Abstract
Background: Approximately 20–30% of obese patients do not achieve successful weight outcomes after bariatric surgery.
Objective: We examined whether short-term changes (≤0.5 y postsurgery) in energy intake and macronutrient composition after bariatric surgery could predict 10-y weight change.
Design: Participants were recruited from the Swedish Obese Subjects (SOS) study, which was a matched (nonrandomized) prospective trial that compared bariatric surgery with usual care for obese patients. A total of 2010 patients who underwent bariatric surgery were included in the study. Physical examinations (e.g., weight) and questionnaires (e.g., dietary questionnaire) were completed before and 0.5, 1, 2, 3, 4, 6, 8, and 10 y after surgery. For the main analytic strategy, a linear mixed model was implemented, which included repeated measures with a random intercept and an unstructured covariance matrix.
Results: Short-term changes in energy intake (P < 0.001) and in relative proportions of energy from carbohydrates (P < 0.001), fat (P < 0.001), and protein (P < 0.05) were associated with 10-y weight change after bariatric surgery. At the 10-y follow-up, men and women with the largest reductions in energy intake had lost 7.3% and 3.9% more weight, respectively, compared with that of subjects with the smallest intake reductions (P < 0.001). Greater weight loss was achieved in men and women who favored protein and carbohydrates over fat and in subjects who favored protein over carbohydrates than in individuals who favored the opposite changes in macronutrient composition (P < 0.05).
Conclusions: The level of energy restriction that is achieved at 0.5 y after bariatric surgery predicts long-term weight loss. Weight loss is also associated with a changing dietary macronutrient composition. This trial was registered at clinicaltrials.gov as NCT01479452.
Keywords: bariatric surgery, energy intake, macronutrients, Swedish Obese Subjects study, weight loss
INTRODUCTION
Bariatric surgery is the most effective treatment option for patients with morbid obesity. Bariatric surgery results in sustained weight loss and reduced risk of comorbidities (1, 2). However, some patients may rely solely on the surgical procedure with the belief that surgery will bring about the behavioral changes that are necessary to lower body weight. This belief may increase the risk of weight regain (3, 4). Approximately 20–30% of patients who are treated with bariatric surgery experience inadequate weight loss, and some patients even experience weight increases (5). Furthermore, ∼20–25% of the weight that is lost after bariatric surgery can be regained over a period of 10 y (1). It has been suggested that the long-term weight outcome after bariatric surgery may be predicted by presurgical patient characteristics, postsurgical eating habits, physical activity, and psychological variables, including a postsurgical follow-up at a clinic (6).
Postsurgical eating behaviors may partly explain the disparity in weight loss between different procedures and different patients (7, 8). Short-term studies have implied that the level of dietary adherence within the first year after the surgery may play an important role in achieving and maintaining the expected weight loss (2, 9). For example, Sarwer et al. (10) showed that self-reported ratings of overall adherence to nutritional guidelines at 20 wk after bariatric surgery predicted weight outcomes at 92 wk. The immediate caloric decrease after bariatric surgery is caused by a substantial reduction in hunger and an increase in satiation (10). The role of anatomical restrictions of the ventricle and nonmechanical factors, such as gut hormones, bile acids, and gut microbiota, remain under investigation (11–13). However, little is known about the associations between changes in total energy intake and energy-contributing nutrients (i.e., the macronutrients carbohydrates, fat, and protein) and the long-term weight change in patients who have undergone bariatric surgery (14). Most studies have been based on few participants (n < 100) (7, 15–18), short follow-ups (<3 y) (7, 17, 19), and only postsurgery dietary intakes (4, 7, 8, 15, 16, 18). Thus, in most studies, it has not been possible to analyze the changes between dietary intakes before and after surgery. On the basis of the reported data, current recommendations for patients after bariatric surgery include a diet that is high in protein (35% of total energy intake), low in carbohydrates (45% of total energy intake), and low in fat (20% of total energy intake) to prevent weight regain over the short term (20, 21).
In this study, we examined whether changes in total energy intake and macronutrient composition after bariatric surgery were associated with a long-term weight change. On the basis of data from the literature, we hypothesized that the changes in energy intake and macronutrient composition ≤0.5 y after surgery would reflect the ability to adapt to dietary changes; therefore, we reasoned that these short-term changes could be used to predict weight outcomes ≤10 y after the surgery.
METHODS
The Swedish Obese Subjects (SOS) study was a prospective, matched (nonrandomized), surgical intervention trial that compared the long-term effects of bariatric surgery with conventional obesity treatment (22). The current study included the surgical group only (n = 2010). Patients had been recruited through recruitment campaigns that were organized in mass media and at primary health care centers. The patients were recruited over a 13.4-y period (1 September 1987 to 31 January 2001) from 25 surgical departments and 480 primary health care centers all over the country. Seven regional ethics committees approved the study protocol. Written or oral informed consent was obtained from all participants. This trial was registered at clinicaltrials.gov as NCT01479452.
In total, 11,453 subjects sent standardized application forms to the SOS secretariat, and 6095 subjects completed a matching examination. Inclusion criteria for entering the study were age 37–60 y and BMI (in kg/m2) ≥34 for men and ≥38 for women. Exclusion criteria were a previous surgery for a gastric or duodenal ulcer, a previous bariatric surgery, the presence of a gastric ulcer or myocardial infarction in the past 6 mo, an ongoing malignancy, an active malignancy in the past 5 y, a bulimic eating pattern, drug or alcohol abuse, psychiatric or cooperative problems that contraindicated bariatric surgery, and other contraindicating conditions (e.g., chronic glucocorticoid or anti-inflammatory treatment). Of patients who were eligible for inclusion in the study, 2010 participants constituted the surgery group. Within the surgery group, 376 participants underwent nonadjustable or adjustable banding, 1369 subjects underwent vertical banded gastroplasty, and 265 subjects underwent gastric bypass. Adjustable banding was performed with the Swedish Adjustable Gastric Band (Obtech Medical), which is similar to the American lap band.
In the current study, analyses were restricted to 10 y after the start of the study because only a low number of participants had completed the dietary questionnaire at the 15- and 20-y follow-ups. We excluded patients who received reoperations or reverse operations during the 10-y follow-up (n = 315) and those who did not return the dietary questionnaire at both baseline and the 0.5-y follow-up (n = 85). We finally included 1610 subjects who received surgical treatments (Supplemental Figure 1). In these subjects, information was available on body weight for 91.9% (n = 1480), 77.0% (n = 1240), and 74.0% (n = 1192) of subjects at the 2-, 6-, and 10-y follow-ups, respectively.
Baseline examinations were conducted 4 wk before surgery. Time points for follow-up visits (0.5, 1, 2, 3, 4, 6, 8, and 10 y) were calculated on the basis of the date of surgery. Follow-ups included out-patient clinical visits and return-mail questionnaires. Each visit included measurements of height, weight, waist circumference, and other anthropometric measurements (22). The baseline questionnaire included questions about socioeconomic status, lifestyle, psychological characteristics, and medical health.
Dietary data were collected with a validated, semi-quantitative diet questionnaire (23). The questionnaire was completed at registration for the study, at baseline, and at 0.5, 1, 2, 3, 4, 6, 8, and 10 y after surgery during follow-up visits. The estimated 24-h energy expenditure (basal metabolic rate that was determined with the use of an open-circuit indirect calorimetry system plus reported physical activity) served as an objective reference for energy intake; on the basis of that reference, the questionnaire has been judged to give valid energy intakes for both obese and nonobese individuals (23). Furthermore, energy-adjusted Pearson’s product-moment correlations between the questionnaire responses and 4-d food records were 0.59 for carbohydrates, 0.52 for total fat, 0.48 for protein, 0.59 for dietary fiber, and 0.50 for alcohol (23). Pearson’s product-moment correlation coefficients for reproducibility were 0.72 for carbohydrate, total fat, and protein intakes, 0.61 for fiber intake, and 0.89 for alcohol intake.
The questionnaire was adapted from a diet-history interview that was developed for the general population in Sweden. Furthermore, the clinical experience of problematic eating characteristics in obese individuals was used in developing the questionnaire (24). The questionnaire included 49 questions, which covered habitual dietary intake over the past 3 mo. The questionnaire was fairly similar to the food-frequency approach. The descriptions of main meals assumed that specific foods that are consumed in a Swedish main meal generally fall into 3 broad categories of potatoes and rice, meat and fish, and vegetables; the emphasis was placed on the total amount of food consumed rather than on the individual constituents. The portion sizes of the main meals were described separately for each of the 3 categories with the aid of color photographs.
All completed food questionnaires were linked to Swedish food-composition tables to provide energy and nutrient information. From the recorded frequencies of foods and drinks, it was possible to calculate average daily intakes of total energy and 29 nutrients. These calculated intakes were used in the present study to represent total energy intake (kilocalories per day) and macronutrient intakes (grams per day of carbohydrates, total fat, protein, fiber, and alcohol). Furthermore, rather than calculating absolute changes in macronutrient intake, we evaluated changes in macronutrients that were proportional to the total energy intake and expressed as the percentage of energy.
We examined whether short-term changes (0.5 y) in total energy intake and macronutrient composition after bariatric surgery could predict 10-y changes in body weight. The 0.5-y follow up was selected on the basis of the following: 1) the observation that this was the time when maximal changes in dietary intake after the surgery were achieved; 2) earlier evidence (19); and 3) that this was the time point when the highest possible number of participants were available for analysis. First, the degree of the short-term change for each variable was calculated as 0.5-y intake per day minus baseline intake per day. Positive values represented increased intake, and negative values represented reduced intake. The range of changes was divided into tertiles, and patients were grouped according to tertiles. For energy intake, group 3 represented the largest negative (intake-reduction) change; group 2 represented an intermediate negative change; and group 1 represented the smallest negative change. The average energy intake reductions compared with initial intakes were 2500 kcal in group 3 and 300 kcal in group 1 (Supplemental Table 1). For the macronutrients, group 3 represented the largest negative change; group 2 represented either a small positive or a small negative change; and group 1 represented the largest positive change. For example, men in group 3 reported reductions in carbohydrate, fat, and protein intakes of 5.0%, 10.3%, and 3.5% of total energy intake, respectively; and men in group 1 reported increases of 10.6%, 2.9%, and 3.6% of total energy intake, respectively, compared with initial intakes (Supplemental Table 1). The change in body weight between baseline and each follow-up assessment was calculated as a percentage of the initial body weight as follows:
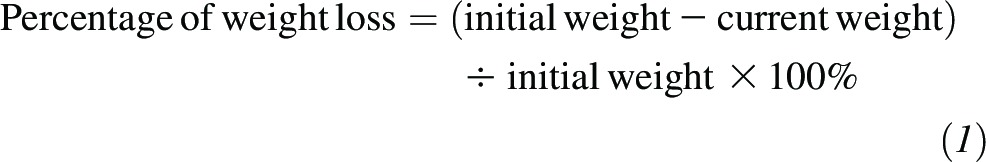 |
Time (years after surgery) was used as categorical variable; each follow-up point represented its own category.
Data were analyzed separately for men and women with the use of R statistics software (version 3.2.5; R Foundation for Statistical Computing) (25). The main analytic strategy that we used was a linear mixed model (lmer procedure in the lme4 package; R Foundation for Statistical Computing) (26), which included repeated measures with a random intercept and an unstructured covariance matrix. The mixed-model repeated-measures analysis was able to accommodate missing data. The subgroups of change in energy intake and macronutrient composition were entered univariably into the model as fixed effects with or without an interaction term with time (for associations with the percentage of weight loss over time). In addition to independent associations between macronutrients and the percentage of weight loss, we also examined associations between isoenergetic changes in macronutrient composition and the percentage of weight loss (substitution analysis). Confounding variables were selected on the basis of previous publications that examined demographic predictors of weight loss after bariatric surgery and statistical testing for confounding as proposed by Rothman (27). All analyses were adjusted for the treatment type and for presurgical age, height, weight, and energy, macronutrient intakes, and their interactions with time. Furthermore, in substitution analyses, when the substitution of 2 macronutrients (carbohydrates, fat, or protein) was examined, changes in the third main macronutrient were adjusted for simultaneously.
The significance of each final model variable was derived from ANOVA tables (ANOVA procedure in the package base) (25). P < 0.05 was considered to indicate a statistically significant association between the predictors and the percentage of weight loss. On the basis of the linear mixed model, the least-square means and 95% CIs were calculated for each group of postsurgery change for each time point (lsmeans procedure in the lsmeans package; R Foundation for Statistical Computing) (28). These values were used to create summary trajectories of the percentage of weight loss.
RESULTS
The baseline patient characteristics are shown in Table 1. The majority of participants in the SOS surgical group were women (Table 1). Approximately 40–50% of both men and women reported a low educational level (comparable to elementary school), a current smoking habit, and sedentary behavior. Self-reported macronutrient intakes were similar between men and women except for alcohol intake, which, on average, was much higher in men than in women. As shown in Supplemental Table 2, participant BMIs and waist circumferences at baseline were only slightly different between the energy- and macronutrient-intake subgroups.
TABLE 1.
Baseline characteristics and macronutrient intakes in patients who subsequently underwent bariatric surgery1
| Characteristic | Men (n = 480) | Women (n = 1130) |
| Age, y | 47.3 ± 5.8 | 47.3 ± 6.1 |
| Treatment type | ||
| Gastric bypass | 14.8 (71) | 15.7 (177) |
| Banding | 18.5 (89) | 15.0 (170) |
| VGB | 66.7 (320) | 69.3 (783) |
| Current smokers | 51.3 (246) | 41.2 (465) |
| Low education | 43.6 (209) | 44.6 (504) |
| Sedentary | 48.9 (234) | 48.9 (552) |
| Height, cm | 179.2 ± 6.49 | 164.3 ± 6.0 |
| Weight, kg | 132.5 ± 17.2 | 115.8 ± 13.7 |
| WC, cm | 130.5 ± 10.7 | 123.6 ± 10.4 |
| Macronutrient intake at baseline | ||
| Energy intake, kcal | 3226 ± 1131 | 2746 ± 1186 |
| Carbohydrates, E% | 43.3 ± 5.8 | 44.4 ± 5.6 |
| Fat, E% | 36.6 ± 5.1 | 36.6 ± 4.8 |
| Protein, E% | 16.3 ± 2.4 | 16.5 ± 2.5 |
| Fiber, E% | 1.5 ± 0.4 | 1.7 ± 0.5 |
| Alcohol, E% | 2.4 ± 2.5 | 0.9 ± 1.3 |
Values are means ± SDs for continuous variables and percentages (numbers of participants) for categorical variables. A low educational level was defined as elementary or primary school. E%, percentage of total energy intake; VBG, vertical banded gastroplasty; WC, waist circumference.
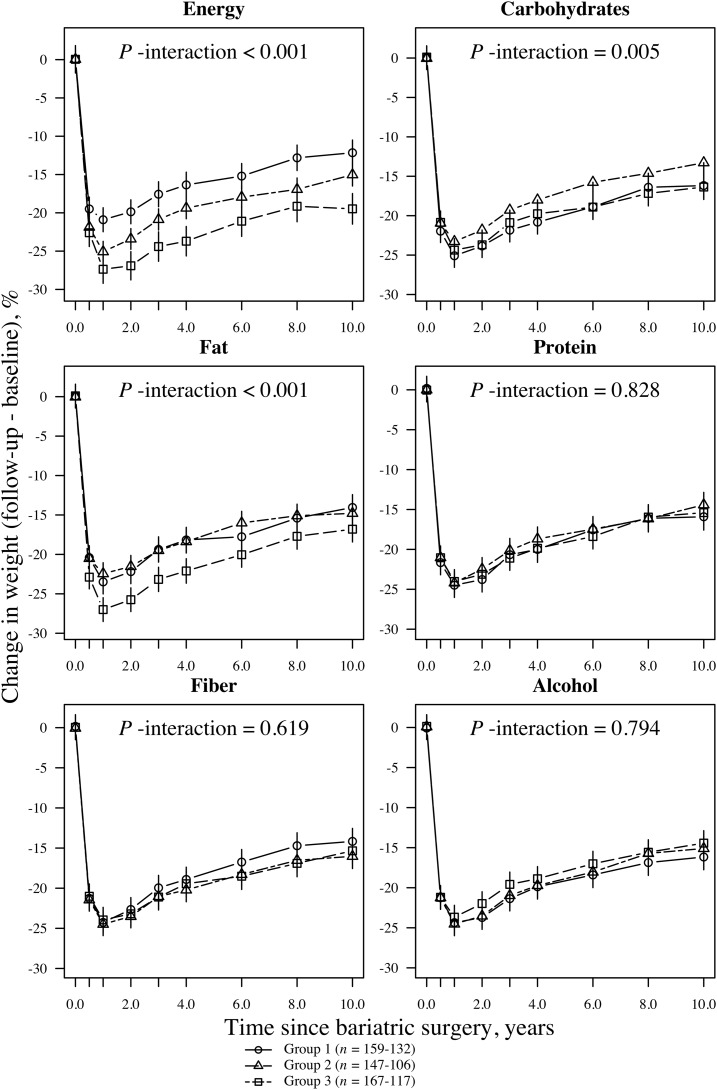
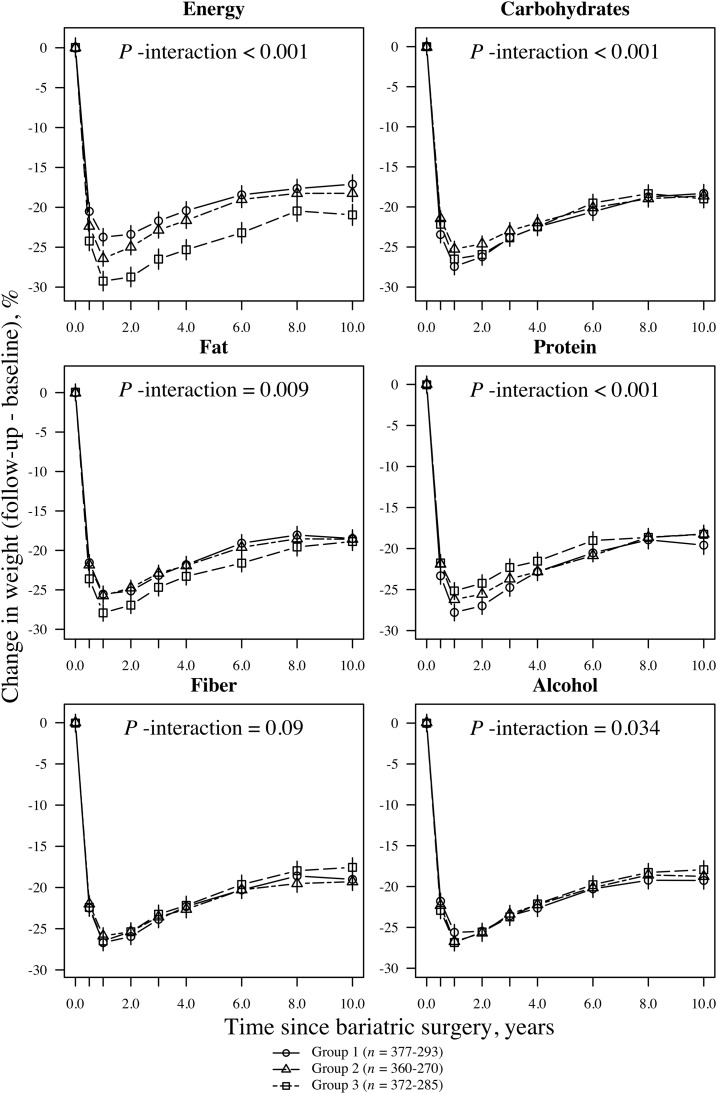
Participants reached their maximum weight loss at the 1-y follow-up. Men had an average ± SD weight loss of 24.0% ± 9.6%, and women had lost 25.8% ± 9.3% of their baseline weight. At the 10-y follow-up, the average weight loss was 14.9% ± 10.5% in men and 16.9% ± 11.8% in women. Figures 1 and 2 illustrate relative weight losses after bariatric surgery that are grouped according to the short-term changes (≤0.5 y postsurgery) in energy and macronutrient intakes. The values that are presented in the figures were based on the mixed-model analysis. Short-term changes in energy intake and macronutrient composition after bariatric surgery were associated with 10-y weight changes (Figures 1 and 2). At the end of the 10-y follow-up, men and women who reported the largest negative changes in energy (energy-group 3) intake had lost 19.5% and 20.9%, respectively, of their initial body weights. In comparison, men and women who reported the smallest negative changes (energy-group 1) lost 12.2% and 17.0%, respectively, of their initial body weights (absolute differences between groups: 7.3% and 3.9%, respectively; P < 0.001). Furthermore, at the end of the follow-up, men who had the largest negative changes in fat intake (fat group 3) had lost 16.8% of their initial weights compared with 14.1% for men with the largest positive changes in fat intake (fat group 1) (absolute differences between groups: 2.7%; P = 0.007). Women showed no significant difference in weight loss between fat groups 3 and 1 by the end of the follow-up (absolute differences between groups: <1%; P = 0.54). We also observed significant associations between changes in carbohydrate intake (in both men and women) or changes in protein and alcohol intake (in women only) and weight loss over time (Figures 1 and 2). However, regarding these nutrients, the differences in weight loss between groups 1 and 3 were small, and thus, they were not clinically meaningful.
FIGURE 1.
Mean (95% CI) relative weight loss after bariatric surgery grouped according to short-term changes (≤0.5 y postsurgery) in energy and macronutrient intakes in men (n = 480). Subgroups were divided according to changes in energy intake and macronutrient composition from baseline to the 0.5-y follow-up. For example, mean intake of carbohydrates was calculated for each time point, and participants were grouped according to tertiles of 0.5-y changes in carbohydrate intake. Similarly, mean intake of fat was calculated for each time point, and participants were grouped according to tertiles of 0.5-y changes in fat intake. Group 3 always represented the largest negative change (i.e., intake reduction; squares with dashed line). Group 1 represents either individuals with the smallest negative change (percentage of energy) or those with the largest positive change (i.e., intake increase; circles with solid lines). Group 2 represents participants with either positive or negative intermediate changes (triangles with solid lines). Symbols represent the weight loss at each follow-up point. Values were based on a mixed model that was adjusted for the treatment type and baseline age, height, weight, energy and macronutrient intakes, and their interactions with time. P-interaction values were derived from ANOVA tables (F statistics). A significant interaction term (P < 0.05) indicates that the 0.5-y change subgroups were associated with a relative weight loss over the entire follow-up time.
FIGURE 2.
Mean (95% CI) relative weight loss after bariatric surgery grouped according to short-term change (≤0.5 y postsurgery) in energy and macronutrient intakes in women (n = 1130). Subgroups were divided according to changes in energy intake and macronutrient composition from baseline to the 0.5-y follow-up. For example, mean intake of carbohydrates was calculated for each time point, and participants were grouped according to tertiles of 0.5-y changes in carbohydrate intake. Similarly, mean intake of fat was calculated for each time point, and participants were grouped according to tertiles of 0.5-y changes in fat intake. Group 3 always represented the largest negative change (i.e., intake reduction; squares with dashed lines). Group 1 represents either individuals with the smallest negative change (percentage of energy) or those with the largest positive change (i.e., intake increase; circles with solid lines). Group 2 represents participants with either positive or negative intermediate changes (triangles with solid lines). Symbols represent the weight loss at each follow-up point. Values were based on a mixed-model that was adjusted for the treatment type and baseline age, height, weight, energy and macronutrient intakes, and their interactions with time. P-interaction values were derived from ANOVA tables (F statistics). A significant interaction term (P < 0.05) indicates that the 0.5-y change subgroups were associated with a relative weight loss over the entire follow-up time.
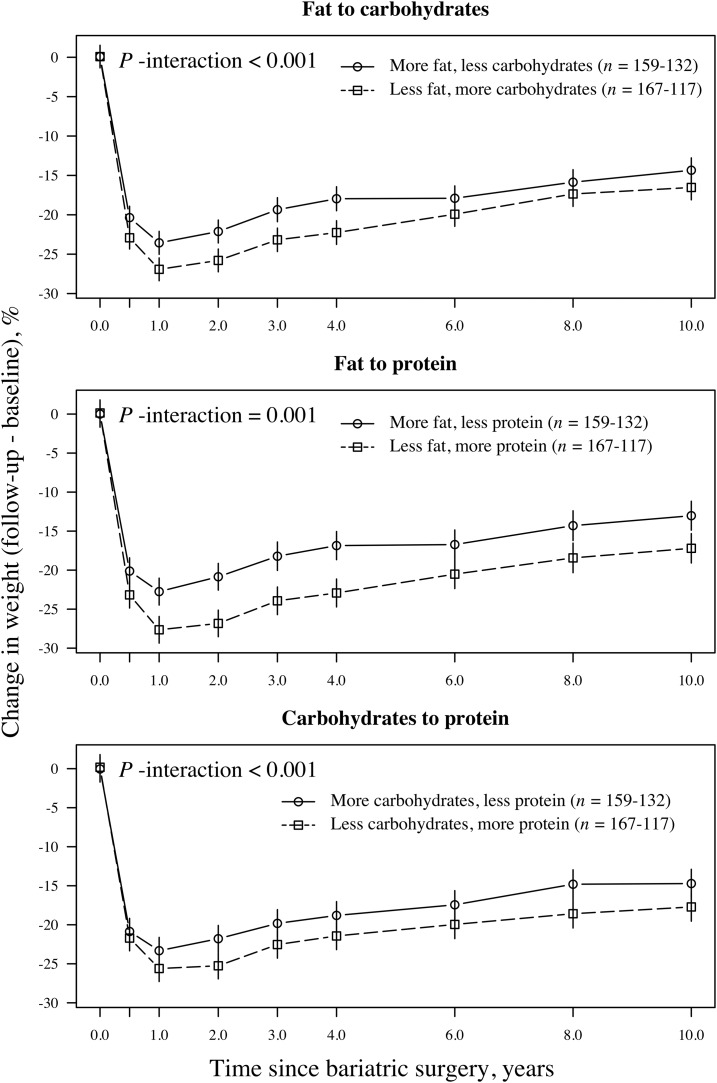
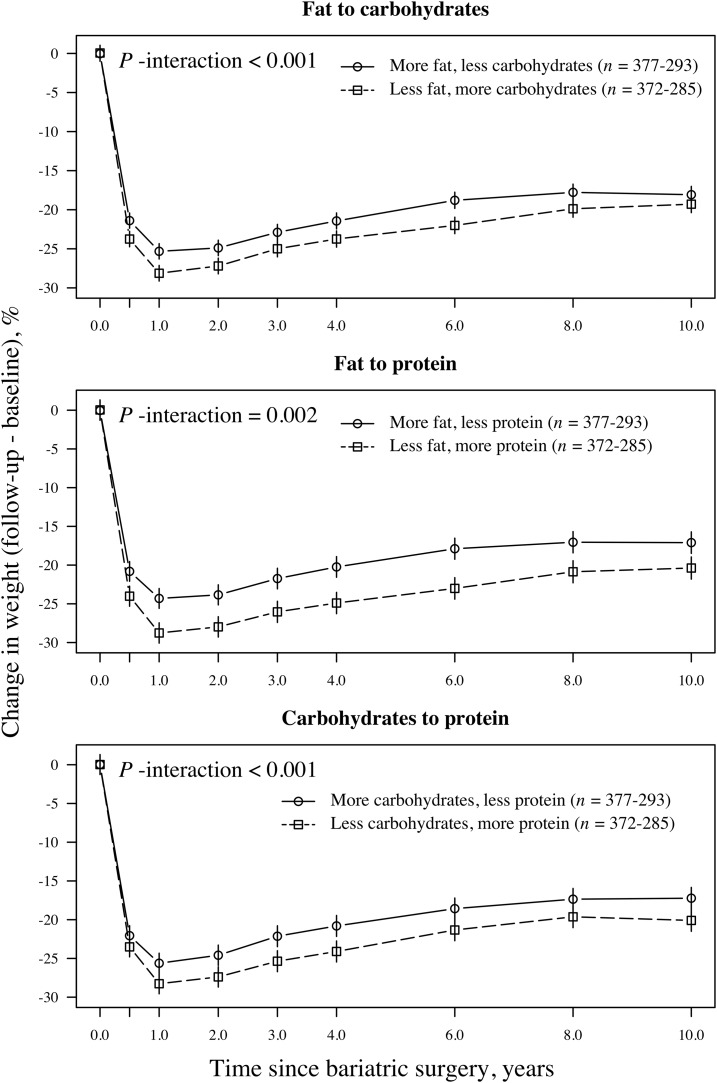
Associations between isoenergetic changes in dietary macronutrient composition and 10-y weight changes are illustrated in Figures 3 and 4. In these figures, the upper plots illustrate isoenergetic changes between fat and carbohydrates, and the middle plots illustrate changes between fat and protein. In the lower plots, changes between carbohydrates and protein are illustrated. Weight loss was greater in individuals who favored protein and carbohydrates over fat and in subjects who favored protein over carbohydrates compared with the opposite changes in macronutrient composition. At the end of follow-up, men and women who consumed a relatively higher proportion of energy from protein and a relatively lower proportion from fat had lost 17.2% and 20.3%, respectively, of their initial weights. In comparison, the opposite changes in macronutrient composition produced weight losses of 13.0% in men and 17.1% in women (absolute differences between groups: 4.2% and 3.2%, respectively; P < 0.01). Men and women who consumed a relatively higher proportion of energy from carbohydrates and a relatively lower proportion of energy from fat had lost 16.5% and 19.3%, respectively, of their initial weights. In comparison, the opposite changes in macronutrient composition produced weight losses of 14.3% in men and 18.0% in women (absolute differences between groups: 2.2% and 1.3%, respectively; P-men = 0.022, P-women = 0.061). At the end of follow-up, men and women who consumed a relatively higher proportion of energy from protein and a relatively lower proportion from carbohydrates had lost 17.7% and 20.0%, respectively, of their initial weights. In comparison, the opposite changes in macronutrient composition produced weight losses of 14.7% in men and 17.2% in women (absolute differences between groups: 3.0% and 2.8%, respectively; P < 0.05).
FIGURE 3.
Mean (95% CI) relative weight loss after bariatric surgery on the basis of a mixed model in men (n = 480) grouped by isoenergetic changes in the proportions of carbohydrates, fat, and protein in the diet. The upper plot illustrates the relative weight loss over time in participants who favored carbohydrates over fat (squares with dashed lines) and in participants with the opposite composition (circles with solid lines). The middle plot illustrates the relative weight loss over time in participants who favored protein over fat (squares with dashed lines) and in participants with the opposite composition (circles with solid lines). In the lower plot, the relative weight loss over follow-up time is illustrated with participants grouped according to those who favored protein over carbohydrates (squares with dashed lines) and those with the opposite composition (circles with solid lines). P-interaction values between the relative weight change over time and the change categories were derived from an ANOVA (F statistics).
FIGURE 4.
Mean (95% CI) relative weight loss after bariatric surgery on the basis of a mixed model in women (n = 1130) grouped by isoenergetic changes in the proportions of carbohydrates, fat, and protein in the diet. The upper plot illustrates the relative weight loss over time in participants who favored carbohydrates over fat (squares with dashed lines) and in participants with the opposite composition (circles with solid lines). The middle plot illustrates the relative weight loss over time in participants who favored protein over fat (squares with dashed lines) and in participants with the opposite composition (circles with solid lines). In the lower plot, the relative weight loss over follow-up time is illustrated with participants grouped according to those who favored protein over carbohydrates (squares with dashed lines) and in those with the opposite composition (circles with solid lines). P-interaction values between the relative weight change over time and the change categories were derived from an ANOVA (F statistics).
DISCUSSION
In this prospective longitudinal study, the self-reported reduction in energy intake ≤0.5 y after bariatric surgery was associated with greater weight loss over 10 y. In addition, an early postsurgery adaptation to a macronutrient composition with a reduced relative proportion of energy from fat in favor of carbohydrates or protein was associated with a larger weight loss. Also, favoring protein over carbohydrates was associated with a greater reduction in weight. To our knowledge, these findings have not been reported in any previous study on bariatric surgery.
Bariatric surgery effectively forces patients to decrease the quantity and increase the quality of the foods eaten to an extent that is extremely difficult to achieve with other methods. Thus, much of the changes in dietary habits after bariatric surgery are due to the surgery itself. Some variation in dietary intake may occur between surgery types, thereby resulting in different degrees of weight loss. We aimed to examine the surgery-independent role of the dietary changes and, therefore, carefully examined the effect modification and possible confounding effects of the surgery type. After conducting these analyses, the changes in energy intake and macronutrient composition 6 mo after surgery were associated with the weight outcome. Because our study was observational, even rigorous analyses could not rule out that the results could have been solely due to surgery-dependent causes. Furthermore, there may be other factors that we were unable to control for that may have affected the dietary change after surgery including personal motivation and external support for a lifestyle change. However, our findings provide evidence to suggest that a dietary intervention in bariatric patients is needed to elucidate the effect of different postsurgery diets on the weight outcome.
Our results suggest that postsurgery weight loss and weight maintenance with energy restriction may be enhanced by changing the macronutrient composition of the diet to include <35% of total energy intake from fat, ≥45% of total energy intake from carbohydrates, and ∼20% of total energy intake from proteins. The proportion of carbohydrates that appeared to be favorable in our study (≥45% of total energy intake) was somewhat higher than the proportions that have been suggested previously for patients who underwent bariatric surgery (40–45% of total energy intake) (20). This observation most likely relates to avoiding the development of dumping syndrome and unpleasant physical symptoms (e.g., nausea) especially after a gastric bypass (21, 29). These symptoms can be limited by decreasing the quantity of food intake, particularly of high-glycemic carbohydrates and sugary foods, consuming a diet with a large proportion of complex carbohydrates, such as whole grains and fiber, and adopting a reduced eating rate and increased chewing frequency (29). Research on overweight and obese populations has linked high protein intake to the induction of satiety, better nutritional status, and weight loss (30, 31). Those findings have led to the recommendation that dietary protein intake should comprise 20–30% of total energy intake for patients who receive bariatric surgery (32). In the present study, the most beneficial protein intake, in terms of weight loss, was at the lower limit of the recommended range (20%). Currently, no specific data are available regarding the optimal proportion of dietary fat, but a low-fat diet is generally recommended for patients after bariatric surgery (20). Our results somewhat support this recommendation because the average of the self-reported fat intake ranged between 28% and 35% of total energy intake during follow-up in participants who decreased their fat intakes the most after the surgery.
After bariatric surgery, the roles of the dietary macronutrient composition and weight change have been somewhat unclear. Several studies have shown that individuals who consume mainly high-glycemic and sugary foods (i.e., high intakes of simple carbohydrates) exhibited less weight loss than did individuals with other dietary habits (15, 19, 33). However, other studies have not supported or showed the opposite associations (34). For example, 2 small-scale, cross-sectional studies did not find an association between the dietary macronutrient composition and postsurgery weight change in patients who received a Roux-en-Y gastric bypass (15, 18). Another study that investigated a 3-mo weight-loss intervention in patients who had experienced weight regain after bariatric surgery (n = 30) showed beneficial results with a diet of low carbohydrates (45% of total energy intake), low fat (20% of total energy intake), and high protein (35% of total energy intake) for weight management (7). The intervention also encouraged patients to increase their physical activity. After the intervention, 86% of patients lost an average of 4.3 kg and significantly reduced the average body fat percentage. However, the study did not report results that were adjusted for physical activity (i.e., CIs and P values); thus, the independent role of the diet remained unclear.
The strengths of our study include its prospective design, its large sample size, and its exceptionally long postsurgery follow-up time. Many previous studies have had a cross-sectional design (8, 15, 16) or considerably shorter follow-ups (7, 17, 19), and they were conducted with relatively few patients (7, 29, 30). Our large sample size allowed for separate analyses of men and women; this approach is important because dietary intake is known to differ between sexes. Earlier, small-scale studies have mainly included female participants, and the studies could not explore differences between sexes. Another strength of our study is that weight was measured, and dietary intake was assessed preoperatively and postoperatively on several occasions during the follow-up. These data made it possible to investigate associations between changes in dietary intakes and weight loss. Most previous studies that have collected dietary data have only assessed dietary intake postsurgery (7, 8, 15, 16, 18). Furthermore, the SOS study included a vast amount of data from questionnaires and clinical measurements. Those data facilitated our statistical analyses because we could control for several confounding factors. Our use of the linear mixed model, including repeated measurements, as the main analytic strategy allowed us to cope with missing data and to take into account changes in associations between the outcome and predictors over time.
Our study also has some limitations. First, the SOS study was initiated almost 30 y ago; thus, a majority of patients who were included in the present study received operations with surgical procedures that are currently used infrequently. This potential limitation may have reduced the generalizability of our results. Another limitation is that dietary intake was based on self-reported data. Thus, inaccurate reporting may have led to overestimations in the observed associations. In particular, obese individuals and, in general, women are known to be more prone to misreporting their dietary intakes (35). However, the dietary questionnaire that was used in the SOS study was validated against laboratory measurements for energy and protein intakes. On the basis of this validation, the questionnaire was judged to provide valid energy intakes for both obese and nonobese individuals (23).
In conclusion, we show that the level of energy restriction that is achieved shortly after bariatric surgery is associated with long-term weight loss. Moreover, our results suggest that weight loss may also be associated with changing the dietary macronutrient composition to conform to general dietary recommendations (decreasing the proportion of fat in favor of carbohydrates and protein). However, an intervention study in which participants are randomly assigned to groups who consume diets with different macronutrient compositions is required to determine the causal effect of dietary changes on long-term weight loss and disease-risk profiles in individuals who have received bariatric surgery. In the meantime, patients should be encouraged to adopt a restricted eating pattern and to self-monitor their dietary intake, physical activity, and weight because these practices have been shown to be protective strategies against weight regain after bariatric surgery (36).
Acknowledgments
The authors’ responsibilities were as follows—LMC, A-KL, IL, and MP: conducted the research; NK: analyzed the data, wrote the manuscript, and had primary responsibility for the final content of the manuscript; and all authors: designed the research and read and approved the final manuscript. LMC has received lecture fees from Johnson & Johnson, AstraZeneca, and MSD. The remaining authors reported no conflicts of interest related to the study.
REFERENCES
- 1.Sjöström L, Narbro K, Sjöström CD, Karason K, Larsson B, Wedel H, Lystig T, Sullivan M, Bouchard C, Carlsson B, et al. Effects of bariatric surgery on mortality in Swedish obese subjects. N Engl J Med 2007;357:741–52. [DOI] [PubMed] [Google Scholar]
- 2.Geraci AA, Brunt A, Marihart C. The work behind weight-loss surgery: a qualitative analysis of food intake after the first two years post-op. ISRN Obes 2014;2014:427062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Klem ML, Wing RR, Chang CC, Lang W, McGuire MT, Sugerman HJ, Hutchison SL, Makovich AL, Hill JO. A case-control study of successful maintenance of a substantial weight loss: individuals who lost weight through surgery versus those who lost weight through non-surgical means. Int J Obes Relat Metab Disord 2000;24:573–9. [DOI] [PubMed] [Google Scholar]
- 4.Bond DS, Raynor HA, McCaffery JM, Wing RR. Salivary habituation to food stimuli in successful weight loss maintainers, obese and normal-weight adults. Int J Obes (Lond) 2010;34:593–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Karlsson J, Taft C, Rydén A, Sjöström L, Sullivan M. Ten-year trends in health-related quality of life after surgical and conventional treatment for severe obesity: the SOS intervention study. Int J Obes (Lond) 2007;31:1248–61. [DOI] [PubMed] [Google Scholar]
- 6.Robinson AH, Adler S, Stevens HB, Darcy AM, Morton JM, Safer DL. What variables are associated with successful weight loss outcomes for bariatric surgery after 1 year? Surg Obes Relat Dis 2014;10:697–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Faria SL, de Oliveira Kelly E, Lins RD, Faria OP. Nutritional management of weight regain after bariatric surgery. Obes Surg 2010;20:135–9. [DOI] [PubMed] [Google Scholar]
- 8.Júnior WS, do Amaral JL, Nonino-Borges CB. Factors related to weight loss up to 4 years after bariatric surgery. Obes Surg 2011;21:1724–30. [DOI] [PubMed] [Google Scholar]
- 9.Sjöström L, Lindroos AK, Peltonen M, Torgerson J, Bouchard C, Carlsson B, Dahlgren S, Larsson B, Narbro K, Sjöström CD, et al. Lifestyle, diabetes, and cardiovascular risk factors 10 years after bariatric surgery. N Engl J Med 2004;351:2683–93. [DOI] [PubMed] [Google Scholar]
- 10.Sarwer DB, Wadden TA, Moore RH, Baker AW, Gibbons LM, Raper SE, Williams NN. Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis 2008;4:640–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Münzberg H, Laque A, Yu S, Rezai-Zadeh K, Berthoud HR. Appetite and body weight regulation after bariatric surgery. Obes Rev 2015;16 Suppl 1:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magouliotis DE, Tasiopoulou VS, Sioka E, Chatedaki C, Zacharoulis D. Impact of bariatric surgery on metabolic and gut microbiota profile: a systematic review and meta-analysis. Obes Surg 2017. (Epub ahead of print; DOI: 10.1007/s11695-017-2595-8). [DOI] [PubMed] [Google Scholar]
- 13.Penney NC, Kinross J, Newton RC, Purkayastha S. The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes (Lond) 2015;39:1565–74. [DOI] [PubMed] [Google Scholar]
- 14.Moizé V, Deulofeu R, Torres F, de Osaba JM, Vidal J. Nutritional intake and prevalence of nutritional deficiencies prior to surgery in a Spanish morbidly obese population. Obes Surg 2011;21:1382–8. [DOI] [PubMed] [Google Scholar]
- 15.Faria SL, Faria OP, Lopes TC, Galvão MV, de Oliveira Kelly E, Ito MK. Relation between carbohydrate intake and weight loss after bariatric surgery. Obes Surg 2009;19:708–16. [DOI] [PubMed] [Google Scholar]
- 16.Freire RH, Borges MC, Alvarez-Leite JI, Toulson Davisson Correia MI. Food quality, physical activity, and nutritional follow-up as determinant of weight regain after Roux-en-Y gastric bypass. Nutrition 2012;28:53–8. [DOI] [PubMed] [Google Scholar]
- 17.Olbers T, Björkman S, Lindroos A, Maleckas A, Lönn L, Sjöström L, Lönroth H. Body composition, dietary intake, and energy expenditure after laparoscopic Roux-en-Y gastric bypass and laparoscopic vertical banded gastroplasty: a randomized clinical trial. Ann Surg 2006;244:715–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.da Silva FB, Gomes DL, de Carvalho KM. Poor diet quality and postoperative time are independent risk factors for weight regain after Roux-en-Y gastric bypass. Nutrition 2016;32:1250–3. [DOI] [PubMed] [Google Scholar]
- 19.Brolin RE, Robertson LB, Kenler HA, Cody RP. Weight loss and dietary intake after vertical banded gastroplasty and Roux-en-Y gastric bypass. Ann Surg 1994;220:782–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moizé VL, Pi-Sunyer X, Mochari H, Vidal J. Nutritional pyramid for post-gastric bypass patients. Obes Surg 2010;20:1133–41. [DOI] [PubMed] [Google Scholar]
- 21.Mechanick JI, Youdim A, Jones DB, Garvey WT, Hurley DL, McMahon MM, Heinberg LJ, Kushner R, Adams TD, Shikora S, et al. Clinical practice guidelines for the perioperative nutritional, metabolic, and nonsurgical support of the bariatric surgery patient–2013 update: cosponsored by American Association of Clinical Endocrinologists, the Obesity Society, and American Society for Metabolic & Bariatric Surgery. Obesity (Silver Spring) 2013;21 Suppl 1:S1–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sjöström L. Review of the key results from the Swedish Obese Subjects (SOS) trial - a prospective controlled intervention study of bariatric surgery. J Intern Med 2013;273:219–34. [DOI] [PubMed] [Google Scholar]
- 23.Lindroos AK, Lissner L, Sjöström L. Validity and reproducibility of a self-administered dietary questionnaire in obese and non-obese subjects. Eur J Clin Nutr 1993;47:461–81. [PubMed] [Google Scholar]
- 24.Abrahamsson M, Isaksson B. Förenklad metodik vid kostvaneundersökningar. [Simplified method for dietary surveys.] Näringsforskning 1988;32:93–9 (in Swedish). [Google Scholar]
- 25.R Core Team. R: a language and environment for statistical computing [Internet]. Vienna (Austria): R Foundation for Statistical Computing; 2016 [cited 2016 Sep 10]. Available from: https://www.R-project.org/.
- 26.Bates D, Maechler M, Bolker B, Walker S. Fitting linear mixed-effects models using lme4. J Stat Softw 2015;67:1–48. [Google Scholar]
- 27.Rothman KJ. Modern epidemiology. 1st ed. Boston: Little, Brown and Company; 1986. [Google Scholar]
- 28.Lenth RV. Least-squares means: the R package lsmeans. J Stat Softw 2016;69:1–33. [Google Scholar]
- 29.Berg P, McCallum R. Dumping syndrome: a review of the current concepts of pathophysiology, diagnosis, and treatment. Dig Dis Sci 2016;61:11–8. [DOI] [PubMed] [Google Scholar]
- 30.Faria SL, Faria OP, Buffington C, de Almeida Cardeal M, Ito MK. Dietary protein intake and bariatric surgery patients: a review. Obes Surg 2011;21:1798–805. [DOI] [PubMed] [Google Scholar]
- 31.Belza A, Ritz C, Sørensen MQ, Holst JJ, Rehfeld JF, Astrup A. Contribution of gastroenteropancreatic appetite hormones to protein-induced satiety. Am J Clin Nutr 2013;97:980–9. [DOI] [PubMed] [Google Scholar]
- 32.Heber D, Greenway FL, Kaplan LM, Livingston E, Salvador J, Still C. Endocrine and nutritional management of the post-bariatric surgery patient: an endocrine society clinical practice guideline. J Clin Endocrinol Metab 2010;95:4823–43. [DOI] [PubMed] [Google Scholar]
- 33.Sugerman HJ, Starkey JV, Birkenhauer RA. Randomized prospective trial of gastric bypass versus vertical banded gastroplasty for morbid obesity and their effects on sweets versus non-sweets eaters. Ann Surg 1987;205:613–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindroos AK, Lissner L, Sjöström L. Weight change in relation to intake of sugar and sweet foods before and after weight reducing gastric surgery. Int J Obes Relat Metab Disord 1996;20:634–43. [PubMed] [Google Scholar]
- 35.Mattisson I, Wirfält E, Aronsson CA, Wallström P, Sonestedt E, Gullberg B, Berglund G. Misreporting of energy: prevalence, characteristics of misreporters and influence on observed risk estimates in the Malmö Diet and Cancer cohort. Br J Nutr 2005;94:832–42. [DOI] [PubMed] [Google Scholar]
- 36.McGrice M, Don Paul K. Interventions to improve long-term weight loss in patients following bariatric surgery: challenges and solutions. Diabetes Metab Syndr Obes 2015;8:263–74. [DOI] [PMC free article] [PubMed] [Google Scholar]