Abstract
Background: The influence of a low-fat dietary pattern on the cardiovascular health of postmenopausal women continues to be of public health interest.
Objective: This report evaluates low-fat dietary pattern influences on cardiovascular disease (CVD) incidence and mortality during the intervention and postintervention phases of the Women’s Health Initiative Dietary Modification Trial.
Design: Participants comprised 48,835 postmenopausal women aged 50–79 y; 40% were randomly assigned to a low-fat dietary pattern intervention (target of 20% of energy from fat), and 60% were randomly assigned to a usual diet comparison group. The 8.3-y intervention period ended in March 2005, after which >80% of surviving participants consented to additional active follow-up through September 2010; all participants were followed for mortality through 2013. Breast and colorectal cancer were the primary trial outcomes, and coronary heart disease (CHD) and overall CVD were additional designated outcomes.
Results: Incidence rates for CHD and total CVD did not differ between the intervention and comparison groups in either the intervention or postintervention period. However, CHD HRs comparing these groups varied strongly with baseline CVD and hypertension status. Participants without prior CVD had an intervention period CHD HR of 0.70 (95% CI: 0.56, 0.87) or 1.04 (95% CI: 0.90, 1.19) if they were normotensive or hypertensive, respectively (P-interaction = 0.003). The CHD benefit among healthy normotensive women was partially offset by an increase in ischemic stroke risk. Corresponding HRs in the postintervention period were close to null. Participants with CVD at baseline (3.4%) had CHD HRs of 1.47 (95% CI: 1.12, 1.93) and 1.61 (95% CI: 1.02, 2.55) in the intervention and postintervention periods, respectively. However, various lines of evidence suggest that results in women with CVD or hypertension at baseline are confounded by postrandomization use of cholesterol-lowering medications.
Conclusions: CVD risk in postmenopausal women appears to be sensitive to a change to a low-fat dietary pattern and, among healthy women, includes both CHD benefit and stroke risk. This trial was registered at clinicaltrials.gov as NCT00000611.
Keywords: cardiovascular disease, coronary heart disease, cholesterol-lowering medications, low-fat dietary pattern, LDL cholesterol, nutritional behavioral intervention, postrandomization confounding, randomized controlled trial, stroke
INTRODUCTION
The Women’s Health Initiative (WHI) Dietary Modification Trial intervention had a principal focus on total fat reduction, motivated by cancer risk reduction hypotheses. In particular, participants were not counseled to substitute unsaturated for saturated fats. However, based on preliminary findings from the antecedent Women’s Health Trial, some modest favorable effects on LDL cholesterol were anticipated, and coronary heart disease (CHD) was listed as the sole secondary trial outcome and principal cardiovascular disease (CVD) outcome, and total CVD was also listed as a trial outcome (1).
We previously reported CVD results over the trial intervention period, noting a modest yet significant reduction in LDL cholesterol in the diet intervention group (2). CHD incidence rates were not significantly reduced during the dietary intervention period, however, largely because of risk elevation among the 3.4% of participants reporting CVD before trial enrollment (P-interaction = 0.006). Many of the women with prior CVD were taking medications related to their diagnosis at enrollment, including cholesterol-lowering medications, the use of which increased dramatically during trial follow-up. It was also observed that the CHD HRs over the intervention period were higher (P = 0.03) for women with baseline hypertension, who accounted for 43.2% of trial participants, than for normotensive women. Among 15 interactions examined, no other interactions with participant characteristics or medications were identified (2).
Outcome data, including mortality outcomes that relied in part on proxy reports, were somewhat incomplete in our earlier reports (2–4). Differential completeness could occur in this unblinded trial because intervention participants had more frequent contact with clinic staff. Here, we provide updated CVD and all-cause mortality results for both the intervention and postintervention periods, including mortality data through 2013 for all enrollees, based on National Death Index (NDI) matches.
METHODS
Dietary Modification Trial methods through the end of the intervention period (31 March 2005) were presented previously (2–4). Intervention participants (40%) were assigned to a dietary behavioral program administered in groups of 8–15, with 18 group sessions in the first year and quarterly sessions thereafter. The dietary goals included a reduction in total fat intake to 20% of total energy, an increase in vegetable and fruit intake to 5 servings/d, and an increase in grain intake to 6 servings/d. The comparison group (60%) received printed health-related materials only.
Dietary intake was monitored by obtaining periodic food frequency questionnaires (FFQs) and by performing laboratory analysis of blood specimens for a subsample of women (5.8%). Reported dietary differences between randomization groups were substantial during the trial’s intervention phase, including lower fat intake by 8–10% of total energy and higher carbohydrate intake by 8–10% of energy over the intervention period. Differences in the percentage of energy from fat were similar for major types of fat between intervention and comparison groups, whereas differences in carbohydrate intake included increases in whole grains and dietary fiber without a change in the glycemic index (5). Dietary differences were small postintervention (6).
Clinical outcomes were identified through biannual medical update questionnaires, followed by a medical record review by trained adjudicators. CHD was defined as nonfatal myocardial infarction or coronary death, to which coronary revascularization was added to define a composite CHD outcome. Stroke comprised ischemic and hemorrhagic stroke, and total CVD was defined as composite CHD plus stroke.
These outcome procedures continued for 81.1% and 84.4%, respectively, of participants in the intervention and comparison groups who consented to additional nonintervention follow-up through 30 September 2010, with annual medical update questionnaires. Unless they were known to be deceased, participants were included in NDI matches at 2- to 3-y intervals during postintervention, and mortality data are included here through the end of 2013 for all randomly assigned women.
Medication inventory data were collected at baseline; at 1, 3, and 6 y postrandomization; and at 1 y before the end of the (active) postrandomization period.
We used Cox regression for data analyses that compared randomized groups, with stratification on age at enrollment, prior CVD status, and randomization status in the WHI hormone therapy trials (1). Analyses over the cumulative intervention and postintervention phases were also stratified on study phase (time-dependent). Results are presented as HRs with 95% CIs and significance levels (P values). Postrandomization risk factor changes, as well as postrandomization medication initiation and cessation rates, were analyzed with generalized estimating equations having unstructured covariance matrices. These results are presented as ORs with 95% CIs and P values. The P values presented are not corrected for multiple testing unless described in the narrative as being Bonferroni corrected.
The WHI is funded by the NIH National Heart, Lung, and Blood Institute as a Trans-NIH Initiative. The study protocol was reviewed and approved by the Fred Hutchinson Cancer Research Center Institutional Review Board (Protocol 6299) in Seattle, Washington, where the WHI Clinical Coordinating Center is located, and by the institutional review boards at each of the 40 participating clinical centers.
RESULTS
An updated Consolidated Standards of Reporting Trials statement is given in Supplemental Figure 1. After our earlier report (2), there were 41 additional CHD deaths in the intervention period (currently 433 CHD deaths), 34% of which were in the intervention group; there were 105 additional deaths total in the intervention period (currently 2509 deaths), 37% of which were in the intervention group. There were an additional 7181 deaths during postintervention follow-up, 40% of which were in the intervention group.
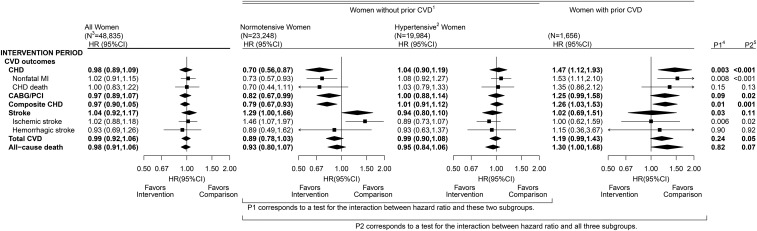
Figure 1 shows randomization group comparisons for CHD, other CVD outcomes, and all-cause mortality during the trial intervention period, including NDI-updated mortality data. See Supplemental Tables 1 and 2 for corresponding information on disease event counts and annualized incidence rates in both the intervention and postintervention periods.
FIGURE 1.
CVD outcomes in the Dietary Modification Trial during the intervention period: overall, by baseline hypertension status among women without a history of CVD, and among women with a history of CVD. Summary statistics and forest plots are shown for the overall cohort (all women randomly assigned; left panel), baseline hypertension subgroups among women without a history of CVD (middle panels), and women with a history of CVD (right panel) during the intervention period. 1Self-report before randomization of MI, CABG/PCI, or stroke; may also have baseline hypertension. 2Self-report of ever taking antihypertensive medication before randomization, or clinic-measured systolic/diastolic blood pressure ≥140/90 mm Hg; excludes women with missing baseline hypertension status data. 3Sample size of the overall cohort and baseline subgroups at randomization. 4P1 corresponds to a 1-df test for the interaction between HR and baseline hypertension status among women without prior CVD. 5P2 corresponds to a 2-df test for the interaction between HR and baseline hypertension or prior CVD strata. CABG/PCI, coronary artery bypass graft/percutaneous coronary intervention; CHD, coronary heart disease; CVD, cardiovascular disease; MI, myocardial infarction.
Overall randomization group comparisons for these outcomes remained nonsignificant in the intervention period with updated outcome data. However, CHD HRs for the low-fat dietary pattern intervention varied strongly across the 3 strata shown, which are defined by baseline hypertension and a prior history of CVD (Figure 1). This stratification builds on our previous report, and CHD HR variations across strata remained highly significant after Bonferroni adjustment for the 15 subgroup variables considered in our previous report (2) (Bonferroni-corrected P = 0.001). In participants without a history of CVD at baseline, the CHD HR was significantly lower (Bonferroni-corrected P = 0.045) for normotensive participants (HR: 0.70; 95% CI: 0.56, 0.87) than for hypertensive participants (HR: 1.04; 95% CI: 0.90, 1.19). The CHD benefit in healthy normotensive women was partially offset by an increase in stroke incidence (HR: 1.29; 95% CI: 1.00, 1.66) and, more specifically, ischemic stroke incidence (HR: 1.46; 95% CI: 1.07, 1.97). The HRs for total CVD incidence were 0.89 (95% CI: 0.78, 1.03) and 0.99 (95% CI: 0.90, 1.08) for normotensive and hypertensive women, respectively. In women with prior CVD, the risk of CHD was increased (HR: 1.47; 95% CI: 1.12, 1.93) but the risk of stroke was not significantly altered, yielding a borderline increase in total CVD risk in the intervention group.
To gain insight into CHD HR variations across the 3 baseline strata, we compared randomization groups within strata according to baseline characteristics and postrandomization changes in dietary intakes and CVD risk factors. Randomization group differences in baseline characteristics were not apparent and did not differ significantly across strata (Table 1). Randomization group differences in targeted dietary variables, as reported on FFQs at 1 y postrandomization, demonstrated substantial intervention effects on dietary fat and carbohydrate intake and were generally consistent across strata but were attenuated for women with prior CVD (Table 2).
TABLE 1.
Baseline characteristics and measures among Dietary Modification Trial participants (N = 48,835) by randomization group, stratified by baseline hypertension and prior history of CVD1
| Women without prior CVD |
|||||||
| Normotensive women |
Hypertensive women |
Women with prior CVD |
|||||
| Baseline variable | Intervention (n = 9381) | Comparison (n = 13,867) | Intervention (n = 7914) | Comparison (n = 12,070) | Intervention (n = 663) | Comparison (n = 993) | P2 |
| Age, y | 61.2 ± 6.6 | 61.2 ± 6.7 | 63.7 ± 6.8 | 63.6 ± 6.8 | 65.7 ± 6.9 | 66.0 ± 6.7 | 0.41 |
| Race/ethnicity3 | 0.07 | ||||||
| White | 7906 (84.3) | 11,800 (85.1) | 6062 (76.6) | 9258 (76.7) | 512 (77.2) | 755 (76.0) | |
| Black | 681 (7.3) | 953 (6.9) | 1238 (15.6) | 1838 (15.2) | 106 (16.0) | 172 (17.3) | |
| Hispanic | 429 (4.6) | 555 (4.0) | 249 (3.1) | 410 (3.4) | 22 (3.3) | 40 (4.0) | |
| American Native | 43 (0.5) | 41 (0.3) | 35 (0.4) | 60 (0.5) | 4 (0.6) | 5 (0.5) | |
| Asian/Pacific | 198 (2.1) | 334 (2.4) | 211 (2.7) | 321 (2.7) | 14 (2.1) | 8 (0.8) | |
| Unknown | 124 (1.3) | 184 (1.3) | 119 (1.5) | 183 (1.5) | 5 (0.8) | 13 (1.3) | |
| Smoking | 0.53 | ||||||
| Never | 4759 (51.3) | 7067 (51.4) | 4124 (52.7) | 6356 (53.2) | 271 (41.4) | 417 (42.8) | |
| Past | 3835 (41.4) | 5678 (41.3) | 3277 (41.9) | 4867 (40.8) | 329 (50.3) | 482 (49.5) | |
| Current | 676 (7.3) | 993 (7.2) | 426 (5.4) | 719 (6.0) | 54 (8.3) | 75 (7.7) | |
| Height, cm (baseline) | 162.5 ± 6.5 | 162.5 ± 6.6 | 161.8 ± 6.4 | 161.5 ± 6.6 | 160.5 ± 6.5 | 160.9 ± 6.3 | 0.05 |
| Hypertension4 | 0 (0.0) | 0 (0.0) | 7914 (100.0) | 12,070 (100.0) | 468 (73.5) | 664 (71.4) | 0.37 |
| Hypertensive medication5 | 588 (6.3) | 834 (6.0) | 4626 (58.5) | 7177 (59.5) | 472 (71.2) | 684 (68.9) | 0.20 |
| High-cholesterol medication6 | 712 (7.7) | 1123 (8.2) | 1071 (14.6) | 1664 (14.9) | 239 (38.5) | 333 (37.1) | 0.50 |
| Statin use | 364 (3.9) | 585 (4.2) | 620 (7.8) | 920 (7.6) | 170 (25.6) | 243 (24.5) | 0.33 |
| Treated for diabetes7 | 196 (2.1) | 277 (2.0) | 523 (6.6) | 840 (7.0) | 104 (15.7) | 159 (16.0) | 0.66 |
| History of MI | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 363 (54.8) | 548 (55.2) | 0.86 |
| History of stroke | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 205 (30.9) | 328 (33.0) | 0.37 |
| History of CABG/PCI | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 241 (36.3) | 321 (32.3) | 0.09 |
| Aspirin use, >80 mg/d | 1367 (14.6) | 2111 (15.2) | 1527 (19.3) | 2449 (20.3) | 297 (44.8) | 476 (47.9) | 0.78 |
| BMI, kg/m2 | 27.9 ± 5.5 | 27.9 ± 5.4 | 30.6 ± 6.2 | 30.6 ± 6.1 | 30.5 ± 5.8 | 30.7 ± 6.1 | 0.73 |
| Weight, kg | 74.0 ± 15.7 | 73.8 ± 15.4 | 80.4 ± 17.5 | 80.2 ± 17.3 | 78.8 ± 15.8 | 79.8 ± 17.5 | 0.34 |
| Waist, cm | 86.0 ± 13.1 | 86.0 ± 13.0 | 92.6 ± 14.0 | 92.7 ± 13.8 | 93.9 ± 14.0 | 93.8 ± 13.7 | 0.95 |
| Systolic BP, mm Hg | 118.1 ± 11.4 | 118.3 ± 11.2 | 139.6 ± 16.1 | 139.9 ± 16.1 | 133.5 ± 18.2 | 133.5 ± 18.5 | 0.91 |
| Diastolic BP, mm Hg | 72.6 ± 7.6 | 72.7 ± 7.4 | 80.1 ± 9.2 | 80.3 ± 9.2 | 75.3 ± 9.8 | 74.9 ± 9.7 | 0.51 |
| LDL-C,8 mg/dL | 131.9 ± 35.8 | 133.4 ± 34.8 | 133.6 ± 34.5 | 134.4 ± 38.2 | 135.2 ± 38.1 | 137.8 ± 33.1 | 0.96 |
| HDL-C,8 mg/dL | 60.5 ± 15.9 | 60.2 ± 15.0 | 56.8 ± 14.8 | 56.4 ± 14.9 | 56.5 ± 14.0 | 53.2 ± 13.0 | 0.70 |
| Triglyceride,9 mg/dL | 118.0 [74.0] | 123.0 [80.5] | 140.0 [91.0] | 139.0 [90.0] | 140.0 [92.0] | 129.0 [76.5] | 0.14 |
| Triglyceride:HDL-C ratio9 | 2.0 [1.7] | 2.1 [1.8] | 2.6 [2.2] | 2.5 [2.1] | 2.4 [2.2] | 2.5 [2.3] | 0.47 |
| Insulin,9 IU/ml | 8.9 [6.2] | 8.9 [5.9] | 11.9 [7.6] | 12.2 [8.7] | 12.6 [7.1] | 13.4 [8.9] | 0.44 |
| Glucose, mg/dL | 93.0 [14.0] | 93.0 [12.0] | 97.0 [19.5] | 96.0 [19.0] | 99.0 [27.0] | 103.0 [40.0] | 0.03 |
| HOMA-IR9 | 2.1 [1.7] | 2.1 [1.6] | 2.9 [2.5] | 3.0 [2.6] | 3.2 [2.4] | 3.8 [3.1] | 0.12 |
| Metabolic syndrome composite9,10 | 1.4 [1.2] | 1.3 [1.2] | 2.6 [1.2] | 2.6 [1.2] | 2.7 [1.2] | 2.8 [1.3] | 0.83 |
Data are presented as n (%), means ± SDs, or medians [IQRs]. BP, blood pressure; CABG/PCI, coronary bypass graft/percutaneous coronary intervention; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol; MI, myocardial infarction.
P value for a test of interaction between randomization group difference and strata, computed from a generalized linear model with the appropriate link function. For the analysis of baseline characteristics, regression models were unadjusted. A significant P value suggests that the association between the characteristic and the randomization group differs by baseline hypertension or prior history of CVD status.
Self-reported race/ethnicity.
Self-report of ever taking antihypertensive medication, or measured blood pressure ≥140/90 mm Hg.
Antihypertensive medications obtained from a medication inventory; medications include diuretics, calcium channel blockers, angiotensin-converting enzyme inhibitors, angiotensin II receptor antagonists, adrenergic receptor antagonists, aldosterone receptor antagonists, vasodilators, β-blockers, other antihypertensive medications, and combinations.
Self-report of hypercholesterolemia requiring medication.
Self-report of treated diabetes requiring oral agents or insulin injections.
Laboratory measurements based on a 5.8% subsample of trial participants.
Because of skewed distributions, medians [IQRs] are presented for these measurements, and P values are based on log-transformed data.
National Cholesterol Education Program's Adult Treatment Panel III definition (0–5 indicates best to worst).
TABLE 2.
Dietary intake and CVD risk factor changes during Dietary Modification Trial follow-up by randomization group, stratified by baseline hypertension and history of CVD1
| Women without prior CVD |
||||||||
| Normotensive women |
Hypertensive women |
Women with prior CVD |
||||||
| Post-randomization variables | Intervention (n = 9381) | Comparison (n = 13,867) | Intervention (n = 7914) | Comparison (n = 12,070) | Intervention (n = 663) | Comparison (n = 993) | P-main2 | P-interaction3 |
| Dietary characteristics at year 1 | ||||||||
| Calories from fat, % | 23.7 ± 7.3 | 34.9 ± 6.9 | 24.8 ± 7.5 | 35.4 ± 6.9 | 26.8 ± 8.0 | 35.2 ± 7.1 | <0.001 | <0.001 |
| Calories from protein, % | 17.6 ± 3.0 | 16.8 ± 3.1 | 17.8 ± 3.1 | 16.9 ± 3.1 | 17.7 ± 3.3 | 16.9 ± 3.2 | <0.001 | 0.77 |
| Calories from carbohydrates, % | 58.8 ± 8.8 | 48.0 ± 7.9 | 57.9 ± 8.9 | 47.7 ± 7.9 | 56.2 ± 9.4 | 48.4 ± 8.4 | <0.001 | <0.001 |
| Vegetable and fruit, medium servings/d | 5.1 ± 2.3 | 3.9 ± 2.0 | 5.1 ± 2.3 | 3.9 ± 2.0 | 4.7 ± 2.3 | 3.7 ± 2.0 | <0.001 | 0.003 |
| Grains, medium servings/d | 5.1 ± 2.6 | 4.2 ± 2.3 | 5.0 ± 2.7 | 4.2 ± 2.3 | 4.8 ± 2.9 | 4.0 ± 2.4 | <0.001 | 0.61 |
| Change in CVD risk factors (baseline to year 1) | ||||||||
| Weight, kg | −2.2 ± 8.7 | 0.1 ± 8.9 | −2.3 ± 8.3 | −0.1 ± 8.3 | −1.1 ± 7.7 | −0.1 ± 9.3 | <0.001 | 0.05 |
| Waist, cm | −1.8 ± 6.5 | −0.1 ± 6.5 | −1.9 ± 6.8 | −0.2 ± 6.5 | −1.6 ± 8.7 | −0.1 ± 6.5 | <0.001 | 0.90 |
| Systolic BP, mm Hg | −0.5 ± 12.2 | 0.4 ± 12.3 | −5.5 ± 17.6 | −5.1 ± 17.4 | −3.2 ± 17.8 | −1.5 ± 17.8 | <0.001 | 0.12 |
| Diastolic BP, mm Hg | −1.2 ± 7.8 | −0.4 ± 7.7 | −2.9 ± 9.4 | −2.3 ± 9.5 | −2.3 ± 9.7 | −0.9 ± 9.6 | <0.001 | 0.34 |
| LDL-C,4 mg/dL | −7.3 ± 23.0 | −5.4 ± 23.1 | −7.5 ± 24.3 | −5.6 ± 22.8 | 3.8 ± 37.3 | −12.1 ± 29.3 | 0.10 | 0.01 |
| HDL-C,4 mg/dL | −0.6 ± 8.7 | 1.1 ± 8.6 | −0.1 ± 8.6 | 1.0 ± 7.9 | −2.1 ± 11.3 | 1.7 ± 9.6 | <0.001 | 0.41 |
| Triglyceride,5 mg/dL | 6.0 [42.0] | 1.0 [47.0] | 3.0 [54.0] | 3.0 [49.0] | 4.5 [78.0] | 1.5 [57.0] | 0.27 | 0.44 |
| Triglyceride:HDL-C ratio5 | 0.11 [0.90] | −0.04 [0.91] | 0.07 [0.96] | 0.02 [1.01] | 0.29 [1.36] | −0.01 [1.35] | 0.009 | 0.27 |
| Insulin,5 IU/ml | −0.6 [3.7] | −0.1 [3.5] | −0.7 [4.8] | −0.1 [4.6] | 0.5 [6.6] | −0.7 [6.1] | <0.001 | 0.59 |
| Glucose,5 mg/dL | −2.0 [10.0] | 0.0 [10.0] | −1.0 [11.0] | 0.0 [11.0] | 0.0 [9.5] | 1.0 [19.0] | 0.004 | 0.62 |
| HOMA-IR5 | −0.2 [0.9] | −0.1 [0.9] | −0.2 [1.3] | −0.0 [1.3] | 0.1 [2.0] | −0.2 [1.7] | <0.001 | 0.69 |
| Metabolic syndrome composite5,6 | −0.0 [0.9] | 0.0 [0.9] | −0.2 [0.9] | −0.1 [0.9] | −0.1 [1.0] | 0.1 [1.0] | 0.03 | 0.51 |
Data are presented as means ± SDs or medians [IQRs]. BP, blood pressure; CVD, cardiovascular disease; HDL-C, HDL cholesterol; LDL-C, LDL cholesterol.
P value for a test of association between randomization group and dietary variables or CVD risk factor changes; computed from a generalized linear model with the appropriate link function.
P value for a test of interaction between randomization group difference and strata, computed from a generalized linear model with the appropriate link function. A significant P value suggests that the association between dietary variables or CVD risk factor changes and the randomization group differs by baseline hypertension or prior history of CVD status.
Laboratory measurements based on a 5.8% subsample of trial participants.
Because of skewed distributions, medians [IQRs] are presented for these measurements, and P values are based on log-transformed data.
National Cholesterol Education Program's Adult Treatment Panel III definition (0–5 indicates best to worst).
Overall changes in CVD risk factors between the intervention and comparison groups over the first year of intervention included reduced HDL cholesterol (P < 0.001) and increased triglyceride:HDL cholesterol (P = 0.009), as one might anticipate for a low-fat, high-carbohydrate diet; however, there were also reductions in insulin (P < 0.001) and glucose (P = 0.004), along with favorable changes in weight, waist circumference, and blood pressure (each P < 0.001). Although the low-fat diet design did not include energy restriction to promote weight loss, some weight loss occurred during the first year of trial participation and averaged (Table 2) ∼1 kg among women with prior CVD, compared with 2 kg for baseline healthy participants (P-interaction = 0.05). Otherwise, randomization group differences in risk factor changes during the first postrandomization year (Table 2) did not differ significantly across strata for a range of CVD risk factors, with one exception: LDL cholesterol at 1 y was lower in the intervention group by an estimated 2 mg/dL in women who were healthy at baseline but was larger by an estimated 15.9 mg/dL in women with prior CVD (P-interaction = 0.01). Evidence for this interaction was apparent in women taking cholesterol-lowering medications at baseline (P-interaction = 0.01) but was absent in women not taking these medications (P = 0.76). Furthermore, additional longitudinal analyses of LDL-cholesterol values for the intervention and comparison groups at 1, 3, and 6 y after randomization gave mean LDL cholesterol differences of −2.5 (95% CI: −4.8, −0.2), −1.8 (95% CI: −4.3, 0.6), and −2.8 (95% CI: −13.0, 7.3), respectively, which were quite similar across the 3 strata after the exclusion of women who were taking cholesterol-lowering medications at baseline, consistent with expectations for this dietary intervention.
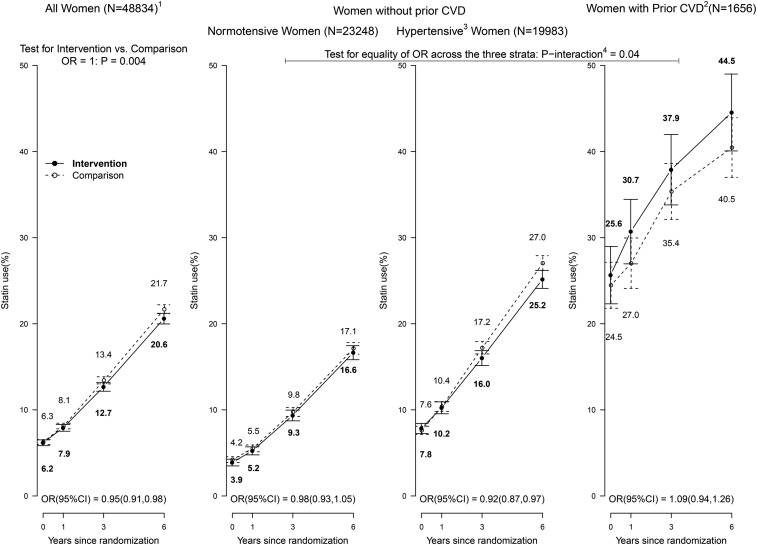
The percentages of women taking statins (β-hydroxy-β-methylglutaryl coenzyme A reductase inhibitors) at baseline were ∼4%, ∼8%, and ∼25% across the 3 strata (Table 1), increasing to ∼17%, ∼26%, and ∼42% by 6 y after randomization. As shown in Figure 2, based on medication inventories collected at 1, 3, and 6 y after randomization, postrandomization use of statins was lower in the intervention group than in the comparison group, with an OR of 0.95 (95% CI: 0.91, 0.98) and P = 0.004 for the null hypothesis. Moreover, the extent of differential statin use varied across the 3 strata (P-interaction = 0.04).
FIGURE 2.
Statin usage in the Dietary Modification Trial during the intervention period: overall, by baseline hypertension status among women without a history of CVD, and among women with a history of CVD. Summary statistics are shown for the overall cohort (all women randomly assigned; left panel), baseline hypertension subgroups among women without a history of CVD (middle panels), and women with a history of CVD (right panel) during the intervention period. Sample size indicates the number of participants with a baseline medication collection. To summarize statin usage during the 3 follow-up visits by randomization group (intervention or comparison), a mean OR (95% CI) was computed for all women and by subgroup through the use of generalized estimating equations with an unstructured correlation matrix. For the visual display, statin usage at each visit was computed as the percentage of women taking statins divided by the number of women with a medication collection; bow ties represent ∼95% confidence limits. 1Sample size of the overall cohort and baseline subgroups at randomization. 2Self-report of MI, CABG/PCI, or stroke before randomization; may also have baseline hypertension. 3Self-report of ever taking antihypertensive medication before randomization, or clinic-measured systolic/diastolic blood pressure ≥140/90 mm Hg; excludes women with missing baseline hypertension status data. 4P-interaction corresponds to a 2-df test for the interaction between OR and baseline hypertension or prior CVD strata. CABG/PCI, coronary artery bypass graft/percutaneous coronary intervention; CVD, cardiovascular disease; MI, myocardial infarction.
Additional analyses of statin use focused separately on statin cessation and statin initiation during the postrandomization period. Statin use was infrequent in healthy normotensive women and appeared to be nondifferential between randomization groups. Specifically, in this stratum, ORs were 0.91 (95% CI: 0.70, 1.19) for statin cessation and 0.97 (95% CI: 0.88, 1.07) for statin initiation for the intervention and comparison groups, respectively. In the baseline hypertensive stratum, women who received the diet intervention stopped statins more frequently (cessation OR: 1.33; 95% CI: 1.08, 1.63) and initiated statins less frequently (initiation OR: 0.91; 95% CI: 0.83, 0.99) than women in the comparison group. Statin use rates during follow-up did not differ significantly between randomization groups in women with prior CVD, but rates of change in statin use, combined cessation, or initiation were higher in the intervention group (OR: 1.22; 95% CI: 1.01, 1.48).
Additional analyses were carried out to further characterize the CHD risk reduction in the normotensive stratum. This CHD HR did not depend significantly on baseline diabetes status, with an HR of 0.67 (95% CI: 0.53, 0.84) for women without (treated) diabetes compared with 0.80 (95% CI: 0.44, 1.80) for women with diabetes at baseline (P-interaction = 0.46); however, there were only 32 CHD cases among women treated for diabetes at baseline. In addition, some women became hypertensive postrandomization. Incident (treated) hypertension was observed to be a CHD risk factor (P < 0.001), but it did not appear to mediate the dietary intervention effect on CHD. Specifically, an intervention compared with comparison group HR of 0.70 (95% CI: 0.56, 0.87) was obtained from analyses that included incident hypertension as a time-dependent variable. Yet further analyses estimated HRs separately according to this time-dependent incident hypertension variable. Women without incident hypertension had a CHD HR of 0.64 (95% CI: 0.50, 0.83) compared with 0.97 (95% CI: 0.62, 1.50) for those with incident hypertension (P-interaction = 0.11). The latter is similar to the CHD HR of 1.04 (Figure 1) in the baseline hypertension stratum.
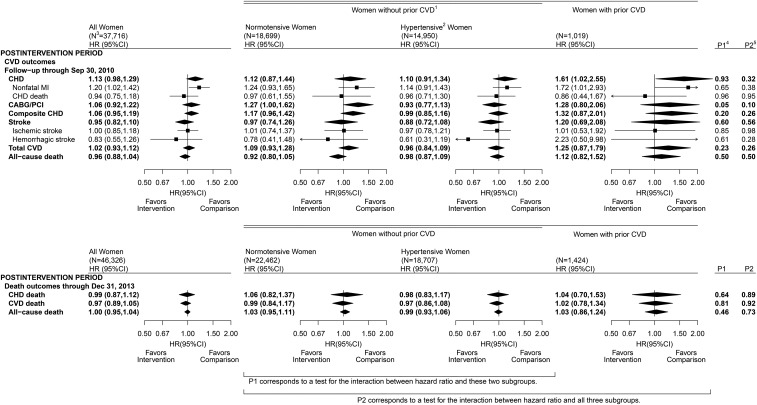
Figure 3 shows contrasts for the intervention and comparison groups for CVD outcomes and all-cause mortality during the postintervention period. HRs comparing randomization groups were mainly close to null and were not significantly different postintervention. As an exception, CHD incidence continued to be elevated in the intervention group in the postintervention period among women with prior CVD. This elevation depends on the use of cholesterol-lowering medications. For example, the CHD HR in women with prior CVD in the intervention group was nonsignificant (1.15; 95% CI: 0.63, 2.11) in the postintervention period after participants who were taking cholesterol-lowering medications at baseline were excluded.
FIGURE 3.
CVD outcomes in the Dietary Modification Trial during the postintervention period: overall, by baseline hypertension status among women without a history of CVD, and among women with a history of CVD. Summary statistics and forest plots are shown for the overall cohort (all randomly assigned women; left panel), baseline hypertension subgroups among women without a history of CVD (middle panels), and women with a history of CVD (right panel) during the postintervention period. 1Self-report of MI, CABG/PCI, or stroke before randomization; may also have baseline hypertension. 2Self-report before randomization of ever taking antihypertensive medication, or clinic-measured systolic/diastolic blood pressure ≥140/90 mm Hg; excludes women with missing baseline hypertension status data. 3Sample size of the overall cohort with active (upper panel) or passive (i.e., NDI linkage; lower panel) follow-up and baseline subgroups at randomization. 4P1 corresponds to a 1-df test for the interaction between HR and baseline hypertension status among women without prior CVD. 5P2 corresponds to a 2-df test for the interaction between HR and baseline hypertension or prior CVD strata. CABG/PCI, coronary artery bypass graft/percutaneous coronary intervention; CHD, coronary heart disease; CVD, cardiovascular disease; MI, myocardial infarction; NDI, National Death Index.
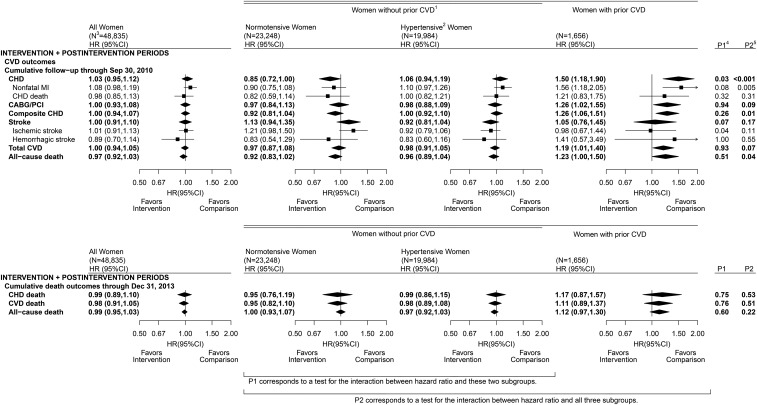
Finally, Figure 4 shows contrasts between the intervention and comparison groups for CVD outcomes and all-cause mortality over the cumulative intervention and postintervention phases. Although the overall CVD HRs did not differ significantly from unity, the CHD HRs varied strongly across the 3 strata (P < 0.001) over the combined intervention and postintervention trial phases.
FIGURE 4.
CVD outcomes in the Dietary Modification Trial during the cumulative intervention and postintervention periods: overall, by baseline hypertension status among women without a history of CVD, and among women with history of CVD. Summary statistics and forest plots are shown for the overall cohort (all women randomly assigned; left panel), baseline hypertension subgroups among women without a history of CVD (middle panels), and women with a history of CVD (right panel) during the postintervention period. 1Self-report of MI, CABG/PCI, or stroke before randomization; may also have baseline hypertension. 2Self-report of ever taking antihypertensive medication before randomization, or clinic-measured systolic/diastolic blood pressure ≥140/90 mm Hg; excludes women with missing baseline hypertension status data. 3Sample size of the overall cohort and baseline subgroups at randomization. 4P1 corresponds to a 1-df test for the interaction between HR and baseline hypertension status among women without prior CVD. 5P2 corresponds to a 2-df test for the interaction between HR and baseline hypertension or prior CVD strata. CABG/PCI, coronary artery bypass graft/percutaneous coronary intervention; CHD, coronary heart disease; CVD, cardiovascular disease; MI, myocardial infarction.
DISCUSSION
Intervention and comparison groups in this low-fat dietary pattern intervention trial overall did not differ in the incidence of CHD, total CVD, or total mortality, during either the trial intervention or postintervention periods. This is useful information, given the absence of previous clinical trial data on the effect of replacing dietary fat with carbohydrates on CVD risk and given related differences of opinion (7, 8).
However, randomization group contrasts differed strongly among subsets defined by baseline CVD and hypertension status. It is well known that subset analyses in clinical trials are fraught with challenges, especially regarding multiple testing issues. Here, however, the CHD HR variations are much greater than can be attributed to chance or to multiple testing, with P-interaction = 0.001 after conservative Bonferroni adjustment for the 15-subset comparison considered in our original report (2). Multiple testing concerns may also relate to the clinical outcomes considered. Breast and colorectal cancer were the designated primary outcomes, whereas CHD was the sole designated secondary outcome. A further multiple testing correction for these 3 outcome variables would yield a highly significant HR P-interaction = 0.003 for CHD.
The 3 subsets used in our analyses (normotensive, no prior CVD; hypertensive, no prior CVD; and prior CVD) had differing evidence for postrandomization confounding by statin use, thereby offering a potential explanation for some or all of the differences among strata in intervention effects on CHD risk.
The evidence for postrandomization confounding is substantial for women with prior CVD. Many of these women took statins at baseline, and statin use increased substantially during trial follow-up with women in the diet intervention group more likely to report changes in statin use (either cessation or initiation) postrandomization than women in the comparison group. Importantly, although comparison group participants in this stratum experienced the expected reduction in LDL cholesterol after this temporal increase in statin use, intervention group participants did not. Rather, the comparatively unfavorable LDL cholesterol change in the intervention group (Table 2), although based on measurements in only 73 women, likely reflects inappropriate cessation of cholesterol-lowering medications (9, 10). Consistent with this, much of the evidence for CHD risk elevation in women in the intervention group in this stratum, especially in the postintervention period, derived from women who were taking cholesterol-lowering medications at baseline. Participants who first initiated statins in the postintervention period had an LDL-cholesterol reduction that was ∼2 mg/dL greater in the intervention group than in the comparison group. A lack of interaction between the low-fat diet intervention and statin use with respect to LDL cholesterol reduction is consistent with results from other studies (11, 12). We concluded that trial results for CHD were uninterpretable in this prior CVD subset. We were not able to rule out the possibility that dietary changes in intervention group participants could have contributed to their unfavorable CHD experience. Others have hypothesized an unfavorable CHD effect based on studies in other contexts (13–15).
Hypertensive participants without prior CVD had CHD, CVD, and total mortality results that were essentially null in both the intervention and postintervention periods. Higher rates of statin cessation and modestly lower rates of statin initiation in the intervention group during trial follow-up could have masked a small CHD benefit in this group; alternatively, hypertension-related CVD risks may have simply outweighed any detectable benefit from the intervention diet. Unfortunately, CHD results in this rather large subset may also be confounded by differential patterns of statin use during the postintervention, although confounding effects on HRs are likely to be small.
In participants without prior CVD, diet intervention HRs for CHD were significantly lower in normotensive than in hypertensive women, even after (Bonferroni) multiple testing correction (P = 0.045). The CHD HR of 0.70 (95% CI: 0.56, 0.87) in normotensive women during the diet intervention period was unlikely to have been affected by postrandomization statin use, which remained comparatively infrequent and nondifferential. Mediation analyses were conducted to examine the influence of postrandomization differences in dietary variables and CVD risk factors on CHD HRs that contrast the intervention group with the comparison group. The HR was attenuated from 0.70 (95% CI: 0.57, 0.88) to 0.79 (95% CI: 0.62, 1.02) when the (time-varying) FFQ percentage of energy from total fat was added to an HR model that also included the baseline percentage of energy from fat. Hence, 29% (0.09 ÷ 0.30) of the reduction in CHD HR for the intervention group compared with the other group is explained by differences in the percentage of energy from fat. This percentage was unchanged when time-dependent differences in the percentage of energy from saturated fat, monounsaturated fat, or polyunsaturated fat were also added to the HR model, but it increased slightly to 32% when daily fruit and vegetable servings were added. These percentages, which are undoubtedly biased downward by dietary assessment measurement error (16), suggest that total fat reduction is an important driver of the observed CHD risk reduction. Similar analyses of body weight and blood pressure changes gave CHD HRs of 0.69 (95% CI: 0.55, 0.86) and 0.70 (95% CI: 0.56, 0.87), respectively, suggesting little ability of these changes to mediate a CHD intervention effect. Hence, to summarize various analyses, it may be that favorable effects on LDL cholesterol, insulin, and glucose (Table 2), mediated by dietary fat reduction, contribute importantly to explaining the observed CHD risk reduction in this stratum.
The value of a CHD benefit for this dietary pattern change, however, may be tempered by a corresponding increase in stroke risk during the intervention period, with HRs of 1.29 (95% CI: 1.00, 1.66) for total stroke and 1.46 (95% CI: 1.07, 1.97) for ischemic stroke. These results may be more susceptible to multiple testing biases because stroke was not a designated primary or secondary trial outcome. Mediation analyses indicate that only a nonsignificant 4% of the ischemic stroke elevation can be attributed to differences in the percentages of energy from fat between the intervention and comparison groups, whereas differences in fruit and vegetable servings, body weight, blood pressure, and incident hypertension have essentially no mediation potential. Gillman et al. (17) reported a reduction in ischemic stroke risk in men in the Framingham Study who consumed a high-fat diet. In response, Sherwin and Price (18) wrote that only the WHI “might have the potential to determine if a major reduction in total fat (i.e., to approximately 20% of calories) would result in an increase in the rate of ischemic stroke.” Our analyses offer some support for this hypothesis, but chance provides another explanation, particularly because the estimated risk elevation seems unrelated to the measured dietary changes.
These analyses, in which there is little potential for postrandomization confounding by cholesterol-lowering medications, suggest that CVD incidence rates in healthy postmenopausal women in the United States are quite sensitive to moderate dietary change. Intervention participants chose varying approaches toward achieving dietary goals, with a corresponding range of potential cardiovascular benefits and risks. Trial data are currently undergoing further analyses in an attempt to identify dietary pattern changes by women in the intervention group that retain CHD benefit while avoiding any adverse effects.
Study strengths include the large sample size, randomized controlled design, and acceptable adherence and retention estimates for the study sample. Study limitations include the inability of these analyses to fully dissect the influence of specific dietary pattern changes beyond intervention goals, some reduction in dietary adherence later in the intervention period, and, especially, unplanned and differential changes in cholesterol-lowering medication use during the trial follow-up period.
Acknowledgments
We thank the following WHI program investigators—Program Office: Jacques E Rossouw, Shari Ludlam, Dale Burwen, Joan McGowan, Leslie Ford, and Nancy Geller (National Heart, Lung, and Blood Institute, Bethesda, MD); Clinical Coordinating Center: Women's Health Initiative Clinical Coordinating Center: (Public Health Sciences, Fred Hutchinson Cancer Research Center, Seattle, WA) Garnet L. Anderson, Ross L. Prentice, Andrea Z. LaCroix, and Charles L. Kooperberg; Investigators and Academic Centers: (Brigham and Women's Hospital, Harvard Medical School, Boston, MA) JoAnn E. Manson; (MedStar Health Research Institute/Howard University, Washington, DC) Barbara V. Howard; (Stanford Prevention Research Center, Stanford, CA) Marcia L. Stefanick; (The Ohio State University, Columbus, OH) Rebecca Jackson; (University of Arizona, Tucson/Phoenix, AZ) Cynthia A. Thomson; (University at Buffalo, Buffalo, NY) Jean Wactawski-Wende; (University of Florida, Gainesville/Jacksonville, FL) Marian C. Limacher; (University of Iowa, Iowa City/Davenport, IA) Robert M. Wallace; (University of Pittsburgh, Pittsburgh, PA) Lewis H. Kuller; (Wake Forest University School of Medicine, Winston-Salem, NC) Sally A. Shumaker Women's Health Initiative Memory Study: (Wake Forest University School of Medicine, Winston-Salem, NC) Sally A. Shumaker. A list of all of the investigators who have contributed to WHI science is available online (https://cleo.whi.org/researchers/SitePages/Write%20a%20Paper.aspx).
The authors’ responsibilities were as follows—RLP, AKA, LVH, and BVH: drafted the manuscript; RLP and AKA: had full access to WHI data and took responsibility for the accuracy of data and analyses presented; and all authors: contributed to critical evaluation of the manuscript and to the interpretation of results, and read and approved the final manuscript. In addition, all authors participated actively in the conduct of the WHI program, including its data gathering, quality control, and funding aspects. None of the authors reported a conflict of interest related to the study.
Footnotes
Abbreviations used: CHD, coronary heart disease; CVD, cardiovascular disease; FFQ, food frequency questionnaire; NDI, National Death Index; WHI, Women’s Health Initiative.
REFERENCES
- 1.Women’s Health Initiative Study Group. Design of the Women’s Health Initiative clinical trial and observational study. Control Clin Trials 1998;19:61–109. [DOI] [PubMed] [Google Scholar]
- 2.Howard BV, Van Horn L, Hsia J, Manson JE, Stefanick ML, Wassertheil-Smoller S, Kuller LH, LaCroix AZ, Langer RD, Lasser NL, et al. . Low-fat dietary pattern and risk of cardiovascular disease: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:655–66. [DOI] [PubMed] [Google Scholar]
- 3.Prentice RL, Caan B, Chlebowski RT, Patterson R, Kuller LH, Ockene JK, Margolis KL, Limacher MC, Manson JE, Parker LM, et al. . Low-fat dietary pattern and risk of invasive breast cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:629–42. [DOI] [PubMed] [Google Scholar]
- 4.Beresford SA, Johnson KC, Ritenbaugh C, Lasser NL, Snetselaar LG, Black HR, Anderson GL, Assaf AR, Bassford T, Bowen D, et al. . Low-fat dietary pattern and risk of colorectal cancer: the Women’s Health Initiative Randomized Controlled Dietary Modification Trial. JAMA 2006;295:643–54. [DOI] [PubMed] [Google Scholar]
- 5.Howard BV, Curb JD, Eaton CB, Kooperberg C, Ockene J, Kostis JB, Pettinger M, Rajkovic A, Robinson JG, Rossouw J, et al. . Low-fat dietary pattern and lipoprotein risk factors: the Women’s Health Initiative Dietary Modification Trial. Am J Clin Nutr 2010;91:860–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomson CA, Van Horn L, Caan BJ, Aragaki AK, Chlebowski RT, Manson JE, Rohan TE, Tinker LF, Kuller LH, Hou L, et al. . Cancer incidence and mortality during the intervention and postintervention periods of the Women’s Health Initiative Dietary Modification Trial. Cancer Epidemiol Biomarkers Prev 2014;23:2924–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siri-Tarino PW, Sun Q, Hu FB, Krauss RM. Saturated fat, carbohydrate, and cardiovascular disease. Am J Clin Nutr 2010;91:502–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stamler J. Diet–heart: a problematic revisit. Am J Clin Nutr 2010;91:497–9. [DOI] [PubMed] [Google Scholar]
- 9.Ho PM, Rumsfeld JS, Masoudi FA, McClure DL, Plomondon ME, Steiner JF, Magid DJ. Impact of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med 2006;166:1836–41. [DOI] [PubMed] [Google Scholar]
- 10.Nielsen SF, Nordestgaard BG. Negative statin-related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur Heart J 2016;37:908–16. [DOI] [PubMed] [Google Scholar]
- 11.Hunninghake DB, Stein EA, Dujovne CA, Harris WS, Feldman EB, Miller VT, Tobert JA, Laskarzewski PM, Quiter E, Held J, et al. . The efficacy of intensive dietary therapy alone or combined with lovastatin in outpatients with hypercholesterolemia. N Engl J Med 1993;328:1213–9. [DOI] [PubMed] [Google Scholar]
- 12.Clifton PM, Wight MB, Nestel PJ. Is fat reduction needed with HMGCoA reductase inhibitor treatment? Atherosclerosis 1992;93:59–70. [DOI] [PubMed] [Google Scholar]
- 13.Mozaffarian D, Rimm EB, Herrington DM. Dietary fats, carbohydrate, and progression of coronary atherosclerosis in postmenopausal women. Am J Clin Nutr 2004;80:1175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mozaffarian D. Low-fat diet and cardiovascular disease. JAMA 2006;296:279–80. [DOI] [PubMed] [Google Scholar]
- 15.Anderson CA, Appel LJ. Dietary modification and CVD prevention: a matter of fat. JAMA 2006;295:693–5. [DOI] [PubMed] [Google Scholar]
- 16.Zhao S, Prentice RL. Covariate measurement error correction methods in mediation analyses with failure time data. Biometrics 2014;70:835–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gillman MW, Cupples LA, Millen BE, Ellison RC, Wolf PA. Inverse association of dietary fat with development of ischemic stroke in men. JAMA 1997;278:2145–50. [PubMed] [Google Scholar]
- 18.Sherwin R, Price TR. Fat chance: diet and ischemic stroke. JAMA 1997;278:2185–6. [DOI] [PubMed] [Google Scholar]