ABSTRACT
Fascinating earlier evidence suggests an intrinsic capacity of human natural killer (NK) cells to acquire adaptive immune features in the context of cytomegalovirus (CMV) infection or pro-inflammatory cytokine stimulation. Since the role of memory NK cells in cancer has so far remained elusive and adoptive NK cell transfer in relapsing pediatric acute B cell precursor leukemia (BCP-ALL) patients awaits improvement, we asked the question whether tumor-priming could promote the generation of memory NK cells with enhanced graft-vs.-leukemia (GvL) reactivity. Here, we provide substantial evidence that priming of naive human NK cells with pediatric acute B cell leukemia or acute myeloid leukemia specimens induces a functional conversion to tumor-induced memory-like (TIML)-NK cells displaying a heightened tumor-specific cytotoxicity and enhanced perforin synthesis. Cell cycles analyses reveal that tumor-priming sustainably alters the balance between NK cell activation and apoptosis in favor of survival. In addition, gene expression patterns differ between TIML- and cytokine-induced memory-like (CIML)-NK cells with the magnitude of regulated genes being distinctly higher in TIML-NK cells. As such, the tumor-induced conversion of NK cells triggers the emergence of a so far unacknowledged NK cell differentiation stage that might promote GvL effects in the context of adoptive cell transfer.
KEYWORDS: Acute B cell precursor leukemia, adoptive cell transfer, children, natural killer cells
Abbreviations
- BCP-ALL
Acute B cell precursor leukemia
- CIML-NK cells
Cytokine-induced memory-like NK cells
- GMP
Good Manufacturing Process
- GO
Gene ontology
- GvL
Graft-versus-Leukemia
- IS
Immunological synapse
- KIR
Killer-immunoglobulin like receptor
- NCR
NK cell receptor
- NSG
NOD.Cg-Prkdcscid IL2rgtmWjl/Sz mice
- TIML-NK cells
Tumor-induced memory-like NK cells
Introduction
Recent data indicate that pediatric BCP-ALL may under certain conditions, such as low MHC class I expression,1 high expression of ligands to major activating NK cell receptors2 and existing mismatches on the killer-immunoglobulin like receptor (KIR)-KIR ligand (KIRL) level,3,4 emerge as a target of “alloreactive” NK cells. However, the results of early phase I/II clinical studies using the adoptive transfer of allogeneic NK cells in the non-transplant or transplant setting to pediatric BCP-ALL patients have so far been disappointing, and adoptive NK cell transfer protocols warrant further evaluation and presumably optimization.5
Intriguingly, earlier reports suggest an intrinsic capacity of NK cells to acquire adaptive immune features and to display augmented functionality.6-8 Hallmarks of such adaptive immune responses have been the detection of NK cell-mediated recall responses in hapten-sensitized T and B cell-deficient Rag2−/− mice,6,7 and the appreciation of a protective transferable NK cell-mediated immunity in a murine cytomegalovirus (MCMV)-infection model.8 In line with this, human CMV-specific memory NK cells have been detected in healthy carrier individuals9 or in transplant recipients upon HCMV reactivation.10,11 Moreover, adaptive immune features of NK cells have also been elicited independent from prior exposure to viral antigens by conditioning mouse or human NK cells in the presence of IL12 and IL18, resulting in the generation of cytokine-induced memory-like (CIML)-NK cells.12-14
As the role of memory NK cells in cancer has indeed been postulated but so far remained elusive, we questioned whether tumor-priming in the absence of vigorous pro-inflammatory cytokine stimulation might promote the generation of memory NK cells that could sustain graft-vs.-leukemia (GvL) reactivity. Here, we provide substantial evidence that tumor-priming induces a functional conversion of NK cells that triggers a tumor-specific NK cell recall response, which is distinct from other forms of immunologic NK cell memory. Collectively, our data strongly support the proof of concept that the overall modest GvL effects of NK cells toward pediatric BCP-ALL or AML can experimentally be augmented, and as such the generation of TIML-NK cells adds an exciting new perspective to the design of future experimental adoptive NK cell transfer protocols.
Results
TIML-NK cells exhibit superior, tumor-specific in vitro functionality
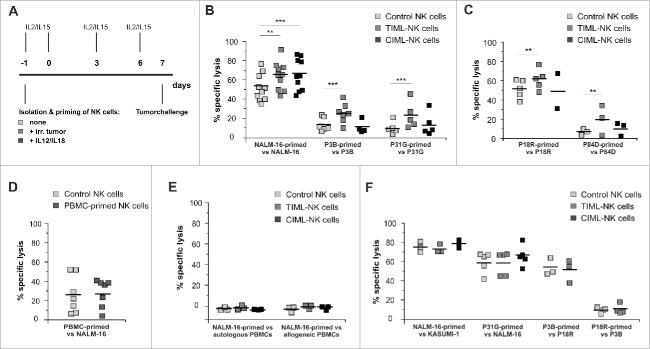
To examine the exploitation of adaptive immune features of NK cells, we started our experiments by priming primary NK cells with pediatric BCP-ALL or AML specimens (Fig. 1A). Our protocol included priming with irradiated specimens such as the pediatric BCP-ALL cell line NALM-16, the primary BCP-ALL specimens P3B and P31G or primary AML specimens P18R and P84D as well as cultivation in the presence of low dose, good manufacturing process (GMP)-compatible IL2 and IL15 to facilitate the implementation of a tumor-priming step into future adoptive cell transfer protocols. We chose these primary specimens as the clinical course of the patients was judged to be representative of high-risk pediatric BCP-ALL and AML (early death after first relapse). Phenotypic analyses revealed that the specimens differed with respect to the expression of important NK cell receptor (NCR) ligands, namely NKG2D ligands (NKG2D-L), ICAM-1, HLA-E, HLA-class I and DNAM-1 ligands (Fig. S1). To assess the potential clinical efficacy in case of experimental adoptive cell transfer, we included IL12/18-primed CIML-NK cell preparations12-14 as a “gold standard” in all experiments.
Figure 1.
Tumor-priming induces TIML-NK cells to elicit a superior, tumor-restricted functionality against pediatric BCP-ALL and AML. (A) Experimental layout for in vitro generation of TIML-NK cells. Freshly isolated NK cells were primed on d-1 with different irradiated tumor specimens, irradiated PBMCs or with a mixture of 10 ng/mL IL12 and 50 ng/mL IL18. All NK cell preparations were cultured in medium supplemented with low dose (100 IU/mL) IL2 and low dose (1 ng/mL) IL15. Cytotoxicity was tested on d7. (B) BCP-ALL-primed TIML-NK cells exhibit heightened anti-tumor functionality toward pediatric BCP-ALL. In vitro cytotoxicity assays on d7. Unprimed (“control NK cells”), BCP-ALL (NALM-16-, P3B- or P31G)-primed (“TIML-NK cells”) and IL12/IL18-primed (“CIML-NK cells”) NK cells were used as effectors and the identical tumor specimen was used as a target for re-stimulation on d7. Data represent 10 (NALM-16 priming/re-stimulation), 7 (P3B-priming/re-stimulation) or 5 (P31G-priming/re-stimulation) different donors (E:T ratio 3:1 in NALM-16 and P3B experiments, E:T ratio 9:1 in P31G experiments). (C) AML-primed TIML-NK cells exhibit heightened anti-tumor functionality toward the identical pediatric AML. In vitro cytotoxicity assays on d7. Unprimed, AML (P18R- or P84D)-primed and IL12/IL18-primed NK cells were used as effectors and the identical tumor specimen was used as a target for re-stimulation on d7. Data represent 5 (P18R priming/re-stimulation) or 3 (P84D-priming/re-stimulation) different donors (E:T ratio 3:1 in all experiments). (D) Priming-induced NK cell conversion requires exposure to malignant cells. NK cells from donors depicted in Fig. 1B (NALM-16-priming) were primed with irradiated allogeneic PBMCs at a ratio of 1:3. In vitro cytotoxicity assays performed on d7 with control or PBMC-primed NK cells as effectors and NALM-16 cells as targets. Results represent data from six different NK cell-donors primed with 5 different PBMC specimens (E:T ratio 1:1). (E) NALM-16-primed TIML-NK cells do not exert cytotoxicity toward non-malignant PBMCs. In vitro cytotoxicity assays were performed on d7 with NALM-16-primed NK cells as effectors and autologous or allogeneic PBMCs as targets. Data represent three different donors (E:T ratio 1:1). (F) TIML-NK cells show heightened cytotoxicity only toward the original priming tumor entity. Unprimed, NALM-16-, P31G-, P3B- or P18R-primed and IL12/IL18-primed NK cells were used as effectors; as indicated other tumor specimens were used targets for re-stimulation on d7 to test functional TIML-NK cell specificity. Note, that the donors shown in Fig. 1F are identical to the respective donors tested in Fig. 1B and C, i.e., the efficacy of the priming effect was documented for every donor shown in Fig. 1F. Data represent 3 (NALM-16 priming/Kasumi-1 re-stimulation), 5 (P31G priming/NALM-16 re-stimulation), 3 (P3B priming/P18R re-stimulation) or 4 (P18R priming/P3B re-stimulation) different donors. E:T ratio 3:1 (all experiments). All experiments were performed in triplicates. **p < 0.01, ***p < 0.001.
In vitro cytotoxicity assays on day 7 (d7, see Supplemental Methods for details) demonstrated that tumor-primed primary NK cells exhibit a significantly enhanced cytotoxic function not only toward the BCP-ALL cell line NALM-16, but also toward the two different primary pediatric BCP-ALL specimens P3B and P31G when compared with control NK cells (Fig. 1B). Importantly, improved NK cell function was also demonstrable upon priming with the primary pediatric acute myeloid leukemia (AML) samples P18R or P84D (Fig. 1C). As early phase I/II clinical trials exploit either adoptive transfer of feeder-expanded NK cells15,16 or occasionally the transfer of the bona fide large granular lymphocyte lymphoma cell line NK-92,17 we next tested whether generation of memory-like features would also be feasible in these NK cell preparations. Therefore, we used K562-(mb)IL15-41BBL-expanded NKAES cells (see Supplemental Methods for details on the NK cell activation and expansion system18) or the NK-92 cell line and performed NALM-16 priming and re-stimulation on d7 (see Supplemental Methods). Although a certain increase in NK cell cytotoxicity was demonstrable for both preparations (Fig. S2), this effect was not statistically significant, indicating that memory-like cell conversion is unlikely to occur in extensively cytokine-stimulated or in malignantly-transformed NK cells.
As peripheral blood mononuclear cells (PBMCs) of various donors tested in Fig. 1B were not able to convert NK cells to memory-like NK cells (Fig. 1D), and as TIML-NK cells did not exert cytotoxicity toward autologous or allogeneic PBMCs (Fig. 1E), we consider the initiation of uncontrolled NK cell activation or the induction of relevant autoimmune responses of NK cells toward non-malignant cells to be highly unlikely. Most interestingly, NK cells exhibited improved antitumor properties on d7 only toward the original priming-tumor entity NALM-16, P3B, P31G or P18R, respectively, but not toward pediatric BCP-ALL and AML specimens other than the one used for priming (Fig. 1F). Thus, we conclude from these data that tumor-priming induces memory NK cell conversion in a tumor-specific manner.
TIML-NK cells confer anti-leukemic activity toward pediatric BCP-ALL and AML in vivo
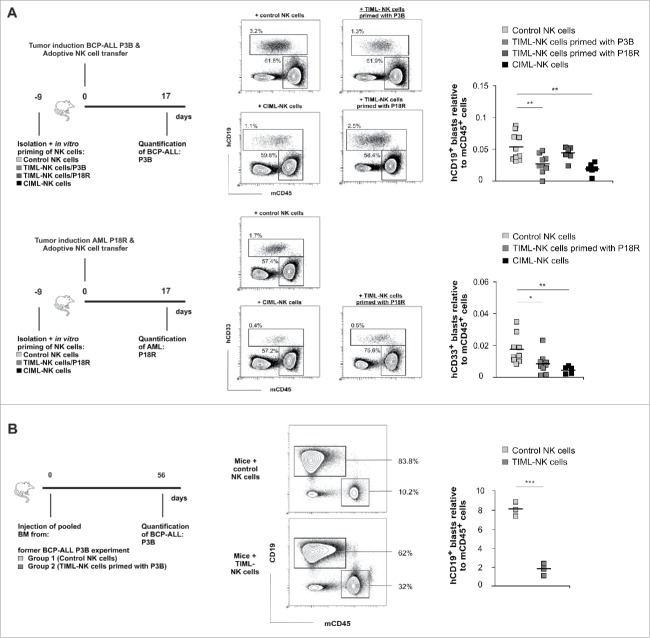
Having shown that tumor-priming initiates the generation of TIML-NK cells, which exhibit a superior tumor-specific in vitro cytotoxicity, we next assessed the efficacy of TIML-NK cell-mediated GvL responses in vivo. To this end, we adoptively transferred in vitro primed TIML-NK cells into immune-deficient NOD.Cg-Prkdcscid IL2rgtmWjl/Sz (NSG) mice that had been previously injected with P3B or P18R and quantified the resulting leukemic burden. In primary (Fig. 2A) as well as in serial engraftment experiments (Fig. 2B), TIML-NK cells primed with P3B significantly reduced leukemic burden in P3B-bearing mice. In contrast, TIML-NK cells primed with the AML P18R did not reduce leukemic load in P3B-bearing mice but instead reduced P18R load, thus confirming the tumor-specificity of TIML-NK cells in vivo (Fig. 2A). Interestingly enough, IL12/IL18-primed CIML-NK cells exhibited a significant cytotoxic function both in P3B-bearing but also in P18R-bearing mice even in the absence of whole body irradiation19 or systemic IL2 supplementation16 (Fig. 2A). In line with the assumption that the clinically relevant effect of adoptive NK cell transfer occurs very early after cell injection, NK cells were not any longer present at the time of euthanasia. Although TIML-NK cells did not sustain tumor regression as shown by serial engraftment experiments, the observed in vivo efficacy is nevertheless striking given that cytotoxicity toward P3B in our in vitro experiments was about 30% (Fig. 1B) and GvL activity of NK cells toward BCP-ALL is known to be low.3,4
Figure 2.
TIML-NK cells confer anti-leukemic activity toward pediatric BCP-ALL and AML in vivo. (A) Experimental layouts for in vivo experiments. Blasts of the donors P3B (BCP-ALL) or P18R (AML) were injected on d0 into NSG mice followed by a second injection of the varying NK cell preparations as indicated several hours later on d0. Leukemic burden was quantified in the bone marrow 17 d later. Exemplified gating strategy for identification of the leukemic load shown as human CD19+ blasts (for the BCP-ALL P3B) or human CD33+ (for the AML P18R) relative to murine CD45+ cells, respectively. Data from BCP-ALL (P3B)-tumor induction represent n = 10 mice injected with control NK cells (three different donors), n = 9 mice injected with TIML-NK cells (three different donors) primed with P3B blasts, n = 6 mice injected with TIML-NK cells (two different donors) primed with P18R blasts and n = 6 mice injected with CIML-NK cells (two different donors). Note, that the P18R-primed TIML-NK cells used in the P3B experiment (first row) exhibited a significant priming effect when tested toward P18R (second row). Data from AML (P18R)-tumor induction represent n = 9 mice injected with control NK cells (three different donors), n = 9 mice injected with TIML-NK cells (three different donors) primed with P18R blasts and n = 5 mice injected with CIML-NK cells (two different donors). (B) Experimental layout for serial transplantations to address residual leukemic load after adoptive NK cell transfer. Pooled BM samples of one of the donors from adoptive NK cell transfer experiments in P3B-induced mice depicted in Fig. 2A was re-injected into groups of naive mice (n = 3; 20 × 106 per mouse). Shown is the gating strategy and the leukemic P3B-burden in the BM on d56. Data represent 1 experiment. *p < 0.05, **p < 0.01, ***p < 0.001.
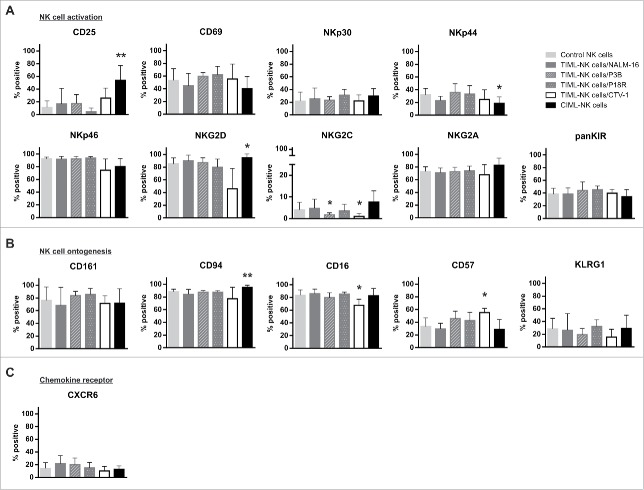
TIML-NK cells show phenotypical characteristics of advanced maturity
Examination of surface markers revealed a CD56brCD94hiCD16hiCD57intKLRG1intinhKIRint phenotype and as such TIML-NK cells comprise an intermediate stage between the less mature CD94hiCD57−inhKIR− and the fully mature CD94−CD57+inhKIR+ NK cell subset20,21 irrespective of the priming tumor entity as shown for NALM-16, P3B and P18R (Fig. 3A–C). In line with published data reporting phenotypical similarities between resting and memory NK cells22 but in contrast to NK cells primed with the T-ALL cell line CTV-1,23 TIML-NK cells exhibited a quiescent phenotype overall resembling features of control NK cells, indicating that phenotypical conversion is obviously not mandatory for functional re-programming. Interestingly, TIML-NK cells lacked significant upregulation of NK cell markers like CD25, which is abundantly expressed in CIML-NK cells14,24 and HCMV-specific memory NK cells,25 CD94/NKG2C and/or CD57 which have been associated with memory NK cell formation in CMV-infected humans10,26 or KLRG1 and CXCR6 observed in murine virus/hapten-specific memory NK cells.7,8 Thus, we do not find any evidence for a clonal expansion of one specific NK cell compartment in the context of tumor encounter.
Figure 3.
TIML-NK cells show phenotypical signs of advanced maturity. Phenotypical characterization of (A) NK cell activation, (B) ontogenesis and (C) chemokine receptors of unprimed, NALM-16-, P3B-, P18R-, CTV-1 and IL12/IL18-primed NK cells on d7. NK cell preparations were subjected to flow cytometric quantification of the proportion of cells expressing the given receptors. Bars represent the mean percentage of the respective receptor+CD56+CD3− NK cell subsets, error bars display SD. Note, that the CD56 receptor is not included in this diagram as all cells were gated on CD56 before subset analysis. Results represent data from 14 different donors for control, 7 donors for NALM-16-primed, 4 donors for P3B-primed, 4 donors for P18R-primed, 4 donors for CTV-1-primed and 6 donors for IL12/IL18-primed NK cells. Displayed p values refer to comparison between primed NK cell preparations and control NK cells. *p < 0.05, **p < 0.01.
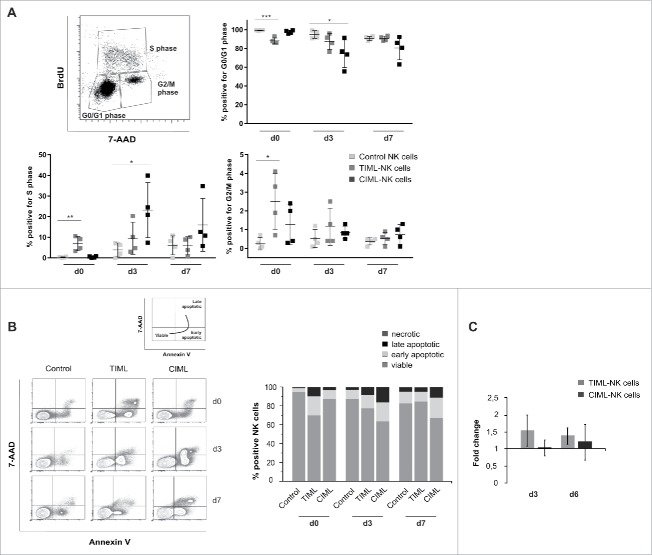
“Tumor-priming” promotes NK cell survival
To further examine TIML-NK cell homeostasis and the capability for dynamic responses, we performed cell-based assays and analyzed cell cycle, viability rates and resulting cell numbers during cultivation. Cell cycle analysis provided evidence that tumor-priming rapidly induces TIML-NK cells to leave the G0/G1 interphase and enter the S-phase, where DNA replication occurs (Fig. 4A). As a result, TIML-NK cells are as early as on d0 able to proceed to mitosis (G2/M phase), i.e. to successfully proliferate, and rapidly return to non-diving cells as demonstrated by the low frequency of cells in the G2/M phase on d7. In contrast, the response of CIML-NK cells is delayed until d3 and although CIML-NK cells indeed leave the interphase and are able to enter the S phase, only few CIML-NK cells accomplish to proceed to the G2/M phase (Fig. 4A). Classifying viable NK cells as 7-AAD−/AnnexinV−, early apoptotic NK cells as 7-AAD−/AnnexinV+ and late apoptotic NK cells as 7-AAD+/AnnexinV+, we were able to demonstrate that TIML-NK cells exhibited/maintained a high rate of viability with little proneness to apoptosis on d7 of culture, whereas a high proportion of CIML-NK cells were rendered apoptotic (Fig. 4B). In line with previously published data27 and with the notion that high expression of the activation marker CD25 is per se associated with increased AnnexinV binding,28 we attribute the extensive rate of apoptosis in CIML-NK cells to the induction of an IL12-mediated “activation-induced cell death” (AICD). As a result of this apparently shifted cell balance between proliferation and apoptosis the total cell number of TIML-NK cells was upregulated (d3: fold increase 1.53 ± 0.47, proliferation >> apoptosis) but virtually unchanged in CIML-NK cells (d3: fold increase 1.0 ± 0.25, proliferation = apoptosis) (Fig. 4C). Overall, the amplitude of TIML-NK cell expansion as determined by cell counting was comparatively modest but mirrors analogous responses recorded in classical models of MCMV infection8 or in poly I:C-stimulated mice bearing adoptively transferred CIML-NK cells.29 Collectively, our data promote the concept that tumor-priming does initiate mitosis, prevents apoptosis and strongly alters the balance between NK cell activation and apoptosis in favor of survival. Based on this characteristic kinetics, we therefore propose the term tumor-induced “memory-like” instead of “memory” NK cells to avoid a potentially misleading comparison to in vivo generated virus or hapten-specific NK cells or memory CD8+ T cells.
Figure 4.
“Tumor-priming” promotes NK cell survival. (A–C) Cell cycle analyses, viability assays and cell numbers were obtained on control, NALM-16-primed TIML- and CIML-NK cells at the indicated time points. (A) Cell cycle analyses evaluating BrdU incorporation. Cells incorporating BrdU are shown relative to their phase in the cell cycle (i.e., G0/G1, S, or G2/M phase) as assessed by quantifying 7-AAD staining intensities. Results represent data from four different donors (mean ± SD). (B) Cell viability assays using 7-AAD/AnnexinV staining. Cell populations are characterized as being viable (7-AAD-/AnnexinV-), early apoptotic (7-AAD−/AnnexinV+) and late apoptotic (7-AAD+/AnnexinV+). Exemplified gating strategy of one representative donor and mean values of the % positive NK cells in the 7-AAD/AnnexinV experiments. Data display four different donors. (C) Cell counts. Shown is the number of CD56+CD3− cells normalized to the number of control NK cells as “fold increase”; error bars represent SD. Data represent 10 donors on d3 and 17 donors on d6. *p < 0.05, **p < 0.01, ***p < 0.001.
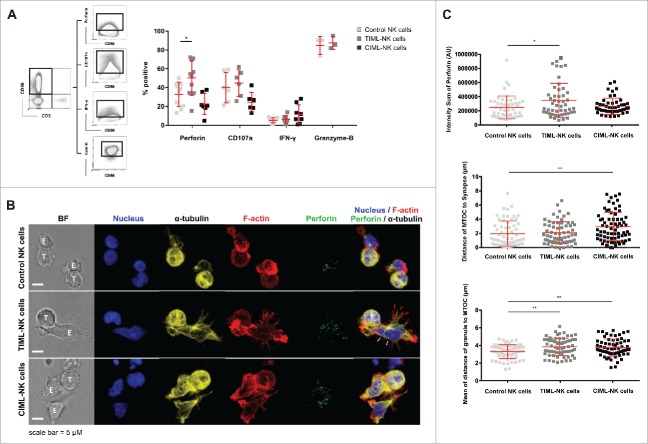
TIML-NK cells display enhanced perforin synthesis
Intrigued by the finding of increased antitumor functionality conferred by TIML-NK cells, we next performed intracellular staining to examine the capacity of TIML-NK cells to express IFNγ, synthesize perforin or granzyme-B and trigger degranulation. Boolean gating revealed an increased number of one and two responses in TIML-NK cells (Fig. S4). Interestingly and in contrast to virus-specific memory NK cells,8,30,31 and CIML-NK cells12,14,19,24 that preferably secrete IFNγ following re-activation, TIML-NK cells synthesized more perforin but not IFNγ upon tumor re-encounter (Fig. 5A, Fig. S5). In support of this finding confocal imaging (see Supplemental Methods) provided evidence for elevated total perforin content per cell in TIML- as compared with control NK cells (Fig. 5B and C). TIML-NK cells maintained microtubule-organizing center (MTOC) polarization, however, the mean distance of lytic granules to the MTOC (convergence) increased in both TIML- and CIML-NK cells (Fig. 5C). As granule convergence to the MTOC is an early step in the preparation of an NK cell for cytotoxicity32,33 this is an unexpected finding; however, movement and exocytosis of secretory lysosomes in NK cells can occur independently from MTOC polarization.32,34-36 As direct visualization of conjugate formation clearly documents an extension and flattening of TIML-NK cell shape toward the immunological synapse (IS) (Fig. 5B, white arrowheads) and formation of multiple F-actin-rich cytoplasmic projections (Fig. 5B, red arrowheads), our observations indeed argue for a more efficient IS formation by TIML-NK cells, particularly in view of the propensity for degranulation and the documented improved cytotoxicity.
Figure 5.
Perforin synthesis is enhanced in TIML-NK cells following contact to pediatric BCP-ALL. (A) NALM-16-primed d7 NK cells together with the respective controls were stained after re-stimulation with NALM-16 to determine the functional response in terms of cytokine synthesis, ability for degranulation and perforin synthesis. CD56+CD3− NK cells were gated and the relative frequency of cells positive for perforin, CD107a, IFNγ and granzyme-B was analyzed. Data represent 10 donors for perforin (except CIML-NK cells with 7 donors), 6 for CD107a, 8 for IFNγ and 3 for granzyme-B. (B) Representative confocal microscopy images of conjugates formed between NALM-16 cells and control NK cells (top panel), NALM-16-primed TIML-NK cells (middle panel) or between NALM-16 cells and CIML-NK cells (bottom panel), E:T ratio of 3:1. Images are z-projections, except the BF, which is a single image taken at the plane of the glass; scale bar = 5 µm; BF = bright field. Note that the NK cell shape is extended and flattened toward the immunological synapse (white arrowheads) and forms multiple F-actin rich, cytoplasmic projections (red arrowheads). Data are representative of three independent experiments using three different NK cell donors. (C) Quantitative analysis of conjugate formation demonstrating total perforin per cell measured by intensity sum of perforin fluorescence, distance of MTOC to synapse per cell (Polarization) and mean distance of lytic granule to MTOC (Convergence) per cell. Data in C represent three different NK cell donors in three independent experiments (mean ± SD). *p < 0.05, **p < 0.01.
TIML-NK cells express a distinct transcriptome signature
To identify contingent differences in the process of TIML- or CIML-NK cell conversion, we further performed transcriptome analysis of all NK cell preparations (Fig. 6, Fig. S5). In line with the notion that NK cell functionality may significantly differ between various donors, we observed a significant inter-individual variability irrespective of any preceding priming in selected genes encoding for particular interleukins, interleukin receptors, chemokine ligands, CD86, MHC class II and killer immunoglobulin-like receptors (Fig. 6A, Table S1). However, based on the analysis of Euclidean distances, significant differences also existed in the signature of TIML- and CIML-NK cells (Fig. 6B, Table S2–S5, Fig. S5). Consistent with our theoretical assumption that tumor-priming should induce a more complex conversion of NK cells than triggering of pro-inflammatory receptors alone, the extent of gene regulation was distinctly higher in TIML-NK cells (Fig. 6C). Both TIML- and CIML-NK cells showed regulated gene expression in the area of cellular components, particularly in the GO term “membrane part” (i.e., regulation of chemokine or cytokine receptors such as CXCR6, IL17RA, CCR7 or IL18RAP), however, only TIML-NK cells displayed a significant alteration in the regulation of genes that are involved in “metabolic processes” (i.e., CYP1B1, ALDOC, TKTL1, AGMAT, VDR). Collectively, the putative transcriptional changes (Table S2–S5) that emerge during the generation and re-programming of TIML-NK cells reveal a unique gene expression pattern and hence we propose that TIML-NK cells are distinct from other forms of memory-like NK cells.
Figure 6.
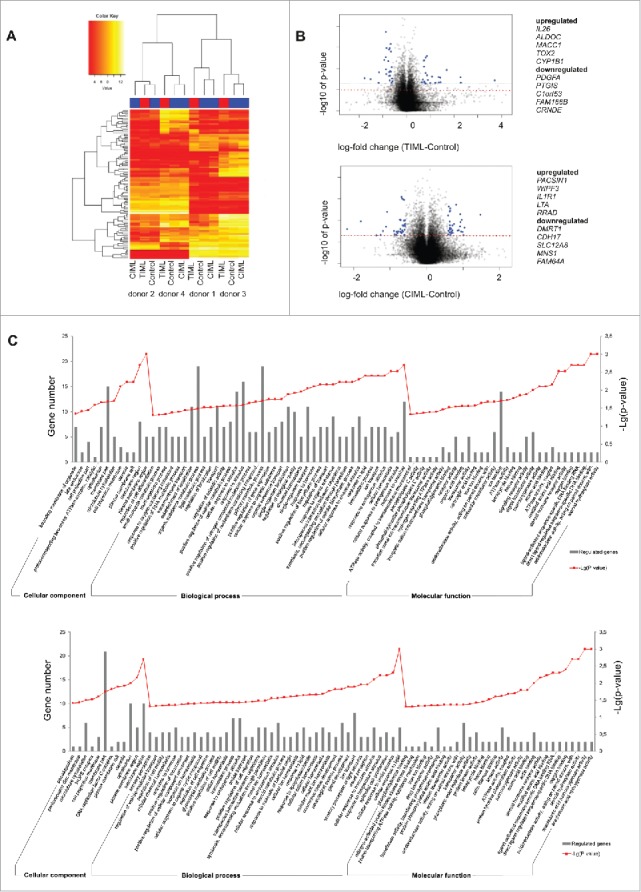
Transcriptome analysis reveals significant differences in the signature of TIML- and CIML-NK cells. (A) Heat map and unsupervised hierarchical clustering. Unsupervised hierarchical cluster analysis using the Euclidean distance of the 100 most variable probes of the data set demonstrates the pronounced inter-donor variability but also the significant differences in the signature of NALM-16-primed TIML- and CIML-NK cells. Red indicates lower and yellow indicates higher expression values. For gene identification see also Table S1. (B) Vulcano Plots. Shown is the log fold change (x-axis) plotted against the significance (y-axis, negative log10), respectively, in NALM-16-primed TIML-NK cells and CIML-NK cells plotted on unprimed NK cells. Probes with significant changes and considerable magnitude of change (log fold change of more than −0.5/0.5) are marked in blue and are identified in the Tables S2-S5. The dashed red line indicates the significance threshold of p = 0.05. (C) GO Terms. Differentially expressed GO terms of NALM-16-primed TIML-NK cells {upper graph} in relation to unprimed NK cells and CIML-NK cells {lower graph} in relation to unprimed NK cells. Shown is a selection of differentially expressed cellular components (top 12), biologic processes (top 40) and molecular functions (top 30). Gray bars represent the regulated gene count and the point plot represents the –Lg (p value) of the respective GO term. Data in A–C represent one experiment performed with four donors. Validation of selected genes is shown in Fig. S5.
Discussion
In light of the modest GvL effects that NK cells are able to exert toward pediatric BCP-ALL and recent evidence suggesting an intrinsic capacity of NK cells to acquire adaptive immune features, we here sought to explore memory-like characteristics of NK cells in the context of tumor-priming. Collectively, our data provide evidence of a tumor-initiated and tumor-specific NK cell recall response, which is associated with enhanced perforin synthesis but not IFNγ secretion. Thus, our data strongly indicate that the formation of TIML-NK cells resembles a so far unacknowledged mode of functional memory-like NK cell conversion.
Adaptive immune features of human NK cells have so far been described in HCMV-infection9-11,25,26,31,37-42 or upon pro-inflammatory cytokine stimulation.13,14,24,31 To our knowledge, an enhancement of NK cell functionality via tumor-priming has only been described in the context of priming with the CTV-1 cell line, where target cell lysis is critically dependent on CD69 triggering23 and STAT5 signaling,43 and for feeder (i.e., RPMI8866, Epstein-Barr lymphoblastoid cell line or K562) expanded NK cells. As enhanced cytotoxicity for tumor-activated NK cells (T-ANKs) and feeder-expanded NK cells is limited to the immediate post-priming period, is critically dependent on extensive activation and is non-selective, we feel that both do not bear features of adaptive NK cells and are therefore not comparable to TIML-NK cells.
Focusing on human CIML-, TIML- and HCMV-specific memory NK cells, we observe certain similarities but more importantly significant differences (Table 1). Most striking are the differences concerning “clonal origin” and “means of functionality." In line with the finding for an antigen-driven oligoclonal expansion of human CMV-specific NK cells,9,37 the phenotype of CMV-induced memory NK cells has reported to be biased toward a CD94/NKG2C+NKG2A−CD57+aKIR+ phenotype.9-11,38,39,41 In contrast, neither CIML- nor TIML-NK cells show signs of a distinct phenotype or clonality (Fig. 3).13,24 Considering that CIML-NK cell conversion is initiated via IL12/IL18 receptor activation,13,24 while TIML-NK cells presumably convert upon recognition of subtle, net changes in activatory, inhibitory and triggering receptor expression, a solely antigen-mediated process is in both instances highly unlikely.
Table 1.
Overview of various forms of human memory NK cells. CNS: conserved non-coding sequence, DAB2: Disabled homolog 2, EAT-2: Ewing's sarcoma-associated transcript 2, IKZF2: IKAROS Family Zinc Finger 2, PLZF: Promyelocytic leukemia zinc finger protein, SYK: Spleen tyrosine kinase.
| CIML-NK cells | TIML-NK cells | HCMV-induced memory NK cells | |
|---|---|---|---|
| Mode of generation | Brief exposure to IL 12/IL15/IL18 (16hrs) | Priming with irritated tumor specimen | CMV infection/reactivation |
| Phenotype | Not distinct | Not distinct | Distinct |
| CD25hi CD94hi NKG2Ahi CD69hi NKp46hi | CD56br CD94hi CD16hi CD57int KLRG1int inhKIRint | CD94/NKG2Chi CD57hi CD25hi NKG2A-KIR2DL2+, KIR2DS2+, KIR2DS4+and/or KIR3DS1+ | |
| Functional characteristics | IFN-γ production ↑ (upon re-stimulation with cytokines or K562) | Multiple cytokine responses ↑ | Capacity to produce IFN-γ ↑ |
| Anti-tumor cytotoxicity vs AML ↑ | In virto cytotoxicity ↑ | Host protection | |
| Tumor growth ↓ | |||
| Involved cytokines | IL12, IL18 | / | IL12, type I IFNs |
| Antigen dependence | / | Unlikely | + (CMV-encoded protiens) |
| Clonal expansion | Unlikely | / | +++ |
| Proliferation | ++ | + | ++ |
| Mode of contraction | ++ (apoptosis) | (+) (apoptosis) | ? |
| Epigenetic modulation | + | ? | + |
| Presumed mechanism | Epigenetic remodeling of the IFNG CNS1 | Perforin production ↑ (upon tumor re-stimulation) | Stochastic epigenetic modulation of the FcRγ– NK cell subset |
| Synapse formation likely to be improved | Antibody-dependent selection / expansion of FcRγ– NK cell subset | ||
| Epigenetic imprinting of the IFN-γ locus | |||
| Involved NCR | ? | ? | NKG2C, NKp46, DNAM1, aKIRs, 2B4 |
| Involved NCR ligands | ? | ? | HLA-E |
| Signalling molecules | IL2-CD25-Stat5 signalling | ? | Multi-protien deficient (SYK, DAB2, EAT-2, PLZF, IKZF2) |
| Quotes | (13, 14, 19, 24, 29, 31) | / | (9, 10, 25, 26, 31, 37 - 42) |
With respect to the means of functionality both human CIML-NK cells and HCMV-specific memory NK cells display a propensity for IFNγ secretion and through this exert antitumor properties 13,14,19 or contribute to anti-viral host defense.26,31,40 In contrast, we detected a higher perforin accumulation upon BCP-ALL tumor re-encounter and an overall increased number of one and two cell responses in TIML-NK cells (Fig. 5A, Fig. S4). Together with the observation that TIML-NK cells flatten toward the IS and form F-actin-rich cytoplasmic projections (Fig. 5B and C), our data favor the concept of a more efficient perforin synthesis as a result of improved IS formation. In fact, we may even have underestimated the propensity of TIML-NK cells for synapse formation as the imaging studies do not accurately allow quantifying the time span that a particular NK cell is in contact with a tumor cell. Hence, it is difficult to draw firm conclusions about whether multiple transient contacts or rather a prolonged interaction might have caused the improved functional response seen in TIML-NK cells. In line with this notion, it has earlier been demonstrated that fixed time-point analyses rather underestimate NK cell cytotoxicity as burst kinetics and spatial coordination during serial killing are not accounted for.44
Here, we document increased functionality of TIML-NK cells toward a repertoire of phenotypically heterogeneous pediatric BCP-ALL, but also AMLs specimens expressing different pools of NK cell receptor ligands at variable levels. Thus, the identification of one individual NCR–NCR ligand axis that might have initiated TIML-NK cell conversion was highly unlikely. And indeed, although we were able to identify mechanisms that sustain improved TIML-NK cell functionality, the intracellular signal transduction mechanisms initiating TIML-NK cell conversion remain elusive. Preliminary own data indicate that the NKG2D-NKG2DL axis might play a role in TIML-NK cell generation (data not shown). However, despite significant NKG2D expression on TIML-NK cells (Fig. 3A) the NKG2D-L expression on our BCP-ALL and AML specimens was variable or in one instance absent (P31G) (Fig. S1), and we did not observe any downregulation of the NKG2D receptor on TIML-NK cells secondary to tumor contact (data not shown). Together with the existing evidence that sustained exposure to NKG2D-L expressing tumor cells may indeed render NK cells dysfunctional,45,46 our data do not support a significant contribution of the NKG2D-NKG2DL axis in the induction of tumor-induced memory NK cell formation. In addition, we were neither able to detect IL15 in our culture supernatants (data not shown) nor did we find any evidence for upregulated pSTAT1, three or five expression in TIML-NK cells (Fig. S3). Considering IL15 being dispensable for the expansion and formation of memory NK cells in MCMV infection8 and the notion that excessive availability of IL15 may even erode the pool of memory progenitors,47 the IL15-JAK3-STAT5-dependent pathway, therefore, is also unlikely responsible for TIML-NK cell conversion.
Consistent with our difficulties to identify a single responsible signal transduction pathway initiating the emergence of TIML-NK cell and our assumption that tumor-priming presumably induces a complex re-programming of NK cells, TIML-NK cells showed a distinct signature in their transcriptome expression, particularly in the areas of “membrane parts” and “metabolic processes” (Fig. 6C). Interestingly, gene annotation revealed IL26 to be the gene most upregulated (Table S2) and PDGF-A to be the gene most downregulated (Table S3) in TIML-NK cells as compared with control NK cells. As IL26 mediates both proliferative as well as anti-apoptotic effects48 and induces TH1 polarization,49 it is tempting to speculate that IL26 secreted by TIML-NK cells might modulate subsequent adaptive immune responses. On the other hand, the platelet-derived growth factor-A (PDGF-A) has so far not been associated with NK cell function; however, downregulation of the corresponding c-Kit / PDGF receptor on dendritic cells indeed effectively improves NK cell-triggering.50 Thus, our data suggest that in addition to enhanced direct anti-tumor cytotoxicity, TIML-NK cells might also sustain successive adaptive immune responses and perpetuate in vivo antitumor immunity.
Confining the definition of memory to a functional consequence and rather not to the recognition of a limitless number of antigens, we here propose to include tumor recognition as sufficient to initiate memory-like cell formation of NK cells, as TIML-NK cells are verifiably unique in terms of cell cycle kinetics (Fig. 4), means of functionality (Fig. 5) and gene expression pattern (Fig. 6). Despite obvious differences to CIML-NK cells and HCMV-induced memory NK cells, TIML-NK cells intriguingly display similarities to the “homeostatic proliferation-induced memory NK cells” growing in lymphopenic hosts.22 Although the evidence for this form of NK cell memory has so far been confined to mice,22and it has not yet been demonstrated that proliferation-induced memory NK cells display a higher functionality upon re-stimulation, the similarities to TIML-NK cells are nevertheless striking. Notably, both populations develop in the absence of other immune cells, may as a result of missing self-MHC class I (i.e., KIR ligands) acquire a somewhat “unlicensed” status, display a quiescent phenotype, similar proliferation and contraction kinetics and a certain longevity. The finding that adaptive NK cells such as TIML-NK cells, “homeostatic proliferation-induced memory NK cells” and murine CIML-NK cells all lack a distinct phenotype is in contrast to memory T cells that differentiate into distinct subsets of either central memory or effector memory phenotype. At this point, we may only speculate whether the current concept of memory cell generation, which is built on studies of adaptive immunity that results from antigen-specific immune recognition is really applicable to innate immune cells.
In view of the fact that our numerous NK cell donors were not selected according to their known basic NK cell cytotoxic performance or according to existing mismatches on the KIR-KIRL level, our data provide strong support for the proof of concept that the overall modest GvL effects of NK cells toward pediatric BCP-ALL can significantly be exploited and augmented (Fig. 1B and C, Fig. 2A). Thus, we introduce an exciting new perspective to future protocols of experimental adoptive NK cell transfer. Previous efforts to improve existing cell transfer strategies harness the potential of somewhat “naive” NK cells for activation and/or expansion, and include the optimization of donor selection, the application of ex vivo or in vivo cytokine stimulation, the use of antibodies to induce antibody-dependent cellular cytotoxicity or to block inhibitory KIRs and the introduction of chimeric antigen receptors.5 In contrast, the exploitation of adaptive immune features of NK cells entails the advantage that it is presumably based on cellular re-programming; however certainly at the expense of having smaller numbers of transferable effector cells at one`s disposal.
Henceforth, various questions remain to be answered as to what the role of antigen-dependent receptor-ligand interactions is, which signal transduction mechanisms realize enhanced perforin secretion/synapse formation and control cell cycle kinetics and, finally, whether unique pathways exist that ensure memory NK cell formation in the context of tumor encounter. Maybe one way to achieve a truly patient-tailored and individualized treatment strategy will be the design of a two-step protocol comprising as a first step memory NK cell conversion via tumor-priming followed by a second step of accomplishing ample activation and expansion.
Materials and methods
Generation of “memory-like” NK cells
PBMCs from healthy volunteer donors were used with informed consent to isolate primary NK cells via negative selection (EasySep Human NK cell Enrichment Kit, STEMCELL Technologies). For tumor-priming, NALM-16 cells or primary blasts of donors P3B, P31G, P18R and P84D were irradiated with 100 Gy and added on day -1 to the respective NK cell preparation (E:T ratio of 1:3). Written informed consent was obtained from the parents of the donating children in accordance with the Declaration of Helsinki and after approval by the local ethics committee. The cell lines were originally obtained from ATCC, Wesel, Germany, and passaged more than 50 times. Thawing and Mycoplasma testing was last performed in September 2016. For cytokine-priming NK cells were pre-activated for 16 h (on day -1) with a mixture of 10 ng/mL IL12 (PeproTech) and 50 ng/mL IL18 (Medical & Biological Laboratories) as described before.13 All NK cell preparations were cultured in medium supplemented with low dose (100 IU/mL) IL2 (Novartis) and low dose (1 ng/mL) IL15 (CellGenix) (from hereon called complete medium) from day -1 to ensure survival. After 16 h, all NK cell preparations were washed and replaced in complete medium, which was refreshed on day 3 and 6 of culture. For microscopy studies, TIML-NK cells were additionally purified by negative selection using the human NK cell isolation kit on day 2 to eliminate potentially surviving NALM-16 cells or cell debris. As cultivation conditions resulted to a small extent (<5%) in the activation and expansion of CD3+ T and CD3+CD56+ NKT cells, all preparations were depleted from residual CD3+ cells on day 6 of culture using EasySep Human CD3 Positive Selection Kit I or EasySep Human NK cell Enrichment Kit (STEMCELL Technologies (≥95% CD56+ cells, ≤0.3% CD3+). Priming was performed in primary NK and NK-92 cells on day -1 of the culture and in expanded NK cells (see below) on day 7 of the 14-day expansion course. In further experiments, primary NK cells were primed with 100 Gy irradiated allogeneic PBMCs on day -1 (E:T ratio of 1:3).
Flow-cytometric analyses
Immunophenotyping of tumor-primed NK cells: The following antibody clones were used for phenotypical NK cell characterization: CD3 (UCHT1), CD16 (3G8), CD25 (2A3), CD56 (HCD56), CD57 (NK-1), CD69 (L78), CD94 (HP-3D9), CD158a/b/e (HP-3E4), CD161 (HP-3G10), CD186 (K041E5), NKp30 (Z25), NKp44 (Z231), NKp46 (9E2/NKp46), NKG2D (BAT221), NKG2A (Z199), NKG2C (134591) and KLRG1 (generated by HP Pircher, Freiburg, Germany). The percentage of CD56+CD3− cells expressing each antigen was determined using cluster analysis.
Cell cycle analyses: NK cell preparations were incubated in the presence of 10 µM BrdU for 16 h in complete medium. Cell cycle analysis was performed using the BD Pharmingen FITC-BrdU Flow Kit according to the manufacturer's protocol (BD Biosciences). Co-staining with 7-AAD hereby allows the enumeration and characterization of cells that are actively synthesising DNA in terms of their respective position in the cell cycle.
Viability assays: NK cell preparations were counted by vital dye exclusion with trypan blue (Sigma-Aldrich) and stained with antibodies against NK cell surface markers, 7-AAD (BD Biosciences) and AnnexinV (BioLegend) according to the manufacturer's protocol.
Functional NK cell response staining: NK cell preparations were co-cultured for 6 h with NALM-16 (E:T ratio of 1:3) in the presence of CD107a antibody (H4A3) and GolgiPlug (BD Biosciences). Subsequently, NK cells were stained with the indicated NK cell surface antibodies, permeabilized and co-stained with the respective intracellular antibodies against perforin (dG9), IFNγ (B27) and granzyme-B (GB11).
Conjugate formation
NK cells (4.5 × 105) were mixed with NALM-16 cells (1.5 × 105) and incubated in FACS tubes at 37 °C and 5% CO2 for 15 min. Conjugates were then transferred to silane-coated microscope slides and stained with the respective antibodies or dyes. For further information concerning staining protocols and confocal microscopy refer to supplemental methods.
Transcriptome analysis
RNA pre-amplification, labeling and hybridization onto Human Genome U133Plus 2.0 GeneChip arrays (Affymetrix) was performed according to standard manufacturer's protocols. Expression array data were background corrected and normalized by the GC robust multi-array Average (GC-RMA) method and log transformed using Bioconductor packages simpleaffy and affy. Unsupervised hierarchical clustering was based on the Euclidean distance. Pairwise comparisons were conducted using t-test controlling at a p value level of 0.05. Pathway and gene ontology analyses were performed using the Database for Annotation, Visualization and Integrated Discovery (DAVID) database system of the NIH.
Adoptive NK cell transfer into NOD.Cg-Prkdcscid IL2rgtmWjl/Sz mice
NOD.Cg-Prkdcscid IL2rgtmWjl/Sz (NSG) mice were purchased at “The Jackson Laboratory” and maintained under specified pathogen-free conditions in the research animal facility of the University of Tuebingen, Germany. All experimental animal procedures were conducted according to German federal and state regulations using unirradiated, 8–12 week-old male NSG mice. For adoptive NK cell transfer, 4 × 106 blasts of patients P3B or P18R were intravenously injected into unirradiated NSG mice on day 0, followed by a second injection of NK cell preparations (5 × 106 cells) later on day 0. On day 17, NSG mice were killed and BM preparations were subjected to flow-cytometric analysis using formerly defined leukemia surface markers. For serial transplantation, pooled 20 × 106 BM cells of group 1 and 2, respectively, of the former P3B-adoptive NK cell transfer experiments shown in Fig. 2A (resembling approximately 6 × 105 leukemic cells in group 1 or 3 × 105 leukemic cells in group 2) was intravenously re-injected into naive NSG mice to verify residual leukemic load after adoptive NK cell transfer. On day 56, NSG mice were killed and BM preparations were subjected to flow-cytometric analysis. The following antibody clones were used in this experiment: CD19 (SJ25C1), CD33 (WM53), CD45 (HI30), CD56 (HCD56) and anti-mouse CD45 (30-F11).
Statistics
Mean values and SD from experiments analyzing two conditions were determined using an unpaired two-tailed Student's t-test. A p value < 0.05 was considered statistically significant. If the p value is not specified, the indicated results have not been statistically significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We thank the many volunteers for repetitively donating large blood volumes for NK cell isolation. We acknowledge the gift of the K562-mbIL15–41BBL cell line by D. Campana, MD, National University of Singapore, Singapore, the NKG2DL antibodies from A. Steinle, PhD, University of Frankfurt, Germany, and the KLRG-1 antibody from H.P. Pircher, PhD, University of Freiburg, Medical Center, Germany. We thank S. Buehler and V. Borgmann for excellent technical assistance, K. Hamprecht, MD PhD, Institute of Medical Virology and Epidemiology of Viral Diseases, University of Tuebingen, Germany, for performing CMV screening of cell lines, and H.G. Rammensee, PhD, Institute for Cell Biology, University of Tuebingen, Germany, for helpful discussions and critical reading of the manuscript.
Funding
This work was supported by grants from the Faculty of Medicine of Tuebingen to MCA (E.05.00301.1 and 2298–0–0) and JW (2146–0–0), the “Jürgen Manchot Stiftung” to LS, “Madeleine-Schickedanz-Kinderkrebs-Stiftung” to MCA, the National Institutes of Health to JSO (R01AI067946), the “Stiftung des Fördervereins für krebskranke Kinder Tübingen” and the “Stefan-Morsch-Stiftung” to RH.
Author contributions
MP designed and performed experiments, interpreted the data and contributed to manuscript writing, LS performed experiments and contributed to data interpretation, AS and EM performed and interpreted confocal microscopy, RC analyzed transcriptome data, ACK performed apoptosis analyses, JSO, JW and RH contributed to data interpretation, UFH critically revised the manuscript, MCA conceptualized the work, designed experiments, interpreted data and wrote the paper.
Data and materials availability
Transcriptome data are deposited in the EMBL-EBI ArrayExpress database, and are accessible through ArrayExpress accession ID E-MTAB-4606.
References
- 1.Pfeiffer M, Schumm M, Feuchtinger T, Dietz K, Handgretinger R, Lang P. Intensity of HLA class I expression and KIR-mismatch determine NK-cell mediated lysis of leukaemic blasts from children with acute lymphatic leukaemia. Br J Haematol 2007; 138(1):97-100; PMID:17555452; https://doi.org/ 10.1111/j.1365-2141.2007.06631.x [DOI] [PubMed] [Google Scholar]
- 2.Pende D, Spaggiari GM, Marcenaro S, Martini S, Rivera P, Capobianco A, Falco M, Lanino E, Pierri I, Zambello R et al. Analysis of the receptor-ligand interactions in the natural killer-mediated lysis of freshly isolated myeloid or lymphoblastic leukemias: Evidence for the involvement of the Poliovirus receptor (CD155) and Nectin-2 (CD112). Blood 2005; 105(5):2066-73; PMID:15536144; https://doi.org/ 10.1182/blood-2004-09-3548 [DOI] [PubMed] [Google Scholar]
- 3.Pende D, Marcenaro S, Falco M, Martini S, Bernardo ME, Montagna D, Romeo E, Cognet C, Martinetti M, Maccario R et al. Anti-leukemia activity of alloreactive NK cells in KIR ligand-mismatched haploidentical HSCT for pediatric patients: Evaluation of the functional role of activating KIR and redefinition of inhibitory KIR specificity. Blood 2009; 113(13):3119-29; PMID:18945967; https://doi.org/ 10.1182/blood-2008-06-164103 [DOI] [PubMed] [Google Scholar]
- 4.Kübler A, Woiterski J, Witte K-E, Bühring H-J, Hartwig UF, Ebinger M, Oevermann L, Mezger M, Herr W, Lang P et al. Both mature KIR+ and immature KIR− NK cells control pediatric acute B-cell precursor leukemia in NOD.Cg-Prkdcscid IL2rgtmWjl/Sz mice. Blood 2014; 124(26):3914-23; PMID:25359989; https://doi.org/ 10.1182/blood-2014-05-572743 [DOI] [PubMed] [Google Scholar]
- 5.Handgretinger R, Lang P, Andre MC. Exploitation of Natural Killer (NK) cells for the treatment of acute leukemia. Blood 2016; 127(26):3341-9; PMID:27207791; https://doi.org/ 10.1182/blood-2015-12-629055 [DOI] [PubMed] [Google Scholar]
- 6.O'Leary JG, Goodarzi M, Drayton DL, von Andrian UH. T cell- and B cell-independent adaptive immunity mediated by natural killer cells. Nat Immunol 2006; 7(5):507-16; PMID:16617337; https://doi.org/ 10.1038/ni1332 [DOI] [PubMed] [Google Scholar]
- 7.Paust S, Gill HS, Wang BZ, Flynn MP, Moseman EA, Senman B, Szczepanik M, Telenti A, Askenase PW, Compans RW et al. Critical role for the chemokine receptor CXCR6 in NK cell-mediated antigen-specific memory of haptens and viruses. Nat Immunol 2010; 11(12):1127-35; PMID:20972432; https://doi.org/ 10.1038/ni.1953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun JC, Beilke JN, Lanier LL. Adaptive immune features of natural killer cells. Nature 2009; 457(7229):557-61; PMID:19136945; https://doi.org/ 10.1038/nature07665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guma M, Angulo A, Vilches C, Gomez-Lozano N, Malats N, Lopez-Botet M. Imprint of human cytomegalovirus infection on the NK cell receptor repertoire. Blood 2004; 104(12):3664-71; PMID:15304389; https://doi.org/ 10.1182/blood-2004-05-2058 [DOI] [PubMed] [Google Scholar]
- 10.Foley B, Cooley S, Verneris MR, Curtsinger J, Luo X, Waller EK, Anasetti C, Weisdorf D, Miller JS. Human cytomegalovirus (CMV)-induced memory-like NKG2C(+) NK cells are transplantable and expand in vivo in response to recipient CMV antigen. J Immunol 2012; 189(10):5082-8; PMID:23077239; https://doi.org/ 10.4049/jimmunol.1201964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Foley B, Cooley S, Verneris MR, Pitt M, Curtsinger J, Luo X, Lopez-Verges S, Lanier LL, Weisdorf D, Miller JS. Cytomegalovirus reactivation after allogeneic transplantation promotes a lasting increase in educated NKG2C+ natural killer cells with potent function. Blood 2012; 119(11):2665-74; PMID:22180440; https://doi.org/ 10.1182/blood-2011-10-386995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cooper MA, Elliott JM, Keyel PA, Yang L, Carrero JA, Yokoyama WM. Cytokine-induced memory-like natural killer cells. Proc Natl Acad Sci USA 2009; 106(6):1915-9; PMID:19181844; 10.1073/pnas.0813192106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Romee R, Schneider SE, Leong JW, Chase JM, Keppel CR, Sullivan RP, Cooper MA, Fehniger TA. Cytokine activation induces human memory-like NK cells. Blood 2012; 120(24):4751-60; PMID:22983442; https://doi.org/ 10.1182/blood-2012-04-419283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, Leong JW, Abdel-Latif S, Schneider SE, Willey S et al. Cytokine-induced memory-like natural killer cells exhibit enhanced responses against myeloid leukemia. Sci Transl Med 2016; 8(357):357ra123; PMID:27655849; https://doi.org/ 10.1126/scitranslmed.aaf2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fehniger TA, Stuart RK, Cooley SA, Miller JS, Curtsinger J, Hillman TM, Silver N, Szarek M, Lowdell MW, Gorelik L et al. Preliminary results of a phase 1/2 clinical trial of Cndo-109-activated allogeneic natural killer cells in high risk acute Myelogenous Leukemia patients in first complete remission. Blood 2014; 124(21):2320.25301333 [Google Scholar]
- 16.Kottaridis PD, North J, Tsirogianni M, Marden C, Samuel ER, Jide-Banwo S, Grace S, Lowdell MW. Two-Stage Priming of allogeneic natural killer cells for the treatment of patients with acute myeloid leukemia: A Phase I Trial. PLoS One 2015; 10(6):e0123416; PMID:26062124; https://doi.org/ 10.1371/journal.pone.0123416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonn T, Schwabe D, Klingemann HG, Becker S, Esser R, Koehl U, Suttorp M, Seifried E, Ottmann OG, Bug G. Treatment of patients with advanced cancer with the natural killer cell line NK-92. Cytotherapy 2013; 15(12):1563-70; PMID:24094496; https://doi.org/ 10.1016/j.jcyt.2013.06.017 [DOI] [PubMed] [Google Scholar]
- 18.Fujisaki H, Kakuda H, Shimasaki N, Imai C, Ma J, Lockey T, Eldridge P, Leung WH, Campana D. Expansion of highly cytotoxic human natural killer cells for cancer cell therapy. Cancer Res 2009; 69(9):4010-7; PMID:19383914; https://doi.org/ 10.1158/0008-5472.CAN-08-3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ni J, Miller M, Stojanovic A, Garbi N, Cerwenka A. Sustained effector function of IL-12/15/18-preactivated NK cells against established tumors. J Exp Med 2012; 209(13):2351-65; PMID:23209317; https://doi.org/ 10.1084/jem.20120944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, Liu S, McClory S, Marcucci G, Trotta R et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood 2010; 115(2):274-81; PMID:19897577; https://doi.org/ 10.1182/blood-2009-04-215491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjorkstrom NK, Riese P, Heuts F, Andersson S, Fauriat C, Ivarsson MA, Bjorklund AT, Flodstrom-Tullberg M, Michaelsson J, Rottenberg ME et al. Expression patterns of NKG2A, KIR, and CD57 define a process of CD56dim NK-cell differentiation uncoupled from NK-cell education. Blood 2010; 116(19):3853-64; PMID:20696944; https://doi.org/ 10.1182/blood-2010-04-281675 [DOI] [PubMed] [Google Scholar]
- 22.Sun JC, Beilke JN, Bezman NA, Lanier LL. Homeostatic proliferation generates long-lived natural killer cells that respond against viral infection. J Exp Med 2011; 208(2):357-68; PMID:21262959; https://doi.org/ 10.1084/jem.20100479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.North J, Bakhsh I, Marden C, Pittman H, Addison E, Navarrete C, Anderson R, Lowdell MW. Tumor-primed human natural killer cells lyse NK-resistant tumor targets: evidence of a two-stage process in resting NK cell activation. J Immunol 2007; 178(1):85-94; PMID:17182543; https://doi.org/ 10.4049/jimmunol.178.1.85 [DOI] [PubMed] [Google Scholar]
- 24.Leong JW, Chase JM, Romee R, Schneider SE, Sullivan RP, Cooper MA, Fehniger TA. Preactivation with IL-12, IL-15, and IL-18 induces CD25 and a functional high-affinity IL-2 receptor on human cytokine-induced memory-like natural killer cells. Biol Blood Marrow Transplant 2014; 20(4):463-73; PMID:24434782; https://doi.org/ 10.1016/j.bbmt.2014.01.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rolle A, Pollmann J, Ewen EM, Le VT, Halenius A, Hengel H, Cerwenka A. IL-12-producing monocytes and HLA-E control HCMV-driven NKG2C+ NK cell expansion. J Clin Invest 2014; 124(12):5305-16; PMID:25384219; https://doi.org/ 10.1172/JCI77440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlums H, Cichocki F, Tesi B, Theorell J, Beziat V, Holmes TD, Han H, Chiang SC, Foley B, Mattsson K et al. Cytomegalovirus infection drives adaptive epigenetic diversification of NK cells with altered signaling and effector function. Immunity 2015; 42(3):443-56; PMID:25786176; https://doi.org/ 10.1016/j.immuni.2015.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ortaldo JR, Winkler-Pickett RT, Nagata S, Ware CF. Fas involvement in human NK cell apoptosis: Lack of a requirement for CD16-mediated events. J Leukoc Biol 1997; 61(2):209-15; PMID:9021927 [DOI] [PubMed] [Google Scholar]
- 28.Stacey MA, Marsden M, Wang EC, Wilkinson GW, Humphreys IR. IL-10 restricts activation-induced death of NK cells during acute murine cytomegalovirus infection. J Immunol 2011; 187(6):2944-52; PMID:21849677; https://doi.org/ 10.4049/jimmunol.1101021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Keppel MP, Yang L, Cooper MA. Murine NK cell intrinsic cytokine-induced memory-like responses are maintained following homeostatic proliferation. J Immunol 2013; 190(9):4754-62; PMID:23530145; https://doi.org/ 10.4049/jimmunol.1201742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, Lanier LL. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J Exp Med 2012; 209(5):947-54; PMID:22493516; https://doi.org/ 10.1084/jem.20111760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luetke-Eversloh M, Hammer Q, Durek P, Nordstrom K, Gasparoni G, Pink M, Hamann A, Walter J, Chang HD, Dong J et al. Human cytomegalovirus drives epigenetic imprinting of the IFNG locus in NKG2Chi natural killer cells. PLoS Pathog 2014; 10(10):e1004441; PMID:25329659; https://doi.org/ 10.1371/journal.ppat.1004441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentlik AN, Sanborn KB, Holzbaur EL, Orange JS. Rapid lytic granule convergence to the MTOC in natural killer cells is dependent on dynein but not cytolytic commitment. Mol Biol Cell 2010; 21(13):2241-56; PMID:20444980; https://doi.org/ 10.1091/mbc.E09-11-0930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.James AM, Hsu HT, Dongre P, Uzel G, Mace EM, Banerjee PP, Orange JS. Rapid activation receptor- or IL-2-induced lytic granule convergence in human natural killer cells requires Src, but not downstream signaling. Blood 2013; 121(14):2627-37; PMID:23380740; https://doi.org/ 10.1182/blood-2012-06-437012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu D, Xu L, Yang F, Li D, Gong F, Xu T. Rapid biogenesis and sensitization of secretory lysosomes in NK cells mediated by target-cell recognition. Proc Natl Acad Sci USA 2005; 102(1):123-7; PMID:15618404; https://doi.org/ 10.1073/pnas.0405737102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu D, Meckel T, Long EO. Distinct role of rab27a in granule movement at the plasma membrane and in the cytosol of NK cells. PLoS One 2010; 5(9):e12870; PMID:20877725; https://doi.org/ 10.1371/journal.pone.0012870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bertrand F, Muller S, Roh KH, Laurent C, Dupre L, Valitutti S. An initial and rapid step of lytic granule secretion precedes microtubule organizing center polarization at the cytotoxic T lymphocyte/target cell synapse. Proc Natl Acad Sci USA 2013; 110(15):6073-8; PMID:23536289; https://doi.org/ 10.1073/pnas.1218640110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guma M, Budt M, Saez A, Brckalo T, Hengel H, Angulo A, Lopez-Botet M. Expansion of CD94/NKG2C+ NK cells in response to human cytomegalovirus-infected fibroblasts. Blood 2006; 107(9):3624-31; PMID:16384928; https://doi.org/ 10.1182/blood-2005-09-3682 [DOI] [PubMed] [Google Scholar]
- 38.Lopez-Verges S, Milush JM, Schwartz BS, Pando MJ, Jarjoura J, York VA, Houchins JP, Miller S, Kang SM, Norris PJ et al. Expansion of a unique CD57(+)NKG2Chi natural killer cell subset during acute human cytomegalovirus infection. Proc Natl Acad Sci USA 2011; 108(36):14725-32; PMID:21825173; https://doi.org/ 10.1073/pnas.1110900108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Della Chiesa M, Falco M, Podesta M, Locatelli F, Moretta L, Frassoni F, Moretta A. Phenotypic and functional heterogeneity of human NK cells developing after umbilical cord blood transplantation: A role for human cytomegalovirus? Blood 2012; 119(2):399-410; PMID:22096237; https://doi.org/ 10.1182/blood-2011-08-372003 [DOI] [PubMed] [Google Scholar]
- 40.Lee J, Zhang T, Hwang I, Kim A, Nitschke L, Kim M, Scott JM, Kamimura Y, Lanier LL, Kim S. Epigenetic modification and antibody-dependent expansion of memory-like NK cells in human cytomegalovirus-infected individuals. Immunity 2015; 42(3):431-42; PMID:25786175; https://doi.org/ 10.1016/j.immuni.2015.02.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Beziat V, Liu LL, Malmberg JA, Ivarsson MA, Sohlberg E, Bjorklund AT, Retiere C, Sverremark-Ekstrom E, Traherne J, Ljungman P et al. NK cell responses to cytomegalovirus infection lead to stable imprints in the human KIR repertoire and involve activating KIRs. Blood 2013; 121(14):2678-88; PMID:23325834; https://doi.org/ 10.1182/blood-2012-10-459545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T, Scott JM, Hwang I, Kim S. Cutting edge: Antibody-dependent memory-like NK cells distinguished by FcRgamma deficiency. J Immunol 2013; 190(4):1402-6; PMID:23345329; https://doi.org/ 10.4049/jimmunol.1203034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sabry M, Tsirogianni M, Bakhsh IA, North J, Sivakumaran J, Giannopoulos K, Anderson R, Mackinnon S, Lowdell MW. Leukemic priming of resting NK cells is killer Ig-like receptor independent but requires CD15-mediated CD2 ligation and natural cytotoxicity receptors. J Immunol 2011; 187(12):6227-34; PMID:22084431; https://doi.org/ 10.4049/jimmunol.1101640 [DOI] [PubMed] [Google Scholar]
- 44.Choi PJ, Mitchison TJ. Imaging burst kinetics and spatial coordination during serial killing by single natural killer cells. Proc Natl Acad Sci USA 2013; 110(16):6488-93; PMID:23576740; https://doi.org/ 10.1073/pnas.1221312110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 2008; 111(7):3571-8; PMID:18198346; https://doi.org/ 10.1182/blood-2007-07-100057 [DOI] [PubMed] [Google Scholar]
- 46.Wang B, Wang Q, Wang Z, Jiang J, Yu SC, Ping YF, Yang J, Xu SL, Ye XZ, Xu C et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res 2014; 74(20):5746-57; PMID:25164008; https://doi.org/ 10.1158/0008-5472.CAN-13-2563 [DOI] [PubMed] [Google Scholar]
- 47.Kamimura Y, Lanier LL. Homeostatic control of memory cell progenitors in the natural killer cell lineage. Cell Rep 2015; 10(2):280-91; PMID:25578733; https://doi.org/ 10.1016/j.celrep.2014.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.You W, Tang Q, Zhang C, Wu J, Gu C, Wu Z, Li X. IL-26 promotes the proliferation and survival of human gastric cancer cells by regulating the balance of STAT1 and STAT3 activation. PLoS One 2013; 8(5):e63588; PMID:23704922; https://doi.org/ 10.1371/journal.pone.0063588 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 49.Wolk K, Kunz S, Asadullah K, Sabat R. Cutting edge: Immune cells as sources and targets of the IL-10 family members? J Immunol 2002; 168(11):5397-402; PMID:12023331; https://doi.org/ 10.4049/jimmunol.168.11.5397 [DOI] [PubMed] [Google Scholar]
- 50.Borg C, Terme M, Taieb J, Menard C, Flament C, Robert C, Maruyama K, Wakasugi H, Angevin E, Thielemans K et al. Novel mode of action of c-kit tyrosine kinase inhibitors leading to NK cell-dependent antitumor effects. J Clin Invest 2004; 114(3):379-88; PMID:15286804; https://doi.org/ 10.1172/JCI21102 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.