Abstract
Background: Refined grains, a major source of dietary carbohydrates, have been related to impaired glucose homeostasis and obesity. Emerging animal data suggest that in utero exposure to dietary refined carbohydrates may predispose offspring to an obese phenotype, indicating a potential role for nutritional programming in the early origins of obesity, but intergenerational human data are lacking.
Objective: We prospectively investigated refined-grain intake during pregnancy in association with offspring growth through age 7 y among high-risk children born to women with gestational diabetes mellitus (GDM).
Design: The analysis included 918 mother–singleton child dyads from the Danish National Birth Cohort. Offspring body mass index z scores (BMIZs) were calculated by using weight and length or height measured at birth, 5 and 12 mo, and 7 y. Overweight or obesity was defined by WHO cutoffs. Linear and Poisson regressions were used, with adjustment for maternal demographic, lifestyle, and dietary factors.
Results: Refined-grain intake during pregnancy was positively associated with offspring BMIZ (adjusted β per serving increase per day: 0.09; 95% CI: 0.02, 0.15) and risk of overweight or obesity at age 7 y [adjusted RR (aRR) comparing the highest with the lowest quartile: 1.80; 95% CI: 1.09, 2.98; P-trend = 0.032]. The association appeared to be more pronounced among children who were breastfed <6 mo. The substitution of 1 serving refined grains/d with an equal serving of whole grains during pregnancy was related to a 10% reduced risk of offspring overweight or obesity at 7 y of age (aRR: 0.90; 95% CI: 0.82, 0.98). No associations were observed between refined-grain intake and infant growth.
Conclusions: Higher maternal refined-grain intake during pregnancy was significantly related to a greater BMIZ and a higher risk of overweight or obesity at age 7 y among children born after pregnancies complicated by GDM. The findings highlight pregnancy as a potential window of susceptibility associated with offspring growth and obesity risk among this high-risk population. Data with longer follow-up are warranted.
Keywords: childhood obesity, diet, gestational diabetes, intrauterine exposure, nutrition
INTRODUCTION
Grains are among the most commonly consumed foods in the world (1), accounting for ∼25% of the daily total energy intake in US adults, including women of reproductive age (2). Accumulating evidence suggests that the sources and types of grains may have a different impact on glycemic response and cardiometabolic outcomes, including obesity, type 2 diabetes, and cardiovascular disease (3–5). In particular, refined grains with a high glycemic index and reduced fiber and nutrient contents have been linked to increased adiposity and a higher risk of metabolic syndrome among adults (6, 7). Despite these differences and the growing body of literature on the link between maternal diet and nutrition during pregnancy and subsequent offspring health consequences throughout the life span (8), little is known about the intergenerational impact of refined-grain intake during pregnancy on long-term cardiometabolic outcomes in the offspring.
Women with gestational diabetes mellitus (GDM), one of the most common pregnancy complications, may predispose their offspring to an increased risk of cardiometabolic disorders, including obesity later in life (9, 10). It is therefore of great public health importance to identify modifiable early-life factors that may inform effective intervention strategies to mitigate childhood obesity in this high-risk group. Emerging data from animal studies suggest that in utero exposure to dietary refined carbohydrates may predispose offspring to an obese phenotype (11), implicating the role of nutritional programming in the developmental origins of obesity. However, epidemiologic studies on the intergenerational association of maternal diet, in particular, the intake of refined grains, during pregnancy with offspring growth and risk of overweight or obesity during infancy and childhood are lacking.
To address this critical data gap, we aimed to investigate the intergenerational association between refined-grain intake during pregnancy and offspring growth and risk of obesity through the first 7 y of life among women with GDM, as a unique model of a high-risk population to study early origins of obesity. We used data from the Danish National Birth Cohort (DNBC), a population who traditionally consume relatively large amounts of grains as staple foods common to the Scandinavian countries.
METHODS
Study population and design
The DNBC is a longitudinal cohort of 91,827 women (101,042 pregnancies) recruited between 1996 and 2002, accounting for ∼36% of pregnancies in Denmark during the study period (12). Briefly, pregnant women who spoke Danish, intended to carry their pregnancy to term, and had a permanent address in Denmark were invited to participate in the study at their first antenatal visit to the general practitioner around weeks 6–12 of gestation. Information on sociodemographic, perinatal, and medical factors was collected from 4 computer-assisted telephone interviews at gestational weeks 12 and 30 and postpartum months 6 and 18. In addition, a semiquantitative food-frequency questionnaire (FFQ) was mailed to the participants at gestational week 25. When the child was 7 y old, a follow-up questionnaire about the child’s health and development was sent by mail or electronically to the parents. The DNBC was approved by the Danish National Committee on Biomedical Research Ethics. Informed consent was obtained from all participants.
Of 101,402 pregnancies in the DNBC cohort, we identified 1379 pregnancies complicated by GDM documented from the 30-wk-of-gestation or 6-mo-postpartum study interviews or the Danish National Patient Registry. During the study period, the WHO criteria (13) or local practices (14) were applied for a diagnosis of GDM. Both verified and self-reported cases were included to maximize the possibility of identifying pregnancies complicated by hyperglycemia. We sequentially excluded pregnancies with missing dietary data from the FFQ (n = 346), implausible daily energy intakes (<4000 or >20,000 kJ/d; n = 14), no data on offspring growth at birth and any follow-up (n = 2), multiple gestations (n = 24), prepregnancy diabetes verified by medical records (n = 57), and recurrent GDM (n = 18), which rendered a total of 918 mother-offspring pairs as our analytic sample. At follow-up, data from offspring with available anthropometric measures at birth (n = 918), 5 mo (n = 589), 12 mo (n = 575), or 7 y (n = 531) were included in the final analysis (see Supplemental Figure 1).
Exposure assessment
Maternal dietary intakes were estimated by using a validated 360-item FFQ distributed at gestational week 25 (15, 16), which included questions about dietary intake during the previous month. Dietary intake and the nutrient content of each food were quantified on the basis of standard portion sizes (17) and the Danish Food-Composition Tables, version 6.02 (18). Nutrient intakes were adjusted for total energy intake by using the residual method (19). By definition, the following foods and ingredients were considered refined grains: white bread, rice, pasta, bread rolls, crisp bread, crackers, and cookies. Whole grains included rye bread, rye flour, whole-wheat bread, whole-wheat flour, barley grouts, wheat kernels, wheat bran, and brown rice.
Outcome measures
Each child’s birth weight and length were extracted from the Danish Medical Birth Registry. To assess birth size, macrosomia was defined as a birth weight >4500 g. Ponderal index at birth was calculated as birth weight (in kilograms) divided by cubed birth length (in meters). Child’s weight and recumbent length at 5 and 12 mo were measured by the general practitioner at the respective postnatal visits and recorded in the Child’s Book, which was kept by the parent or parents. During the 18-mo postpartum interview, the parent or parents referred to the Child’s Book and reported their child’s anthropometric measures obtained by the general practitioner at 5 and 12 mo, respectively. The child’s weight and height at mean age 7.0 y (SD = 0.3 y) were reported by the parent or parents from the 7-y follow-up questionnaire on the basis of measurements obtained by general practitioners, school nurses, or parents. Age- and sex-specific BMI z scores (BMIZs) were calculated by using the WHO Child Growth Standards for infants and children aged <5 y (20) and the WHO Growth Reference for those aged ≥5 y (21). We further classified children as overweight or obese by using the corresponding age- and sex-specific WHO cutoffs [i.e., ≥85 percentile for children aged <5 y (20) and ≥2 SDs for those aged ≥5 y (21)].
Covariates
Data on parity (nulliparous or multiparous), socioeconomic status [high (high- or medium-level professionals), middle (skilled workers), or low (unskilled workers and others); determined by the highest level within the couple], prepregnancy BMI categories [in kg/m2; <25.0, 25.0–29.9, or ≥30.0; calculated as self-reported prepregnancy weight (kilograms) divided by squared height (meters)], smoking during pregnancy (yes or no), and supplements during pregnancy (yes or no) were obtained from interviews at gestational weeks 12 and 30. Data on moderate-to-vigorous physical activity (yes or no) during the third trimester were obtained from the interview at gestational week 30. Information on age at index childbirth (year) was extracted from medical records. Dietary covariates significantly correlated with refined-grain intake were assessed by the FFQ, including red meat; processed or mixed meat; desserts and sweets; oil, margarine, or butter; whole grains; sugar-sweetened beverages; and potatoes (all in grams per day). The overall glycemic index was calculated by summing the products of the available carbohydrate content of each food item and the glycemic index of that food, divided by the total intake of available carbohydrate (22). With regard to early-life factors in the offspring, information on breastfeeding duration (≥6 mo or <6 mo) was collected from interviews at 6 and 18 mo postpartum, whereas offspring physical activity (≥2 h/weekday or <2 h/weekday) and intake frequency of sugar-sweetened beverages (≥1 time/wk or <1 time/wk) and other dietary factors (intakes of vegetables, fruit, meat, and desserts and sweets) were obtained from the 7-y follow-up questionnaire.
Statistical analysis
Descriptive statistics are presented as means ± SDs for continuous variables and percentages for categorical variables. P values for group comparisons across quartiles of refined-grain intakes during pregnancy were obtained by ANOVA for continuous variables and by chi-square test for categorical variables. Linear regression models were used to estimate β coefficients and 95% CIs for continuous measures of offspring growth (i.e., Ponderal index at birth and BMIZ at follow-up) in association with maternal refined-grain intake during pregnancy [per serving (1 ounce, 28.35-g equivalent)/d], after adjustment for maternal sociodemographic, lifestyle, and dietary factors. RRs and 95% CIs for offspring risk of macrosomia at birth and overweight or obesity at 5 mo, 12 mo, and 7 y in association with refined-grain intake in quartiles were assessed by using Poisson regression with robust SEs (23). Tests for linear trend were conducted across quartiles of refined-grain consumption by using the median value in each quartile as a continuous variable in the models. We also assessed these associations comparing women with extremely high intakes of refined grains during pregnancy (i.e., in the top decile) with their counterparts in the lowest quartile. To explore the potential mechanisms underlying the associations between refined-grain intake and offspring risk of overweight or obesity, we further adjusted for glycemic index and cereal fiber intake during pregnancy.
We estimated the effect of substituting 1 serving refined grains/d with an equivalent serving of alternative foods (i.e., whole grains, vegetables excluding potatoes, fruit, or legumes) on offspring risk of overweight or obesity by including both food intakes (refined grains and an alternative food) as continuous variables in the same multivariable model, after adjustment for the above-mentioned covariates. RRs and 95% CIs for the substitution associations were estimated by using the computed difference in their β coefficients, difference in their variances, and their covariance (24).
To test the potential effect modification by early-life factors that have been linked to the risk of childhood obesity (25–27), we added a cross-product of the potential modifier and maternal refined-grain intake. Furthermore, we conducted stratified analyses by breastfeeding duration (≥6 mo or <6 mo), offspring physical activity (≥2 h/weekday or <2 h/weekday), and intake of sugar-sweetened beverages (≥1 time/wk or <1 time/wk) and other dietary factors (vegetables, fruit, meat, and desserts and sweets) at age 7 y. We also conducted sensitivity analyses restricted to women who did not use any medications [insulin (n = 58) or other/unspecified (n = 10)] for GDM treatment during pregnancy (n = 850, 93%), who did not have hypertensive complications during pregnancy (n = 839, 91%), who delivered term births (n = 862, 94%), and whose children did not have congenital anomalies (2 with orofacial defects) or chronic diseases (i.e., 54 with asthma, allergy, cerebral palsy, or type 1 diabetes during childhood; n = 862, 94%). In addition, we examined the impact of nonresponse on offspring anthropometric measures by examining whether participant characteristics differed between mother-offspring pairs lost to follow-up (n1) and those retained at each follow-up (n2; n1/n2: 589/329, 575/343, and 531/387 at the 5-mo, 12-mo, and 7-y follow-ups, respectively). Last, to assess the potential impact of residual confounding due to unmeasured dietary changes or other GDM treatment effect during the third trimester, we conducted sensitivity analyses additionally adjusting for fasting plasma glucose concentrations measured during the diagnostic oral-glucose challenge test and gestational weight gain, as indicators of GDM severity and management, respectively. All of the analyses were conducted by using SAS version 9.4 (SAS Institute) with 2-tailed P values <0.05 considered significant for main effects.
RESULTS
Among the 918 women with pregnancies complicated by GDM, the mean ± SD age at the index childbirth was 31.3 ± 4.6 y (Table 1). The mean ± SD intake of refined grains during pregnancy was 86.2 ± 49.4 g/d. Across increasing quartiles of maternal refined-grain intake during pregnancy, women tended to have a higher intake of total energy and a higher percentage of energy from total fat but a lower percentage of energy from protein. In addition, a higher intake of refined grains was associated with higher intakes of red meat; processed or mixed meat; desserts and sweets; oil, butter, and margarine; and potatoes but lower intakes of cereal fiber. Offspring characteristics at baseline, including sex, gestational age at delivery, birth weight, and other measures of birth size, did not vary significantly across quartiles of maternal refined-grain intake during pregnancy, whereas women with higher intakes of refined grains during pregnancy were more likely to have preterm deliveries.
TABLE 1.
Maternal and offspring characteristics at baseline according to maternal refined-grain intake during pregnancy1
| Overall (n = 918) | Quartile 1 (n = 229) | Quartile 2 (n = 230) | Quartile 3 (n = 230) | Quartile 4 (n = 229) | P2 | |
| Refined grains, g/d | 86.2 ± 49.4 | 36.5 ± 10.3 | 61.0 ± 6.6 | 91.4 ± 13.4 | 156.2 ± 37.9 | <0.001 |
| Maternal characteristics | ||||||
| Age at index child's birth, y | 31.3 ± 4.6 | 31.4 ± 4.7 | 31.7 ± 4.2 | 31.6 ± 4.5 | 30.6 ± 4.8 | 0.08 |
| Prepregnancy BMI (in kg/m2), n (%) | 0.80 | |||||
| <25.0 | 342 (37.3) | 80 (34.9) | 84 (36.5) | 88 (38.3) | 90 (39.3) | |
| 25.0–29.9 | 247 (26.9) | 64 (27.9) | 58 (25.2) | 67 (29.1) | 58 (25.3) | |
| ≥30.0 | 276 (30.1) | 69 (30.1) | 77 (33.5) | 65 (28.3) | 65 (28.4) | |
| Missing or unknown | 53 (5.8) | 16 (7.0) | 11 (4.8) | 10 (4.3) | 16 (7.0) | |
| Socioeconomic status, n (%) | 0.31 | |||||
| High | 415 (45.2) | 101 (44.1) | 104 (45.2) | 120 (52.2) | 90 (39.3) | |
| Middle | 267 (29.1) | 65 (28.4) | 71 (30.9) | 59 (25.7) | 72 (31.4) | |
| Low | 199 (21.7) | 52 (22.7) | 46 (20.0) | 46 (20.0) | 55 (24.0) | |
| Missing or unknown | 37 (4.0) | 11 (4.8) | 9 (3.9) | 5 (2.2) | 12 (5.2) | |
| Nulliparity, n (%) | 354 (37.8) | 101 (44.10) | 80 (34.78) | 81 (35.22) | 92 (40.17) | 0.20 |
| Smoking during pregnancy, n (%) | 148 (16.1) | 32 (14.0) | 40 (17.4) | 36 (15.7) | 40 (17.5) | 0.62 |
| Hypertensive complications,3 n (%) | 49 (5.3) | 14 (6.1) | 8 (3.5) | 13 (5.7) | 14 (6.1) | 0.73 |
| Any MVPA during the third trimester, n (%) | 222 (24.2) | 62 (27.1) | 60 (26.1) | 49 (21.3) | 51 (22.3) | 0.67 |
| Supplements during pregnancy, n (%) | 840 (91.5) | 206 (90.0) | 217 (94.3) | 213 (92.6) | 204 (89.1) | 0.16 |
| GDM medication therapy, n (%) | 68 (7.4) | 19 (8.3) | 14 (6.1) | 17 (7.4) | 18 (7.9) | 0.82 |
| Gestational weight gain, kg | 11.8 ± 8.2 | 11.4 ± 8.2 | 12.1 ± 7.5 | 10.9 ± 9.5 | 12.8 ± 7.4 | 0.07 |
| Cesarean delivery, n (%) | 244 (24.4) | 63 (27.5) | 58 (25.2) | 50 (21.7) | 53 (23.1) | 0.50 |
| Dietary intakes during pregnancy | ||||||
| Total energy, kcal/d | 2390.6 ± 634.1 | 1990.9 ± 570.7 | 2273.1 ± 487.6 | 2561.5 ± 576.3 | 2736.8 ± 628.3 | <0.001 |
| Carbohydrate, % of energy | 53.9 ± 6.0 | 53.9 ± 6.2 | 54.1 ± 6.2 | 54.1 ± 5.4 | 53.3 ± 6.0 | 0.41 |
| Protein, % of energy | 15.7 ± 2.5 | 16.5 ± 2.7 | 15.9 ± 2.4 | 15.5 ± 2.4 | 14.7 ± 2.3 | <0.001 |
| Total fat, % of energy | 30.1 ± 6.0 | 29.2 ± 6.1 | 29.5 ± 6.1 | 30.0 ± 5.4 | 31.6 ± 6.2 | <0.001 |
| Saturated fat, % of energy | 12.0 ± 33 | 11.6 ± 3.4 | 11.7 ± 3.2 | 11.8 ± 3.1 | 12.8 ± 3.4 | <0.001 |
| Monounsaturated fat, % of energy | 9.3 ± 2.1 | 9.1 ± 2.2 | 9.1 ± 2.1 | 9.2 ± 1.9 | 9.8 ± 2.2 | 0.001 |
| Polyunsaturated fat, % of energy | 4.5 ± 0.9 | 4.2 ± 0.9 | 4.5 ± 0.8 | 4.6 ± 0.8 | 4.5 ± 0.9 | <0.001 |
| trans Fat, % of energy | 0.6 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.5 ± 0.2 | 0.6 ± 0.3 | <0.001 |
| Cholesterol,4 mg/d | 311.0 ± 96.9 | 314.2 ± 76.0 | 319.2 ± 104.4 | 308.6 ± 99.1 | 302.0 ± 104.9 | 0.26 |
| Cereal fiber,4 g/d | 1.8 ± 2.3 | 2.3 ± 2.5 | 2.0 ± 2.4 | 1.7 ± 2.0 | 1.2 ± 2.2 | <0.001 |
| Glycemic index4 | 79.5 ± 71.9 | 76.3 ± 76.6 | 71.8 ± 81.2 | 74.1 ± 49.5 | 96.0 ± 73.9 | <0.001 |
| Glycemic load4 | 252.9 ± 295.1 | 209.8 ± 279.5 | 218.0 ± 317.9 | 245.5 ± 220.5 | 338.4 ± 333.7 | <0.001 |
| Whole grains, g/d | 152.8 ± 88.2 | 114.8 ± 68.4 | 159.6 ± 77.6 | 198.8 ± 95.6 | 137.8 ± 86.6 | <0.001 |
| Red meat, g/d | 71.9 ± 36.9 | 65.1 ± 46.5 | 72.0 ± 33.3 | 74.9 ± 30.1 | 75.5 ± 35.0 | 0.01 |
| Processed and mixed meat, g/d | 17.1 ± 12.9 | 13.1 ± 10.4 | 16.0 ± 10.7 | 20.0 ± 14.7 | 19.3 ± 14.2 | <0.001 |
| Eggs, g/d | 14.1 ± 11.3 | 11.6 ± 7.8 | 14.1 ± 12.1 | 15.7 ± 12.0 | 14.9 ± 12.2 | <0.001 |
| Desserts and sweets, g/d | 35.6 ± 28.8 | 32.6 ± 28.0 | 32.0 ± 24.2 | 36.1 ± 30.3 | 41.9 ± 31.5 | <0.001 |
| Oil/butter/margarine, g/d | 26.9 ± 20.9 | 19.6 ± 15.1 | 22.4 ± 15.3 | 27.9 ± 19.2 | 37.9 ± 27.0 | <0.001 |
| Vegetables (excluding potatoes), g/d | 129.4 ± 101.3 | 129.0 ± 99.9 | 132.6 ± 106.6 | 141.7 ± 109.6 | 114.3 ± 86.1 | 0.03 |
| Potatoes, g/d | 135.9 ± 88.9 | 124.2 ± 80.2 | 133.8 ± 87.8 | 147.1 ± 95.2 | 138.5 ± 90.7 | 0.04 |
| Legumes, g/d | 13.2 ± 21.1 | 13.3 ± 21.1 | 13.3 ± 26.6 | 15.0 ± 17.9 | 11.0 ± 17.6 | 0.24 |
| Fruit, g/d | 145.3 ± 108.1 | 141.4 ± 116.2 | 153.6 ± 107.2 | 149.1 ± 107.9 | 137.3 ± 100.5 | 0.36 |
| Dairy products, g/d | 654.7 ± 430.9 | 640.5 ± 493.8 | 618.4 ± 347.1 | 671.4 ± 424.4 | 688.7 ± 445.0 | 0.30 |
| Sugar-sweetened beverages, g/d | 456.9 ± 445.6 | 423.2 ± 429.5 | 438.1 ± 429.7 | 480.2 ± 460.3 | 486.1 ± 461.3 | 0.34 |
| Offspring characteristics | ||||||
| Male sex, n (%) | 469 (50.1) | 108 (47.2) | 117 (50.9) | 122 (53.0) | 111 (48.5) | 0.06 |
| Gestational age at delivery, wk | 39.5 ± 1.7 | 39.6 ± 1.6 | 39.5 ± 1.7 | 39.6 ± 1.6 | 39.5 ± 1.9 | 0.64 |
| Preterm birth (<37 wk of gestation), n (%) | 56 (6.1) | 9 (3.9) | 15 (6.5) | 10 (4.3) | 22 (9.6) | 0.04 |
| Birth weight, kg | 3.7 ± 0.6 | 3.7 ± 0.5 | 3.7 ± 0.6 | 3.7 ± 0.6 | 3.7 ± 0.7 | 0.39 |
| Ponderal index, kg/m3 | 25.6 ± 2.8 | 25.6 ± 2.6 | 25.6 ± 2.5 | 25.2 ± 2.7 | 25.8 ± 2.7 | 0.08 |
| Macrosomia (>4500 g), n (%) | 79 (8.6) | 17 (7.4) | 19 (8.3) | 25 (10.9) | 18 (7.9) | 0.56 |
| Small-for-gestational age,5 n (%) | 42 (4.6) | 7 (3.1) | 11 (4.8) | 7 (3.0) | 17 (7.4) | 0.08 |
| Large-for-gestational age,5 n (%) | 276 (30.1) | 66 (28.8) | 72 (31.3) | 73 (31.7) | 65 (28.4) | 0.81 |
Values are means ± SDs unless otherwise indicated; n = 918. GDM, gestational diabetes mellitus; MVPA, moderate-to-vigorous physical activity [≥3.0 metabolic task equivalents according to CDC guidelines (28)].
P values for group comparisons across quartiles of refined-grain intakes during pregnancy were obtained by ANOVA for continuous variables and by chi-square test for categorical variables.
Included pregestational hypertension, gestational hypertension, pre-eclampsia, and eclampsia.
Values were energy-adjusted using the residual method (19).
According to gestational age– and sex-specific distributions of birth weight in the entire Danish National Birth Cohort. Small-for-gestational age: <10th percentile; large-for-gestational age: ≥90th percentile.
After adjustment for maternal sociodemographic, lifestyle, and dietary factors including total energy intake, every increment of 1 serving refined grains/d during pregnancy was associated with an increase of 0.09 SD (95% CI: 0.02, 0.15 SD) in offspring BMIZ at age 7 y (Table 2). No associations were observed with offspring Ponderal index at birth or BMIZ at 5 or 12 mo. Furthermore, maternal refined-grain intake during pregnancy was significantly associated with a higher risk of overweight or obesity at age 7 y (Table 3), whereas null associations were observed with macrosomia at birth or overweight or obesity in infancy (see Supplemental Table 1). Specifically, offspring born to women with refined-grain intakes in the highest quartile (quartile 4: ≥4.3 servings/d) were 1.8-fold (95% CI: 1.09-, 2.98-fold) more likely to be overweight or obese at age 7 y than their counterparts in the lowest quartile (quartile 1: <1.8 servings/d) (model 2, Table 3). The association was more pronounced when comparing women with extremely high intakes in the top decile (≥5.3 servings/d) with those in quartile 1 (RR: 2.57; 95% CI: 1.32, 5.00). To explore the potential mechanism, we further adjusted for glycemic index, but the results did not materially change (RR comparing quartile 4 with quartile 1: 1.80; 95% CI: 1.08, 2.98; P-trend across quartiles = 0.034; data not shown), whereas the association was attenuated after further adjustment for cereal fiber intake (RR comparing quartile 4 with quartile 1: 1.63; 95% CI: 0.96, 2.75; P-trend across quartiles = 0.119; data not shown). Sensitivity analysis showed similar, robust results restricted to pregnancies in women not taking medications for GDM (93%), without hypertensive complications during pregnancy (91%), with term deliveries (94%), or with births without congenital anomalies or other chronic diseases (94%) (Figure 1; see point estimates in Supplemental Tables 2 and 3). Furthermore, in sensitivity analyses with additional adjustment for fasting plasma glucose concentrations measured during the oral-glucose challenge test and gestational weight gain, results remained robust and materially unchanged (see Supplemental Table 4).
TABLE 2.
Associations between maternal intakes of refined grains per daily serving during pregnancy and offspring growth through the first 7 y of life1
| β (95% CI) | P2 | |
| Ponderal index, kg/m3 | ||
| Energy-adjusted model3 | 0.02 (−0.08, 0.13) | 0.66 |
| Model 14 | 0.03 (−0.08, 0.14) | 0.62 |
| Model 25 | 0.03 (−0.09, 0.14) | 0.67 |
| BMIZ at 5 mo | ||
| Energy-adjusted model3 | 0.02 (−0.04, 0.08) | 0.43 |
| Model 14 | 0.02 (−0.04, 0.08) | 0.54 |
| Model 25 | 0.02 (−0.05, 0.08) | 0.60 |
| BMIZ at 12 mo | ||
| Energy-adjusted model3 | 0.01 (−0.05, 0.07) | 0.72 |
| Model 14 | 0.001 (−0.06, 0.06) | 0.99 |
| Model 25 | 0.003 (−0.06, 0.07) | 0.92 |
| BMIZ at 7 y | ||
| Energy-adjusted model3 | 0.07 (0.01, 0.13) | 0.02 |
| Model 14 | 0.08 (0.02, 0.14) | 0.009 |
| Model 25 | 0.09 (0.02, 0.15) | 0.007 |
BMIZ, BMI z score.
P values were obtained by linear regression models.
Adjusted for energy (kilocalories per day).
Adjusted for energy (kilocalories per day), maternal age (years), parity (nulliparous or multiparous), socioeconomic status (low, middle, or high), prepregnancy BMI categories (kg/m2; <25.0, 25.0–29.9, or ≥30.0), smoking during pregnancy (yes or no), moderate- to vigorous-intensity physical activity during pregnancy (yes or no), and supplements during pregnancy (yes or no).
Adjusted for covariates in model 1 and intakes of red meat, processed and mixed meat, desserts and sweets, whole grains, sugar-sweetened beverages, potatoes, and oil, margarine, and butter (all in grams per day).
TABLE 3.
Adjusted RRs (95% CIs) for offspring risk of overweight or obesity at age 7 y in association with maternal refined-grain intake during pregnancy1
| Cases/total, n/n | Energy-adjusted model2 | Model 13 | Model 24 | |
| Quartile5 | ||||
| 1 | 25/136 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 33/121 | 1.48 (0.89, 2.46) | 1.55 (0.93, 2.60) | 1.53 (0.92, 2.55) |
| 3 | 34/139 | 1.59 (0.94, 2.71) | 1.67 (0.98, 2.85) | 1.60 (0.92, 2.79) |
| 4 | 35/135 | 1.76 (1.05, 2.96) | 1.76 (1.06, 2.92) | 1.80 (1.09, 2.98) |
| P-trend across quartiles6 | 0.050 | 0.049 | 0.032 | |
| Top decile7 compared with quartile 1 | 20/528 | 2.60 (1.23, 5.50) | 2.62 (1.29, 5.32) | 2.57 (1.32, 5.00) |
n = 531.
Adjusted for energy (kilocalories per day).
Model 1 was adjusted for energy (kilocalories per day), maternal age (years), parity (nulliparous or multiparous), socioeconomic status (low, middle, or high), prepregnancy BMI categories (kg/m2; <25.0, 25.0–29.9, or ≥30.0), smoking during pregnancy (yes or no), moderate- to vigorous-intensity physical activity during pregnancy (yes or no), and supplements during pregnancy (yes or no).
Model 2 was adjusted for covariates in model 1 and intakes of red meat, processed and mixed meat, desserts and sweets, whole grains, sugar-sweetened beverages, potatoes, and oil, margarine, and butter (all in grams per day).
Quartile 1: <1.8 servings/d; quartile 2: 1.8–2.5 servings/d; quartile 3: 2.6–4.2 servings/d; quartile 4: ≥4.3 servings/d.
Tests for linear trend were conducted across quartiles of refined-grain consumption by using the median value in each quartile as a continuous variable in the models.
Top decile: ≥5.3 servings/d.
Cases/total, n/n in the top decile of maternal refined-grain intake during pregnancy.
FIGURE 1.
Sensitivity analyses for the associations between maternal intake of refined grains during pregnancy and offspring risk of overweight or obesity at age 7 y restricted to women without GDM medications (A) or hypertensive complications during pregnancy (B) or to children born at term (C) or without congenital anomalies or chronic diseases (D) by using Q1 as the reference group. Q1–4 and the top decile: (A) n = 118, 126, 125, 125, and 50; (B) n = 123, 129, 126, 126, and 51; (C) n = 127, 124, 129, 118, and 47; (D) n = 115, 116, 120, 125, and 50, respectively. RR was adjusted for energy (kilocalories per day), maternal age (years), parity (nulliparous or multiparous), socioeconomic status (low, middle, or high), prepregnancy BMI categories (kg/m2; <25.0, 25.0–29.9, or ≥30.0), smoking during pregnancy (yes or no), moderate- to vigorous-intensity physical activity during pregnancy (yes or no), supplements during pregnancy (yes or no), and intakes of red meat, processed or mixed meat, desserts and sweets, whole grains, sugar-sweetened beverages, potatoes, and oil, margarine, and butter (grams per day). *P < 0.05. GDM, gestational diabetes mellitus; Q, quartile; ref, reference.
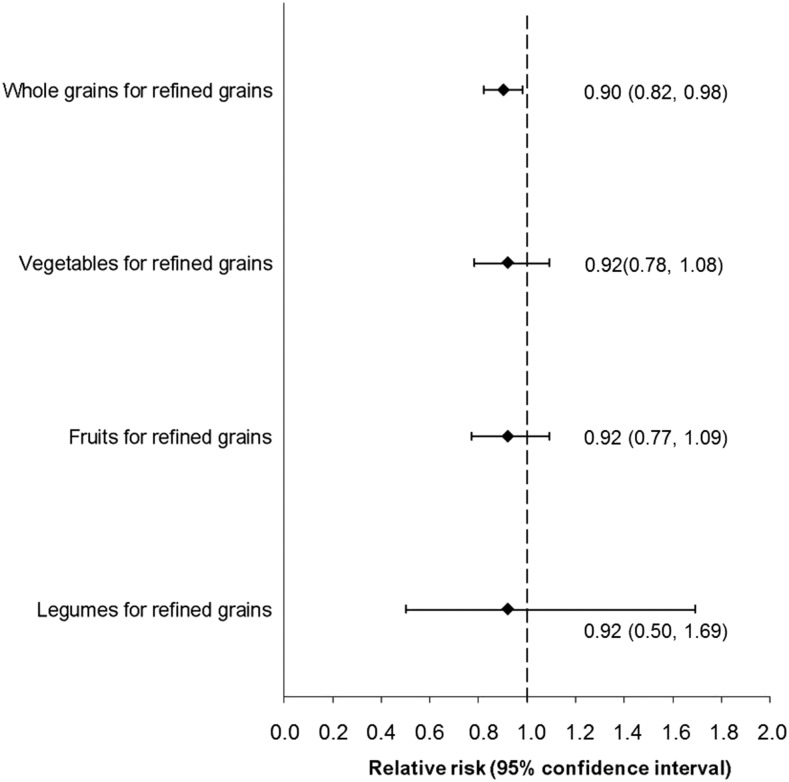
Although no significant independent association was observed between whole-grain intake and the risk of overweight or obesity at age 7 y (RR comparing quartile 4 with quartile 1: 0.97; 95% CI: 0.58, 1.61; data not shown), substituting 1 serving refined grains/d with 1 serving whole grains/d was associated with a lower risk of offspring overweight or obesity at age 7 y by 10% (RR: 0.90; 95% CI: 0.82, 0.98; Figure 2). No significant risk reduction was observed for substituting 1 serving refined grains/d with intakes of vegetables (excluding potatoes; per 100 g), fruit (per 100 g), or legumes (per 100 g) daily.
FIGURE 2.
Risk of overweight or obesity at age 7 y in the offspring associated with the substitution of maternal intakes of whole grains (per serving, 1-ounce equivalent), vegetables (excluding potatoes; per 100 g), fruit (per 100 g), or legumes (per 100 g) for 1 serving (1 ounce, 28.35 g equivalent) refined grains/d during pregnancy (n = 518). RRs were adjusted for energy (kilocalories per day), maternal age (years), parity (nulliparous or multiparous), socioeconomic status (low, middle, or high), prepregnancy BMI categories (kg/m2; <25.0, 25.0–29.9, or ≥30), smoking (yes or no), moderate- to vigorous-intensity physical activity during pregnancy (yes or no), supplements during pregnancy (yes or no), and intakes of red meat, processed or mixed meat, desserts and sweets, sugar-sweetened beverages, potatoes, and oil, margarine, and butter (all in grams per day). The vertical dashed line indicates a null association.
In the stratified analyses by early-life factors, the association tended to be more prominent among children who were breastfed <6 mo after adjustment for maternal covariates (Table 4), although the test of interaction was not significant (P-interaction = 0.14). The associations did not significantly vary by child’s physical activity, intake of sugar-sweetened beverages (Table 4), or other childhood dietary factors at age 7 y (intakes of vegetables, fruit, meat, dairy, and desserts and sweets; data not shown).
TABLE 4.
Adjusted RRs (95% CIs) for offspring risk of overweight or obesity at age 7 y according to maternal intake of refined grains during pregnancy stratified by offspring early-life factors1
| Breastfeeding duration |
Child’s physical activity at age 7 y |
Child’s intake of sugar-sweetened beverages at age 7 y |
||||
| <6 mo (n = 276) | ≥6 mo (n = 255) | <2 h/weekday (n = 340) | ≥2 h/weekday (n = 189) | ≥1/wk (n = 205) | <1/wk (n = 323) | |
| Quartile2 | ||||||
| 1 | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) | 1.00 (reference) |
| 2 | 2.24 (0.92, 5.46) | 1.13 (0.57, 2.24) | 1.43 (0.72, 2.81) | 1.53 (0.72, 3.23) | 1.47 (0.72, 3.03) | 1.22 (0.63, 2.37) |
| 3 | 3.80 (1.38, 10.5) | 0.99 (0.47, 2.07) | 1.71 (0.82, 3.55) | 1.52 (0.67, 3.43) | 1.92 (0.93, 3.98) | 1.09 (0.43, 2.74) |
| 4 | 3.13 (1.24, 7.93) | 1.29 (0.66, 2.51) | 1.92 (0.92, 4.00) | 1.44 (0.68, 3.07) | 1.91 (0.92, 3.97) | 1.20 (0.54, 2.68) |
| Top decile3 compared with quartile 1 | 7.11 (1.46, 34.5) | 1.04 (0.44, 2.47) | 4.50 (1.17, 17.3) | 1.56 (0.43, 5.63) | 5.14 (1.67, 15.9) | 2.35 (0.69, 8.01) |
n = 531. RRs were adjusted for energy (kilocalories per day), maternal age (years), parity (nulliparous or multiparous), socioeconomic status (low, middle, or high), prepregnancy BMI categories (kg/m2; <25.0, 25.0–29.9, or ≥30.0), smoking during pregnancy (yes or no), moderate- to vigorous-intensity physical activity during pregnancy (yes or no), supplements during pregnancy (yes or no), and intakes of red meat, processed and mixed meat, desserts and sweets, whole grains, sugar-sweetened beverages, potatoes, and oil, margarine, and butter (all in grams per day). P-interaction for maternal refined-grain intake by breastfeeding duration, child’s physical activity, and intake of sugar-sweetened beverages at age 7 y in relation to offspring risk of overweight or obesity at age 7 y = 0.14, 0.54, and 0.82, respectively.
Quartile 1: <1.8 servings/d; quartile 2: 1.8–2.5 servings/d; quartile 3: 2.6–4.2 servings/d; quartile 4: ≥4.3 servings/d. Quartile 1 was the reference group for quartiles 2–4 and the top decile.
Top decile: ≥5.3 servings/d.
Moreover, baseline characteristics of mother-offspring pairs lost to follow-up due to missing offspring anthropometric measures did not differ substantially from those included in the analytic sample, with the exception that those lost to the 7-y follow-up were slightly more likely to have a low socioeconomic status (see Supplemental Table 5). Furthermore, maternal dietary intakes of total energy, refined grains, and other major food groups during pregnancy did not differ between those lost to follow-up and those retained at the 7-y follow-up.
DISCUSSION
In this prospective study, we found that a higher intake of refined grains during pregnancy was significantly associated with a higher BMIZ and a greater risk of overweight or obesity at age 7 y among offspring born to women with GDM, even after adjustment for maternal sociodemographic, lifestyle, and dietary factors. The associations appeared to be more pronounced among children who were breastfed for <6 mo. Furthermore, the substitution of 1 serving refined grains/d with an equivalent serving of whole grains was significantly associated with a lower risk of offspring overweight or obesity at age 7 y.
Despite the limited data on refined-grain intake during pregnancy, the median daily intake of refined grains in this population of Danish women with GDM (median: 73.3 g; range: 3.6–355.5 g) was similar to the intake observed among adults aged 40–69 y in another Scandinavian country, Finland (29), but greater than that among nonpregnant women in the United States (30). To the best of our knowledge, we are unaware of previous prospective epidemiologic studies on the intergenerational association of refined-grain intake during pregnancy with offspring growth and the risk of overweight or obesity, either among the general population or within the context of a high-risk population of children born to women with GDM. However, our findings are biologically plausible. Emerging yet limited animal evidence suggests that in utero exposure to refined carbohydrates is associated with an increased activity of the sympathetic nervous system and dysregulated gene expression for the insulin-regulated glucose transporter type 4 in the offspring (11, 31), which, in turn, is related to hyperinsulinemia and increased adiposity (32).
Of note, the positive association between refined-grain intake during pregnancy and offspring risk of overweight or obesity was not present during infancy but only at age 7 y. The specific mechanisms underpinning the age-specific association remain to be elucidated. Nonetheless, it is possible that the clustered diet, family environment, and other obesity-related risk behaviors shared between women and their offspring could potentially play a role, which may become more apparent at a later age among the offspring as indicated by the obesity phenotype (33). Also notably, the validity of BMIZ as an indicator of infant adiposity remains controversial and to be further evaluated (34, 35). Future studies with more direct measures of infant adiposity via body-composition assessment tools are warranted.
Our findings are biologically plausible, although the underlying mechanisms remain largely unknown. The milling during food processing of refined-grain products reduces fiber, bioactive phytochemicals, and other nutrient contents, thus potentially lowering their nutritional quality (36). In particular, the inherent low-fiber content of refined-grain foods may alter profiles of satiety hormones and expression of genes involved in glucose and lipid metabolism (37) and increase adiposity in offspring (38). As shown in our study, further adjustment for cereal fiber intake attenuated the association between maternal refined-grain intake during pregnancy and offspring risk of overweight or obesity at age 7 y, which suggests that cereal fiber intake might partially explain the association. In addition, a diet rich in whole grains and low in refined grains appears to be effective in normalizing blood glucose concentrations among women with GDM (39), whereas maternal glucose concentrations during pregnancy have been positively related to the risk of childhood obesity, as observed in our own study population (40). Collectively, these data are consistent with our finding that dietary substitution of refined grains with whole grains during pregnancy is associated with a lower risk of offspring overweight or obesity at 7 y of age. Future prospective dietary intervention studies to confirm these findings and to explore mechanisms underlying these intergenerational associations are warranted.
Notable strengths of this study include the prospective design with repeated measures of childhood anthropometric variables, which represents a unique opportunity to investigate the intergenerational association of refined-grain intake during pregnancy with offspring obesity after adjustment for important confounders. Furthermore, given the available data on childhood lifestyle factors, we were able to explore the effect modification by these early-life factors as indicators of shared family lifestyle. However, we acknowledge that there were several potential limitations. First, childhood weight and height at age 7 y were reported by the parent or parents on the basis of measurements from health professionals or from parents with inevitable measurement errors. Nonetheless, previous data showed high correlations between parent-reported and measured anthropometric variables among 7- to 9-y-old children (r = 0.942 for height, r = 0.925 for weight, r = 0.813 for BMI; P < 0.001) (41). Furthermore, due to the prospective nature of the data, the potential measurement errors should be unrelated to the dietary exposure. Second, prepregnancy weight and height were self-reported. However, as shown by previous studies (42, 43), women’s prepregnancy weight status based on BMI calculated by self-reported prepregnancy weight and height was in substantial agreement with measurements obtained during the first trimester (Pearson's correlation r = 0.98, P < 0.001; κ statistic = 0.78). Third, maternal dietary intakes were self-reported by using the FFQ at gestational week 25, with potential recall bias and exposure misclassification. However, the FFQ was validated against a 7-d weighed food record and was shown to be applicable to analyses on a food-group level (16). Moreover, we did not have data on maternal diet in the third trimester after GDM diagnosis. Despite the potential overadjustment bias, we conducted sensitivity analyses with additional adjustment for fasting plasma glucose concentrations measured during the diagnostic oral-glucose challenge test and gestational weight gain as indicators of GDM severity and management. Reassuringly, results remained robust and similar. Last, the loss to follow-up could have reduced the statistical power and introduced selection bias. Notably, our results at birth and at 5 and 12 mo did not seem to be largely limited by statistical power given the significant and robust associations observed between maternal refined-grain intake during pregnancy and offspring growth outcomes at age 7 y. Nonetheless, there is no certain way of knowing the effect of dropouts on growth outcomes at follow-ups. The loss to follow-up may, in fact, have either underestimated or overestimated the true effect sizes, including those at 7 y. Furthermore, selection bias seems unlikely or minimal given that the major maternal and child characteristics at baseline and maternal dietary factors including intake of refined grains during pregnancy did not differ substantially between participants lost to follow-up and those retained at follow-up.
In conclusion, we found significant and positive associations of maternal refined-grain intake during pregnancy with offspring BMIZ and the risk of overweight or obesity at age 7 y among the high-risk children born to women with GDM. Our study adds to the emerging, yet limited, data on the possible intergenerational association of refined-grain intake during pregnancy with adverse offspring cardiometabolic outcomes and suggests that these associations may become more apparent after infancy. It also underlines the need for future prospective studies with longer follow-up through later childhood, adolescence, and adulthood to evaluate whether our findings persist in later life.
Acknowledgments
The authors’ responsibilities were as follows—YZ: conceptualized and designed the analysis, researched the data, and wrote the manuscript; SFO and CZ: contributed to the design and analysis of the study and reviewed and edited the manuscript; PM, TIH, EHY, AAB, SR, JEC, FBH, and CZ: interpreted the results and reviewed and edited the manuscript; CG and JW: contributed to the data analysis; YZ and CZ: are the guarantors of this work and, as such, had full access to all of the data in the study and took responsibility for the integrity of the data and the accuracy of the data analysis; and all authors: read and approved the final manuscript. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: BMIZ, BMI z score; DNBC, Danish National Birth Cohort; FFQ, food-frequency questionnaire; GDM, gestational diabetes mellitus.
REFERENCES
- 1.FAO. FAOstat [Internet]. Most produced commodities, world average 1994 - 2014. [cited 2016 Mar 25]. Available from: http://www.fao.org/faostat/en/#data/QC/visualize.
- 2.O’Neil CE, Nicklas TA, Keast DR, Fulgoni VL. Ethnic disparities among food sources of energy and nutrients of public health concern and nutrients to limit in adults in the United States: NHANES 2003-2006. Food Nutr Res 2014;58:15784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aune D, Norat T, Romundstad P, Vatten LJ. Whole grain and refined grain consumption and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis of cohort studies. Eur J Epidemiol 2013;28:845–58. [DOI] [PubMed] [Google Scholar]
- 4.Liu S. Intake of refined carbohydrates and whole grain foods in relation to risk of type 2 diabetes mellitus and coronary heart disease. J Am Coll Nutr 2002;21:298–306. [DOI] [PubMed] [Google Scholar]
- 5.Liu S, Willett WC, Manson JE, Hu FB, Rosner B, Colditz G. Relation between changes in intakes of dietary fiber and grain products and changes in weight and development of obesity among middle-aged women. Am J Clin Nutr 2003;78:920–7. [DOI] [PubMed] [Google Scholar]
- 6.Song S, Lee JE, Song WO, Paik HY, Song Y. Carbohydrate intake and refined-grain consumption are associated with metabolic syndrome in the Korean adult population. J Acad Nutr Diet 2014;114:54–62. [DOI] [PubMed] [Google Scholar]
- 7.McKeown NM, Troy LM, Jacques PF, Hoffmann U, O’Donnell CJ, Fox CS. Whole- and refined-grain intakes are differentially associated with abdominal visceral and subcutaneous adiposity in healthy adults the Framingham Heart Study. Am J Clin Nutr 2010;92:1165–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barker DJP. Intrauterine programming of adult disease. Mol Med Today 1995;1:418–23. [DOI] [PubMed] [Google Scholar]
- 9.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, Hadden DR, McCance DR, Hod M, McIntyre HD, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008;358:1991–2002. [DOI] [PubMed] [Google Scholar]
- 10.Lawlor DA. The Society for Social Medicine John Pemberton Lecture 2011: developmental overnutrition—an old hypothesis with new importance? Int J Epidemiol 2013;42:7–29. [DOI] [PubMed] [Google Scholar]
- 11.Young JB. Developmental origins of obesity: a sympathoadrenal perspective. Int J Obes (Lond) 2006;30 Suppl 4:S41–9. [DOI] [PubMed] [Google Scholar]
- 12.Olsen J, Melbye M, Olsen SF, Sorensen TI, Aaby P, Andersen AM, Taxbol D, Hansen KD, Juhl M, Schow TB, et al. The Danish National Birth Cohort—its background, structure and aim. Scand J Public Health 2001;29:300–7. [DOI] [PubMed] [Google Scholar]
- 13.WHO. Definition, diagnosis and classification of diabetes mellitus and its complications: report of a WHO consultation. Part 1, Diagnosis and classification of diabetes mellitus. [Internet] [cited 2016 Mar 25]. Available from: http://apps.who.int/iris/handle/10665/66040.
- 14.Kühl C. Glucose metabolism during and after pregnancy in normal and gestational diabetic women. 1. Influence of normal pregnancy on serum glucose and insulin concentration during basal fasting conditions and after a challenge with glucose. Acta Endocrinol (Copenh) 1975;79:709–19. [PubMed] [Google Scholar]
- 15.Mikkelsen TB, Olsen SF, Rasmussen SE, Osler M. Relative validity of fruit and vegetable intake estimated by the food frequency questionnaire used in the Danish National Birth Cohort. Scand J Public Health 2007;35:172–9. [DOI] [PubMed] [Google Scholar]
- 16.Mikkelsen TB, Osler M, Olsen SF. Validity of protein, retinol, folic acid and n-3 fatty acid intakes estimated from the food-frequency questionnaire used in the Danish National Birth Cohort. Public Health Nutr 2006;9:771–8. [DOI] [PubMed] [Google Scholar]
- 17.Andersen LT, Jensen H, Haraldsdottir J. Typiske vægte for madvarer. [Typical weights for food.] Scand J Nutr 1996;40(4 Suppl 32):S129–52 (in Danish). [Google Scholar]
- 18.National Food Institute (Denmark). Danish Food Composition Databank. [Internet] [cited 2106 Mar 25]. Available from: http://www.foodcomp.dk/v6/fcdb_default.asp.
- 19.Willett W, Stampfer MJ. Total energy intake: implications for epidemiologic analyses. Am J Epidemiol 1986;124:17–27. [DOI] [PubMed] [Google Scholar]
- 20.WHO Multicentre Growth Reference Study Group. WHO child growth standards: length/height-for-age, weight-for-age, weight-for-length, weight-for-height and body mass index-for-age: methods and development. Geneva (Switzerland): WHO; 2006. [Google Scholar]
- 21.de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull World Health Organ 2007;85:660–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wolever TM, Nguyen PM, Chiasson JL, Hunt JA, Josse RG, Palmason C, Rodger NW, Ross SA, Ryan EA, Tan MH. Determinants of diet glycemic index calculated retrospectively from diet records of 342 individuals with non-insulin-dependent diabetes mellitus. Am J Clin Nutr 1994;59:1265–9. [DOI] [PubMed] [Google Scholar]
- 23.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol 2004;159:702–6. [DOI] [PubMed] [Google Scholar]
- 24.Pan A, Sun Q, Bernstein AM, Schulze MB, Manson JE, Stampfer MJ, Willett WC, Hu FB. Red meat consumption and mortality: results from 2 prospective cohort studies. Arch Intern Med 2012;172:555–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wijnhoven TM, van Raaij JM, Yngve A, Sjoberg A, Kunesova M, Duleva V, Petrauskiene A, Rito AI, Breda J. WHO European Childhood Obesity Surveillance Initiative: health-risk behaviours on nutrition and physical activity in 6-9-year-old schoolchildren. Public Health Nutr 2015;18:3108–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malik VS, Pan A, Willett WC, Hu FB. Sugar-sweetened beverages and weight gain in children and adults: a systematic review and meta-analysis. Am J Clin Nutr 2013;98:1084–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cameron AJ, Spence AC, Laws R, Hesketh KD, Lioret S, Campbell KJ. A review of the relationship between socioeconomic position and the early-life predictors of obesity. Curr Obes Rep 2015;4:350–62. [DOI] [PubMed] [Google Scholar]
- 28.CDC. General physical activities defined by level of intensity [table] [Internet] [cited 2016 Oct 21]. Available from: http://www.cdc.gov/nccdphp/dnpa/physical/pdf/PA_Intensity_table_2_1.pdf.
- 29.Montonen J, Knekt P, Jarvinen R, Aromaa A, Reunanen A. Whole-grain and fiber intake and the incidence of type 2 diabetes. Am J Clin Nutr 2003;77:622–9. [DOI] [PubMed] [Google Scholar]
- 30.Zhang C, Schulze MB, Solomon CG, Hu FB. A prospective study of dietary patterns, meat intake and the risk of gestational diabetes mellitus. Diabetologia 2006;49:2604–13. [DOI] [PubMed] [Google Scholar]
- 31.Young JB, Weiss J, Boufath N. Effects of dietary monosaccharides on sympathetic nervous system activity in adipose tissues of male rats. Diabetes 2004;53:1271–8. [DOI] [PubMed] [Google Scholar]
- 32.Bonzón-Kulichenko E, Fernández-Agulló T, Moltó E, Serrano R, Fernández A, Ros M, Carrascosa JM, Arribas C, Martínez C, Andrés A, et al. Regulation of insulin-stimulated glucose uptake in rat white adipose tissue upon chronic central leptin infusion: effects on adiposity. Endocrinology 2011;152:1366–77. [DOI] [PubMed] [Google Scholar]
- 33.Cameron AJ, Crawford DA, Salmon J, Campbell K, McNaughton SA, Mishra GD, Ball K. Clustering of obesity-related risk behaviors in children and their mothers. Ann Epidemiol 2011;21:95–102. [DOI] [PubMed] [Google Scholar]
- 34.Horan M, Gibney E, Molloy E, McAuliffe F. Methodologies to assess paediatric adiposity. Ir J Med Sci 2015;184:53–68. [DOI] [PubMed] [Google Scholar]
- 35.Prentice AM, Jebb SA. Beyond body mass index. Obes Rev 2001;2:141–7. [DOI] [PubMed] [Google Scholar]
- 36.Seal CJ, Brownlee IA. Whole-grain foods and chronic disease: evidence from epidemiological and intervention studies. Proc Nutr Soc 2015;74:313–9. [DOI] [PubMed] [Google Scholar]
- 37.Maurer AD, Reimer RA. Maternal consumption of high-prebiotic fibre or -protein diets during pregnancy and lactation differentially influences satiety hormones and expression of genes involved in glucose and lipid metabolism in offspring in rats. Br J Nutr 2011;105:329–38. [DOI] [PubMed] [Google Scholar]
- 38.Maurer AD, Chen Q, McPherson C, Reimer RA. Changes in satiety hormones and expression of genes involved in glucose and lipid metabolism in rats weaned onto diets high in fibre or protein reflect susceptibility to increased fat mass in adulthood. J Physiol 2009;587:679–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Asemi Z, Samimi M, Tabassi Z, Esmaillzadeh A. The effect of DASH diet on pregnancy outcomes in gestational diabetes: a randomized controlled clinical trial. Eur J Clin Nutr 2014;68:490–5. [DOI] [PubMed] [Google Scholar]
- 40.Zhu Y, Olsen SF, Mendola P, Yeung EH, Vaag A, Bowers K, Liu A, Bao W, Li S, Madsen C, et al. Growth and obesity through the first 7 y of life in association with levels of maternal glycemia during pregnancy: a prospective cohort study. Am J Clin Nutr 2016;103:794–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Cauwenberghe J, Delvaux I, Michels N, Den Hond E, Schoeters G, Nelen V, Croes K, Van Larebeke N, Sioen I. Validity of parentally reported versus measured weight, length and waist in 7- to 9-year-old children for use in follow-up studies. Eur J Pediatr 2014;173:921–8. [DOI] [PubMed] [Google Scholar]
- 42.Shin D, Chung H, Weatherspoon L, Song WO. Validity of prepregnancy weight status estimated from self-reported height and weight. Matern Child Health J 2014;18:1667–74. [DOI] [PubMed] [Google Scholar]
- 43.Tomeo CA, Rich-Edwards JW, Michels KB, Berkey CS, Hunter DJ, Frazier AL, Willett WC, Buka SL. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology 1999;10:774–7. [PubMed] [Google Scholar]