ABSTRACT
We have previously reported that direct injection of dendritic cells (DC) engineered to express the Type-1 transactivator Tbet (i.e., DC.Tbet) into murine tumors results in antitumor efficacy in association with the development of structures resembling tertiary lymphoid organs (TLO) in the tumor microenvironment (TME). These TLO contained robust infiltrates of B cells, DC, NK cells, and T cells in proximity to PNAd+ blood vessels; however, they were considered incomplete, since the recruited B cells failed to organize into classic germinal center-like structures. We now report that antitumor efficacy and TLO-inducing capacity of DC.Tbet-based i.t. therapy is operational in peripheral lymph node-deficient LTA−/− mice, and that it is highly dependent upon a direct Tbet target gene product, IL-36γ/IL-1F9. Intratumoral DC.Tbet fails to provide protection to tumor-bearing IL-36R−/− hosts, or to tumor-bearing wild-type recipient mice co-administered rmIL-1F5/IL-36RN, a natural IL-36R antagonist. Remarkably, the injection of tumors with DC engineered to secrete a bioactive form of mIL-36γ (DC.IL36γ) also initiated therapeutic TLO and slowed tumor progression in vivo. Furthermore, DC.IL36γ cells strongly upregulated their expression of Tbet, suggesting that Tbet and IL-36γ cooperate to reinforce each other's expression in DC, rendering them competent to promote TLO formation in an “immunologically normalized,” therapeutic TME.
KEYWORDS: Dendritic cells, immunotherapy, interleukin (IL)-36γ, tbet, tertiary lymphoid organ, tumor
Introduction
Preferred clinical endpoints for cancer immunotherapies include the activation and recruitment of Type-1 T effector cells into tumors, the sustained (poly)functionality of these T cell populations, and the coordinate reduction of operational antagonism mediated by regulatory cells (Treg and MDSC) and tumor cells in the immunosuppressive tumor microenvironment (TME).1-6 Antitumor T cell (cross) priming is conventionally believed to occur in tumor-draining secondary lymph nodes7-9; however, recent findings suggest that the induction of T effector cells from naïve precursors can occur in and around tumor lesions in vivo, within the so-called tertiary lymphoid organs (TLO, aka tertiary lymphoid structures or ectopic lymphoid structures).10-14 The presence of TLO in a broad range of cancer types has been reported to represent a harbinger of improved clinical prognosis (i.e., extended OS, PFS, RFS), in association with the presence of robust populations of TIL.10-17
In contrast to tissue-draining secondary lymphoid organs (SLO), TLO are not encapsulated and can be found embedded within almost any non-lymphoid tissue,18 typically under conditions of persistent inflammation, as seen in the settings of chronic infectious disease, autoimmunity, transplantation, and cancer.18-21 A canonical feature of TLO is the presence of PNAd+ high-endothelial venules (HEV),18,22 specialized vascular structures that allow for the preferential adhesion and recruitment of CD62L(L-selectin)+ naïve and central memory T cells and dendritic cells (DC) into peripheral lymphoid sites.22,23 Notably, in a study of 225 primary melanomas, Martinet and colleagues reported that the density of HEVs correlated positively with the degree of tumor infiltration by naïve and type-1-polarized T cells, as well as, DC-LAMP+ mature DC,24 with these recruited immune cell populations found to loosely cluster around HEV.25 Furthermore, B cell infiltrating primary melanomas were not organized into formal germinal centers (GC) as is typical of SLO, suggesting a state of “incomplete” lymphoid organogenesis in the observed TLO.25
We have previously reported that DC engineered to express the type-1 transactivator protein Tbet/TBX21 (i.e., DC.Tbet) exhibits type-1 functional polarization,26 and that when delivered directly into tumor lesions in mice, these biologic products promote rapid (within hours) infiltration by lymphocytes and NK cells, and the development of TLO within days.13,27 We now show that the abilities of i.t.-delivered DC.Tbet to slow tumor growth and to promote TLO within the TME are highly IL-36γ dependent, as this therapy fails to achieve either end point when applied in wild-type hosts that are co-administered IL-36R antagonist IL-1F5 or when applied to tumor-bearing IL-36R−/− recipient mice.
IL-36γ (aka IL-1F9) is a recently identified member of the IL-1 family that we now report to be profoundly upregulated in DC after infection with rAd.Tbet (but not control Ad), presumably due to the direct transcriptional action of Tbet on the IL-36γ promoter.28 Interestingly, we noted that DC engineered to express a secreted, bioactive form of mIL-36γ (i.e., DC.IL36γ) upregulate their expression of Tbet, suggesting a positive feedback loop formed between Tbet and IL-36γ. Like DC.Tbet, when delivered into the established TME, DC.IL36γ also promoted the rapid development of TLO and slowed tumor growth. These results support the utility of Tbet- and IL-36γ-based therapeutics in the cancer setting and may provide clues for the roles of IL-36R agonist cytokines (i.e., IL-36α, IL-36β and IL-36γ) in disease-associated TLO formation in alternate chronic inflammatory states, such as autoimmune arthritis, diabetes, and psoriasis,19,21,29,30 among others.
Results
DC.Tbet overexpress pro-inflammatory gene products, including IL-36γ
Day 5 cultured, bone-marrow-derived DC from C57BL/6 mice were infected with rAd.mTbet to generate DC.Tbet. To investigate the effect of Tbet overexpression in DC, molecular profiling of DC.Tbet versus control DC.null was performed, leading to the identification of a range of differentially expressed gene transcripts, including several pro-inflammatory cytokines and chemokines (Fig. 1A). Notably, IL-36γ and IL-12p40 were two of the most overexpressed gene transcripts in DC.Tbet when compared with control DC in both mice and humans (Figs. 1A and S1), whereas other transcripts such as CXCL9 and IL-1β were not consistently elevated in DC.Tbet in an evolutionarily conserved manner (thus limiting their likely translational relevance). Both Tbet and IL-36γ protein expression could be detected in murine DC.Tbet by IFM (Fig. 1B), and DC.Tbet secreted higher levels of mIL-12p70 (Fig. 1C) and mIL-36γ (Fig. 1D) than control engineered DC. We then confirmed that the injection of DC.Tbet cells into established s.c. MCA205 sarcomas on days 7 and 14 post-tumor inoculation resulted in slowed tumor growth when compared with i.t. therapy using PBS or control DC.ψ5 cells (Fig. 1E).
Figure 1.
Characterization of therapeutic DC.Tbet. Bone marrow-derived DC were generated in GM-CSF + IL-4 cultures for 5–6 d, before being infected for 48 h with rAd.mTbet or control rAd.EGFP or empty rAd.ψ5, or they were left untransduced, as indicated. In (A), DC were treated, as indicated, with the addition of LPS + IFNγ for the second 24 h of infection. Affymetrix gene array analyses were performed on DC.Tbet (A), with transcripts increased >5-fold compared with control DC reported. DC.Tbet generated from Tbet-ZsG (H-2b) reporter mice were then analyzed by IFM for intracellular expression of the Tbet reporter (green) and IL-36γ (red), with DAPI staining of nuclei (B). DC.Tbet or control DC were cultured at 4 × 105 cells/mL. After 48 h of infection, supernatant was harvested and mIL-12p70 (C) or mIL-36γ (D) production was analyzed by ELISA. **p < 0.05 for DC.Tbet vs. control DC (t-test). In E, DC.Tbet, control DC.ψ5, or PBS were then injected intratumorally into mice bearing d7 established s.c. MCA205 sarcomas on days 7 and 14 post-tumor inoculation. Tumor size was measured over time. **p < 0.05 for DC.Tbet vs. PBS or control DC.ψ5 treatment on days ≥ 11 (ANOVA). Data are representative of those obtained in two independent experiments performed in each case.
i.t. delivery of DC.Tbet results in rapid infiltration of lymphocytes and development of TLO in vivo
To investigate the longitudinal cellular and molecular changes occurring within the therapeutic TME, MCA205 sarcomas were harvested from tumor-bearing wild-type C57BL/6 mice or syngenic (H-2b) Tbet-ZsG reporter mice at various time points after i.t. delivery of DC.Tbet cells. Immunofluorescence microscopy analyses revealed early (by 4–10 h post-treatment) infiltration of treated tumors by T cells (both CD4+ and CD8+) that continued to increase in their abundance through 24 h post-treatment (Fig. 2A). Many of these TILs appeared to exhibit Type-1 functional polarization, based on their expression of the Tbet reporter in Tbet-ZsG recipient models (Fig. 2A). In stark contrast, B cells (identified by their CD19+ or B220+ phenotypes) though present in the TME by 5 d following treatment with DC.Tbet, did not contribute significantly to TIL composition early or late (Fig. 2A–C). Interestingly, although not evident at 4–24 h post-i.t. injection of DC.Tbet, PNAd+ vessels became readily detectable in MCA205 tumors beginning on day 5 after treatment and persisted until at least 5 d following the second treatment with DC.Tbet (i.e., d12 after first injection of DC.Tbet; Fig. 2B and C). Immunofluorescence imaging also revealed strong local production of IL-36γ in MCA205 tumors beginning at 4 h after i.t.-based DC.Tbet therapy, with sustained intratumoral IL-36γ observed through 5 d following the second treatment with DC.Tbet (Fig. 2D and E). Since PNAd+ HEV in peripheral tissues have been linked to the formation of TLO in chronically inflamed tissues,8,18,20,31 we next analyzed whether injected DC.Tbet and/or the therapeutic TME expressed a transcript profile consistent with TLO formation. We observed that DC.Tbet at the time of injection into mice expressed higher levels of mRNA encoding CCL19, CCL21, LIGHT, and LTA (but not CXCL13), when compared with control DC.ψ5 (Fig. S2A). Notably, we found that each of these transcripts was differentially upregulated in the TME within 4–10 d of DC.Tbet vs. control DC.ψ5 injection (Fig. S2B), at least circumstantially implicating the involvement of additional DC.Tbet-conditioned tumor stromal cells (or tumor cells themselves) as principal pro-TLO transcript sources over time in vivo.
Figure 2.
DC.Tbet injected i.t. promote the rapid infiltration of lymphocytes and the development of PNAd+ blood vessels in association with enhanced locoregional induction of IL-36γ. MCA205 tumor-bearing wild-type C57BL/6 mice or Tbet-ZsG reporter mice were treated by i.t. delivery of 1 × 106 DC.Tbet or DC.ψ5 7 d post-tumor inoculation. In (A), tumors were harvested from Tbet-ZsG mice at 4h, 10h or 24h after treatment with DC.Tbet and tissue sections analyzed by IFM for CD4+ T cells, CD8+ T cells, CD19+ B cells, and PNAd+ HEV. In (B) and (C), expression and localization of peripheral node addressin (PNAd), CD11c+ DC, CD3+ T cells, and B220+ B cells were analyzed in tumor sections from Tbet reporter mice on days 5 (B) and 12 (C) post-treatment with DC.Tbet. In (D), day 5 (post-DC.Tbet treatment) tumor sections from Tbet-ZsG mice were analyzed by IFM for co-expression of the Tbet reporter (green) and IL-36γ (red, using a specific polyclonal antibody). In (E), IL-36γ protein levels were assayed by quantification of fluorescence from IFM, or transcript levels were assayed by real-time PCR in total tumor RNA isolated from wild-type C57BL6/J hosts, at the indicated time points following DC.Tbet or DC.ψ5 treatment. Data are representative of those obtained in two to three independent experiments performed in each case.
Therapeutic antitumor efficacy of i.t. DC.Tbet is not dependent on host LTA
Given evidence for the development of TLO in the TME of DC.Tbet-treated C57BL/6 wild-type mice, we next examined whether SLO were necessary for the observed therapy benefits of this treatment strategy. MCA205 sarcomas were established in peripheral lymph node-deficient LTA−/− mice,32 and treated with i.t. delivered DC.Tbet or control DC.ψ5 as outlined in Fig. 1D. We noted that the treatment of MCA205 sarcomas with DC.Tbet was comparably efficacious in both LTA−/− and C57BL/6 wild-type host animals (Fig. 3A). Furthermore, based on the results of repeat experiments involving the co-administration of depleting anti-CD4+ or anti-CD8+ antibodies, we conclude that therapeutic protection mediated by DC.Tbet in LTA−/− mice is both CD4+ T cell- and CD8+ T cell dependent (Fig. 3B).
Figure 3.

I.t.-delivered DC.Tbet mediates antitumor efficacy in secondary lymph node-deficient LTA−/− mice that is both CD4+ and CD8+ T cell-dependent. In (A), lymphotoxin-α (LTA)−/− mice bearing established day 7 MCA205 sarcomas were left untreated, or they were treated (D) on days 7 and 14 by i.t. injection of 106 DC.Tbet or control DC.ψ5 cells alone, with tumor size (mean +/− SD from five mice/group) then monitored over time. In (B), this same model was left untreated, or treated on days 7 and 14 with DC.ψ5 or DC.Tbet cells (+/− systemic i.p. administration of depleting anti-CD4+ or anti-CD8+ mAbs) and time-to-euthanasia (as an index of overall survival) reported over time. **p < 0.05 for DC.Tbet vs. all other cohorts (ANOVA). Data are representative of those obtained in two independent experiments performed.
Ablation of IL-36R signaling abrogates the therapeutic antitumor efficacy of i.t. DC.Tbet
Although mouse and human DC.Tbet produce high levels of IL-36γ and IL-12 transcripts and protein (Figs. 1 and S1), our previous work demonstrated that the therapeutic benefits of such treatments were maintained even if the injected DC.Tbet were generated from syngenic IL-12p35−/− or IL-12p40−/− mice.27 Subsequent mechanistic analyses were therefore focused on the study of DC.Tbet-associated IL-36γ and its receptor IL-36R. To determine whether the therapeutic benefits of i.t.-delivered DC.Tbet were IL-36R-dependent, wild-type C57BL/6 mice were treated on days 7 and 14 after s.c. injection of MCA205 sarcomas, in the absence or presence of (i.t.) co-delivered rmIL-1F5 (aka IL-36RA or IL-36RN), a natural IL-36R antagonist.33,34 As depicted in Fig. 4, we observed that rmIL-1F5 co-delivery effectively blocked the ability of i.t. DC.Tbet to slow tumor growth (Fig. 4A), to sponsor the development of PNAd+ HEV in the TME (Fig. 4B), or to recruit/retain CD4+ and CD8+ TIL (Fig. 4C).
Figure 4.

The antitumor efficacy and TLO promoting activity associated with i.t. DC.Tbet-based therapy is ablated by the IL-36R antagonist IL-1F5 in vivo. WT C57BL6/J mice bearing established day 7 MCA205 sarcomas were left untreated, or they were treated on days 7 and 14 by i.t. injection of 106 DC.Tbet or DC.ψ5 alone or with DC.Tbet plus co-injection (i.t.) of rmIL-1F5 (0.1 μg or 1 μg in 50 μL PBS) followed by (i.t.) injections of the respective doses of IL-1F5 alone in 50 μL PBS on days 8, 9, 15, and 16, with tumor growth (A) monitored over time (n = 5 mice/group). **p < 0.05 for DC.Tbet vs. all other cohorts (ANOVA) on days ≥ 17. In (B)and (C), tumors were harvested from the indicated treatment cohorts on day 25 post-tumor inoculation (i.e., 9 d following the final injection of rmIL-1F5) and analyzed by IFM for the presence of PNAd+ vessels (B) and CD4+ T cells and CD8+ T cells (C). Data are representative of three independent assays performed in each case.
To further confirm the importance of IL-36R on host (tumor stromal/immune) cells to the antitumor efficacy of i.t.-delivered DC.Tbet in an alternate tumor model, we established s.c. MC38 colon carcinomas in syngenic (H-2b) C57BL/6 wild-type or IL-36R−/− mice, before treating them i.t. on days 7 and 14 with DC.Tbet/EGFP or control DC.EGFP (i.e., rAd.EGFP was used to engineer both DC cohorts to mark these injected cells for subsequent tissue imaging studies). As shown in Fig. 5A, consistent with data obtained in the MCA205 sarcoma model, treatment of established MC38 tumors with DC.Tbet/EGFP but not DC.EGFP resulted in significant slowing in tumor growth. Also consistent with data obtained in the MCA205 model, we noted that transcripts associated with PNAd+ HEV/TLO development and immune cell trafficking (particularly LTA and CCL21) were increased in the TME of MC38-bearing mice treated i.t. with DC.Tbet/EGFP vs. control DC/EGFP (Fig. S3). Although data from MCA205 models in LTA−/− recipient animals argued against a major role for host LTA in the therapeutic efficacy of i.t.-delivered DC.Tbet (Fig. 3), to determine the broader role(s) of LTβR agonist cytokines (from either the injected DC.Tbet cells or the host TME) on the antitumor action of DC.Tbet, we established s.c. MC38 tumors in C57BL/6 mice, before treating them i.t. on days 7 and 14 with LTβR-Ig or an isotype control antibody in concert with control DC.EGFP or DC.Tbet/EGFP. As shown in Fig. 5B, MC38 tumors in mice co-treated with LTβR-Ig and DC.Tbet/EGFP exhibited similar rates of slowed progression (through day 18 of the experiment) when compared with mice treated with DC.Tbet/EGFP plus an isotype control antibody. These data suggest that LTβR agonists do not play a pivotal role in DC.Tbet-associated antitumor efficacy.
Figure 5.
The antitumor efficacy and TLO promoting activity associated with i.t. DC.Tbet-based therapy is absent in the IL-36R−/− recipient mice. 105 MC38 colon carcinoma cells were injected into the flanks of syngenic wild-type C57BL/6 (A) mice and allowed to establish. Tumor-bearing mice were randomized into groups of five animals/cohort on day 7 post-implantation, with all cohorts exhibiting comparable mean tumor size. These animals were then left untreated, or were treated on days 7 and 14 by i.t. injection of 106 control DC.EGFP or DC.Tbet/EGFP, with tumor growth subsequently monitored over time (n = 5 mice/group). **p < 0.05 for DC.Tbet vs. all other cohorts on days ≥ 13 for C57BL/6 recipients. In (B), the experiment from (A)was repeated, with the addition of co-treatment cohorts including LTβR-Ig (100 μg) or an isotype control antibody (100 μg), injected i.t. 3 h before each injection of DC.Tbet. *p < 0.05 for DC.Tbet + Iso-Ig vs. DC.EGFP on days ≥ 11; **p < 0.05 for DC.Tbet + LTβR-Ig vs. DC.EGFP on days 11–18 (NS on day 20); ***p < 0.05 for DC.Tbet + Iso-Ig vs. DC.Tbet + LTβR-Ig on day 20. In (C), tumors harvested 5 d following i.t. injections of DC.Tbet/EGFP or control DC.EGFP were cryosectioned and analyzed by IFM for the presence of PNAd+ HEV, CD3+ T cells, and CD11c+ DC (C). In (D), 105 MC38 colon carcinoma cells were injected into the flanks of syngenic IL-36R−/− and allowed to establish and were treated as described in (A). p = NS for DC.Tbet vs. control cohorts in IL-36R−/− mice at all time points (ANOVA). In (E), day 18–21 tumors harvested from the indicated treatment cohorts were cryosectioned and then H&E stained as described in Materials and Methods. Robust TIL populations (C) and (E) were observed in cortical regions of MC38 tumors, only in DC.Tbet-treated C57BL/6 hosts. In (F)and (G), DC.Tbet/EGFP-treated tumors were harvested from wild-type C57BL6/J hosts at day 20 and analyzed by IFM for the presence of PNAd+ HEV, CD3+ T cells, and CD11c+ DC (F) or PNAd+ HEV, CD3+ T cells, and DC.Tbet/EGFP (G). Data are representative of those obtained inthree independent experiments performed in each case.
As was the case in our MCA205 tumor models, i.t. injections of DC.Tbet into established s.c. MC38 tumors in C57BL/6 mice led to the development of PNAd+ HEV surrounded by CD3+ T cell and CD11c+ DC infiltrates within 5 d of initiating treatment with DC.Tbet/EGFP, but not control DC.EGFP (Fig. 5C). We also confirmed that the therapeutic benefit and TLO formation associated with i.t. delivery of DC.Tbet/EGFP into MC38 tumors was negated in IL-36R−/− recipient mice (Fig. 5D and E), whereas TLO were readily apparent in H&E stains of DC.Tbet/EGFP-treated tumors in wild-type C57BL/6 recipient animals (Fig. 5E). In MC38-bearing C57BL/6 wild-type hosts, TLO persisted within the TME for at least 6 d following the second injection of DC.Tbet/EGFP (Fig. 5F), and these TLO contained injected EGFP+ DC located proximal to PNAd+ vessels (Fig. 5G). Prior reports in the field have documented the proximity of lymphocytes to PNAd+ HEV in SLO and, critically, identified a role for PNAd in maintaining lymphocyte proximity to HEV.35 Consistent with previous observations for approximately 30–40% of lymphocytes residing within 20 µm of an HEV in SLO, we observed a similar distribution pattern in tumor-associated TLO after i.t. treatment with DC.Tbet/EGFP cells (Fig. S4A–E). Specifically, we noted that 31.99% of CD3+ T cells, 48.46% of B220+ B cells, and 21.65% of CD11c+ DC within the TME treated with DC.Tbet were located within 20 µm of a PNAd+ vessel (Fig. S4F). However, as we also discerned in the MCA205 model, B cells in this therapeutic TME failed to organize into formal GC-like structures (Fig. S4A). Tumors treated with DC.Tbet/EGFP also contained a higher frequency of CD3+ T cells vs. tumors treated with control DC.EGFP (Fig. S5A), with increased numbers of Tbet+ T cells within the TME after i.t. delivery of DC.Tbet/EGFP (Fig. S5B). Interestingly, CD3+ TIL in DC.Tbet/EGFP-treated tumors contained a lower abundance of exhausted/anergic T cells as suggested by their decreased expression profiles for the PD-1, CTLA4, and TIM-3 checkpoint molecules, vs. CD3+ TIL isolated from control treated mice (Fig. S5C).
DC engineered to secrete bioactive rmIL-36γ (DC.IL36γ) recapitulate the effects of i.t. DC.Tbet treatment in vivo
We next asked whether ectopic IL-36γ production by DC would allow these cells to phenocopy DC.Tbet in their ability to effectively treat established tumors after i.t. injection, in association with the promotion of “non-classical” TLO within the TME. We first designed and produced a recombinant adenoviral vector encoding a fusion protein consisting of the human CD8+ signal sequence followed by a full agonist form of mIL-36γ chain (i.e., amino acid positions G13-S164 found in the processed, bioactive form of this cytokine).33 This vector was then used to generate and characterize DC.IL36γ (Fig. 6), before subsequently applying them as an i.t.-delivered cellular therapy (Fig. 7).
Figure 6.
DC.IL36γ produce/secrete bioactive IL-36γ and upregulate intrinsic transcription of Tbet. Real-time PCR (A) and Western blot analysis (B) for IL-36γ were performed on lysates of DC.IL36γ/EGFP vs. control DC.null or DC.EGFP to confirm transduction efficacy. In (C), Affymetrix gene array analyses were performed as outlined in Materials and Methods, with transcripts increased >5-fold compared in DC.IL36γ vs. control DC.null reported. In (D) and (E), real-time PCR (D) and Western blot analysis (E) for Tbet were performed on lysates of DC.IL36γ/EGFP and/or DC.Tbet/EGFP and/or control DC.EGFP and/or control DC.null. In (A) and (D), mean ± SD data are reported; **p < 0.05 (t-test). In (F), DC were differentiated for 5 d in vitro, with CD11c+ cells then isolated and either transduced with rAd to express EGFP, mTbet, and/or mIL-36γ, or untransfected control DC were treated for 24 h with the indicated TLR agonists or agonist anti-CD40 (FGK45) antibody. Cell-free supernatants were then analyzed by mIL-36γ ELISA. *p < 0.05 for DC.Tbet and DC.IL36γ vs. all other treatment groups (ANOVA). In (G), cell-free supernatants were recovered from engineered DC and analyzed for bioactivity by addition to cultures of bulk splenocytes isolated from Tbet (ZsG) reporter mice; i.e., 106 splenocytes were cultured in 200 μL of basal media (negative control), media containing LPS + IFNγ (positive control) or cell-free media harvested from DC.null or DC.IL36γ cells 48 h after rAd infection. After overnight culture, splenocytes were analyzed by flow cytometry for upregulation of intracellular Tbet reporter expression. In (H), DC.IL36γ cells were analyzed by IFM as described in Fig. 1(B) to detect coordinate expression of Tbet and IL-36γ protein. All data are representative of those obtained in two to three independent experiments performed in each case.
Figure 7.
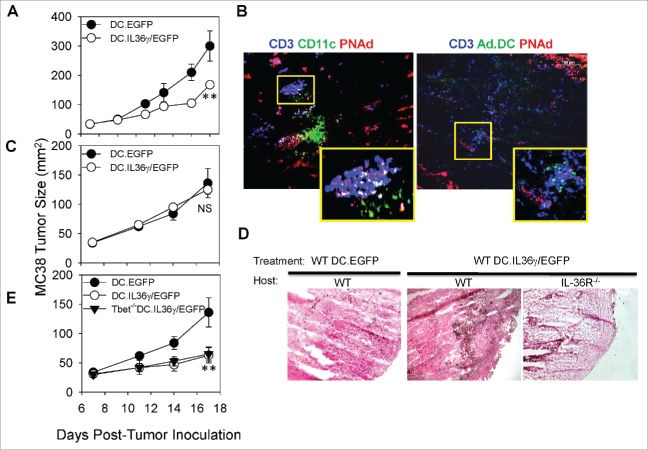
I.t. delivery of DC.IL36γ is therapeutic and promotes TLO in wild type, but not in IL-36R−/−hosts. 105 MC38 colon carcinoma cells were established s.c. in the flanks of syngenic wild-type C57BL/6 (A) mice. After randomization of the tumor-bearing animals on day 7 to cohorts (five mice/group) exhibiting comparable mean tumor sizes, mice were treated by i.t. injection with 106 control DC.EGFP or DC.IL36γ/EGFP on days 7 and 14, and tumor growth monitored over time. **p < 0.05 for DC.IL36γ/EGFP vs. DC.EGFP on days ≥ 11 (ANOVA). After euthanasia on day 20 post-tumor inoculation, tumors were cryosectioned and analyzed by IFM for the presence of TLO based on the presence of PNAd+ HEV surrounded by CD3+ T cells, CD11c+ DC, and DC.IL36γ/EGFP in the tumor cortex (B). In C, the experiment performed in (A)was repeated in IL-36R−/− host animals. NS for DC.IL36γ/EGFP vs. DC.EGFP at all time points (ANOVA). In (D), MC38 tumor sections harvested on d18 (IL-36R−/− hosts) or d20 (WT C56BL/6J hosts) post-treatment with DC.IL36γ/EGFP or control DC.EGFP were then H&E stained to identify TIL in the cortical regions of the tumors. In (E), BMDC generated from wild-type or Tbet−/− mice were infected with control rAd.EGFP or rAd.mIL36γ/EGFP and injected i.t. into established MC38 tumors in wild-type C57BL/six mice on days 7 and 14 and tumor growth (mean +/− SEM in mm2) was monitored over time. **p < 0.05 for WT or Tbet−/− DC.IL36γ/EGFP vs. DC.EGFP on days ≥11; NS for WT vs. Tbet−/− DC.IL36γ/EGFP at all time points (ANOVA). Data are representative of those obtained in two to three independent experiments performed in each case.
Predictably, DC.IL36γ displayed a robust increase in mIL-36γ mRNA transcript (Fig. 6A) and protein (Fig. 6B) levels when compared with control DC. Molecular profiling of DC.IL36γ vs. control DC was performed, leading to the identification of a panel of upregulated chemokines and cytokines (Fig. 6C). Consistent with data from DC.Tbet cells, DC.IL36γ at the time of injection expressed elevated levels of CCL21 and LTA, as well as LIGHT (Fig. S6). Remarkably, DC.IL36γ strongly upregulated their expression of Tbet when compared with control DC, although this level of elevated transcription (Fig. 6D) and protein production (Fig. 6E) did not quite reach that observed in DC.Tbet. IL-36γ was secreted from DC.IL36γ (and DC.Tbet) but not control DC (Fig. 6F), and the IL-36γ secreted by DC.IL36γ was bioactive based on its ability to upregulate Tbet reporter expression in splenic T cells isolated from Tbet-ZsG mice (Fig. 6G). When visualized by IFM, DC.IL36γ cells were determined to co-express Tbet and IL-36γ (Fig. 6H).
We subsequently determined that i.t. delivery of DC.IL36γ slows MC38 tumor growth in C57BL/6 wild-type hosts (Fig. 7A) and promotes the development of TLO with MC38 tumors (Fig. 7B) to a degree similar to that observed for DC.Tbet-based therapy (Fig. 5). Euclidean distance analyses of fluorescence microscopy images revealed that 39.62% of CD3+ TIL, 60.09% of B220+ B cells, and 37.32% of CD11c+ DC were located within 20 μm of PNAd+ vessels within the TME of animals treated with DC.IL36γ (Fig. S7). Transcripts associated with TLO formation, including LTA, LIGHT, CCL19, CCL21, and CXCL13, were coordinately upregulated within 1–5 d following DC.IL36γ treatment. As expected, DC.IL36γ-based therapy was ineffective in limiting tumor progression or in facilitating TLO formation in MC38 tumors established in IL-36R−/− recipient animals (Fig. 7C and D). Given the coordinate upregulation of Tbet transcription and protein production in DC.IL36γ (Fig. 6D and E), we next analyzed whether DC.IL36γ developed from Tbet−/− bone marrow remained effective as an antitumor agent in wild-type recipients. As shown in Fig. 7E, DC.IL36γ/EGFP developed from wild-type or Tbet−/− bone marrow was equally efficacious as a therapy vs. DC.EGFP when injected directly into MC38 tumors. Thus, DC expressing ectopic IL-36γ does not require intrinsic Tbet expression to mediate anti-MC38 benefit after i.t. delivery.
Discussion
A major novel finding in our report is that the therapeutic benefits of i.t. delivered DC.Tbet therapy are tied to the ability of these gene-modified cells to promote TLO formation in the cortical region of progressive tumors via a process that is strictly dependent on IL-36γ, an IL-1 family member cytokine associated with a range of chronic inflammatory diseases including psoriasis, arthritis, and diabetes, among others.19,21,29,30 Indeed, while development of TLO within tumors and slowed tumor growth resulted from the injection of DC.Tbet directly into established MCA205 sarcoma or MC38 colon carcinoma lesions in syngenic C57BL/6 mice, these processes failed to occur after DC.Tbet treatment of tumors in IL-36R−/− hosts or in wild-type mice that received co-injections of the IL-36R antagonist IL-1F5 (aka IL-36Ra/IL-36RN). Surprisingly, IL-36γ-dependent TLO formation in the TME did not appear to require significant participation by LTβR ligand cytokines based on the results of studies integrating i.t. co-delivery of antagonist LTβR-Ig (Fig. 5B) or the use of LTA−/− host animals (Fig. 3). In this regard, recent results from Engelhard's group suggest that induction of HEV and early stages of TLO development in i.p. B16.OVA melanomas are LTβR-independent, but LTα3/TNFR-dependent36. Future studies using TNFR1/2−/− recipient animals will help in delineating the role of the LTα3/TNFR signaling axis in the antitumor efficacy of i.t.-delivered DC.Tbet (or DC.IL36γ).
The antitumor benefits mediated by i.t. delivery of DC.Tbet were associated with the rapid infiltration of tumors (within hours) by Tbet+ CD4+ and CD8+ T cells and by locoregional production of cytokines (LTA, LIGHT) and chemokines (CCL19, CCL21 >> CXCL13) classically linked to TLO formation.10,11,18,20,36 Tumor infiltration by B cells and the development of PNAd+ HEV in the tumor cortex were subsequently observed by 5 d after treatment with DC.Tbet (but not with control DC). Notably, though present in the TME, B cells were not observed to coalesce into formal GC in treated tumors at any time during the performance of our studies. This result is consistent with previous reports of “incomplete”/”non-classical” lymphoid neogenesis of TLO detected in primary human melanoma25 and oral squamous cell carcinoma.37 These TLO are instead defined by the presence of PNAd+ HEV and the proximity of DC and B/T cells to the therapeutically induced HEV (Fig. S4), based on a paradigm established for SLO by Tsuboi et al.35
The presence of regulatory T cells (Tregs) in TLO has been recently described by Joshi et al.,38 where these suppressive cells were observed to localize proximal to DC, B cells, and other T cell subsets within these structures. In our studies, Tregs (i.e., CD4+FoxP3+) were notably rare events in MC38 tumors, and the frequency of tumor-infiltrating Tregs was statistically equivalent in DC.Tbet/EGFP- vs. DC.EGFP-treated tumors (Fig. S8A). In striking contrast, we observed that levels of CD11b+Gr1+ MDSC in MC38 tumors were reduced after i.t. treatment with DC.Tbet/EGFP vs. DC.EGFP (Fig. S8B). When taken in the context of our findings for reduced levels of immune checkpoint molecules associated with TIL exhaustion/anergy in our treatment models (Fig. S5C), this may support the general capacity of i.t.-delivered DC.Tbet to promote TLO in the context of an “immunologically normalized” TME.
In “classic” TLO formation, B cell nucleation/GC formation appears to critically depend on the action of T follicular-helper (Tfh) cells18-20,39 and follicular dendritic cells (fDC).37 As such, we would hypothesize that B cell nucleation/GC formation may fail after treatment with DC.Tbet or DC.IL36γ based on the absence or dysfunction of Tfh and fDC within the TME. We will evaluate this supposition in prospective studies in each of our tumor models. In this same vein, we also previously reported early infiltration of NK cells into the TME of DC.Tbet-treated MCA205 tumors.13 We are prospectively evaluating whether any deficiency in Tfh cell numbers/function may be related to the recruitment of NK cells that have been reported to inhibit Tfh numbers/function40 or alternatively to the inability of DC.Tbet or DC.IL36γ cells, or an IL-36γ-rich microenvironment, to sponsor the differentiation or survival of Tfh cells in TLO induced within the treated TME.
An additional major finding in our studies reflects the apparent cooperativity of Tbet and IL-36γ in therapeutic DC, with ectopic expression of either gene product promoting the robust upregulation of the alternate gene product in transfected DC. Hence, in addition to the aforementioned transactivation of IL-36γ by Tbet in DC.Tbet (Fig. 1A and ref. 28), we also observed strong upregulation of Tbet mRNA/protein expression in DC.IL36γ vs. control DC (Fig. 6C–E). This is suggestive of a positive feed-back loop in their molecular regulation, at least in myeloid DC. Like DC.Tbet, DC.IL36γ was capable of mediating potent antitumor effects and promoting TLO formation when injected directly into established tumors in wild-type C57BL/6 host animals. We also observed that the therapeutic antitumor benefits of DC.Tbet were lost when applied to tumor-bearing IL-36R−/− mice and that DC.IL36γ derived from Tbet−/− bone marrow remained effective in suppressing the progressive growth of tumors in wild-type C57BL/6 mice. These findings support the cooperativity of Tbet and IL-36γ underlying the antitumor efficacy observed for this DC-based cellular therapy, with IL-36γ being down-stream of Tbet in the underlying operational paradigm.
While our findings suggest the Tbet- and IL-36γ-dependency of therapeutic efficacy (TLO formation and slowed tumor growth in vivo) associated with i.t. delivery of DC.Tbet or DC.IL36γ, it remains unclear as to whether DC provide more than a “vessel” to deliver the key Tbet-/IL-36γ-dependent signals into the therapeutic TME. We are currently investigating the comparative treatment benefits associated with mTbet and mIL-36γ recombinant protein/gene (rAd)-based therapies applied locoregionally in the setting of well-established MCA205 and MC38 murine tumor models. It is also both practical and pertinent to ask whether in vitro conditioning of DC (in the absence of genetic engineering) is capable of yielding IL-36γ-secreting cells that might be competent to recapitulate the therapeutic benefits of DC.Tbet or DC.IL36γ upon delivery into the TME. As shown in Fig. 6F, DC cultured with agonists for TLR3, TLR4, and TLR7 (but not TLR2, TLR9 or CD40) were capable of stimulating increased secretion of IL-36γ from DC, albeit to far lower levels than those achieved from DC.Tbet or DC.IL36γ. Nevertheless, it will be important that prospective translational modeling includes a comparison of DC.Tbet/DC.IL36γ vs. TLR-agonized DC as therapeutic agents. Furthermore, it has also been reported that cathelicidin anti-microbial peptides including LL37 (in humans) and CRAMP (in mice) promote robust production of IL-36γ by keratinocytes in the skin,41 with both proteins found prevalently in psoriatic plaques.42,43 As a consequence, it will be informative to determine whether local application of CRAMP to our tumor models begets IL-36/IL-36R-dependent TLO development as well as therapeutic benefit.
Even though DC.Tbet preferentially produce IL-36γ (over alternate IL-36R agonists IL-36α and IL-36β), an additional remaining question is whether i.t. administration of IL-36α or IL-36β, or neutralizing antibodies against natural IL-36R antagonists (i.e., IL-1F5/IL-36RA/IL-36RN or IL-1F10/IL-38; ref. 44), which might be used to agonize IL-36R-dependent signaling in the TME, would prove therapeutic against well-established tumors. In our murine gene array analyses of DC.Tbet and DC.IL36γ (Fig. 1A and C), IL-36α transcription is upregulated, albeit to a lesser extent than is IL-36γ (8–20-fold vs. 105–316 fold, respectively), supporting the reported co-regulation of these 2 IL-36R agonists45 and suggesting that administration of IL-36α might lead to effects similar to those observed following IL-36γ-based therapy. Interestingly, a meta-analysis has recently revealed an association between tumor expression of IL-1F5 and poor clinical prognosis in cancer patients,46 and IL-1F5 (and to a lesser extent IL-38) appears to be commonly expressed at the protein level in the TME of a broad range of cancer histologies (Fig. S9). Prospective experiments using i.t. delivery of DC.IL36α, DC.IL36β, or (positive control) DC.IL36γ +/− blocking anti-IL1F5 or anti-IL-1F10 antibodies will attempt to clarify these issues in our murine tumor models. Future studies will also elucidate which tumor stromal/immune cell populations respond to IL-36R agonism in the therapeutically effective TME.
Although not critical to the therapeutic benefits observed in our tumor models,27 it is also notable that DC.Tbet and DC.IL36γ secrete high-levels of IL-12p70 (Fig. 1C and H and ref.26), which is known to be induced by IL-36R-mediated signaling.45 IL-12 has recently been reported to upregulate IL-36R expression on CD8+ T cells and to promote the development of improved Type-1 antigen-specific immunity under conditions of aerobic glycolysis46 (a characteristic of the progressor TME),44 providing further support for the reinforcing nature of Tbet- and IL-36γ-associated (immune) biologies within the purview of cancer immunotherapy. Tsurutani et al.46 also suggest that IL-2 and costimulation potently upregulate CD8+ T cell expression of IL-36R, which could foreshadow the superior antitumor activity of combined immunotherapies integrating DC.Tbet or DC.IL36γ with (i) systemic or locoregional administration of rIL-2 and/or (ii) costimulatory agonists or immune checkpoint inhibitors (i.e., to improve the ratio of co-stimulatory/co-inhibitory signals into antitumor effector cells), and/or (iii) adoptive T cell or CAR-T cell-based therapy (to promote extended Type-1 polarization and functionality of the transferred antitumor effector cells).
In conclusion, our findings support the utility of Tbet- and IL-36γ-driven, IL-36R-dependent therapies to recondition the TME by fostering the development of non-classical TLO and an “immunogically normalized” microenvironment (i.e., fewer MDSC, lower expression of immune checkpoint molecules on CD3+ TIL) in association with delayed tumor growth in both wild-type and peripheral lymph node-deficient hosts. Such approaches would be expected to promote improved cross-priming of antitumor T effector cells within the TME, as well as to condition the TME to preferentially recruit vaccine-primed or ACT T cells into tumor sites when applied in combination protocols. The further inclusion of immune checkpoint blockade or regulatory cell depletion/antagonists would also be expected to improve the fate of the TLO-sponsored antitumor T cell repertoire. Such combination regimens are currently being investigated in our pre-clinical models, with the intent to inform future clinical trial designs for the treatment of patients with solid forms of cancer.
Materials and methods
Study design
The objectives of these studies were to test the hypothesis that early infiltration of immune cells into the TME and formation of TLO correlated with delayed tumor progression as a consequence of DC.Tbet-based therapy, and to identify downstream mediators of these effects. Once IL-36γ was identified as a potential candidate (Fig. 1A), we sought to characterize its role in mediating antitumor immunity and immune cell recruitment to the TME. The sample sizes and endpoints were determined based on previously published work,13,27 and Institutional Animal Care and Use Committee (IACUC) guidelines. In all in vivo experiments, cohorts of mice were randomized 7 d following tumor inoculation. Experiments were replicated as described in individual figure legends. All experiments were performed in an unblinded fashion.
Mice
Female 6–8 week old wild-type C57BL/6 (H-2b) mice, as well as, LTA−/− and Tbet/TBX21−/− mice (all on the B6 background) were purchased from the Jackson Laboratory (Bar Harbor, ME). IL-36R−/− and Tbet-ZsGreen (Tbet-ZsG) reporter mice47 were kindly provided under MTAs from AMGEN and Dr. Jinfang Zhu (NIH/NIAID), respectively, from stocks maintained at Taconic. All animals were handled under aseptic conditions per an IACUC-approved protocol and in accordance with recommendations for the proper care and use of laboratory animals.
Tumor cell lines and culture
The MCA205 sarcoma and MC38 colon carcinoma (H-2b) cell lines were purchased from the American Type Culture Collection (ATCC). These cell lines were free of Mycoplasma contamination and were maintained in complete medium (CM: RPMI-1640 media supplemented with 10% heat-inactivated fetal bovine serum, 100 μg/mL streptomycin, 100 U/mL penicillin, and 10 mmol/L L-glutamine, all reagents purchased from Invitrogen, at 5% CO2 tension in a 37°C humidified incubator.
Recombinant adenoviruses (rAd)
E1/E3-substituted, replication-defective (Ad5-derived) recombinant adenoviruses encoding EGFP (rAd.EGFP), murine Tbet (rAd.mTbet), as well as the empty control Ad.ψ5 vector, have been described previously.48 To generate the rAd.mIL36γ vector, the nucleotide sequence encoding a fusion protein composed of the hCD8α signal peptide fused to the N-terminus of the bioactive mIL-36γ (G13–S164) protein was isolated by PCR amplification from the pcDEF3.hCD8/mIL36γ plasmid49 using specific primers (forward: 5′-AAAGTCGACGCCATGGCCTTACCAGTGAC-3′ and reverse: 5′-AAAGGATCCTTAAGACTTTATATCTAA-3′), and then ligated into the SalI-BamHI cloning site in the pAdLox shuttle vector,48 yielding pAdlox.mIL36γ. After sequence validation of the plasmid, rAd.mIL36γ was generated by co-transfection of pAdLox.mIL36γ and ψ5 helper virus DNA into the adenoviral packaging cell line CRE8.48 rAd.mIL36γ was purified from specific CRE8 lysates by cesium chloride density-gradient centrifugation and subsequent dialysis before storage in 3% threalose at −80°C. Titers of viral particles were determined by optical densitometry. As needed, rAd vectors were further expanded, qualified, and supplied by the University of Pittsburgh Cancer Institute's Vector Core Facility (a Shared Resource).
Generation of BM-derived DC and transduction with adenoviral vectors in vitro
DC were generated from the tibias/femurs of mice, and infected with recombinant adenovirus as described previously48 for 48 h to produce control DC (i.e., DC.ψ5 or DC.EGFP), DC.Tbet, or DC.IL36γ. In cases where DC.EGFP was used as the control, DC.Tbet and DC.IL36γ were cotransduced with Ad.EGFP to produce DC.Tbet/EGFP and DC.IL36γ/EGFP, respectively. Western blotting and qPCR were used to document expression of mTbet in transduced DC as previously reported,48 while the presence of IL-36γ in transfected DC was detected by Western blotting using a polyclonal rabbit anti-mIL36γ (IL-1F9) antibody49 or by qPCR using primers described in Table S1. For ELISA analyses, cell-free supernatant was harvested 48 h following infection. For gene array analyses only, DC.Tbet, DC.IL36γ, or uninfected DC (i.e., DC.null) were activated in vitro for 24 h in media containing 10 μg/mL LPS (Sigma-Aldrich, part number L4516) and 10 ng/mL rmIFNγ (Peprotech, part number 315–05), or left untreated, before mRNA isolation.
In vitro stimulation of DC
In selected experiments as indicated, DC were generated from the tibias/femurs of mice as described previously48 and were then stimulated in vitro using TLR2 agonist HKLM (108 cells/mL), TLR3 agonist polyI:C (1 µg/mL), TLR7 agonist CLO97 (1 µg/mL) (all from Invitrogen, part number tlrl-kit1hw), TLR4 agonist LPS (10 µg/mL) with or without rmIFNγ (10 ng/mL), TLR9 agonist ODN1585 (5 nmol; Invitrogen, part number tlrl1585), or anti-CD40 antibody FGK45 (1 µg/mL), in complete media containing 1,000 U/mL granulocyte/macrophage colony-stimulating factor (GMCSF) and 1,000 U/mL recombinant murine IL-4 (Peprotech). After 24 h of stimulation, cell-free supernatant was harvested for subsequent analysis by ELISA.
ELISA
Murine IL-36γ ELISA kit (Aviva Systems Biology Corporation, part number OKEH03002) was used per the manufacturer's instructions.
DC-based therapy
Recipient wild-type, mutant, or transgenic (H-2b) mice received s.c. injections of 5 × 105 MCA205 sarcoma cells or 1 × 105 MC38 colon carcinoma cells in the right flank on day 0. On day 7 post-tumor inoculation, mice were randomized into treatment cohorts of five mice, with each cohort exhibiting comparable mean tumor sizes (i.e., approximately 40–50 mm2). One million DC (i.e., control DC.ψ5, control DC.EGFP, DC.Tbet, or DC.IL36γ) developed from wild-type C57BL/6 or syngenic mutant (IL-36R−/− or Tbet−/−) or transgenic Tbet-ZsG mice were then injected i.t. in a total volume of 100 μL (in PBS) on day 7 post-tumor inoculation, and then again 1 week later. In some experiments, where noted, IL-36γ function was blocked by i.t. injection of rmIL-1F5 (an IL-36R antagonist also known as IL-36RA or IL-36RN; purchased from Life Technologies, part number 50213-MNAE) at the time of therapeutic DC delivery (and then daily x 2). In other experiments, where noted, LTβR signaling was blocked by i.t. injection of 100 µg LTβR-Ig or control antibody (Sigma-Aldrich, part number AG714) 3 h before each delivery of DC. Mean tumor size (± SEM) was monitored every 3–4 d and recorded in mm2 by determining the product of the largest orthogonal diameters measured using vernier calipers. Mice were killed when tumors became ulcerated or if they exceeded a size of 400 mm2, in accordance with IACUC guidelines.
In vivo depletion of CD4+ or CD8+ T cells
In selected experiments, as indicated, mice were injected i.p. with 50–100 μg rat isotype control Ab (Sigma-Aldrich), 50 μg anti-CD4+ mAb GK1.5 (eBioscience, part number 16–0041) or 100 μg anti-CD8+ mAb53–6.7 (Biolegend, part number 100716) on days 6, 13, and 20 after tumor inoculation. Specific cell depletion was > 95% effective in vivo based on flow cytometry analysis of peripheral blood mononuclear cells obtained by tail venipuncture from treated mice 24–48 h after Ab administration (data not shown).
Imaging of tumor tissues
Tumor samples were prepared and sectioned as previously reported.47 Briefly, tumor tissues were harvested and fixed in 2% paraformaldehyde (Sigma-Aldrich) at 4°C for 2 h, and then cryoprotected in 30% sucrose for 24 h. Tumor tissues were then frozen in liquid nitrogen and 6 µm cryosections prepared. Hematoxylin and Eosin (H&E) stains were performed as described previously.50 For immunofluorescence microscopy (IFM) analysis of TLO, sections were stained as described previously using primary and secondary antibodies as indicated in Table S2. In tumors where a biotinylated primary antibody was used, the following protocol modification was made: after blocking with BSA, slides were treated with Avidin Blocking Buffer for 15 min, washed in 0.5% BSA in PBS, and treated with Biotin Blocking Buffer (both from R&D Systems, part number CTS002) for 15 min, before the addition of the primary antibody. Cell nuclei were then stained with DAPI as described previously.27 After washing, sections were then covered in Gelvatol (Monsanto) and a coverslip applied. Slide images were acquired using an Olympus 500 scanning confocal microscope or an Olympus Provis AX70 fluorescence microscope (both from Olympus America). Positively stained cells were quantified by analyzing images at a final magnification of ×20 using Metamorph Imaging software (Molecular Devices) or NIS-Elements software (Nikon Instruments, Inc.).
Flow cytometric analyses
Tumors were isolated from C57BL/6 mice 20 d following initial tumor inoculation, mechanically minced, and enzymatically digested with 0.5 mg/mL collagenase IA (Sigma-Aldrich, part number C5894), 0.5 mg/mL collagenase II (Sigma-Aldrich, part number C1764), 0.5 mg/mL collagenase IV (Sigma-Aldrich, part number C1889), and 20 U/mL DNase I (Sigma-Aldrich, part number D5025). The resulting single cell suspensions were labeled using fluorescently labeled antibodies as indicated in Table S2. Cell staining for cell surface markers was performed in PBS in the presence of anti-CD16/CD32 (i.e., Mouse BD Fc Block; BD Biosciences, part number 553142). To stain for intracellular transcription factors, FoxP3/Transcription factor staining buffer set (eBioscience, part number 00–5523–00) was used with the anti-Tbet APC (Biolegend, part number 644814).
RNA purification and PCR analyses
Total RNA was isolated from control or rAd-infected DC using Trizol reagents (Invitrogen, part number 15596018) or Buffer RLT (Quiagen, part number 1030963). Total RNA was further purified using the RNeasy Plus Micro Kit (Qiagen, part number 74034) including the gDNA Eliminator spin column. The purity and quantity of the total RNA was assessed using Nanodrop ND-1000 (CelBio SpA). For gene array analyses, gene expression analyses were performed on total RNA using the mouse Clariom D Assay (Affymetrix, part number 902513) according to the manufacturer's instructions by the University of Pittsburgh's Genomics Research Core (a Shared Resource), and data were analyzed using Transcriptome Analysis Console 3.0 (Affymetrix). Otherwise, total RNA (1 μg) was reversed transcribed into cDNA using the high-capacity RNA to cDNA Kit (Qiagen, part number 4387406) and the cDNA added to RT2 SYBR Green ROX™ qPCR Mastermix (Qiagen, part number 4385612) and used for quantitative PCR using specific primer pairs (Table S1). Reactions were performed on a StepOnePlus™ real-time PCR thermocycler (Applied Biosystems) using the recommended cycling conditions. mRNA expression levels were normalized to expression of control β-Actin or HPRT mRNA and analyzed using the 2−ΔΔCT method.
Statistical analyses
Comparisons between groups were performed using Student's t-test or one-way Analysis of Variance (ANOVA) with post-hoc analysis, as indicated. All data were analyzed using GraphPad software (La Jolla, CA). Differences with a p-value < 0.05 were considered significant.
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors wish to thank Dr. Adrian Morelli and William J. Shufesky for their thoughtful input during the design of experiments. We also wish to thank Dr. Ron Fecek for his careful review and helpful comments provided during the preparation of this manuscript.
Funding
This project used the UPCI Cell and Tissue Imaging Core and Vector Core Facilities supported in part by NIH P30 CA047904. The performance of this work was also supported by the NIH under Grants R01 CA169118 and R01 CA204419 (both to W.J.S.) and NIH T32 AI089443 (to A.M.W.).
Author contributions
A.M.W., B.L., T.L.D., P.A.C., S.C.W. and W.J.S. designed experiments. A.M.W., L.C., E.A.B., P.R.P., J.L.T., K.L.F., C.T.W., SDJ., and D.F.S. performed experiments. A.M.W., J.L.T., B.L., S.C.W., T.L.D., Y.X.F., P.A.C., and W.J.S. interpreted data. A.M.W. and W.J.S. wrote the paper. All authors read and agreed on the final version of the submitted manuscript.
References
- 1.Roychoudhuri R, Eil RL, Restifo NP. The interplay of effector and regulatory T cells in cancer. Curr Opin Immunol 2015; 33:101-11; PMID:25728990; https://doi.org/ 10.1016/j.coi.2015.02.003 [DOI] [PubMed] [Google Scholar]
- 2.Parker KH, Beury DW, Ostrand-Rosenberg S. Myeloid-derived suppressor cells: critical cells driving immune suppression in the tumor microenvironment. Adv Cancer Res 2015; 128:95-139; PMID:26216631; https://doi.org/ 10.1016/bs.acr.2015.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whiteside TL. Clinical impact of regulatory T cells (Treg) in cancer and HIV. Cancer Microenviron 2015;8:201-7; PMID:25385463; https://doi.org/ 10.1007/s12307-014-0159-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teng MW, Ngiow SF, Ribas A, Smyth MJ. Classifying cancers based on T-cell infiltration and PD-L1. Cancer Res 2015; 75:2139-45; PMID:25977340; https://doi.org/ 10.1158/0008-5472.CAN-15-0255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruno TC, French JD, Jordan KR, Ramirez O, Sippel TR, Borges VF, Haugen BR, McCarter MD, Waziri A, Slansky JE. Influence of human immune cells on cancer: studies at the University of Colorado. Immunol Res 2013; 55:22-33; PMID:22941561; https://doi.org/ 10.1007/s12026-012-8346-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nosho K, Baba Y, Tanaka N, Shima K, Hayashi M, Meyerhardt JA, Giovannucci E, Dranoff G, Fuchs CS, Ogino S. Tumour-infiltrating T-cell subsets, molecular changes in colorectal cancer, and prognosis: cohort study and literature review. J Pathol 2010; 222:350-66; PMID:20927778; https://doi.org/ 10.1002/path.2774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Josien R, Li HL, Ingulli E, Sarma S, Wong BR, Vologodskaia M, Steinman RM, Choi Y. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J Exp Med 2000; 191:495-502; PMID:10662795; https://doi.org/ 10.1084/jem.191.3.495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Angeli V, Randolph GJ. Inflammation, lymphatic function, and dendritic cell migration. Lymphat Res Biol 2006; 4:217-28; PMID:17394405; https://doi.org/ 10.1089/lrb.2006.4406 [DOI] [PubMed] [Google Scholar]
- 9.Knight SC. Dendritic cells in contact sensitivity. Res Immunol 1989; 140:907-10; PMID:2697914; https://doi.org/ 10.1016/0923-2494(89)90053-9 [DOI] [PubMed] [Google Scholar]
- 10.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res 2011; 71:6391-9; PMID:21900403; https://doi.org/ 10.1158/0008-5472.CAN-11-0952 [DOI] [PubMed] [Google Scholar]
- 11.Goc J, Germain C, Vo-Bourgais TK, Lupo A, Klein C, Knockaert S, de Chaisemartin L, Ouakrim H, Becht E, Alifano M et al.. Dendritic cells in tumor-associated tertiary lymphoid structures signal a Th1 cytotoxic immune contexture and license the positive prognostic value of infiltrating CD8+ T cells. Cancer Res 2014; 74:705-15; PMID:24366885; https://doi.org/ 10.1158/0008-5472.CAN-13-1342 [DOI] [PubMed] [Google Scholar]
- 12.Coppola D, Nebozhyn M, Khalil F, Dai H, Yeatman T, Loboda A, Mulé JJ. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol 2011; 179:37-45; PMID:21703392; https://doi.org/ 10.1016/j.ajpath.2011.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen L, Fabian KL, Taylor JL, Storkus WJ. Therapeutic use of dendritic cells to promote the extranodal priming of anti-tumor immunity. Front Immunol 2013: 4:388; PMID:24348473; https://doi.org/ 10.3389/fimmu.2013.00388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pagès F, Galon J, Dieu-Nosjean MC, Tartour E, Sautès-Fridman C, Fridman WH. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 2010; 29:1093-102; PMID:19946335; https://doi.org/ 10.1038/onc.2009.416 [DOI] [PubMed] [Google Scholar]
- 15.Dieu-Nosjean MC, Antoine M, Danel C, Heudes D, Wislez M, Poulot V, Rabbe N, Laurans L, Tartour E, de Chaisemartin L et al.. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol 2008; 26:4410-7; PMID:18802153; https://doi.org/ 10.1200/JCO.2007.15.0284 [DOI] [PubMed] [Google Scholar]
- 16.Fridman WH, Dieu-Nosjean MC, Pagès F, Cremer I, Damotte D, Sautès-Fridman C, Galon J. The immune microenvironment of human tumors: general significance and clinical impact. Cancer Microenviron 2013; 6:117-22; PMID:23108700; https://doi.org/ 10.1007/s12307-012-0124-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tseng WW, Malu S, Zhang M, Chen J, Sim GC, Wei W, Ingram D, Somaiah N, Lev DC, Pollock RE, Lizée G et al.. Analysis of the intratumoral adaptive immune response in well differentiated and dedifferentiated retroperitoneal liposarcoma. Sarcoma 2015; 2015:547460; PMID:25705114; https://doi.org/ 10.1155/2015/547460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ruddle NH. Lymphatic vessels and tertiary lymphoid organs. J Clin Invest 2014; 124:953-9; PMID:24590281; https://doi.org/ 10.1172/JCI71611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Astorri E, Bombardieri M, Gabba S, Peakman M, Pozzilli P, Pitzalis C. Evolution of ectopic lymphoid neogenesis and in situ autoantibody production in autoimmune nonobese diabetic mice: cellular and molecular characterization of tertiary lymphoid structures in pancreatic islets. J Immunol 2010; 185:3359-68; PMID:20713891; https://doi.org/ 10.4049/jimmunol.1001836 [DOI] [PubMed] [Google Scholar]
- 20.Dieu-Nosjean MC, Goc J, Giraldo NA, Sautès-Fridman C, Fridman WH. Tertiary lymphoid structures in cancer and beyond. Trends Immunol 2014; 35:571-80; PMID:25443495; https://doi.org/ 10.1016/j.it.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 21.Noort AR, van Zoest KP, van Baarsen LG, Maracle CX, Helder B, Papazian N, Romera-Hernandez M, Tak PP, Cupedo T, Tas SW. Tertiary lymphoid structures in rheumatoid arthritis: NF-κB-inducing kinase-positive endothelial cells as central players. Am J Pathol 2015; 185:1935-43; PMID:25963989; https://doi.org/ 10.1016/j.ajpath.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 22.Drayton DL, Ying X, Lee J, Lesslauer W, Ruddle NH. Ectopic LTα/β directs lymphoid organ neogenesis with concomitant expression of peripheral node addressin and a HEV-restricted sulfotransferase. J Exp Med 2003; 197:1153-63; PMID:12732657; https://doi.org/ 10.1084/jem.20021761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Warnock RA, Askari S, Butcher EC, von Andrian UH. Molecular mechanisms of lymphocyte homing to peripheral lymph nodes. J Exp Med 1998; 187:205-16; PMID:9432978; https://doi.org/ 10.1084/jem.187.2.205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, Garrido I, Girard JP. High endothelial venules (HEVs) in human melanoma lesions: major gateways for tumor-infiltrating lymphocytes. Oncoimmunology 2012; 1:829-839; PMID:23162750; https://doi.org/ 10.4161/onci.20492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cipponi A, Mercier M, Seremet T, Baurain JF, Théate I, van den Oord J, Stas M, Boon T, Coulie PG, van Baren N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res 2012; 72:3997-4007; PMID:22850419; https://doi.org/ 10.1158/0008-5472.CAN-12-1377 [DOI] [PubMed] [Google Scholar]
- 26.Lipscomb MW, Chen L, Taylor JL, Goldbach C, Watkins SC, Kalinski P, Butterfield LH, Wesa AK, Storkus WJ. Ectopic T-bet expression licenses dendritic cells for IL-12-independent priming of Type-1 T cells in vitro. J Immunol 2009; 183:7250-8; PMID:19915058; https://doi.org/ 10.4049/jimmunol.0901477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chen L, Taylor JL, Sabins NC, Lowe DB, Qu Y, You Z, Storkus WJ. Extranodal induction of therapeutic immunity in the tumor microenvironment after intratumoral delivery of Tbet gene-modified dendritic cells. Cancer Gene Ther 2013; 20:469-77; PMID:23846252; https://doi.org/ 10.1038/cgt.2013.42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bachmann M, Scheiermann P, Härdle L, Pfeilschifter J, Mühl H. IL-36γ/IL-1F9, an innate T-bet target in myeloid cells. J Biol Chem 2012; 287:41684-96; PMID:23095752; https://doi.org/ 10.1074/jbc.M112.385443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cañete JD, Santiago B, Cantaert T, Sanmartí R, Palacin A, Celis R, Graell E, Gil-Torregrosa B, Baeten D, Pablos JL. Ectopic lymphoid neogenesis in psoriatic arthritis. Ann Rheum Dis 2007; 66:720-6; PMID:17223654; https://doi.org/ 10.1136/ard.2006.062042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carrier Y, Ma HL, Ramon HE, Napierata L, Small C, O'Toole M, Young DA, Fouser LA, Nickerson-Nutter C, Collins M et al.. Inter-regulation of Th17 cytokines and the IL-36 cytokines in vitro and in vivo: implications in psoriasis pathogenesis. J Invest Dermatol 2011; 131:2428-37; PMID:21881584; https://doi.org/ 10.1038/jid.2011.234 [DOI] [PubMed] [Google Scholar]
- 31.Michie SA, Streeter PR, Bolt PA, Butcher EC, Picker LJ. The human peripheral lymph node vascular addressin. An inducible endothelial antigen involved in lymphocyte homing. Am J Pathol 1993; 143:1688-98; PMID:8256856 [PMC free article] [PubMed] [Google Scholar]
- 32.Banks TA, Rouse BT, Kerley MK, Blair PJ, Godfrey VL, Kuklin NA, Bouley DM, Thomas J, Kanangat S, Mucenski ML. Lymphotoxin-α-deficient mice. Effects on secondary lymphoid organ development and humoral immune responsiveness. J Immunol 1995; 155:1685-93; PMID:7636227 [PubMed] [Google Scholar]
- 33.Towne JE, Renshaw BR, Douangpanya J, Lipsky BP, Shen M, Gabel CA, Sims JE. Interleukin-36 (IL-36) ligands require processing for full agonist (IL-36α, IL-36β, and IL-36γ) or antagonist (IL-36RA) activity. J Biol Chem 2011; 286:42594-602; PMID:21965679; https://doi.org/ 10.1074/jbc.M111.267922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blumberg H, Dinh H, Trueblood ES, Pretorius J, Kugler D, Weng N, Kanaly ST, Towne JE, Willis CR, Kuechle MK et al.. Opposing activities of two novel members of the IL-1 ligand family regulate skin inflammation. J Exp Med 2007; 204:2603-14; PMID:17908936; https://doi.org/ 10.1084/jem.20070157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tsuboi K, Hirakawa J, Seki E, Imai Y, Yamaguchi Y, Fukuda M, Kawashima H. Role of high endothelial venule-expressed heparin sulfate in chemokine presentation and lymphocyte homing. J Immunol 2013; 191:448-55; PMID:23733868; https://doi.org/ 10.4049/jimmunol.1203061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Peske JD, Thompson ED, Gemta L, Baylis RA, Fu YX, Engelhard VH. Effector lymphocyte-induced lymph node-like vasculature enables naive T-cell entry into tumours and enhanced anti-tumour immunity. Nat Commun 2015; 6:7114; PMID:25968334; https://doi.org/ 10.1038/ncomms8114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wirsing AM, Rikardsen OG, Steigen SE, Uhlin-Hansen L, Hadler-Olsen E. Characterization and prognostic value of tertiary lymphoid structures in oral squamous cell carcinoma. BMC Clin Pathol 2014; 14:38; PMID:25177210; https://doi.org/ 10.1186/1472-6890-14-38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Joshi NS, Akama-Garren EH, Lu Y, Lee DY, Chang GP, Li A, DuPage M, Tammela T, Kerper NR, Farago AF et al.. Regulatory T cells in tumor-associated tertiary lymphoid structures suppress anti-tumor T cell responses. Immunity 2015; 43:579-90; PMID:26341400; https://doi.org/ 10.1016/j.immuni.2015.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity 2014; 41:529-42; PMID:25367570; https://doi.org/ 10.1016/j.immuni.2014.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cook KD, Kline HC, Whitmire JK. NK cells inhibit humoral immunity by reducing the abundance of CD4+ T follicular helper cells during a chronic virus infection. J Leukoc Biol 2015; 98:153-62; PMID:25986014; https://doi.org/ 10.1189/jlb.4HI1214-594R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li N, Yamasaki K, Saito R, Fukushi-Takahashi S, Shimada-Omori R, Asano M, Aiba S. Alarmin function of cathelicidin antimicrobial peptide LL37 through IL-36γ Induction in human epidermal keratinocytes. J Immunol 2014; 193:5140-8; PMID:25305315; https://doi.org/ 10.4049/jimmunol.1302574 [DOI] [PubMed] [Google Scholar]
- 42.Lande R, Chamilos G, Ganguly D, Demaria O, Frasca L, Durr S, Conrad C, Schröder J, Gilliet M. Cationic antimicrobial peptides in psoriatic skin cooperate to break innate tolerance to self-DNA. Eur J Immunol 2015; 45:203-13; PMID:25332209; https://doi.org/ 10.1002/eji.201344277 [DOI] [PubMed] [Google Scholar]
- 43.Towne JE, Sims JE. IL-36 in psoriasis. Curr Opin Pharmacol 2012; 12:486-90; PMID:22398321; https://doi.org/ 10.1016/j.coph.2012.02.009 [DOI] [PubMed] [Google Scholar]
- 44.van de Veerdonk FL, Stoeckman AK, Wu G, Boeckermann AN, Azam T, Netea MG, Joosten LA, van der Meer JW, Hao R, Kalabokis V et al.. IL-38 binds to the IL-36 receptor and has biological effects on immune cells similar to IL-36 receptor antagonist. Proc Natl Acad Sci USA 2012; 109:3001-5; PMID:22315422; https://doi.org/ 10.1073/pnas.1121534109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vigne S, Palmer G, Lamacchia C, Martin P, Talabot-Ayer D, Rodriguez E, Ronchi F, Sallusto F, Dinh H, Sims JE et al.. IL-36R ligands are potent regulators of dendritic and T cells. Blood 2011; 118:5813-23; PMID:21860022; https://doi.org/ 10.1182/blood-2011-05-356873 [DOI] [PubMed] [Google Scholar]
- 46.Tsurutani N, Mittal P, St Rose MC, Ngoi SM, Svedova J, Menoret A, Treadway FB, Laubenbacher R, Suárez-Ramírez JE, Cauley LS et al.. Costimulation endows immunotherapeutic CD8 T cells with IL-36 responsiveness during aerobic glycolysis. J Immunol 2016; 196:124-34; PMID:26573834; https://doi.org/ 10.4049/jimmunol.1501217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu J, Jankovic D, Oler AJ, Wei G, Sharma S, Hu G, Guo L, Yagi R, Yamane H, Punkosdy G et al.. The transcription factor T-bet is induced by multiple pathways and prevents an endogenous Th2 cell program during Th1 cell responses. Immunity 2012; 37:660-73; PMID:23041064; https://doi.org/ 10.1016/j.immuni.2012.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Qu Y, Chen L, Lowe DB, Storkus WJ, Taylor JL. Combined Tbet and IL12 gene therapy elicits and recruits superior antitumor immunity in vivo. Mol Ther 2012; 20:644-51; PMID:22215017; https://doi.org/ 10.1038/mt.2011.283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang X, Zhao X, Feng C, Weinstein A, Xia R, Wen W, Lv Q, Zuo S, Tang P, Yang X et al.. IL-36γ Transforms the tumor microenvironment and promotes Type-1 lymphocyte-mediated antitumor immune responses. Cancer Cell 2015; 28:296-306; PMID:26321222; https://doi.org/ 10.1016/j.ccell.2015.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardiff RD, Miller CH, Munn RJ. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb Protoc 2014; 2014:655-8; PMID:24890205; https://doi.org/ 10.1101/pdb.prot073411 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.