL-17A to affect VEGF-A production by GBM cells was not known. We show here that IL-17A is able to trigger VEGF-A secretion by GBM cells leading to angiogenesis. Interestingly, with IHC procedures, we observed that IL-17A-positive cells were preferentially found next to capillary blood vessels and may favor their proangiogenic functions. These results were also confirmed with transcriptomic data where we found strong correlations between Th17 and angiogenesis metagenes. However, the correlation between VEGF-A and Th17 was less strong, suggesting that other proangiogenic factors, such as angiopoietin or PDGF could also be involved in Th17-induced angiogenesis.31
In conclusion, our results underlined that on one hand GBM benefits from CD8+ immuno-surveillance like many other human cancers and that a high CD8+ infiltrate is clearly correlated with a favorable prognosis. On the other hand, Th17 and IL-17A seemed to be deleterious either by their ability to inhibit immune response or by activating angiogenesis in GBM.
Beyond the prognosis role of these biomarkers, our study shows that GBM bearing patients can also be classified on the immune infiltrate aspects which could definitely help clinicians to deal with targeted therapy administration. Thus, our data raised the hypothesis that bevacizumab and generally speaking antiangiogenic therapy could be considered in Th17-infiltrated GBM before recurrence. Such hypothesis should be assayed in clinical trials testing the efficacy of bevacizumab in GBM expressing high Th17 metagene. A very promising brief report12 showed that brain tumors can benefit from CAR-T cells immunotherapy. Consistent with this paper, our data also suggest that other immunotherapeutic interventions, such as PD-L1/PD-1 blockade for instance, could only be considered for patients with a potent cytotoxic infiltrate (i.e., a high CD8+ infiltrate). As IL-17A is known to promote angiogenesis in GBM, our data support the rational that IL-17 blockers should be tested in combination with angiogenesis inhibitor at least for patients displaying a combined high Th17 and angiogenesis metagenes expression. The right understanding of immunity in GBM may appear to be a decisive step for major improvements in care for patients having a devastating cancer.
Supplementary Material
Disclosure of potential conflict of interest
No potential conflicts of interest were disclosed.
Funding
FG was supported by La Ligue Contre le Cancer (grant #EL2013.LNCC/FG).
References
- 1.Adamson C, Kanu OO, Mehta AI, Di C, Lin N, Mattox AK, Bigner DD. Glioblastoma multiforme: a review of where we have been and where we are going. Expert Opin Investig Drugs 2009; 18(8):1061-83; PMID:19555299; https://doi.org/ 10.1517/13543780903052764 [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn U et al.. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 2005; 352(10):987-96; PMID:15758009; https://doi.org/ 10.1056/NEJMoa043330 [DOI] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455(7216):1061-8; PMID:18772890; https://doi.org/ 10.1038/nature07385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Phillips HS, Kharbanda S, Chen R, Forrest WF, Soriano RH, Wu TD, Misra A, Nigro JM, Colman H, Soroceanu L et al.. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006; 9(3):157-73; PMID:16530701; https://doi.org/ 10.1016/j.ccr.2006.02.019 [DOI] [PubMed] [Google Scholar]
- 5.Gil-Gil MJ, Mesia C, Rey M, Bruna J. Bevacizumab for the treatment of glioblastoma. Clin Med Insights Oncol 2013; 7:123-35; PMID:23843722; https://doi.org/ 10.4137/CMO.S8503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chinot OL, Wick W, Mason W, Henriksson R, Saran F, Nishikawa R, Carpentier AF, Hoang-Xuan K, Kavan P, Cernea D et al.. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med 2014; 370(8):709-22; PMID:24552318; https://doi.org/ 10.1056/NEJMoa1308345 [DOI] [PubMed] [Google Scholar]
- 7.Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol 2011; 29:235-71; PMID:21219185; https://doi.org/ 10.1146/annurev-immunol-031210-101324 [DOI] [PubMed] [Google Scholar]
- 8.Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C et al.. Towards the introduction of the ‘Immunoscore’ in the classification of malignant tumours. J Pathol 2014; 232(2):199-209; PMID:24122236; https://doi.org/ 10.1002/path.4287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Louveau A, Harris TH, Kipnis J. Revisiting the mechanisms of CNS immune privilege. Trends Immunol 2015; 36(10):569-77; PMID:26431936; https://doi.org/ 10.1016/j.it.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sayour EJ, McLendon P, McLendon R, De Leon G, Reynolds R, Kresak J, Sampson JH. Mitchell DA increased proportion of foxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol Immunother 2015; 64(4):419-27; PMID:25555571; https://doi.org/ 10.1007/s00262-014-1651-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doucette T, Rao G, Rao A, Shen L, Aldape K, Wei J, Dziurzynski K, Gilbert M. Heimberger AB immune heterogeneity of glioblastoma subtypes: extrapolation from the cancer genome atlas. Cancer Immunol Res 2013; 1(2):112-22; PMID:24409449; https://doi.org/ 10.1158/2326-6066.CIR-13-0028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, Ostberg JR, Blanchard MS, Kilpatrick J, Simpson J et al.. Regression of glioblastoma after chimeric antigen receptor T-cell therapy. N Engl J Med 2016; 375(26):2561-9; PMID:28029927; https://doi.org/ 10.1056/NEJMoa1610497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azari H, Millette S, Ansari S, Rahman M, Deleyrolle LP, Reynolds BA. Isolation and expansion of human glioblastoma multiforme tumor cells using the neurosphere assay. J Vis Exp 2011; (56):e3633; PMID:22064695; https://doi.org/ 10.3791/3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Budczies J, Klauschen F, Sinn BV, Győrffy B, Schmitt WD, Darb-Esfahani S, Denkert C. Cutoff finder: a comprehensive and straightforward web application enabling rapid biomarker cutoff optimization. PLoS One 2012; 7(12):e51862; PMID:23251644; https://doi.org/ 10.1371/journal.pone.0051862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Irizarry RA, Hobbs B, Collin F, Beazer-Barclay YD, Antonellis KJ, Scherf U, Speed TP. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics 2003; 4(2):249-64; PMID:12925520; https://doi.org/ 10.1093/biostatistics/4.2.249 [DOI] [PubMed] [Google Scholar]
- 16.Angelova M, Charoentong P, Hackl H, Fischer ML, Snajder R, Krogsdam AM, Waldner MJ, Bindea G, Mlecnik B, Galon J et al.. Characterization of the immunophenotypes and antigenomes of colorectal cancers reveals distinct tumor escape mechanisms and novel targets for immunotherapy. Genome Biol 2015; 16:64; PMID:25853550; https://doi.org/ 10.1186/s13059-015-0620-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Becht E, Giraldo NA, Lacroix L, Buttard B, Elarouci N, Petitprez F, Selves J, Laurent-Puig P, Sautès-Fridman C, Fridman WH et al.. Estimating the population abundance of tissue-infiltrating immune and stromal cell populations using gene expression. Genome Biol 2016; 17(1):218; PMID:27765066; https://doi.org/ 10.1186/s13059-016-1070-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu X, Yang T, Liu X, Guo JN, Xie T, Ding Y, Lin M, Yang H. IL-17 promotes tumor angiogenesis through Stat3 pathway mediated upregulation of VEGF in gastric cancer. Tumour Biol 2016; 37(4):5493-501; PMID:26566627; https://doi.org/ 10.1007/s13277-015-4372-4 [DOI] [PubMed] [Google Scholar]
- 19.Pan B, Shen J, Cao J, Zhou Y, Shang L, Jin S, Cao S, Che D, Liu F, Yu Y. Interleukin-17 promotes angiogenesis by stimulating VEGF production of cancer cells via the STAT3/GIV signaling pathway in non-small-cell lung cancer. Sci Rep 2015; 5:16053; PMID:26524953; https://doi.org/ 10.1038/srep16053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han S, Zhang C, Li Q, Dong J, Liu Y, Huang Y, Jiang T, Wu A. Tumour-infiltrating CD4(+) and CD8(+) lymphocytes as predictors of clinical outcome in glioma. Br J Cancer 2014; 110(10):2560-68; PMID:24691423; https://doi.org/ 10.1038/bjc.2014.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Waziri A, Killory B, Ogden AT 3rd, Canoll P, Anderson RC, Kent SC, Anderson DE, Bruce JN. Preferential in situ CD4+CD56+ T cell activation and expansion within human glioblastoma. J Immunol 2008; 180(11):7673-80; PMID:18490770; https://doi.org/ 10.4049/jimmunol.180.11.7673 [DOI] [PubMed] [Google Scholar]
- 22.Rooney MS, Shukla SA, Wu CJ, Getz G, Hacohen N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 2015; 160(1–2):48-61; PMID:25594174; https://doi.org/ 10.1016/j.cell.2014.12.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang I, Tihan T, Han SJ, Wrensch MR, Wiencke J, Sughrue ME, Parsa AT. CD8+ T-cell infiltrate in newly diagnosed glioblastoma is associated with long-term survival. J Clin Neurosci 2010; 17(11):1381-5; PMID:20727764; https://doi.org/ 10.1016/j.jocn.2010.03.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yue Q, Zhang X, Ye HX, Wang Y, Du ZG, Yao Y, Mao Y. The prognostic value of Foxp3+ tumor-infiltrating lymphocytes in patients with glioblastoma. J Neurooncol 2014; 116(2):251-9; PMID:24276989; https://doi.org/ 10.1007/s11060-013-1314-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas AA, Fisher JL, Rahme GJ, Hampton TH, Baron U, Olek S, Schwachula T, Rhodes CH, Gui J, Tafe LJ et al.. Regulatory T cells are not a strong predictor of survival for patients with glioblastoma. Neuro Oncol 2015; 17(6):801-9; PMID:25618892; https://doi.org/ 10.1093/neuonc/nou363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodriguez-Perea AL, Arcia ED, Rueda CM, Velilla PA. Phenotypical characterization of regulatory T cells in humans and rodents. Clin Exp Immunol 2016; 185(3):281-91; PMID:27124481; https://doi.org/ 10.1111/cei.12804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martin F, Apetoh L, Ghiringhelli F. Controversies on the role of Th17 in cancer: a TGF-beta-dependent immunosuppressive activity? Trends Mol Med 2012; 18(12):742-9; PMID:23083809; https://doi.org/ 10.1016/j.molmed.2012.09.007 [DOI] [PubMed] [Google Scholar]
- 28.Thibaudin M, Chaix M, Boidot R, Végran F, Derangère V, Limagne E, Berger H, Ladoire S, Apetoh L, Ghiringhelli F. Human ectonucleotidase-expressing CD25high Th17 cells accumulate in breast cancer tumors and exert immunosuppressive functions. Oncoimmunology 2016; 5(1):e1055444; PMID:26942062; https://doi.org/ 10.1080/2162402X.2015.1055444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paladugu M, Thakur A, Lum LG, Mittal S, Parajuli P. Generation and immunologic functions of Th17 cells in malignant gliomas. Cancer Immunol Immunother 2013; 62(1):75-86; PMID:22752645; https://doi.org/ 10.1007/s00262-012-1312-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui X, Xu Z, Zhao Z, Sui D, Ren X, Huang Q, Qin J, Hao L, Wang Z, Shen L et al.. Analysis of CD137L and IL-17 expression in tumor tissue as prognostic indicators for gliblastoma. Int J Biol Sci 2013; 9(2):134-41; PMID:23411595; https://doi.org/ 10.7150/ijbs.4891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dunn IF, Heese O, Black PM. Growth factors in glioma angiogenesis: FGFs, PDGF, EGF, and TGFs. J Neurooncol 2000; 50(1–2):121-37; PMID:11245272; https://doi.org/ 10.1023/A:1006436624862 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


