Abstract
The purpose of this study was to evaluate for differences in variations in pro- and anti-inflammatory cytokine genes between participants who were classified as having low and high levels of morning and evening fatigue and to evaluate for differences in phenotypic characteristics between these two groups. In a sample of 167 oncology outpatients with breast, prostate, lung, or brain cancer and 85 of their family caregivers, growth mixture modeling (GMM) was used to identify latent classes of individuals based on ratings of morning and evening fatigue obtained prior to, during, and for 4 months following completion of radiation therapy. Differences in single nucleotide polymorphisms (SNPs) and haplotypes in 15 cytokine genes were evaluated between the latent classes. Multiple logistic regression was used to assess the effect of phenotypic and genotypic characteristics on morning and evening fatigue class membership. Associations were found between morning fatigue and number of comorbidities as well as variations in TNFA rs1800629 and rs3093662. Evening fatigue was associated with caring for children at home and variations in IL4 rs2243248 and TNFA rs2229094. Younger age and lower performance status was associated with both morning and evening fatigue. These findings suggest that inflammatory mediators are associated with the development of morning and evening fatigue. However, because different phenotypic characteristics and genomic markers are associated with diurnal variations in fatigue, morning and evening fatigue may be distinct but related symptoms.
Keywords: cytokines, genetics, morning fatigue, evening fatigue, breast cancer, tumor necrosis factor alpha, interleukin 4
Fatigue is a frequent and disabling symptom (Lawrence, Kupelnick, Miller, Devine, & Lau, 2004) that occurs in approximately 80% of patients receiving radiation therapy (Henry et al., 2008; Hofman, Ryan, Figueroa-Moseley, Jean-Pierre, & Morrow, 2007) and in 24–30% of family caregivers of patients with cancer (Swore Fletcher, Dodd, Schumacher, & Miaskowski, 2008). Although authors have reported on the high prevalence and negative impact of this symptom for over 30 years, little is known about the mechanisms that underlie fatigue.
While the etiology of fatigue is undoubtedly multifactorial, a growing body of evidence suggests that inflammatory pathways are important in the development of this symptom (Barsevick, Frost, Zwinderman, Hall, & Halyard, 2010; Jager, Sleijfer, & van der Rijt, 2008; Reyes-Gibby et al., 2008; Schubert, Hong, Natarajan, Mills, & Dimsdale, 2007). In fact, several studies have evaluated for associations between serum cytokine levels and fatigue occurrence or severity. The findings of a quantitative review (Schubert et al., 2007) suggested a positive correlation between fatigue and circulating levels of inflammatory markers. However, of the 19 circulating inflammatory markers evaluated, only interleukin (IL)-6, IL1-receptor alpha (IL-1rα), and neopterin remained significant in the final analyses. These inconclusive findings may be due to the challenges inherent in the measurement of circulating cytokines and to circadian variability in cytokine levels (Gilbertson-White, Aouizerat, & Miaskowski, 2011).
In addition to the studies of serum cytokines, several studies have documented associations between variations in cytokine genes and fatigue (Aouizerat et al., 2009; Bower, Ganz, Irwin, Arevalo, & Cole, 2011; Collado-Hidalgo, Bower, Ganz, Irwin, & Cole, 2008; Hong et al., 1995; Miaskowski et al., 2010; Reinertsen et al., 2011). In a study of 33 fatigued and 14 nonfatigued breast cancer survivors, Collado-Hidalgo et al. (2008) found that, while IL6 was not associated with fatigue, IL1B did show an association. In a study conducted by our research team (Miaskowski et al., 2010), oncology patients (n = 288) and family caregivers (n = 103) who were homozygotes for the common allele in IL6 rs4719714 reported higher levels of morning and evening fatigue and sleep disturbance. In addition, in this same sample, individuals who were homozygous for the common allele in tumor necrosis factor alpha (TNFA) rs1800629 reported higher levels of morning fatigue and sleep disturbance (Aouizerat et al., 2009). The same single nucleotide polymorphism (SNP) in TNFA was associated with symptoms of exhaustion and higher C-reactive protein levels in patients with chronic fatigue syndrome (Jeanmonod, von Kanel, Maly, & Fischer, 2004). In another study, in which researchers compared fatigued (n = 11) and nonfatigued (n = 10) breast cancer survivors, nuclear factor kappa beta (NFKB) transcripts were shown to be increased among fatigued survivors (Bower et al., 2011). However, in a large study of breast cancer survivors, only one of seven SNPs in five cytokine genes (i.e., C-reactive protein [CRP] rs3091244) was associated with fatigue (Reinertsen et al., 2011).
One of the limitations of the research studies conducted to date is that diurnal variations in fatigue severity and associated phenotypic and genotypic characteristics were not evaluated in detail. In a recent paper from our research team that used growth mixture modeling (GMM) to identify latent classes of individuals with distinct morning and evening fatigue trajectories among oncology patients and their family caregivers (Dhruva et al., 2013), we found phenotypic characteristics that distinguished among the morning and evening fatigue classes. For example, participants in the High Morning Fatigue class were more likely to be younger and have a lower functional status than participants in the Low Morning Fatigue class. In contrast, participants in the High Evening Fatigue class were more likely to be younger, female, caring for children at home, and a family caregiver than participants in the Low Evening Fatigue class. In addition, only 10.3% of the sample was classified as being in both the lowest morning and evening fatigue classes and only 41.3% of the sample was classified in both the highest morning and evening fatigue classes. Based on these findings, we suggested that morning and evening fatigue may be distinct but related symptoms.
In this paper, we extend these findings and evaluate for differences in a number of pro- and anti-inflammatory cytokine genes among those participants who were classified as having low and high levels of morning and evening fatigue.
Methods
Participants and Settings
Details of this study are published elsewhere (Aouizerat et al., 2009; Carney et al., 2011; Dhruva et al., 2012; Dunn et al., 2012; Miaskowski et al., 2010; Miaskowski et al., 2011). In brief, patients and their family caregivers (FCs) were recruited from two radiation-therapy departments located in a comprehensive cancer center and a community-based oncology program at the time of the patients' simulation visit.
Patients were eligible to participate if they were ≥18 years of age; were scheduled to receive primary or adjuvant radiation therapy for one of four cancer diagnoses (i.e., breast, prostate, lung, or brain); were able to read, write, and understand English; gave written informed consent; and had a Karnofsky Performance Status (KPS) score of ≥ 60. Patients were excluded if they had metastatic disease, more than one cancer diagnosis, or a diagnosed sleep disorder. FCs were eligible to participate if they were ≥18 years of age; were able to read, write, and understand English; gave written informed consent; had a KPS score of ≥ 60; were living with the patient; and did not have a diagnosed sleep disorder.
Instruments
We used a demographic questionnaire to obtain information on age, gender, marital status, education, ethnicity, employment status, and the presence of a number of comorbid conditions. We also reviewed medical records for disease and treatment information.
The Lee Fatigue Scale (LFS), which we used in the present study, consists of 13 items designed to assess physical fatigue (K. A. Lee, Hicks, & Nino-Murcia, 1991). A total fatigue score is calculated as the mean of the 13 items, with higher scores indicating greater fatigue severity. Participants are asked to rate each item based on how they feel “right now” within 30 min of awakening (morning fatigue) and prior to going to bed (evening fatigue). The LFS has well established validity and reliability. In the present study, Cronbach's alphas for evening and morning fatigue at enrollment were 0.96 and 0.95 for patients and 0.95 and 0.96 for FCs, respectively.
Study Procedures
The study was approved by the Committee on Human Research at the University of California, San Francisco, and the Institutional Review Board at the second site. We invited patients to participate in the study approximately 1 week prior to the start of radiation therapy (i.e., simulation visit when the measurements for radiation therapy are made). If the FC was present, a research nurse explained the study protocol to both the patient and FC, determined eligibility, and obtained written informed consent. FCs who were not present were contacted by phone to determine their interest in participation. These FCs completed the enrollment procedures at home. Participants completed the LFS at enrollment, 4 weeks after the initiation of radiation therapy, at the completion of radiation therapy, and at 4, 8, 12, and 16 weeks after the completion of radiation therapy (i.e., seven assessments over 6 months).
Methods of Analysis for Phenotypic Data
Data were analysed using SPSS, version 19 (SPSS, 2010), and Mplus, version 6.11 (L. K. Muthen & Muthen, 1998-2010). Descriptive statistics and frequency distributions were generated on sample characteristics and fatigue severity scores. Independent sample t-tests, analyses of variance (ANOVA), and Chi-square analyses were done to evaluate for differences in demographic and clinical characteristics between the fatigue classes.
GMM with robust maximum likelihood estimation was used to identify latent classes (i.e., subgroups of participants) with distinct morning and evening fatigue trajectories over the 6 months of the study (B. O. Muthen & Kaplan, 2004). Because 65% of the participants were in patient–caregiver dyads, models were estimated with “dyad” as a clustering variable to ensure that any dependency between the morning and evening fatigue scores for patients and FCs in the same dyad was controlled for in the GMM analysis. It should be noted that after taking any dependency within dyads into account, no significant differences were found between patients and FCs in the parameter estimates for the various morning and evening fatigue GMM trajectories that were identified in the initial analysis.
As reported previously (Dhruva et al., 2013), three distinct latent classes were identified for morning and evening fatigue. For the candidate gene analyses reported in the present paper, the three morning fatigue classes were collapsed into two groups (i.e., Very Low [32.5%] versus Low and High [67.5%]) as were the three evening fatigue classes (i.e., Low [11.1%] versus Moderate and High [88.9%]). The rationale for this categorization is that very low levels of morning fatigue and low levels of evening fatigue might be expected in the general population. This extreme-phenotype approach is an effective strategy to identify potential candidate genes associated with symptoms or clinical conditions (Li, Lewinger, Gauderman, Murcray, & Conti, 2011).
Adjustments were not made for missing data. Therefore, the cohort for each analysis was dependent on the largest set of available data across groups. A p-value of < .05 was considered statistically significant.
Methods of Analysis for Genomic Data
Gene selection
Pro-inflammatory cytokines promote systemic inflammation and include interferon gamma (IFNγ), IFNγ receptor 1 (IFNγR1), IL-1R1, IL-2, IL-8, IL-17A, NFKB1, NFKB2, and TNF-α. Anti-inflammatory cytokines suppress the activity of pro-inflammatory cytokines and include IL-1R2, IL-4, IL-10, and IL-13. Of note, IFNγ1, IL-1β, and IL-6 possess pro- and anti-inflammatory characteristics (Seruga, Zhang, Bernstein, & Tannock, 2008).
Blood collection and genotyping
Genomic deoxyribonucleic acid (DNA) was extracted from archived buffy coats using the PUREGene DNA Isolation System (Invitrogen, Carlsbad, CA). Of the 287 participants recruited, DNA was recovered from 253 participants (i.e., 168 patients and 85 FCs). Samples were genotyped using the GoldenGate genotyping platform (Illumina, San Diego, CA) and processed using GenomeStudio (Illumina, San Diego, CA).
Single nucleotide polymorphism (SNP) selection
We selected a combination of tagging SNPs and SNPs suggested by the literature for analyses. Tagging SNPs were required to be common (i.e., defined as having a rare allele frequency ≥ 0.05) in public databases. In order to ensure robust genetic association analyses, we performed quality control filtering of the SNPs. SNPs with call rates of < 95% or Hardy-Weinberg p-values of < .001 were excluded.
As shown in supplemental Table 1 <PRODUCTION: Please add link here to online table, if possible>, a total of 92 SNPs among the 15 candidate genes passed all quality control filters and were included in the genetic association analyses (IFNG1: 5 SNPs; IFNGR1: 1 SNP; IL1B: 12 SNPs; IL1R1: 5 SNPs; IL1R2: 3 SNPs; IL2: 5 SNPs; IL4: 8 SNPs; IL6: 9 SNPs; IL8: 3 SNPs; IL10: 8 SNPs; IL13: 4 SNPs; IL17A: 5 SNPs; NFKB1: 11 SNPs; NFKB2: 4 SNPs; TNFA: 9 SNPs). Potential functional roles of SNPs associated with specific symptoms were examined using PUPASuite 2.0 (Conde et al., 2006).
Statistical analyses
Allele and genotype frequencies were determined by gene counting. Hardy-Weinberg equilibrium was assessed by the Chi-square or Fisher's exact tests. Measures of linkage disequilibrium (LD; i.e., D' and r2) were computed from the participants' genotypes with Haploview 4.2. LD-based haplotype block definition was based on D' confidence interval (Gabriel et al., 2002).
Haplotypes were constructed using the program PHASE, version 2.1 (Stephens, Smith, & Donnelly, 2001). Only haplotypes that were inferred with probability estimates of ≥ .85 across five iterations were retained for downstream analyses. Haplotypes were evaluated assuming a dosage model.
Ancestry informative markers (AIMS) were used to minimize confounding due to population stratification (Halder, Shriver, Thomas, Fernandez, & Frudakis, 2008; Hoggart et al., 2003; Tian, Gregersen, & Seldin, 2008). Homogeneity in ancestry among participants was verified by principal component analysis (Price et al., 2006) using Helix Tree (Golden Helix, Bozeman, MT). Included in the analysis were 106 AIMs. The first three PCs were selected to adjust for potential confounding due to population substructure (i.e., race/ethnicity) by including the three covariates in all regression models.
For association tests, three genetic models were assessed for each SNP: additive, dominant, and recessive. Barring trivial improvements (i.e., delta < 10%), the genetic model that best fit the data, by maximizing the significance of the p-value, was selected for each SNP. Logistic regression analysis, which controlled for significant covariates as well as for race/ethnicity, was used to evaluate the association between genotype and fatigue-group membership. A backwards stepwise approach was used to create a parsimonious model. Except for self-reported race/ethnicity and AIMS, only predictors with a p-value of < .05 were retained in the final model. Genetic model fit and both unadjusted and covariate-adjusted odds ratios were estimated using STATA version 9.
As was done in our previous studies (Illi et al., 2012; McCann et al., 2012; C Miaskowski et al., 2012), based on recommendations in the literature (Hattersley & McCarthy, 2005; Rothman, 1990), the implementation of rigorous quality controls for genomic data, the non-independence of SNPs/haplotypes in LD, and the exploratory nature of the analyses, adjustments were not made for multiple testing. In addition, significant SNPs identified in the bivariate analyses were evaluated further using regression analyses that controlled for differences in phenotypic characteristics, potential confounding due to population stratification, and variation in other SNPs/haplotypes within the same gene. Only those SNPs that remained significant were included in the final presentation of the results. Therefore, the significant independent associations reported are unlikely to be due solely to chance. Unadjusted associations are reported for all SNPs passing quality control criteria in supplemental Table 1 to allow for subsequent comparisons and meta-analyses.
Results
Participant Characteristics
The total sample, which was 46.2% male and 53.8% female, consisted of 167 oncology outpatients and 85 FCs. The majority of the participants were well educated and Caucasian with a mean age of 61.5 years. The mean KPS score was 92 and the average participant had more than four comorbid conditions. Approximately 49% of the patients had prostate cancer, 38% had breast cancer, 7% had a brain tumor, and 6% had lung cancer. We found no significant differences between patients' and FCs' ratings of morning fatigue (2.3 ± 2.0 versus 2.3 ± 1.9, respectively) and evening fatigue (4.2 ± 2.0 versus 4.5 ± 2.0, respectively) at enrollment.
Phenotypic Differences Between the Morning Fatigue Classes
Compared to those in the Very Low morning fatigue class (n = 82 [32.5%]), participants in the Low and High classes (combined n = 170 [67.5%]) were significantly younger and had a higher number of comorbidities and a significantly lower KPS score (Table 1). Within the Very Low morning fatigue class, we found no differences between patients' (0.7 ± 1.0) and FCs' (0.9 ± 0.9) ratings of morning fatigue at enrollment. Within the Low and High morning fatigue classes, we found no differences between in patients' (3.1 ± 1.8) and FCs' (3.0 ± 2.0) ratings of morning fatigue at enrollment.
Table 1. Differences in demographic and clinical characteristics at enrollment between participants (N = 167 patients and 85 FCs) by fatigue class.
| Characteristic | Very Low Morning
Fatigue (n = 82) Mean (SD) |
Low and High Morning
Fatigue (n = 170) Mean (SD) |
Statistics for Morning Fatigue | Low Evening
Fatigue (n = 28) Mean (SD) |
Moderate and High Evening
Fatigue (n = 224) Mean (SD) |
Statistics for Evening Fatigue |
|---|---|---|---|---|---|---|
| Age (years) | 64.9 (10.6) | 59.0 (11.2) | t = 3.36, p = .001 | 66.5 (9.0) | 60.9 (11.4) | t = 2.52, p = .012 |
| Education (years) | 15.8 (3.0) | 16.0 (3.0) | NS | 15.4 (3.2) | 16.0 (3.0) | NS |
| Number of comorbid conditions | 4.1 (2.7) | 4.9 (2.7) | t = -2.10, p = .029 | 3.9 (2.9) | 4.7 (2.8) | NS |
| Weight (lb) | 178.5 (34.1) | 173.6 (41.2) | NS | 180.0 (36.3) | 174.8 (39.5) | NS |
| KPS score | 96.5 (7.9) | 89.8 (12.4) | t = 5.14, p < .0001 | 95.7 (8.4) | 91.4 (11.8) | t = 2.40, p = .020 |
|
|
|
|||||
| n (%) | n (%) | n (%) | n (%) | |||
|
|
|
|||||
| Gender (female) | 39 (47.6) | 96 (56.5) | NS | 11 (39.3) | 124 (55.4) | NS |
| Ethnicity (White) | 66 (80.5) | 122 (71.8) | NS | 19 (67.9) | 169 (75.4) | NS |
| Lives alone (yes) | 14 (26.4) | 39 (34.2) | NS | 10 (52.6) | 43 (29.1) | NS |
| Married or partnered (yes) | 61 (74.4) | 113 (67.3) | NS | 17 (60.7) | 157 (70.7) | NS |
|
| ||||||
| Children at home (yes) | 9 (12.9) | 27 (19.1) | NS | 0 (0.0) | 36 (19.1) | FE = .017 |
| Older adult at home (yes) | 3 (4.3) | 4 (2.8) | NS | 2 (8.7) | 5 (2.6) | NS |
| Works for pay (yes) | 34 (42.0) | 81 (48.8) | NS | 10 (35.7) | 105 (47.9) | NS |
| Patient/FC (patient) | 53 (64.6) | 114 (67.1) | NS | 19 (67.9) | 148 (66.1) | NS |
Note. FC = family caregiver; FE = Fisher's Exact; KPS = Karnofsky Performance Status; NS = not significant; SD = standard deviation.
Candidate Gene Analyses for the Morning Fatigue Classes
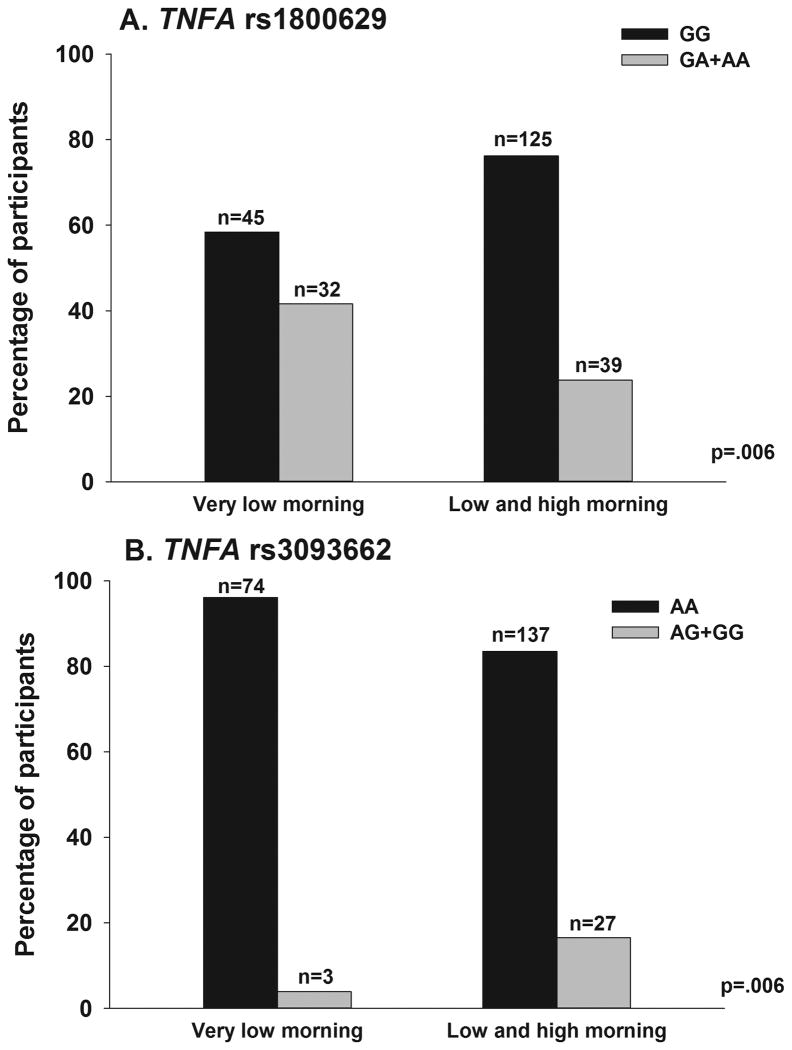
Of the five SNPs that differed significantly between the two morning fatigue classes (Supplementary Table 1), two associations in one gene remained significant in the multivariate regression analyses (i.e., TNFA rs1800629, TNFA rs3093662). For both SNPs, a dominant model fit the data best (both p = 0.006).
Regression Analyses of Candidate Genes for Morning Fatigue Classes
In order to better estimate the magnitude (i.e., odds ratio, OR) and precision (95% confidence interval, CI) of genotype on morning fatigue class membership, multiple variable logistic regression models were fit to compare the two classes. In addition to genotype, the phenotypic characteristics that were included in the models were age, KPS score, and self-reported and genomic estimates of race/ethnicity (Table 2).
Table 2. Multiple logistic regression analyses for morning fatigue groups and TNFA candidate genes.
| Predictor | Odds Ratio | Standard Error | 95% CI | Z | p-value |
|---|---|---|---|---|---|
| TNFA rs1800629 | 0.48 | 0.157 | 0.252, 0.910 | -2.25 | 0.025 |
| Age | 0.79 | 0.063 | 0.673, 0.922 | -2.97 | 0.003 |
| KPS score | 0.54 | 0.102 | 0.371, 0.782 | -3.26 | 0.001 |
| Overall model fit: χ2 = 39.39, p < 0.0001, R2 = 0.1342 | |||||
| TNFA rs3093662 | 6.59 | 4.369 | 1.796, 24.171 | 2.84 | 0.004 |
| Age | 0.76 | 0.062 | 0.645, 0.889 | -3.39 | 0.001 |
| KPS score | 0.53 | 0.103 | 0.365, 0.780 | -3.24 | 0.001 |
| Overall model fit: χ2 = 45.43, p < 0.0001, R2 = 0.1548 | |||||
Note. For each model, the first three principal components identified from the analysis of ancestry informative markers as well as self-reported race/ethnicity (White, Asian/Pacific Islander, Black, Hispanic/mixed background/other) were retained in all models to adjust for potential confounding due to race or ethnicity (data not shown). Predictors evaluated in the model included genotype (TNFA rs1800629: GG versus GA+AA; TNFA rs3093662: AA versus AG + GG), age (5-year increments), and functional status (KPS score, 10-point increments). CI = confidence interval; KPS = Karnofsky Performance Status; TNFA = tumor necrosis factor alpha gene.
In the regression analysis for TNFA rs1800629, being heterozygous or homozygous for the rare A allele (GG versus GA+AA) was associated with a 52% decrease in the odds of belonging to the higher morning fatigue group (Figure 1A). In the regression analysis for TNFA rs309662, being heterozygous or homozygous for the rare G allele (AA versus AG+GG) was associated with a 6.59-fold increase in the odds of belonging to the higher morning fatigue group (Figure 1B).
Figure 1.
Differences between the morning fatigue latent classes in the percentages of participants who were A) homozygous for the common allele (GG) or heterozygous or homozygous for the rare allele (GA+AA) for rs1800629 and B) homozygous for the common allele (AA) or heterozygous or homozygous for the rare allele (AG+GG) for rs3093662 in tumor necrosis factor alpha (TNFA). Values are plotted as unadjusted proportions with corresponding p-value.
Phenotypic Differences Between the Evening Fatigue Classes
Compared to the Low evening fatigue class, participants in the Moderate and High classes were significantly younger, had a significantly lower KPS score, and were more likely to have children living at home (Table 2). Within the Low evening fatigue class, we found no differences between patients' (1.2 ± 1.2) and FCs' (1.2 ± 0.8) ratings of evening fatigue at enrollment. Likewise, we found no differences within the Moderate and High evening fatigue classes between patients' (4.5 ± 1.8) and FCs' (4.8 ± 1.8) ratings of evening fatigue at enrollment.
Candidate Gene Analyses for the Evening Fatigue Classes
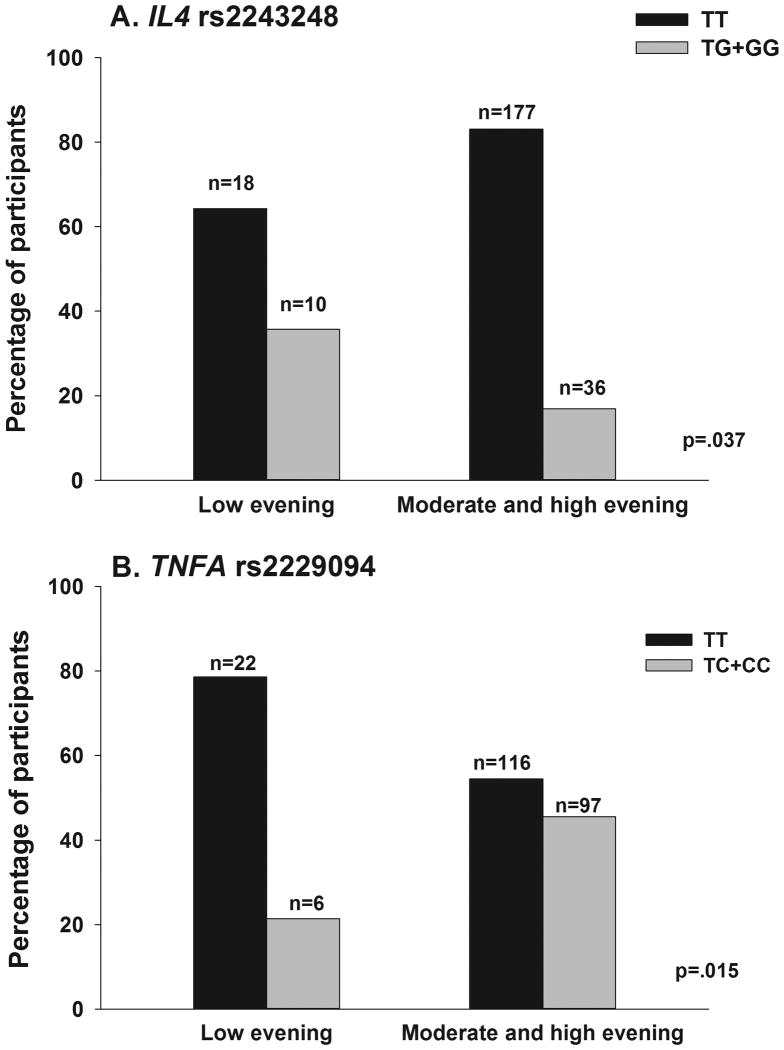
Of the eight SNPs that differed significantly between the two evening fatigue classes (Supplementary Table 1), one association in each of two genes remained significant in the multivariate regression analyses (i.e., IL4 rs2243248, TNFA rs2229094). For both SNPs, a dominant model fit the data best.
Regression Analyses of Candidate Genes for Evening Fatigue Classes
In order to better estimate the magnitude (i.e., OR) and precision (95% CI) of genotype on evening fatigue class membership, multiple variable logistic regression models were fit to compare the two classes. In addition to genotype, the phenotypic characteristics that were included in the models were age and self-reported and genomic estimates of race/ethnicity (Table 3).
Table 3. Multiple logistic regression analyses for evening fatigue groups and cytokine candidate genes.
| Predictor | Odds Ratio | Standard Error | 95% CI | Z | p-value |
|---|---|---|---|---|---|
| IL4 rs2243248 | 0.30 | 0.143 | 0.120, 0.762 | -2.54 | 0.011 |
| Age | 0.73 | 0.082 | 0.583, 0.910 | -2.79 | 0.005 |
| Overall model fit: χ2 = 15.86, p = 0.04, R2 = 0.0917 | |||||
| TNFA rs2229094 | 3.75 | 1.897 | 1.389, 10.110 | 2.61 | 0.009 |
| Age | 0.71 | 0.081 | 0.571, 0.891 | -2.98 | 0.003 |
| Overall model fit: χ2 = 17.86, p = 0.02, R2 = 0.1033 | |||||
Note. For each model, the first three principal components identified from the analysis of ancestry informative markers as well as self-reported race/ethnicity (White, Asian/Pacific Islander, Black, Hispanic/Mixed background/other) were retained in all models to adjust for potential confounding due to race or ethnicity (data not shown). Predictors evaluated in the model included genotype (IL4 rs2243248: TT versus TG+GG; TNFA rs2229094: TT versus TC+CC) and age (5-year increments). CI = confidence interval; IL4 = interleukin 4 gene; TNFA = tumor necrosis factor alpha gene.
In the regression analysis for IL4 rs2243248, being heterozygous or homozygous for the rare G allele (TT versus TG+GG) was associated with a 70% decrease in the odds of belonging to the higher evening fatigue class (Figure 2A). In the regression analysis for TNFA rs2229094, being heterozygous or homozygous for the rare C allele (TT versus TC+CC) was associated with a 3.75-fold increase in the odds of belonging to the higher evening fatigue class (Figure 2B).
Figure 2.
Differences between the evening fatigue latent classes in the percentages of participants who were A) homozygous for the common allele (TT) or heterozygous or homozygous for the rare allele (TG+GG) for rs2243248 in interleukin 4 (IL4) and B) homozygous for the common allele (TT) or heterozygous or homozygous for the rare allele (TC+CC) for rs2229094 in tumor necrosis factor alpha (TNFA). Values are plotted as unadjusted proportions with corresponding p-value.
Discussion
Findings from the present study support our prior work (Dhruva et al., 2013) that suggested that morning and evening fatigue are distinct but related symptoms. While we did find some overlap, different phenotypic and genotypic characteristics were associated with morning and evening fatigue. Among the phenotypic characteristics, we found that having a higher number of comorbid conditions was associated with more severe morning fatigue. In contrast, caring for children at home was associated with more severe evening fatigue. While these phenotypic differences warrant confirmation in future studies, they suggest that different biological and lifestyle factors are associated with diurnal variations in fatigue severity. The identification of distinct modifiable risk factors for morning and evening fatigue may lead to the development and testing of more targeted interventions.
This study is the first to identify genotypic differences in morning and evening fatigue. While the TNFA gene was associated with both morning and evening fatigue, we found that different SNPs were associated with morning (i.e., rs1800629, rs3093662) versus evening (i.e., rs2229094) fatigue. The presence of the rare allele in TNFA rs1800629, which is located in the promoter region of the gene, is known to alter gene expression. However, studies that evaluated the direction and magnitude of the changes in TNF-α due to the rare “A” allele have yielded conflicting results (Aouizerat et al., 2009; Kroeger, Carville, & Abraham, 1997; Kroeger, Steer, Joyce, & Abraham, 2000). In the current study, carrying the rare “A” allele decreased the odds of being categorized in the Moderate or High morning fatigue classes. Given the role that TNF-α plays in a number of inflammatory conditions (Bishehsari et al., 2012; Cerri et al., 2009; Y. H. Lee et al., 2008; Leung & Cahill, 2010; Raison et al., 2013), this finding suggests that changes in TNF-α may play an important role in the occurrence, severity, and maintenance of morning fatigue.
TNFA rs2229094 lies in the promoter region of TNFA as well as within an exon of lymphotoxin alpha (LTA). This SNP results in a missense mutation (i.e., an amino acid change from cysteine to arginine) in LTA. In addition, this SNP occurs in a DNase 1 hypersensitivity region that may influence both TNFA and LTA gene expression (Encode Project Consortium et al., 2012). Previous research has identified associations between this polymorphism and increased risks for proliferative vitreoretinopathy (Rojas et al., 2010) and cancer (Gallicchio et al., 2008; Takei et al., 2008). In addition, research has found associations between the rare “C” allele and increased risks for coronary artery disease (Y. Liu et al., 2011) and type 2 diabetes (Mahajan et al., 2010) and it appears to be a systemic marker of inflammation (i.e., C-reactive protein; Mahajan et al., 2010). In the aggregate, findings across these studies suggest that the rare “C” allele in TNFA is associated with increases in inflammation.
Findings from the current study suggest that carriers of the rare “C” allele in TNFA rs2229094 have a 3.75-fold increase in the odds of being classified in the two higher morning fatigue classes. Findings from studies done to date suggest that the biology of TNFA/LTA is extremely complex (Gallicchio et al., 2008; Mahajan et al., 2010; Oikari et al., 2013). This complexity may be due, in part, to the fact that the SNP lies both in the promoter region of TNFA and within an exon of LTA. Future studies are needed to determine if the association between higher levels of morning fatigue and rs2229094 is due to functional changes in LTα, differential gene expression of TNF-α, or both these mechanisms.
In terms of evening fatigue, participants who were heterozygous or homozygous for the rare “G” allele at rs3093662 were 3.8 times more likely to be in the higher evening fatigue classes. This SNP lies in an intronic region of TNFA, and research has found it to be associated with inflammation and poorer outcomes in renal transplant patients (Israni et al., 2008). In addition, this SNP occurs in both a DNase I hypersensitivity region and in a region that is differentially methylated (Encode Project Consortium et al., 2012). Finally, this region is differentially bound by DNA polymerase, which is required for gene expression (Encode Project Consortium et al., 2012). Taken together, our findings suggest that TNF-α plays a role in the mechanisms that underlie both morning and evening fatigue. Further study may elucidate the role of this complex multifunctional pro-inflammatory cytokine in the development and maintenance of morning and evening fatigue.
The other genetic association with evening fatigue was for IL4 rs2243248. This SNP lies within the promoter region of the IL4 gene, which suggests that it may have functional significance. In the current study, carrying one or two doses of the rare G allele was associated with a 70% decrease in the odds of being in the higher evening fatigue classes. While factors such as the target cell influence its biological effect (Biedermann & Rocken, 2005), IL-4 is primarily an anti-inflammatory cytokine. In previous studies (Brenner et al., 2007; Erdei et al., 2010), the rare “G” allele of IL4 rs2243248 was associated with an increased risk for glioma and breast cancer. In addition, the “G” allele was associated with higher lytic titers (i.e., causing viral infected cell lysis) in patients with HHV-8 infection (Brown et al., 2006). Our own group reported an association with the “G” allele and an increased risk for belonging to an “All High” symptom cluster subgroup (i.e., oncology patients and their FCs who reported high levels of pain, fatigue, sleep disturbance and depression; Illi et al., 2012). In addition, this SNP occurs in a region of the IL4 gene that displays differential heterochromatin structure. This region is differentially bound by RNA polymerase, which is required for gene expression (Encode Project Consortium et al., 2012). Taken together, these data suggest that this SNP influences the expression of IL-4. Of note, in several studies (Atkins et al., 1992; Gilleece et al., 1992; Majhail et al., 2004; Taylor et al., 2000; Vokes, Figlin, Hochster, Lotze, & Rybak, 1998; Whitehead et al., 2002; Whitehead et al., 1998), the therapeutic administration of IL-4 resulted in fatigue, which suggests that the association of IL-4 with evening fatigue may have clinical implications.
Prior studies (Bower et al., 2009; L. Liu et al., 2012; Wang et al., 2010), including work from our group (C. Miaskowski et al., 2010) have suggested that IL-6 is associated with fatigue. While in the bivariate analyses, only one SNP in IL6 was significant for morning fatigue (i.e., rs4719714) and one SNP in IL6 was significant for evening fatigue (i.e., rs1800796), none remained significant in the multivariate analyses. Studies with larger samples may identify SNPs in IL6 that are associated with diurnal variations in fatigue.
Several limitations are worth noting. The relatively small sample size precluded the examination of gene–gene interactions. In addition, the genetic associations identified in this study require validation in an independent cohort. Finally, serum cytokine levels were not measured. Future studies need to evaluate for serum cytokines and changes in gene expression associated with these polymorphisms.
Overall, the results of the current study suggest that polymorphisms in TNFA influence both morning and evening fatigue. However, only evening fatigue was associated with a polymorphism in IL4. Many cytokines display circadian variability in serum levels (Schubert et al., 2007). It may be that variability in cytokine genes leads to circadian variability in serum levels. Further research will help to clarify the relationships among circadian variability in serum cytokine levels, genetic variability, and the severity of morning and evening fatigue. In addition, while numerous studies have suggested a role for a number of pro- and anti-inflammatory cytokines in the development of fatigue (Bower, Ganz, Irwin, Kwan, et al., 2011; Bower et al., 2009; Dantzer et al., 2008; Myers, 2008), the fact that only two out of the fifteen candidate genes evaluated in the current study were associated with morning and/or evening fatigue suggests that additional mechanisms (e.g., neurotransmitter pathways) may be involved in this clinically significant symptom. We are evaluating this hypothesis in another study.
Supplementary Material
Acknowledgments
This research was supported by a grant from the National Institute of Nursing Research (NINR; NR04835). Dr. Miaskowski is funded by the American Cancer Society (ACS) as a Clinical Research Professor. Dr. Dhruva is funded through an NIH Mentored Patient-Oriented Research Career Development Award (K23 AT005340). Dr. Aouizerat received funding through the National Institutes of Health Roadmap for Medical Research Grant (KL2 RR624130). Dr. Dunn received funding from the Mount Zion Health Fund and the UCSF Academic Senate. Dr. Langford is supported by a Department of Defense Breast Cancer Research Program postdoctoral fellowship. Dr. Merriman was supported by an NINR fellowship (F31 NR012604), an ACS Doctoral Degree Scholarship (DSCN-10-087), an Oncology Nursing Society Doctoral Scholarship, and a UCSF Nursing Alumni Association Scholarship. Dr. Baggott is supported by an ACS Mentored Research Scholar Grant (MRSG-12-01-PCSM). Dr. Leutwyler is funded by the KL2 Program (RR624130). Dr. Cataldo was supported by an Oncology Nursing Society Fellowship. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Aouizerat BE, Dodd M, Lee K, West C, Paul SM, Cooper BA, Miaskowski C. Preliminary evidence of a genetic association between tumor necrosis factor alpha and the severity of sleep disturbance and morning fatigue. Biological Research for Nursing. 2009;11:27–41. doi: 10.1177/1099800409333871. [DOI] [PubMed] [Google Scholar]
- Atkins MB, Vachino G, Tilg HJ, Karp DD, Robert NJ, Kappler K, Mier JW. Phase I evaluation of thrice-daily intravenous bolus interleukin-4 in patients with refractory malignancy. Journal of Clinical Oncology. 1992;10:1802–1809. doi: 10.1200/JCO.1992.10.11.1802. [DOI] [PubMed] [Google Scholar]
- Barsevick A, Frost M, Zwinderman A, Hall P, Halyard M. I'm so tired: Biological and genetic mechanisms of cancer-related fatigue. Quality of Life Research. 2010;19:1419–1427. doi: 10.1007/s11136-010-9757-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biedermann T, Rocken M. Pro- and anti-inflammatory effects of IL-4: From studies in mice to therapy of autoimmune diseases in humans. Ernst Schering Research Foundation Workshop. 2005;50:235–242. doi: 10.1007/3-540-26811-1_13. [DOI] [PubMed] [Google Scholar]
- Bishehsari F, Sharma A, Stello K, Toth C, O'Connell MR, Evans AC, Whitcomb DC. TNF-alpha gene (TNFA) variants increase risk for multi-organ dysfunction syndrome (MODS) in acute pancreatitis. Pancreatology. 2012;12:113–118. doi: 10.1016/j.pan.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Arevalo JM, Cole SW. Fatigue and gene expression in human leukocytes: Increased NF-kappaB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain, Behavior, and Immunity. 2011;25:147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Irwin MR, Kwan L, Breen EC, Cole SW. Inflammation and behavioral symptoms after breast cancer treatment: Do fatigue, depression, and sleep disturbance share a common underlying mechanism? Journal of Clinical Oncology. 2011;29:3517–3522. doi: 10.1200/JCO.2011.36.1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bower JE, Ganz PA, Tao ML, Hu W, Belin TR, Sepah S, Aziz N. Inflammatory biomarkers and fatigue during radiation therapy for breast and prostate cancer. Clinical Cancer Research. 2009;15:5534–5540. doi: 10.1158/1078-0432.CCR-08-2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner AV, Butler MA, Wang SS, Ruder AM, Rothman N, Schulte PA, Inskip PD. Single-nucleotide polymorphisms in selected cytokine genes and risk of adult glioma. Carcinogenesis. 2007;28:2543–2547. doi: 10.1093/carcin/bgm210. [DOI] [PubMed] [Google Scholar]
- Brown EE, Fallin MD, Goedert JJ, Hutchinson A, Vitale F, Lauria C, Chanock SJ. Host immunogenetics and control of human herpesvirus-8 infection. Journal of Infectious Diseases. 2006;193:1054–1062. doi: 10.1086/501470. [DOI] [PubMed] [Google Scholar]
- Carney S, Koetters T, Cho M, West C, Paul SM, Dunn L, Miaskowski C. Differences in sleep disturbance parameters between oncology outpatients and their family caregivers. Journal of Clinical Oncology. 2011;29:1001–1006. doi: 10.1200/JCO.2010.30.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerri AP, Arosio B, Viazzoli C, Confalonieri R, Teruzzi F, Annoni G. 308(G/A) TNF-alpha gene polymorphism and risk of depression late in the life. Archives of Gerontology and Geriatrics. 2009;49(Suppl 1):29–34. doi: 10.1016/j.archger.2009.09.009. [DOI] [PubMed] [Google Scholar]
- Collado-Hidalgo A, Bower JE, Ganz PA, Irwin MR, Cole SW. Cytokine gene polymorphisms and fatigue in breast cancer survivors: early findings. Brain Behavior and Immunity. 2008;22:1197–1200. doi: 10.1016/j.bbi.2008.05.009. doi:S0889-1591(08)00270-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde L, Vaquerizas JM, Dopazo H, Arbiza L, Reumers J, Rousseau F, Dopazo J. PupaSuite: Finding functional single nucleotide polymorphisms for large-scale genotyping purposes. Nucleic Acids Research. 2006;34(Web Server issue):W621–625. doi: 10.1093/nar/gkl071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Capuron L, Irwin MR, Miller AH, Ollat H, Perry VH, Yirmiya R. Identification and treatment of symptoms associated with inflammation in medically ill patients. Psychoneuroendocrinology. 2008;33(1):18–29. doi: 10.1016/j.psyneuen.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhruva A, Aouizerat BE, Cooper B, Paul SM, Dodd M, West C, Miaskowski C. Differences in morning and evening fatigue in oncology patients and their family caregivers. European Journal of Oncology Nursing. 2013;16:841–848. doi: 10.1016/j.ejon.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhruva A, Lee K, Paul SM, West C, Dunn L, Dodd M, Miaskowski C. Sleep-wake circadian activity rhythms and fatigue in family caregivers of oncology patients. Cancer Nursing. 2012;35:70–81. doi: 10.1097/NCC.0b013e3182194a25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LB, Aouizerat BE, Cooper BA, Dodd M, Lee K, West C, Miaskowski C. Trajectories of anxiety in oncology patients and family caregivers during and after radiation therapy. European Journal of Oncology Nursing. 2012;16:1–9. doi: 10.1016/j.ejon.2011.01.003. doi:S1462-3889(11)00007-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Encode Project Consortium. Dunham I, Kundaje A, Aldred SF, Collins PJ, Davis CA, Birney E. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdei E, Kang H, Meisner A, White K, Pickett G, Baca C, Berwick M. Polymorphisms in cytokine genes and serum cytokine levels among New Mexican women with and without breast cancer. Cytokine. 2010;51:18–24. doi: 10.1016/j.cyto.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Altshuler D. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- Gallicchio L, Chang H, Christo DK, Thuita L, Huang HY, Strickland P, Helzlsouer KJ. Single nucleotide polymorphisms in inflammation-related genes and mortality in a community-based cohort in Washington County, Maryland. American Journal of Epidemiology. 2008;167:807–813. doi: 10.1093/aje/kwm378. [DOI] [PubMed] [Google Scholar]
- Gilbertson-White S, Aouizerat BE, Miaskowski C. Methodologic issues in the measurement of cytokines to elucidate the biological basis for cancer symptoms. Biological Research for Nursing. 2011;13:15–24. doi: 10.1177/1099800410379497. [DOI] [PubMed] [Google Scholar]
- Gilleece MH, Scarffe JH, Ghosh A, Heyworth CM, Bonnem E, Testa N, Dexter TM. Recombinant human interleukin 4 (IL-4) given as daily subcutaneous injections—a phase I dose toxicity trial. British Journal of Cancer. 1992;66:204–210. doi: 10.1038/bjc.1992.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halder I, Shriver M, Thomas M, Fernandez JR, Frudakis T. A panel of ancestry informative markers for estimating individual biogeographical ancestry and admixture from four continents: Utility and applications. Human Mutation. 2008;29:648–658. doi: 10.1002/humu.20695. [DOI] [PubMed] [Google Scholar]
- Hattersley AT, McCarthy MI. What makes a good genetic association study? Lancet. 2005;366:1315–1323. doi: 10.1016/S0140-6736(05)67531-9. [DOI] [PubMed] [Google Scholar]
- Henry DH, Viswanathan HN, Elkin EP, Traina S, Wade S, Cella D. Symptoms and treatment burden associated with cancer treatment: Results from a cross-sectional national survey in the U.S. Supportive Care in Cancer. 2008;16:791–801. doi: 10.1007/s00520-007-0380-2. [DOI] [PubMed] [Google Scholar]
- Hofman M, Ryan JL, Figueroa-Moseley CD, Jean-Pierre P, Morrow GR. Cancer-related fatigue: The scale of the problem. Oncologist. 2007;12(Suppl 1):4–10. doi: 10.1634/theoncologist.12-S1-4. [DOI] [PubMed] [Google Scholar]
- Hoggart CJ, Parra EJ, Shriver MD, Bonilla C, Kittles RA, Clayton DG, McKeigue PM. Control of confounding of genetic associations in stratified populations. America Journal of Human Genetics. 2003;72:1492–1504. doi: 10.1086/375613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong JH, Chiang CS, Campbell IL, Sun JR, Withers HR, McBride WH. Induction of acute phase gene expression by brain irradiation. International Journal of Radiation Oncology Biology Physics. 1995;33:619–626. doi: 10.1016/0360-3016(95)00279-8. [DOI] [PubMed] [Google Scholar]
- Illi J, Miaskowski C, Cooper B, Levine JD, Dunn L, West C, Aouizerat BE. Association between pro- and anti-inflammatory cytokine genes and a symptom cluster of pain, fatigue, sleep disturbance, and depression. Cytokine. 2012;58:437–447. doi: 10.1016/j.cyto.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israni AK, Li N, Cizman BB, Snyder J, Abrams J, Joffe M, Feldman HI. Association of donor inflammation- and apoptosis-related genotypes and delayed allograft function after kidney transplantation. American Journal of Kidney Disease. 2008;52:331–339. doi: 10.1053/j.ajkd.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jager A, Sleijfer S, van der Rijt CC. The pathogenesis of cancer related fatigue: Could increased activity of pro-inflammatory cytokines be the common denominator? European Journal of Cancer. 2008;44:175–181. doi: 10.1016/j.ejca.2007.11.023. doi:S0959-8049(07)00968-9. [DOI] [PubMed] [Google Scholar]
- Jeanmonod P, von Kanel R, Maly FE, Fischer JE. Elevated plasma C-reactive protein in chronically distressed subjects who carry the A allele of the TNF-alpha -308 G/A polymorphism. Psychosomatic Medicine. 2004;66:501–506. doi: 10.1097/01.psy.0000130903.78444.7d. [DOI] [PubMed] [Google Scholar]
- Kroeger KM, Carville KS, Abraham LJ. The -308 tumor necrosis factor-alpha promoter polymorphism effects transcription. Molecular Immunology. 1997;34:391–399. doi: 10.1016/s0161-5890(97)00052-7. [DOI] [PubMed] [Google Scholar]
- Kroeger KM, Steer JH, Joyce DA, Abraham LJ. Effects of stimulus and cell type on the expression of the -308 tumour necrosis factor promoter polymorphism. Cytokine. 2000;12:110–119. doi: 10.1006/cyto.1999.0529. [DOI] [PubMed] [Google Scholar]
- Lawrence DP, Kupelnick B, Miller K, Devine D, Lau J. Evidence report on the occurrence, assessment, and treatment of fatigue in cancer patients. Journal of the National Cancer Institute. 2004;32:40–50. doi: 10.1093/jncimonographs/lgh027. [DOI] [PubMed] [Google Scholar]
- Lee KA, Hicks G, Nino-Murcia G. Validity and reliability of a scale to assess fatigue. Psychiatry Research. 1991;36:291–298. doi: 10.1016/0165-1781(91)90027-m. [DOI] [PubMed] [Google Scholar]
- Lee YH, Woo JH, Rho YH, Choi SJ, Ji JD, Song GG. Meta-analysis of the combination of TNF inhibitors plus MTX compared to MTX monotherapy, and the adjusted indirect comparison of TNF inhibitors in patients suffering from active rheumatoid arthritis. Rheumatology International. 2008;28:553–559. doi: 10.1007/s00296-007-0475-6. [DOI] [PubMed] [Google Scholar]
- Leung L, Cahill CM. TNF-alpha and neuropathic pain—a review. Journal of Neuroinflammation. 2010;7:27. doi: 10.1186/1742-2094-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Lewinger JP, Gauderman WJ, Murcray CE, Conti D. Using extreme phenotype sampling to identify the rare causal variants of quantitative traits in association studies. Genetic Epidemiology. 2011;35:790–799. doi: 10.1002/gepi.20628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Mills PJ, Rissling M, Fiorentino L, Natarajan L, Dimsdale JE, Ancoli-Israel S. Fatigue and sleep quality are associated with changes in inflammatory markers in breast cancer patients undergoing chemotherapy. Brain, Behavior, and Immunity. 2012;26:706–713. doi: 10.1016/j.bbi.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Sheng H, Lu L, Wu Z, Chen Q, Xiao H, Jin W. Haplotype-based association of four lymphotoxin-alpha gene polymorphisms with the risk of coronary artery disease in Han Chinese. Tohoku Journal of Experimental Medicine. 2011;224:119–125. doi: 10.1620/tjem.224.119. [DOI] [PubMed] [Google Scholar]
- Mahajan A, Tabassum R, Chavali S, Dwivedi OP, Chauhan G, Tandon N, Bharadwaj D. Obesity-dependent association of TNF-LTA locus with type 2 diabetes in North Indians. Journal of Molecular Medicine. 2010;88:515–522. doi: 10.1007/s00109-010-0594-5. [DOI] [PubMed] [Google Scholar]
- Majhail NS, Hussein M, Olencki TE, Budd GT, Wood L, Elson P, Bukowski RM. Phase I trial of continuous infusion recombinant human interleukin-4 in patients with cancer. Investigational New Drugs. 2004;22:421–426. doi: 10.1023/B:DRUG.0000036684.67675.fe. [DOI] [PubMed] [Google Scholar]
- McCann B, Miaskowski C, Koetters T, Baggott C, West C, Levine JD, Aouizerat BE. Associations between pro- and anti-inflammatory cytokine genes and breast pain in women prior to breast cancer surgery. Journal of Pain. 2012;13:425–437. doi: 10.1016/j.jpain.2011.02.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Cooper BA, Dhruva A, Dunn LB, Langford DJ, Cataldo JK, Aouizerat BE. Evidence of associations between cytokine genes and subjective reports of sleep disturbance in oncology patients and their family caregivers. PLoS One. 2012;7:e40560. doi: 10.1371/journal.pone0040560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Dodd M, Lee K, West C, Paul SM, Cooper BA, Aouizerat BE. Preliminary evidence of an association between a functional interleukin-6 polymorphism and fatigue and sleep disturbance in oncology patients and their family caregivers. Journal of Pain and Symptom Management. 2010;40:531–544. doi: 10.1016/j.jpainsymman.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miaskowski C, Lee K, Dunn L, Dodd M, Aouizerat BE, West C, Swift P. Sleep-wake circadian activity rhythm parameters and fatigue in oncology patients before the initiation of radiation therapy. Cancer Nursing. 2011;34:255–268. doi: 10.1097/NCC.0b013e3181f65d9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen BO, Kaplan DW. Latent variable analysis: Growth mixture modeling and related techniques for longitudinal data. In: Kaplan D, editor. The Sage handbook of quantitative methodology for the social sciences. Newbury Park, CA: Sage Publications; 2004. pp. 345–368. [Google Scholar]
- Muthen LK, Muthen BO. Mplus user's guide. 6th ed. Los Angeles, CA: Muthen & Muthen; 1998-2010. [Google Scholar]
- Myers JS. Proinflammatory cytokines and sickness behavior: Implications for depression and cancer-related symptoms. Oncology Nursing Forum. 2008;35:802–807. doi: 10.1188/08.ONF.802-807. [DOI] [PubMed] [Google Scholar]
- Oikari LE, Stuart S, Okolicsanyi RK, Cox HC, Dixit S, Lea RA, Griffiths LR. Investigation of lymphotoxin alpha genetic variants in migraine. Gene. 2013;512:527–531. doi: 10.1016/j.gene.2012.09.116. [DOI] [PubMed] [Google Scholar]
- Price AL, Patterson NJ, Plenge RM, Weinblatt ME, Shadick NA, Reich D. Principal components analysis corrects for stratification in genome-wide association studies. Nature Genetics. 2006;38:904–909. doi: 10.1038/ng1847. [DOI] [PubMed] [Google Scholar]
- Raison CL, Rutherford RE, Woolwine BJ, Shuo C, Schettler P, Drake DF, Miller AH. A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: The role of baseline inflammatory biomarkers. JAMA Psychiatry. 2013;70:31–41. doi: 10.1001/2013.jamapsychiatry.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinertsen KV, Grenaker Alnaes GI, Landmark-Hoyvik H, Loge JH, Wist E, Kristensen VN, Edvardsen H. Fatigued breast cancer survivors and gene polymorphisms in the inflammatory pathway. Brain, Behavior, and Immunity. 2011;25:1376–1383. doi: 10.1016/j.bbi.2011.04.001. [DOI] [PubMed] [Google Scholar]
- Reyes-Gibby CC, Wu X, Spitz M, Kurzrock R, Fisch M, Bruera E, Shete S. Molecular epidemiology, cancer-related symptoms, and cytokines pathway. Lancet Oncology. 2008;9:777–785. doi: 10.1016/S1470-2045(08)70197-9. doi:S1470-2045(08)70197-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas J, Fernandez I, Pastor JC, Garcia-Gutierrez MT, Sanabria MR, Brion M, Carracedo A. A strong genetic association between the tumor necrosis factor locus and proliferative vitreoretinopathy: The retina 4 project. Ophthalmology. 2010;117:2417–2423.e2. doi: 10.1016/j.ophtha.2010.03.059. [DOI] [PubMed] [Google Scholar]
- Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. [PubMed] [Google Scholar]
- Schubert C, Hong S, Natarajan L, Mills PJ, Dimsdale JE. The association between fatigue and inflammatory marker levels in cancer patients: A quantitative review. Brain, Behavior, and Immunity. 2007;21:413–427. doi: 10.1016/j.bbi.2006.11.004. doi:S0889-1591(06)00357-6. [DOI] [PubMed] [Google Scholar]
- Seruga B, Zhang H, Bernstein LJ, Tannock IF. Cytokines and their relationship to the symptoms and outcome of cancer. Nature Reviews Cancer. 2008;8:887–899. doi: 10.1038/nrc2507. [DOI] [PubMed] [Google Scholar]
- SPSS. IBM SPSS for Windows. Chicago, IL: SPSS, Inc.; 2010. (Version 19) [Google Scholar]
- Stephens M, Smith NJ, Donnelly P. A new statistical method for haplotype reconstruction from population data. American Journal of Human Genetics. 2001;68:978–989. doi: 10.1086/319501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swore Fletcher BA, Dodd MJ, Schumacher KL, Miaskowski C. Symptom experience of family caregivers of patients with cancer. Oncology Nursing Forum. 2008;35:E23–44. doi: 10.1188/08.ONF.E23-E44. [DOI] [PubMed] [Google Scholar]
- Takei K, Ikeda S, Arai T, Tanaka N, Muramatsu M, Sawabe M. Lymphotoxin-alpha polymorphisms and presence of cancer in 1,536 consecutive autopsy cases. BMC Cancer. 2008;8:235. doi: 10.1186/1471-2407-8-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor CW, LeBlanc M, Fisher RI, Moore DF, Sr, Roach RW, Elias L, Miller TP. Phase II evaluation of interleukin-4 in patients with non-Hodgkin's lymphoma: A Southwest Oncology Group trial. Anticancer Drugs. 2000;11:695–700. doi: 10.1097/00001813-200010000-00004. [DOI] [PubMed] [Google Scholar]
- Tian C, Gregersen PK, Seldin MF. Accounting for ancestry: Population substructure and genome-wide association studies. Human Molecular Genetics. 2008;17:R143–150. doi: 10.1093/hmg/ddn268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vokes EE, Figlin R, Hochster H, Lotze M, Rybak ME. A phase II study of recombinant human interleukin-4 for advanced or recurrent non-small cell lung cancer. Cancer Journal Scientific America. 1998;4:46–51. [PubMed] [Google Scholar]
- Wang XS, Shi Q, Williams LA, Mao L, Cleeland CS, Komaki RR, Liao Z. Inflammatory cytokines are associated with the development of symptom burden in patients with NSCLC undergoing concurrent chemoradiation therapy. Brain, Behavior, and Immunity. 2010;24:968–974. doi: 10.1016/j.bbi.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead RP, Lew D, Flanigan RC, Weiss GR, Roy V, Glode ML, Crawford ED. Phase II trial of recombinant human interleukin-4 in patients with advanced renal cell carcinoma: A Southwest Oncology Group study. Journal of Immunotherapy. 2002;25:352–358. doi: 10.1097/00002371-200207000-00007. [DOI] [PubMed] [Google Scholar]
- Whitehead RP, Unger JM, Goodwin JW, Walker MJ, Thompson JA, Flaherty LE, Sondak VK. Phase II trial of recombinant human interleukin-4 in patients with disseminated malignant melanoma: A Southwest Oncology Group study. Journal of Immunotherapy. 1998;21:440–446. doi: 10.1097/00002371-199811000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.