ABSTRACT
NHS-IL12 is an immunocytokine, a fusion protein of IL12's functional domains and a necrosis-targeting antibody, which has shown significant effects against human rhabdomyosarcoma xenografts in a humanized tumor model, including terminal growth arrest and differentiation of the tumor cells. Here, we locally irradiated the tumors, increasing necrosis and consequently intratumoral immune cytokine availability, and asked whether this effect may surmount efficacy of single treatment modality.
Humanized mice bearing bilateral rhabdomyosarcoma xenografts were evaluated for tumor burden and survival after irradiation, systemic NHS-IL12 therapy or a combination of both. Intratumoral immune compartments were characterized by immunohistochemistry and molecular methods. TH1-cytokine dependency of underlying effector mechanisms were investigated in vitro in several human tumor cell lines.
NHS-IL12 when combined with irradiation terminally arrested tumor growth and significantly improved survival. Combination treatment induced dense intratumoral T-cell infiltrates, clonal epitope-specific T-cell expansions, expression of cytotoxins, decreased pro-tumorigenic cytokines and induced senescence and differentiation in the cancer cells. Senescence and differentiation were reproduced in vitro and confirmed to be dependent on TH1 cytokines IFNγ and TNF-α.
NHS-IL12 and irradiation together induced broad intratumoral TH1 biased NK and T-cell compartments, established antitumoral cytokine profiles and irreversibly growth arrested tumor cells, leading to systemic cancer control and improved survival. For the first time, we describe immune-induced senescence as a novel mechanism resulting from a treatment regimen combining irradiation with immunotherapy.
KEYWORDS: Differentiation, glioma, IL12, immunocytokine, radiotherapy, rhabdomyosarcoma, senescence
Introduction
Immunotherapy for cancer is on the rise with astounding results. In malignant melanoma and other entities, immune checkpoint inhibition leads to long-term remission in subgroups of metastasized patients.1-3 Also, in other tumor entities like prostate cancer, non-small-cell lung cancer, brain tumors and gynecological malignancies, immunotherapy is an emerging treatment option (reviewed in Refs. [4-7]). For childhood sarcomas, the tested immunotherapy regimens, such as cytokines, antibodies, checkpoint blockade or T-cell-based therapies, have not shown striking efficacy, yet the available data are limited (reviewed in Ref. [8]).
Whereas single modality immunotherapy is effective in only few tumor types, such as melanoma,9 non-small-cell lung cancer10 or renal cell carcinoma,11 its combination with radiotherapy or immunogeneic cell death inducing chemotherapies shows promising results and thus strongly suggests combined therapies for treatment in tumor types in which immunotherapy alone showed limited success.12 The rationale for the synergy is bidirectional with immunotherapy enabling abscopal effects of radiotherapy outside the radiation field and with radiotherapy altering the tumor microenvironment in a pro-immunogeneic way as well as a release of tumor antigens and danger signals.13-15 Dose and fractionation of irradiation in combination with immunotherapy are still a major area of debate.16 We chose 8.0 Gy single dose as this regimen had shown significant necrosis induction and accumulation of immunotherapeutics in our previous study.17
Interleukin 2 (IL-2) and interleukin 12 (IL-12) are cytokines that polarize the immune system toward TH1 antitumor response18 while simultaneously blocking the pro-tumorigenic TH2 response.19 Yet, their systemic administration in humans was associated with severe toxicity.20-22 To circumvent the limitations of systemic cytokine treatment, tumor-targeted immunocytokines were developed consisting of functional domains of the cytokine fused to an antibody directing it to the tumor. Due to their accumulation in the tumor, these constructs require lower systemic concentrations and show reduced toxicity.23 In consequence, promising results were obtained in preclinical and clinical therapeutic approaches with anti-GD2-IL2,24,25 F8-IL226,27 and L19-IL2.28,29 The latter has already been studied in combination with irradiation showing significant efficacy even in MHC class I deficient cancers.30,31
The major TH1 polarizing cytokine IL-12 acts species specific. Therefore, NHS-IL12 was tested in an in vivo model consisting of CD34+ stem cell humanized mice bearing aggressive human rhabdomyosarcoma. As shown before, NHS-IL12 alone or combined with engineered IL2 induced massive intratumoral immune compartments, including M1 macrophages, NK, γδ T cells and clonally expanded αβ T cells, that were associated with tumor eradication or halted tumor growth and significantly prolonged survival. The underlying immunologic mechanisms were found to be TH1 cytokine induced terminal growth arrest (senescence) and differentiation of growth arrested tumor cells into cross-striated muscle fibers.32 Radiolabelled NHS-IL12 strongly accumulated in irradiated rhabdomyosarcoma xenografts in comparison to unirradiated control tumors, which was associated with necrosis induction in the tumors. In contrast, the control tumor model, which did not exhibit necrotic areas, did not show enhanced NHS-IL12 binding after irradiation.17
In this study, we sought to evaluate whether local irradiation may enhance the efficacy of NHS-IL12 in the model of fully humanized NSG mice bearing aggressive human rhabdomyosarcoma xenografts. To this end, we examined the emanated impact from local irradiation, systemic NHS-IL12 and a combination of both on the infiltration of the tumor by immune effector cells, tumor size, abscopal effect and survival. Induced immune responses were comprehensively characterized on molecular and histologic level to elucidate the underlying mechanism of action. Different cell lines in vitro were used to establish the cellular mechanisms of the combination of TH1 cytokines and irradiation.
Results
Humanization and treatment
The treatment consisted of either NHS-IL12 weekly (I) or a single radiation dose of 8.0 Gy at the tumor site (II) or a combination of both (III) (Fig. 1A). The treatment schedule led to five groups of tumors with different treatments: Unirradiated control tumors (I1), irradiation only (I2), NHS-IL12 (II3), unirradiated tumors in animals receiving the combination treatment with irradiation of the contralateral tumor (III4) and irradiated tumors in animals receiving the combination treatment (III5). All treatment regimens were well tolerated.
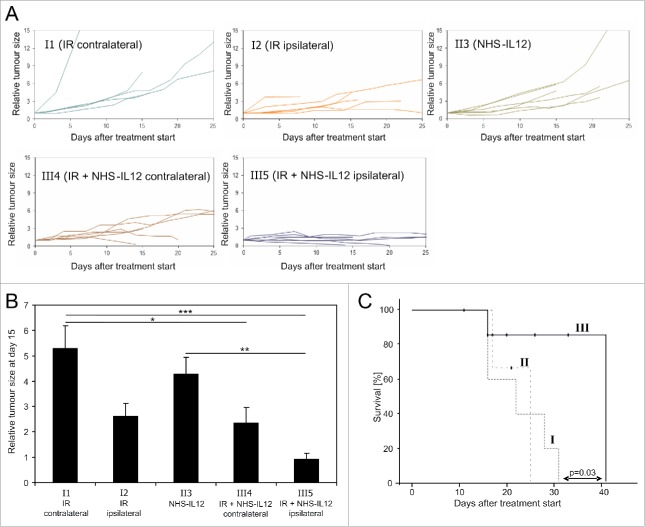
Figure 1.
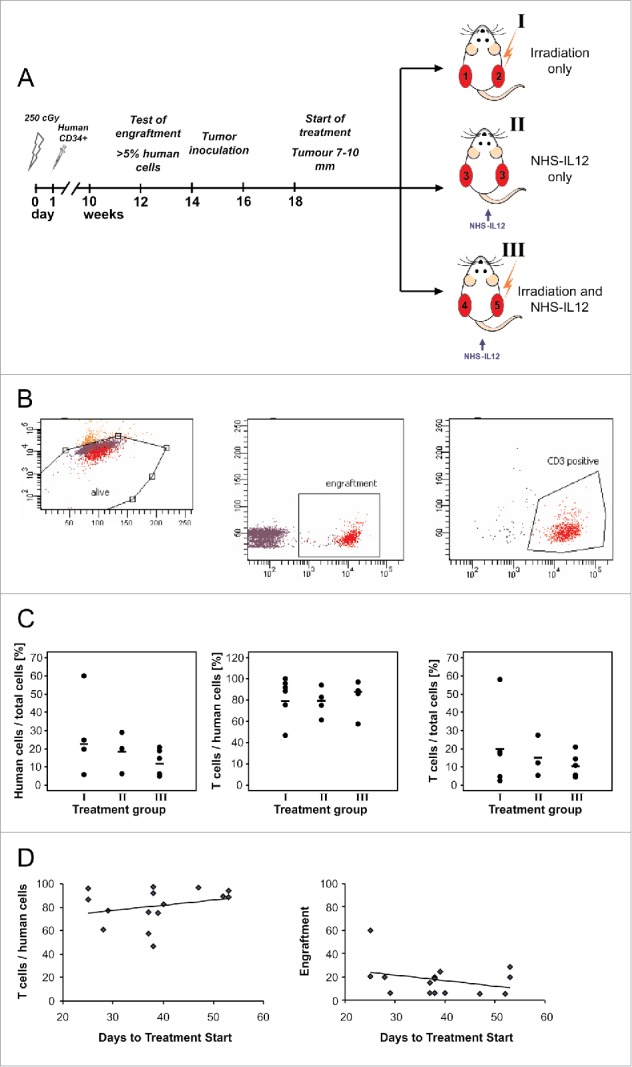
Preparation of the animals with human CD34+ stem cell transplantation and engraftment and animal and tumor treatment groups are shown in (A). Engraftment was tested by FACS analysis of peripheral blood mononuclear cells (B) and did not differ between treatment groups (C). Engraftment did not influence tumor growth before start of treatment (D).
To ensure sufficient human engraftment peripheral blood of the animals was tested 12–16 weeks after receiving CD34+ stem cell grafts. Mice with at least 5% human cells within peripheral lymphocyte pool were included in the experiment. As engraftment differed between 5.3% and 60.0% human lymphoid cells per peripheral blood lymphocytes in humanized mice the mean value of engraftment of human leukocytes (CD45+) and T cells (CD3+) in the different treatment groups was analyzed (example of flow cytometry in Fig. 1B). Cohorts were balanced for the engraftment (Fig. 1C). The level of engraftment did not significantly influence tumor growth before treatment as indicated by the time span between tumor inoculation and tumors reaching requested size not being correlated with the percentage of human cells (Fig. 1D). An effect of different levels of engraftment on tumor growth during treatment was excluded as tumor size after 15 d of treatment did not correlate with engraftment (data not shown).
Tumor growth and survival
Tumor growth differed significantly in the treatment cohorts. In unirradiated controls (I1) and NHS-IL12 only treated cohort (II3) all tumors continued to grow. Also, 5 of 6 irradiated tumors (I2) were continuously growing, whereas one of the tumors regressed. Of the irradiated tumors (III5) in the combination treatment group, 2 of 6 tumors regressed, the other 4 tumors had stable size for at least 7 d before the animals were sacrificed. The respective contralateral tumors (III4) were heterogenous in growth. The two animals with regressing tumors on the irradiated side also had regressing contralateral tumors. That regression started with a time delay compared with the irradiated tumors. One additional contralateral tumor was stabilized in size. Three contralateral tumors showed continued growth, yet with slower kinetics than in the control group (Fig. 2A). The comparison of relative tumor size at day 15 after start of treatment showed no significant effects for irradiation and NHS-IL12 alone in comparison to unirradiated controls. However, NHS-IL12 and irradiation combination treatment highly significantly inhibited tumor growth in irradiated tumors and significantly reduced tumor size in contralateral tumors (abscopal effect). A significant difference was also observed between NHS-IL12 only treated tumors and the irradiated tumors of the combination group (Fig. 2B). The tumor-size-specific survival showed a significant difference between irradiation only (I) and the combination therapy (III). The survival of NHS-IL12 only treated animals (II) was similar to the irradiation only group (Fig. 2C).
Figure 2.
Size of individual tumors in the five treatment groups are depicted in (A). While control tumors showed rapid tumor growth and NHS-IL12 only slowed tumor growth, irradiation controlled one of six tumors. The combination group showed tumor control for all irradiated tumors and deceleration or halt in tumor growth of the contralateral tumors (A). Tumor size at day 15 of treatment differed significantly between controls and combination therapy, as well as NHS-IL12 treated tumors and the irradiated tumors in the combination group (B). Tumor-size-specific survival was significantly longer with combination treatment than with irradiation alone. Single modality NHS-IL12 and single modality irradiation treated animals had similar survival (C).
Histology and immunohistochemistry
Degree of lymphocytic tumor infiltration and the phenotype of immune cells were analyzed by immunohistochemistry. CD64-expressing cells dominated in all irradiated tumors (data not shown). T-cell infiltration (CD3) was marginal in both single treatment groups except for one irradiated tumor. Yet, the number of infiltrating T cells was massively enhanced in all tumors by combination therapy both at contralateral and ipsilateral sites. There, CD4+ as well as CD8+ T cells were present. Granzyme B and perforin expression indicated cytolytic killer phenotype. Combination treatment lead to expression of desmin, a marker indicating myogenic differentiation and the formation of multinucleated myogenic syncytia and cross-striated muscle tissue indicating differentiation toward the cross-striated muscle origin of rhabdomyosarcoma (Figs. 3A and B).
Figure 3.
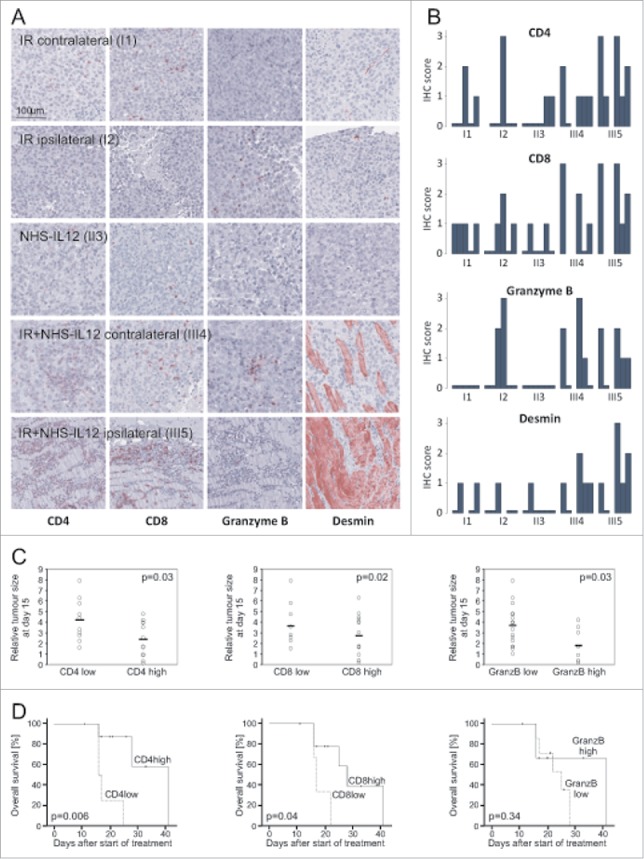
Immunohistochemistry showed marked differences in T-cell infiltration (CD4+, CD8+) and activation (Granzyme B+) leading to differentiation to Desmin+ cross striated muscle cells as shown exemplarily in (A). T cell infiltration and activation were pronounced in the tumors treated with a combination of NHS-IL12 and irradiation. Whereas hardly any infiltration was observed in the contralateral tumors of the irradiation only group, the animals of the NHS-IL12 and irradiation group with extensive infiltrates in the ipsilateral tumors showed also infiltrates contralaterally. Desmin+, differentiated areas were predominantly present in tumors treated with the combination regime (B). Tumor size at day 15 differed significantly for tumors with T-cell infiltrates (CD4high and CD8high) as well as for Granzyme Bhigh tumors (C). For survival analysis, animals were scored with the higher IHC scoring of both tumors and stratified as “high” or “low.” T-cell infiltration (CD4high, CD8high) correlated with tumor-size-specific survival, whereas T cell activation (Granzyme Bhigh) showed no significant correlation with survival (D).
Fifteen days after start of treatment degree of T cell infiltration – demonstrated by intratumoral CD3+, CD4+ and CD8+ cells – and the expression of T cell markers characterizing cytolytic effector function (Granzyme B+) was significantly associated with smaller tumor volume. Interestingly, myogenic differentiation (Desmin+) did not correlate with tumor size (Fig. 3C). For survival analysis, animals were grouped according to the higher IHC scoring of the two tumors. Pronounced CD4+ T cell infiltration was significantly associated with better survival. CD3+ and CD8+ correlated with survival with a trend to statistical significance (Fig. 3D).
Cytokine profile
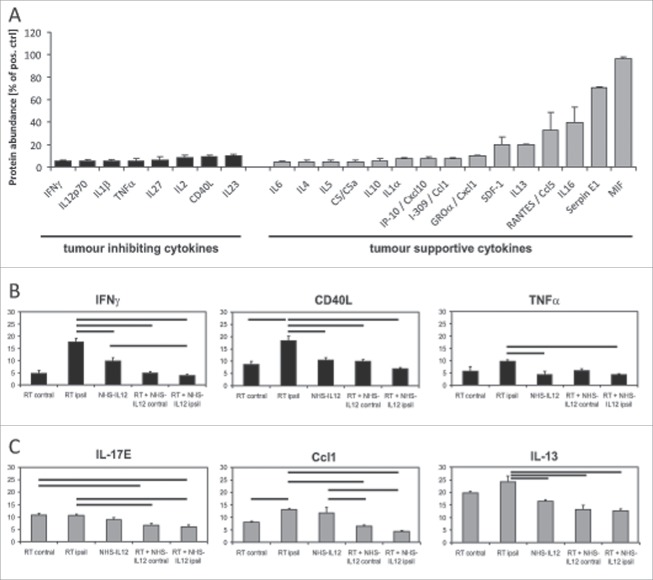
Tumor supportive cytokines, in particular IL13, IL16, macrophage migration inhibitory factor (MIF), plasminogen-activator-inhibitor (PAI, serpin E1) and RANTES (regulated upon activation normal T-cell expressed and presumably secreted), dominated in the control group (Fig. 4A).While irradiation as a single modality therapy lead to an increase of antitumoral cytokines, such as CD40 ligand, IFNγ and TNFα (I2, Fig. 4B), the major change induced by combination treatment was the reduced abundance of pro-tumorigenic cytokines, exemplarily shown for IL17-E, CCL-1 and IL13 (Fig. 4C).
Figure 4.
Cytokine arrays were performed on representative tumors of the different treatment groups and stratified according to the pro- and antitumor properties of the cytokines described in the literature. Untreated tumors showed a strong protumorigenic cytokine profile (A). Results are displayed normalized to the intensity of the positive controls included in the array. Changes in tumor inhibiting cytokines (shown exemplarily for IFNγ, CD40L and TNFα) show an increase after irradiation (I2), but no significant changes after NHS-IL12 (II3) or combination treatment compared with control (III4, III5, B). In contrast, tumor supportive cytokines were significantly reduced after combination treatment (C). Bars indicate significant differences between treatment groups.
T and NK cell compartment
Tumor-infiltrating NK cells, αβ and γδ T-lymphocytes were examined for inherent marker molecules. NK-activating and -inhibitory receptors indicated activated NK cells and lytic potential of immune cells in all tumors as KIR and NKG2 receptors with intracytoplasmic tails that confer activation rather than inhibitory signals and DNAM-1, the receptor for PVR (CD155) and Nectin-2 (CD112), was present in every sample tested. Except for Nkp44, all tested innate receptors were expressed in all samples. In all cohorts, the expression of CD161 indicating IL-17-producing T cells of memory phenotype was detected, a phenotype that is polarized into TH1 cells when exposed to IL-12 (Fig. 5A). In contrast, the T cell compartment differed significantly between cohorts. Unobtrusive intratumoural T-cell receptor repertoires were evoked by NHS-IL12-only treatment in the relatively short treatment period compared with the repertoires induced by local irradiation. There, complex T cell compartments were induced in ipsi- as well as contralateral tumors, interestingly with considerably more complex repertoires in contralateral unirradiated tumors than in the respective irradiated counterparts. Tumors receiving combination therapy showed the broadest αβ TCR repertoire. Here, complex diversity was accompanied with highest peak amplitudes and prominent distinct single peaks in TCR Vα spectratype analysis indicating prominent expression of several variable segments and clonal expansion. Moreover, prominent single peaks of corresponding length in ipsi- and contralateral tumors indicative for systemic distribution of clonal T cell expansions were found only after combination treatment. In addition, lymphocytic infiltration rate, complexity of the TCR repertoires and signs indicating clonal expansions were the same in ipsi- and contralateral tumor sites in the combination therapy cohort (Fig. 5B). Vγ and Vδ spectratypes were similar between treatment groups (Fig. 5C).
Figure 5.

Spectratypes were performed following established protocols. NK cell receptor repertoires showed a homogenous distribution among the treatment groups (A). Vα repertoires showed a broader spectrum for irradiated tumors and after combination treatment (B). Vγ and Vδ spectratypes confirmed these findings (C).
Tumor control mechanism in vitro
As the combination of NHS-IL12 and IL2 also led to differentiation32 and we and others have shown that TH1 effector cytokines (IFNγ combined with TNFα) can induce senescence in tumor cell lines in vitro,32,34 we evaluated the combination effect of TH1 effector cytokines and irradiation in vitro. Senescence induction was not observed after IFNγ application. The combination effect of IFNγ and irradiation was highly significantly greater than the effect of irradiation alone and even superseded the senescence induction of IFNγ combined with TNFα. This effect could be abrogated by incubation with TNFα-blocking antibody before irradiation (Figs. 6A and B). A204 cells secreted significantly more TNFα after being irradiated with 4.0 Gy than under control conditions (Fig. 6C). A204 cells as well as glioma cell lines T98G and Ln229 express the IFNγ receptor and thus are suitable models to evaluate combination effects (Figs. 6D and E). Senescence induction led to significantly reduced clonogenicity in colony formation assay after irradiation combined with IFNγ in comparison to irradiation alone, not only in A204 rhabdomyosarcoma cells, but also in T98G and Ln229 glioma cell lines (Fig. 6F).
Figure 6.
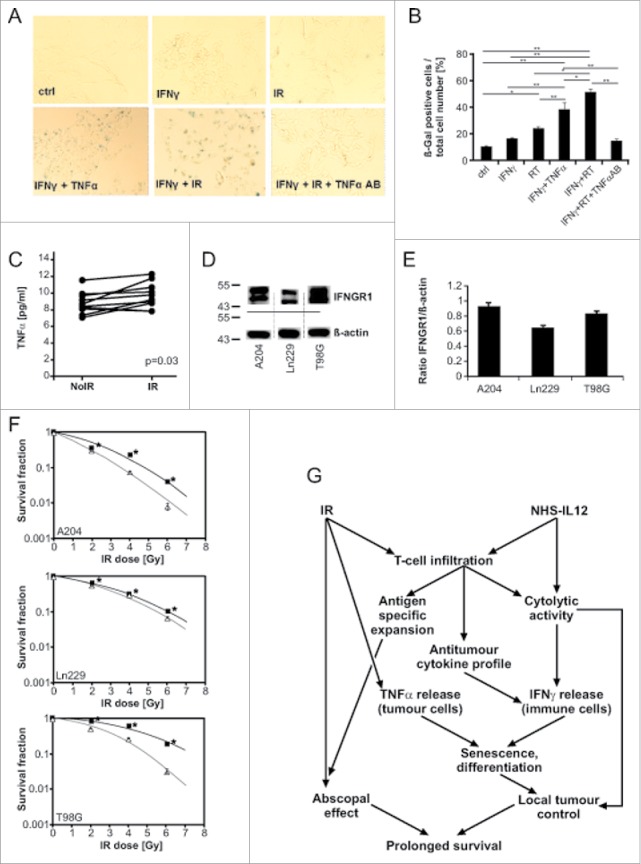
IFNγ combined with irradiation induced senescence in A204 cells. The induction was significantly more pronounced than with single modality treatment or even treatment with IFNγ and TNFα (published previously for senescence induction). The effect could be abrogated by the addition of TNFα-blocking antibody (A, B). TNFα secretion of A204 cells was significantly increased after irradiation (C). Rhabdomyosarcoma A204 as well as glioma cell lines T98G and Ln229 showed IFNγ receptor expression and were used for further investigations (D, E). Senescence induction led to a significant decrease of clonogenicity after treatment with IFNγ and irradiation compared with irradiation alone in all three cancer cell lines (F). The proposed mechanism of interaction of irradiation and NHS-IL12 to promote anticancer immune response superseding the effect of irradiation alone and to increase survival even in the presence of non-irradiated tumors is depicted in (G). *p < 0.05. **p < 0.0001.
Discussion
This study evaluates the combination of the immunocytokine NHS-IL12 with tumor irradiation to investigate the antitumor effect of the combination therapy as well as the mechanisms of action in vivo and in vitro. The humanized NSG mouse model allows for in vivo evaluation of species-specific immune therapeutic interventions in human malignancies. Yet, the limitations of the study lie in the short follow-up time for animals and the limited possibility of rechallenge experiments as well as CD8+ and CD4+ cell depletion. In addition, the immune responses might differ from patients as tumors and engrafted immune cells do not share the same HLA types as discussed in our previous study.32
In contrast to our last study,32 we commenced therapy later, i.e., when tumors had reached a diameter of at least 7 mm, a tumor size that could not be controlled by NHS-IL12 as single agent treatment or in combination with other cytokines. However, when combined with local tumor irradiation, not only irradiated tumors showed halted tumor growth, but also their contralateral counterparts. This led to a significant survival benefit for combination treatment compared with single treatment. The abscopal response was combination treatment specific, yet was not observed in every animal. Abscopal effects were observed when ipsilateral irradiated tumors showed a response (in terms of immune cell infiltration and tumor shrinkage) and occurred subsequently to induction of immune response in the irradiated tumor lesion. Abscopal effects are rare in patients receiving radiation as monotherapy,35-37 yet are one of the main rationales for combining radiotherapy and immunotherapy with the aim of achieving this effect in larger numbers of cancer patients.15 Hypofractionated or large single-dose irradiation as applied in our study has been described as particularly efficient in inducing immune effects such as immunogeneic cell death,38,39 and abscopal tumor responses,16,40,41 and are common for stereotactic radiotherapy of metastases or small primary tumors in the clinical setting.42-44
Histology showed dense immune cell infiltrates predominantly in tumors of those animals that had received combination therapy. Furthermore, their tumors were of smaller size and their infiltrating T cells in general characterized by markers indicating cytolytic activity, while the CD4+ T cells were assumingly of T helper type as they were negative for Foxp3 (data not shown) and associated with better survival. Yet, dense immune infiltrates correlated not only with a significant better survival but also large intratumoral areas of multinucleated desmin+, cross-striated muscle cells arranged in steric order resembling myofbrilles and fibres, indicating induction of myogeneic differentiation. As described in our previous work, the mechanism leading to tumor differentiation from rhabdomyosarcoma to cross-striated muscle fibers was senescence in the xenograft tumors indicated by pHP1γ and p16INK4a positivity.32 In contrast, irradiation-only treated tumors showed only some immune cell infiltration and smaller immune compartments in the corresponding contralateral tumors. NHS-IL12 cohort had even less infiltrates. Both single modality treatment cohorts were not associated with signs of myogenic differentiation neither in ipsi- nor contralateral tumors.
With regard to the cytokine milieu control tumors exhibited a tumor-promoting cytokine profile with hardly any antitumoral cytokines present. NHS-IL12 treatment seemed to reduce pro-tumorigenic cytokines. While irradiation as a single modality therapy led to a slight increase of antitumoral CD40 ligand and TH1 cytokines IFNγ and TNFα, the major change induced by combination treatment was the reduced abundance of pro-tumorigenic cytokines.
The molecular analysis of the intratumoral immune compartments indicated cytolytic activity of T cells and cytokine secretion. NKG2D, a major activating receptor of innate and adaptive immune cells, potentially triggering NK cells, γδ T cells, iNKT, CD8+ αβ T cells and under certain conditions CD4+ T cells' cytolytic activity was expressed in all tumors.45 The marker profile in our study suggests that NK cells might be able to produce TH1 cytokines and cross-talk with dendritic cells paving the way for adaptive T cell responses.46,49 Yet, the respective NK cell compartments seemed functionally homogenous among treatment groups. The complexity of T cell compartments, however, differed significantly between the treatment cohorts indicating a T cell mediated effect through combination treatment supported by initial NK cell response. In concordance with our previous data32 short-term NHS-IL12 treatment induced only a moderate TCR diversity. Irradiation provoked a broad TCR diversity and systemic spreading of the immune response indicated by immune infiltration preferentially in unirradiated contralateral tumors and patterns characterizing identical clones at respective contralateral tumor site. Combining NHS-IL12 with irradiation induced the numerically strongest clonal expansions and the most diverse T cell receptor repertoires with bilaterally equal rates of immune infiltration, corresponding to differentiation and shrinkage of the tumor lesions.
Senescence was induced by IFNγ and as we show in this study also irradiation-induced tumor-derived TNFα in vitro. Irradiation and IFNγ induced senescence even more efficiently than combined IFNγ and TNFα. The blocking of TNFα in cultures of A204 tumor cells that had been supplemented with IFNγ before irradiation abolished senescence induction in these cultures. These findings confirm that the growth arrest is dependent on IFNγ and TNFα. A204 rhabdomyosarcoma cells as well as several IFNγ-receptor expressing glioma cell lines showed significantly reduced clonogenicity when treated with irradiation and IFNγ in comparison to irradiation alone. In vivo, the observed differentiation to muscle tissue is associated with senescence as described previously. NHS-IL12 in combination with IL-2 or IL-7 also led to differentiation via senescence induction as demonstrated by pHP1γ and p16INK4a induction.32
Established concepts of the combination of immunotherapy with irradiation include the antitumor immune response via well-known pathways such as an increase of danger signals,50,51 luring of immune cells into the tumor lesion,52,53 augmenting the release of tumor-associated antigens,54,55 and supporting the priming of T cells.56 For the first time, we show that an effectively TH1 polarized tumor stroma (by IL-12) together with irradiation terminally growth arrested the tumor cells. Reduced clonogenicity has so far mostly been ascribed to radiosensitizing agents like certain chemotherapeutics.57,58 In contrast to these agents that impair DNA repair,59,60 enhance cell death61 or affect the cell cycle,62 IFNγ and TNFα induce senescence. Importantly, IFNγ and TNFα are generated in the tumor lesion with TNFα originating from tumor cells in response to irradiation and M1 macrophages63 polarized by IL-12 while IFNγ is released from IL-12 stimulated NK- and γδ-T cells and αβ-T cells induced in context with this TH1 biased tumor microenvironment.64
Our results are in line with other reports about combinations of immunocytokines and irradiation.30,31 L19-IL2, too, led to synergistic effects with irradiation in vivo, even in MHCI deficient tumors.30,31 The immunocytokine L19-IL2 alone has shown antitumor activity in a phase I trial for mixed solid tumours as well as in malignant melanoma.29,65 NHS-IL2 also has been evaluated in vivo as well as clinically and has shown antitumour activity.66 Immunocytokines with IL2 enable the in vivo evaluation in immunocompetent murine models. However, IL12 might be advantageous in the clinical setting, as it elicits TH1 responses without the risk of fostering Tregs as described for IL2.67
In conclusion, neither local tumor irradiation nor NHS-IL12 alone but the combination demonstrated highly efficient antitumor activity. Even large and fast growing tumors could be controlled and systemic antitumor effects outside the irradiation field were observed. These abscopal effects were based on the induction of massive intratumoral NK and T cell compartments, characterized by the expression of activation markers and cytotoxins and a shift of the cytokine profile into antitumoral profiles, leading to growth arrest and differentiation of the tumor with or without the subsequent elimination of growth arrested cancers. Our data suggest senescence and differentiation as new mechanisms for abscopal effects induced by the combination of irradiation and immunotherapy and build a strong rationale for combining NHS-IL12 with irradiation as a highly attractive concept that aims for efficient and systemic cancer control in clinical settings.
Material and methods
Humanized mouse model
As described previously,32 we used fully humanized NSG mice (JAX mouse stock name NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ; Jackson Laboratory) housed in single airflow cages under specific pathogen-free conditions in the Research Animal Facility at the Children's Hospital, University of Tübingen, Germany. Briefly, 6- to 8-week-old NSG mice received total body irradiation with 2.5 Gy and were injected intravenously (i.v.) with 106 CD34+ human hematopoetic stem cells obtained from surplus of G-CSF mobilized donors with their informed consent. Engraftment was fostered by weekly injections of 20 µg Fc-IL7 (Merck Serono). Twelve weeks after injection of stem cells and at repeated time points thereafter engraftment was tested by flow cytometry (multicolor staining for human CD45, CD3 and live/dead with antibodies PerCP-labeled CD45 (2D1) (BD Biosciences), APC-labeled CD3 (MEM-57) (Exbio) and Alexa Fluor 350). Animals with human engraftment of 5% or more were included in the experiment.
Tumor model and treatment
After confirmation of engraftment, human rhabdomyosarcoma cell line A204 (ATCC®, ATCC®-number: HTB-82) was inoculated subcutaneously on both hind legs of the animals (106 cells) after being cultured in RPMI 1640 plus 10% FBS. Treatment was started at tumor size of 7–10 mm (of the larger tumor in case of asymmetric tumor growth). Tumors were irradiated with a single dose of 8.0 Gy using a linear accelerator (Elekta Oncology Systems®) with 6MV photons with complete lead shielding of the body under anesthesia with 1.5% isoflurane (Abbott) evaporated in air at a flow of 0.5 L/min. Dosimetry of the irradiation field showed good coverage of the irradiated tumor, while the contralateral tumor received a maximum scattered irradiation dose of 0.3% of the applied dose (unpublished data). NHS-IL12 (Merck Serono) was injected i.v. weekly at a dose of 20 µg in 100 µL phosphate-buffered saline (PBS) starting 2 to 3 d after irradiation in the combination group. Tumor diameters were measured every 2 to 3 d by a digital calliper, tumor volume was calculated using the following equation VT = a×b×d×π/6, where a, b, and d describe length, width, and depth of the tumor. Relative tumor size was evaluated 15 ± 2 d after start of treatment. After sacrificing the animals, tumors were excised and aliquoted for histology (formalin-fixed, paraffin embedded) and molecular analysis (fresh frozen in liquid nitrogen, stored at −80°C).
Survival analysis
Mice were sacrificed when the tumors reached a size of 15 mm in the largest diameter according to the humane end point criteria applied at our institution. For the sake of tissue analysis, animals with regressing tumors were sacrificed when reaching a tumor size of 3 mm in the largest diameter. For Kaplan–Meier analysis animals that were sacrificed or died without reaching tumor end point criteria were censored to create a “tumor-size-specific survival.”
Histology and immunohistochemistry
Representative parts of the tumors were formalin fixed and paraffin embedded. Tumor sections were HE stained for histological analysis. Immunohistochemistry (IHC) was performed with antibodies against human CD3 (SP7, 1:50; DCS Innovative Diagnostic Systems GmBH), CD4+ (SP35, 1:50, Zytomed Systems), CD8+ (C8/144B, 1:100), CD56 (123C3-D5, 1:20), CD68 (PG-M1, 1:150), desmin (D33, 1:100) by DAKO and perforin (5810, 1:200) by Novocastra/Leica. Final staining was performed with a Permanent AP Red Kit (Zytomed Systems). Sections were scanned with NanoZoomer (Hamamatsu Photonics K.K., Hamamatsu), evaluated with NDP.view (Hamamatsu Photonics K.K., Hamamatsu) and scored from 0 to 3. “Low” and “high” category for IHC staining refers to a score of 0, 1 and 2, 3, respectively. For survival analysis, animals were grouped according to the higher IHC scoring of the two tumors.
Molecular analysis
TCR-α, -γ and -δ chain repertoires were analyzed with spectratype analysis as described previously.33 KIR typing included Nkp46, CD226, Nkp44, Nkp30, NKG2D, NKG2E, CD161 (primers available on request). The human cytokine array A (R&D systems) was executed according to the manufacturer's instructions. Two samples of each treatment group were analyzed in technical duplicates. Cytokines supporting tumor progression including TH2 cytokines were classified as pro-tumorigenic. TH1 and tumor inhibitory cytokines were classified as antitumor effective.
In vitro analysis
Protein abundance of IFNγ receptor 1 was detected by Western blot with antibody ab25448 (Abcam). Clonogenicity of A204 cells as well as glioma cell lines Ln229 and T98G was evaluated in vitro by clonogeneic assay with irradiation doses of 0 Gy, 2.0 Gy, 4.0 Gy and 6.0 Gy with and without combined rh-IFNγ (Immunotools) treatment. TNFα secretion was measured by ELISA according to the manufacturer's instructions (Thermo Fischer Scientific). Senescence indicated by β-Galactosidase positivity (Assay US Biological) of A204 cells was evaluated after IFNγ treatment alone and in combination with tumor necrosis factor α (TNFα, Immunotools) and irradiation following the manufacturer's instructions. The combination treatment was also tested in the presence of TNFα-neutralizing antibodies (Cell Signaling).
Statistical analysis
In all figures, center values represent mean of data and error bars represent SEM unless indicated otherwise. Statistical significance was analyzed with one-tailed Student's t-tests if the data met the assumptions of this test. Multiple data sets were compared with ANOVA. Dot plots were analyzed with the linear regression model. Survival was evaluated with the Kaplan–Meier method and compared with log-rank test. Animals dying or being killed with tumors < 15 mm were censored for the survival analysis. p-values <0.05 were considered statistically significant, p-values between 0.1 and 0.05 are described as trend to statistical significance.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Funding
The fusion proteins NHS-IL-12 and FcIL-7 were a kind gift of Merck Serono (Merck, Germany). This research was supported by the PATE (2007–0–0) and TÜFF (2154–0–0) programs of the Medical Faculty of the University of Tuebingen.
References
- 1.Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, Gonzalez R, Robert C, Schadendorf D, Hassel JC et al.. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010; 363(8):711-23; PMID:20525992; https://doi.org/ 10.1056/NEJMoa1003466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Postow MA, Chesney J, Pavlick AC, Robert C, Grossmann K, McDermott D, Linette GP, Meyer N, Giguere JK, Agarwala SS et al.. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N Engl J Med 2015; 372(21):2006-17; PMID:25891304; https://doi.org/ 10.1056/NEJMoa1414428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, Hassel JC, Rutkowski P, McNeil C, Kalinka-Warzocha E et al.. Nivolumab in previously untreated melanoma without braf mutation. N Engl J Med 2015; 372(4):320-30; PMID:25399552; https://doi.org/ 10.1056/NEJMoa1412082 [DOI] [PubMed] [Google Scholar]
- 4.Vansteenkiste J, Craps J, De Brucker N, Wauters I. Immunotherapy for non-small-cell lung cancer: The past 10 years. Future Oncol 2015; 11(19):2681-95; PMID:26039564; https://doi.org/ 10.2217/fon.15.116 [DOI] [PubMed] [Google Scholar]
- 5.Silvestri I, Cattarino S, Agliano AM, Collalti G, Sciarra A. Beyond the immune suppression: The immunotherapy in prostate cancer. Biomed Res Int 2015; 2015:794968; PMID:26161414; https://doi.org/ 10.1155/2015/794968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Preusser M, Lim M, Hafler DA, Reardon DA, Sampson JH. Prospects of immune checkpoint modulators in the treatment of glioblastoma. Nat Rev Neurol 2015; 11:504-14; PMID:26260659; https://doi.org/ 10.1038/nrneurol.2015.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De Felice F, Marchetti C, Palaia I, Musio D, Muzii L, Tombolini V, Panici PB. Immunotherapy of ovarian cancer: The role of checkpoint inhibitors. J Immunol Res 2015; 2015:191832; PMID:26236750; https://doi.org/ 10.1155/2015/191832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts SS, Chou AJ, Cheung NK. Immunotherapy of childhood sarcomas. Front Oncol 2015; 5:181; PMID:26301204; https://doi.org/ 10.3389/fonc.2015.00181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ascierto ML, Melero I, Ascierto PA. Melanoma: From incurable beast to a curable bet. Front Oncol 2015; 5:152; PMID:26217587; https://doi.org/ 10.3389/fonc.2015.00152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gettinger S, Rizvi NA, Chow LQ, Borghaei H, Brahmer J, Ready N, Gerber DE, Shepherd FA, Antonia S, Goldman JW et al.. Nivolumab monotherapy for first-line treatment of advanced non-small-cell lung cancer. J Clin Oncol 2016; 34:2980-7; PMID:27354485; https://doi.org/ 10.1200/JCO.2016.66.9929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, Staehler M, Brugger W, Dietrich PY, Mendrzyk R et al.. Multipeptide immune response to cancer vaccine ima901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med 2012; 18(8):1254-61; PMID:22842478; https://doi.org/ 10.1038/nm.2883 [DOI] [PubMed] [Google Scholar]
- 12.Thomas A, Giaccone G. Why has active immunotherapy not worked in lung cancer? Ann Oncol 2015; 26:2213-20; PMID:26232492; https://doi.org/ 10.1093/annonc/mdv323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sharabi AB, Tran PT, Lim M, Drake CG, Deweese TL. Stereotactic radiation therapy combined with immunotherapy: Augmenting the role of radiation in local and systemic treatment. Oncology (Williston Park) 2015; 29(5):331-40; PMID:25979541 [PMC free article] [PubMed] [Google Scholar]
- 14.Shahabi V, Postow MA, Tuck D, Wolchok JD. Immune-priming of the tumor microenvironment by radiotherapy: Rationale for combination with immunotherapy to improve anticancer efficacy. Am J Clin Oncol 2015; 38(1):90-7; PMID:25616204; https://doi.org/ 10.1097/COC.0b013e3182868ec8 [DOI] [PubMed] [Google Scholar]
- 15.Reynders K, Illidge T, Siva S, Chang JY, De Ruysscher D. The abscopal effect of local radiotherapy: Using immunotherapy to make a rare event clinically relevant. Cancer Treat Rev 2015; 41(6):503-10; PMID:25872878; https://doi.org/ 10.1016/j.ctrv.2015.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demaria S, Formenti SC. Radiation as an immunological adjuvant: Current evidence on dose and fractionation. Front Oncol 2012; 2:153; PMID:23112958; https://doi.org/ 10.3389/fonc.2012.00153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eckert F, Schmitt J, Zips D, Krueger MA, Pichler BJ, Gillies SD, Strittmatter W, Handgretinger R, Schilbach K. Enhanced binding of necrosis-targeting immunocytokine nhs-il12 after local tumour irradiation in murine xenograft models. Cancer Immunol Immunother 2016; 65:1003-13; PMID:27376889; https://doi.org/ 10.1007/s00262-016-1863-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haabeth OA, Lorvik KB, Hammarstrom C, Donaldson IM, Haraldsen G, Bogen B, Corthay A. Inflammation driven by tumour-specific th1 cells protects against b-cell cancer. Nat Commun 2011; 2:240; PMID:21407206; https://doi.org/ 10.1038/ncomms1239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harshyne LA, Nasca BJ, Kenyon LC, Andrews DW, Hooper DC. Serum exosomes and cytokines promote a t-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro Oncol 2015; 18:206-15; PMID:26180083; https://doi.org/ 10.1093/neuonc/nov107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Car BD, Eng VM, Lipman JM, Anderson TD. The toxicology of interleukin-12: A review. Toxicol Pathol 1999; 27(1):58-63; PMID:10367675; https://doi.org/ 10.1177/019262339902700112 [DOI] [PubMed] [Google Scholar]
- 21.Motzer RJ, Rakhit A, Schwartz LH, Olencki T, Malone TM, Sandstrom K, Nadeau R, Parmar H, Bukowski R. Phase i trial of subcutaneous recombinant human interleukin-12 in patients with advanced renal cell carcinoma. Clin Cancer Res 1998; 4(5):1183-91; PMID:9607576 [PubMed] [Google Scholar]
- 22.Cohen J. Il-12 deaths: Explanation and a puzzle. Science 1995; 270(5238):908; PMID:7481785; https://doi.org/ 10.1126/science.270.5238.908a [DOI] [PubMed] [Google Scholar]
- 23.Sondel PM, Gillies SD. Current and potential uses of immunocytokines as cancer immunotherapy. Antibodies (Basel) 2012; 1(2):149-71; PMID:24634778; https://doi.org/ 10.3390/antib1020149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribas A, Kirkwood JM, Atkins MB, Whiteside TL, Gooding W, Kovar A, Gillies SD, Kashala O, Morse MA. Phase i/ii open-label study of the biologic effects of the interleukin-2 immunocytokine emd 273063 (hu14.18-il2) in patients with metastatic malignant melanoma. J Transl Med 2009; 7:68; PMID:19640287; https://doi.org/ 10.1186/1479-5876-7-68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang RK, Kalogriopoulos NA, Rakhmilevich AL, Ranheim EA, Seo S, Kim K, Alderson KL, Gan J, Reisfeld RA, Gillies SD et al.. Intratumoral hu14.18-il-2 (ic) induces local and systemic antitumor effects that involve both activated t and nk cells as well as enhanced ic retention. J Immunol 2012; 189(5):2656-64; PMID:22844125; https://doi.org/ 10.4049/jimmunol.1200934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moschetta M, Pretto F, Berndt A, Galler K, Richter P, Bassi A, Oliva P, Micotti E, Valbusa G, Schwager K et al.. Paclitaxel enhances therapeutic efficacy of the f8-il2 immunocytokine to eda-fibronectin-positive metastatic human melanoma xenografts. Cancer Res 2012; 72(7):1814-24; PMID:22392081; https://doi.org/ 10.1158/0008-5472.CAN-11-1919 [DOI] [PubMed] [Google Scholar]
- 27.Wieckowski S, Hemmerle T, Prince SS, Schlienger BD, Hillinger S, Neri D, Zippelius A. Therapeutic efficacy of the f8-il2 immunocytokine in a metastatic mouse model of lung adenocarcinoma. Lung Cancer 2015; 88(1):9-15; PMID:25682318; https://doi.org/ 10.1016/j.lungcan.2015.01.019 [DOI] [PubMed] [Google Scholar]
- 28.Eigentler TK, Weide B, de Braud F, Spitaleri G, Romanini A, Pflugfelder A, Gonzalez-Iglesias R, Tasciotti A, Giovannoni L, Schwager K et al.. A dose-escalation and signal-generating study of the immunocytokine l19-il2 in combination with dacarbazine for the therapy of patients with metastatic melanoma. Clin Cancer Res 2011; 17(24):7732-42; PMID:22028492; https://doi.org/ 10.1158/1078-0432.CCR-11-1203 [DOI] [PubMed] [Google Scholar]
- 29.Johannsen M, Spitaleri G, Curigliano G, Roigas J, Weikert S, Kempkensteffen C, Roemer A, Kloeters C, Rogalla P, Pecher G et al.. The tumour-targeting human l19-il2 immunocytokine: Preclinical safety studies, phase i clinical trial in patients with solid tumours and expansion into patients with advanced renal cell carcinoma. Eur J Cancer 2010; 46(16):2926-35; PMID:20797845; https://doi.org/ 10.1016/j.ejca.2010.07.033 [DOI] [PubMed] [Google Scholar]
- 30.Rekers NH, Zegers CM, Yaromina A, Lieuwes NG, Biemans R, Senden-Gijsbers BL, Losen M, Van Limbergen EJ, Germeraad WT, Neri D et al.. Combination of radiotherapy with the immunocytokine l19-il2: Additive effect in a nk cell dependent tumour model. Radiother Oncol 2015; 116:438-42; PMID:26138057; https://doi.org/ 10.1016/j.radonc.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 31.Zegers CM, Rekers NH, Quaden DH, Lieuwes NG, Yaromina A, Germeraad WT, Wieten L, Biessen EA, Boon L, Neri D et al.. Radiotherapy combined with the immunocytokine l19-il2 provides long-lasting antitumor effects. Clin Cancer Res 2015; 21(5):1151-60; PMID:25552483; https://doi.org/ 10.1158/1078-0432.CCR-14-2676 [DOI] [PubMed] [Google Scholar]
- 32.Schilbach K, Alkhaled M, Welker C, Eckert F, Blank G, Ziegler H, Sterk M, Muller F, Sonntag K, Wieder T et al.. Cancer-targeted il-12 controls human rhabdomyosarcoma by senescence induction and myogenic differentiation. Oncoimmunology 2015; 4(7):e1014760; PMID:26140238; https://doi.org/ 10.1080/2162402X.2015.1014760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sonntag K, Eckert F, Welker C, Muller H, Muller F, Zips D, Sipos B, Klein R, Blank G, Feuchtinger T et al.. Chronic graft-versus-host-disease in cd34(+)-humanized nsg mice is associated with human susceptibility hla haplotypes for autoimmune disease. J Autoimmun 2015; 62:55-66; PMID:26143958; https://doi.org/ 10.1016/j.jaut.2015.06.006 [DOI] [PubMed] [Google Scholar]
- 34.Braumuller H, Wieder T, Brenner E, Assmann S, Hahn M, Alkhaled M, Schilbach K, Essmann F, Kneilling M, Griessinger C et al.. T-helper-1-cell cytokines drive cancer into senescence. Nature 2013; 494(7437):361-5; PMID:23376950; https://doi.org/ 10.1038/nature11824 [DOI] [PubMed] [Google Scholar]
- 35.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol 2006; 45(4):493-7; PMID:16760190; https://doi.org/ 10.1080/02841860600604611 [DOI] [PubMed] [Google Scholar]
- 36.Robin HI, AuBuchon J, Varanasi VR, Weinstein AB. The abscopal effect: Demonstration in lymphomatous involvement of kidneys. Med Pediatr Oncol 1981; 9(5):473-6; PMID:7029238; https://doi.org/ 10.1002/mpo.2950090510 [DOI] [PubMed] [Google Scholar]
- 37.Kingsley DP. An interesting case of possible abscopal effect in malignant melanoma. Br J Radiol 1975; 48(574):863-6; PMID:811297; https://doi.org/ 10.1259/0007-1285-48-574-863 [DOI] [PubMed] [Google Scholar]
- 38.Kepp O, Senovilla L, Vitale I, Vacchelli E, Adjemian S, Agostinis P, Apetoh L, Aranda F, Barnaba V, Bloy N et al.. Consensus guidelines for the detection of immunogenic cell death. Oncoimmunology 2014; 3(9):e955691; PMID:25941621; https://doi.org/ 10.4161/21624011.2014.955691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Adkins I, Fucikova J, Garg AD, Agostinis P, Spisek R. Physical modalities inducing immunogenic tumor cell death for cancer immunotherapy. Oncoimmunology 2015; 3(12):e968434; PMID:25964865; https://doi.org/ 10.4161/21624011.2014.968434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kwon ED, Drake CG, Scher HI, Fizazi K, Bossi A, van den Eertwegh AJ, Krainer M, Houede N, Santos R, Mahammedi H et al.. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (ca184-043): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol 2014; 15(7):700-12; PMID:24831977; https://doi.org/ 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gandhi SJ, Minn AJ, Vonderheide RH, Wherry EJ, Hahn SM, Maity A. Awakening the immune system with radiation: Optimal dose and fractionation. Cancer Lett 2015; 368:185-90; PMID:25799953; https://doi.org/ 10.1016/j.canlet.2015.03.024 [DOI] [PubMed] [Google Scholar]
- 42.Trifiletti DM, Patel N, Lee CC, Romano AM, Sheehan JP. Stereotactic radiosurgery in the treatment of brain metastases from gastrointestinal primaries. J Neurooncol 2015; 124:439-46; PMID:26186901; https://doi.org/ 10.1007/s11060-015-1857-3 [DOI] [PubMed] [Google Scholar]
- 43.Guckenberger M, Allgauer M, Appold S, Dieckmann K, Ernst I, Ganswindt U, Holy R, Nestle U, Nevinny-Stickel M, Semrau S et al.. Safety and efficacy of stereotactic body radiotherapy for stage 1 non-small-cell lung cancer in routine clinical practice: A patterns-of-care and outcome analysis. J Thorac Oncol 2013; 8(8):1050-8; PMID:23817193; https://doi.org/ 10.1097/JTO.0b013e318293dc45 [DOI] [PubMed] [Google Scholar]
- 44.Tang C, Hess K, Bishop AJ, Pan HY, Christensen EN, Yang JN, Tannir N, Amini B, Tatsui C, Rhines L et al.. Creation of a prognostic index for spine metastasis to stratify survival in patients treated with spinal stereotactic radiosurgery: Secondary analysis of mature prospective trials. Int J Radiat Oncol Biol Phys 2015; 93(1):118-25; PMID:26130231; https://doi.org/ 10.1016/j.ijrobp.2015.04.050 [DOI] [PubMed] [Google Scholar]
- 45.Bauer S, Groh V, Wu J, Steinle A, Phillips JH, Lanier LL, Spies T. Activation of nk cells and t cells by nkg2d, a receptor for stress-inducible mica. Science 1999; 285(5428):727-9; PMID:10426993; https://doi.org/ 10.1126/science.285.5428.727 [DOI] [PubMed] [Google Scholar]
- 46.Bottino C, Moretta L, Moretta A. Nk cell activating receptors and tumor recognition in humans. Curr Top Microbiol Immunol 2006; 298:175-82; PMID:16323416 [DOI] [PubMed] [Google Scholar]
- 47.Larsen SK, Gao Y, Basse PH. Nk cells in the tumor microenvironment. Crit Rev Oncog 2014; 19(1–2):91-105; PMID:24941370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Montaldo E, Del Zotto G, Della Chiesa M, Mingari MC, Moretta A, De Maria A, Moretta L. Human nk cell receptors/markers: A tool to analyze nk cell development, subsets and function. Cytometry A 2013; 83(8):702-713; PMID:23650273; https://doi.org/ 10.1002/cyto.a.22302 [DOI] [PubMed] [Google Scholar]
- 49.Della Chiesa M, Vitale M, Carlomagno S, Ferlazzo G, Moretta L, Moretta A. The natural killer cell-mediated killing of autologous dendritic cells is confined to a cell subset expressing cd94/nkg2a, but lacking inhibitory killer ig-like receptors. Eur J Immunol 2003; 33(6):1657-66; PMID:12778484; https://doi.org/ 10.1002/eji.200323986 [DOI] [PubMed] [Google Scholar]
- 50.Krysko DV, Garg AD, Kaczmarek A, Krysko O, Agostinis P, Vandenabeele P. Immunogenic cell death and damps in cancer therapy. Nat Rev Cancer 2012; 12(12):860-75; PMID:23151605; https://doi.org/ 10.1038/nrc3380 [DOI] [PubMed] [Google Scholar]
- 51.Suzuki Y, Mimura K, Yoshimoto Y, Watanabe M, Ohkubo Y, Izawa S, Murata K, Fujii H, Nakano T, Kono K. Immunogenic tumor cell death induced by chemoradiotherapy in patients with esophageal squamous cell carcinoma. Cancer Res 2012; 72(16):3967-76; PMID:22700877; https://doi.org/ 10.1158/0008-5472.CAN-12-0851 [DOI] [PubMed] [Google Scholar]
- 52.Filatenkov A, Baker J, Mueller AM, Kenkel J, Ahn GO, Dutt S, Zhang N, Kohrt H, Jensen K, Dejbakhsh-Jones S et al.. Ablative tumor radiation can change the tumor immune cell microenvironment to induce durable complete remissions. Clin Cancer Res 2015; 21(16):3727-39; PMID:25869387; https://doi.org/ 10.1158/1078-0432.CCR-14-2824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teng F, Meng X, Kong L, Mu D, Zhu H, Liu S, Zhang J, Yu J. Tumor-infiltrating lymphocytes, forkhead box p3, programmed death ligand-1, and cytotoxic t lymphocyte-associated antigen-4 expressions before and after neoadjuvant chemoradiation in rectal cancer. Transl Res 2015; 166:721-32.e1; PMID:26209749; https://doi.org/ 10.1016/j.trsl.2015.06.019 [DOI] [PubMed] [Google Scholar]
- 54.Demaria S, Ng B, Devitt ML, Babb JS, Kawashima N, Liebes L, Formenti SC. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys 2004; 58(3):862-70; PMID:14967443; https://doi.org/ 10.1016/j.ijrobp.2003.09.012 [DOI] [PubMed] [Google Scholar]
- 55.Stamell EF, Wolchok JD, Gnjatic S, Lee NY, Brownell I. The abscopal effect associated with a systemic anti-melanoma immune response. Int J Radiat Oncol Biol Phys 2013; 85(2):293-5; PMID:22560555; https://doi.org/ 10.1016/j.ijrobp.2012.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mortara L, Frangione V, Castellani P, De Lerma Barbaro A, Accolla RS. Irradiated ciita-positive mammary adenocarcinoma cells act as a potent anti-tumor-preventive vaccine by inducing tumor-specific cd4+ t cell priming and cd8+ t cell effector functions. Int Immunol 2009; 21(6):655-65; PMID:19395374; https://doi.org/ 10.1093/intimm/dxp034 [DOI] [PubMed] [Google Scholar]
- 57.Kjellstrom J, Kjellen E, Johnsson A. In vitro radiosensitization by oxaliplatin and 5-fluorouracil in a human colon cancer cell line. Acta Oncol 2005; 44(7):687-93; PMID:16227158; https://doi.org/ 10.1080/02841860500247552 [DOI] [PubMed] [Google Scholar]
- 58.Rave-Frank M, Schmidberger H, Christiansen H, Boll C, Lehmann J, Weiss E. Comparison of the combined action of oxaliplatin or cisplatin and radiation in cervical and lung cancer cells. Int J Radiat Biol 2007; 83(1):41-7; PMID:17357438; https://doi.org/ 10.1080/09553000601121108 [DOI] [PubMed] [Google Scholar]
- 59.Kim EH, Kim MS, Jung WG. The mechanisms responsible for the radiosensitizing effects of sorafenib on colon cancer cells. Oncol Rep 2014; 32(6):2421-8; PMID:25242034; https://doi.org/ 10.3892/or.2014.3497 [DOI] [PubMed] [Google Scholar]
- 60.Kobashigawa S, Morikawa K, Mori H, Kashino G. Gemcitabine induces radiosensitization through inhibition of rad51-dependent repair for DNA double-strand breaks. Anticancer Res 2015; 35(5):2731-7; PMID:25964552 [PubMed] [Google Scholar]
- 61.Dai Y, Liu M, Tang W, DeSano J, Burstein E, Davis M, Pienta K, Lawrence T, Xu L. Molecularly targeted radiosensitization of human prostate cancer by modulating inhibitor of apoptosis. Clin Cancer Res 2008; 14(23):7701-10; PMID:19047096; https://doi.org/ 10.1158/1078-0432.CCR-08-0188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schick U, Kyula J, Barker H, Patel R, Zaidi S, Gregory C, Hafsi H, Roulstone V, Deutsch E, McLaughlin M et al.. Trametinib radiosensitises ras- and braf-mutated melanoma by perturbing cell cycle and inducing senescence. Radiother Oncol 2015; 117:364-75; PMID:26163092; https://doi.org/ 10.1016/j.radonc.2015.06.026 [DOI] [PubMed] [Google Scholar]
- 63.Kerkar SP, Goldszmid RS, Muranski P, Chinnasamy D, Yu Z, Reger RN, Leonardi AJ, Morgan RA, Wang E, Marincola FM et al.. Il-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J Clin Invest 2011; 121(12):4746-57; PMID:22056381; https://doi.org/ 10.1172/JCI58814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol 2003; 3(2):133-46; PMID:12563297; https://doi.org/ 10.1038/nri1001 [DOI] [PubMed] [Google Scholar]
- 65.Weide B, Eigentler TK, Pflugfelder A, Zelba H, Martens A, Pawelec G, Giovannoni L, Ruffini PA, Elia G, Neri D et al.. Intralesional treatment of stage iii metastatic melanoma patients with l19-il2 results in sustained clinical and systemic immunologic responses. Cancer Immunol Res 2014; 2(7):668-78; PMID:24906352; https://doi.org/ 10.1158/2326-6066.CIR-13-0206 [DOI] [PubMed] [Google Scholar]
- 66.van den Heuvel MM, Verheij M, Boshuizen R, Belderbos J, Dingemans AM, De Ruysscher D, Laurent J, Tighe R, Haanen J, Quaratino S. Nhs-il2 combined with radiotherapy: Preclinical rationale and phase ib trial results in metastatic non-small cell lung cancer following first-line chemotherapy. J Transl Med 2015; 13:32; PMID:25622640; https://doi.org/ 10.1186/s12967-015-0397-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Letourneau S, van Leeuwen EM, Krieg C, Martin C, Pantaleo G, Sprent J, Surh CD, Boyman O. Il-2/anti-il-2 antibody complexes show strong biological activity by avoiding interaction with il-2 receptor alpha subunit cd25. Proc Natl Acad Sci U S A 2010; 107(5):2171-6; PMID:20133862; https://doi.org/ 10.1073/pnas.0909384107 [DOI] [PMC free article] [PubMed] [Google Scholar]