Abstract
Microbial resistance to antibiotics is a global concern. The World Health Organization (WHO) has identified antimicrobial resistance as one the three greatest threats for human beings in the 21st century. Without urgent and coordinated action, the world is moving toward a post-antibiotic era, in which normal infections or minor injuries may become fatal. In an effort to find new agents, we report the synthesis and antimicrobial activities of 40 novel 1,3-diphenyl pyrazole derivatives. These compounds have shown zones of growth inhibition up to 85 mm against Acinetobacter baumannii. We tested the active compounds against this Gram-negative bacterium in minimum inhibitory concentration (MIC) tests and found activity with concentration as low as 4 μg/mL.
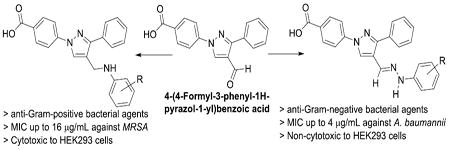
Highlights: Pyrazole, antimicrobials, resistant, hydrazone, antibacterial, Acinetobacter baumannii, methicillin-resistant Staphylococcus aureus (MRSA)
Antibiotic resistance to infection has become a worldwide problem in recent years. According to the latest Center for Disease Control (CDC) report more than two million people are infected every year with antibiotic-resistant infections and at least 23,000 are dying as a result of these diseases in the US alone.[1] In healthcare settings, Gram-negative bacterial infection causes pneumonia, bloodstream infections, wound or surgical site infections, and meningitis. Gram-negative bacteria are increasingly resistant to most of the available antibiotics. Most of the Gram-negative bacterial infections are caused by Klebsiella, Acinetobacter, Pseudomonas aeruginosa, and Escherichia coli.[2]
There are many species of Acinetobacter commonly found in soil and water. All the species of Acinetobacter can cause human disease, but A. baumannii alone accounts for about 80% of Acinetobacter infections. The main victims of Acinetobacter infections are people with weakened immune system, chronic lung disease, and diabetes. Outbreaks of Acinetobacter infections normally happen in intensive care units and healthcare settings. Acinetobacter can colonize tracheostomy sites or open wounds without causing infection. These bacteria can survive on the skin or surfaces for several days and can be spread to susceptible persons by person-to-person contact or contact with contaminated surfaces.[3] A. baumannii infection to US service members has become a major problem since the OPERATION Iraqi Freedom began in 2003.[4, 5] In particular, multidrug resistant (MDR) A. baumannii is a rising class of extremely pathogenic bacteria. The medical community is in a desperate need of finding new antibiotics to treat MDR A. baumannii infection as some of the clinical strains are resistant to all known antibiotics approved to treat infections.[6]
The outer membrane of certain non-fermenting Gram-negative bacteria such as A. baumannii and P. aeruginosa can be highly impermeable to the vast majority of molecules. These non-fermenters are opportunistic and nosocomial pathogens, and many of these pathogens are multi-drug resistant.[7] There are several components associated with the A. baumannii cell wall that prevent the penetration of antibiotics.[8] Therefore, finding novel compounds with growth inhibition properties against non-fermenting bacteria is extremely important and may offer exciting new opportunities to treat these infections.
Phenylpyrazole, a privileged scaffold, is found in a great number of drugs and drug candidates including best selling drugs.[9] Several pyrazole derivatives have been found as analgesic, anti-inflammatory, antimicrobial, anticonvulsant, antidepressant, antimycobacterial, antiviral, and antitumor agents among others.[10-14] Pyrazole derivatives have been reported as antimicrobial agents in a number of publications,[15-17] but as anti-Acinetobacter, they are unknown. In our efforts to get potent antimicrobial agents,[18] we have synthesized several pyrazole-derived terphenyl like novel molecules. We have found pyrazole-derived N-aryl amines as anti-methicillin resistant Staphylococcus aureus (anti-MRSA) agents. Herein, we report the synthesis and antibacterial studies of forty pyrazole derivatives.
We have synthesized the starting material by using our recently published manuscript.[18] Aromatic amine derivatives are an integral part of many potent antimicrobial agents.[19] All these novel molecules are characterized by 1H and 13C NMR spectroscopy. We reported several N-aryl derivatives of the pyrazole as anti-MRSA agents up to 16 μM concentration.[18] To get the N-aryl pyrazole derivatives as potent antimicrobial agents, we synthesized several novel molecules (Scheme 1) to test against Gram-positive and Gram-negative bacteria. Nearly all of the test compounds showed moderate activity against Gram-positive bacteria in zone of inhibition studies. Compounds having electron donating groups such alkyl along with halogen atoms in the N-aryl moiety (1, 2, 3, 4, & 5) have shown moderate activity against Gram-positive bacteria, S. aureus and B. subtilis. These compounds showed slightly better growth inhibition against B. subtilis than S. aureus. N-Aryl moiety with a carboxylic acid functional group has eliminated the activity of the resultant molecule (6). Addition of one more carboxylic acid group (7) showed some activity against Gram-positive bacteria. Replacing carboxylic groups with trifluoromethyl group increased the activity significantly and the resultant molecule (8) showed activity up to 22 mm and 25 mm against S. aureus and B. subtilis respectively. One trifluoro group with a fluoro substituent (9) also showed good activity against Gram-positive bacteria. Trifluoro substituent with other halogen atoms (Cl & Br) also showed moderate activity in zone of inhibition studies (10 & 11). Very strong electron withdrawing group (NO2) substitution also showed moderate activity against Gram-positive bacteria (12 & 13). Dihalogen substituted compounds (14 & 15) also showed some activity against Gram-positive bacteria in zone of inhibition studies (Scheme 1). The positive control, chloramphenicol, showed 25 mm and 32 mm zone of inhibition against S. aureus and B. subtilis respectively.
Scheme 1.
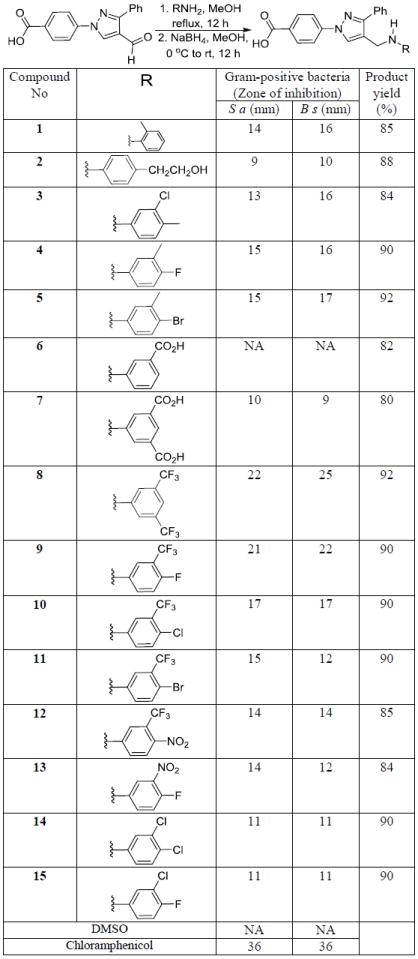
Synthesis and antimicrobial studies of pyrazole derived N-arylamines, Gram-positive bacteria: S. aureus (S a) and B. subtilis (B s), NA = No Activity
Hydrazone derivatives show a wide range of biological properties such as antimicrobial, antitubercular, anti-inflammatory, and anticancer activities.[20] Based on the excellent pharmacological profile in the literature, we synthesized several hydrazones of the aldehyde derivative, 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid. We synthesized the target molecules by the reaction of aldehyde with the corresponding hydrazine in methanol and acetic acid as a catalyst (Scheme 2). Refluxing the reaction mixture for eight hours followed by filtration of the solid precipitate afforded the hydrazones in excellent yield. All hydrazone derivatives are characterized by NMR (1H & 13C) spectroscopy and mass spectrometry.
Scheme 2.
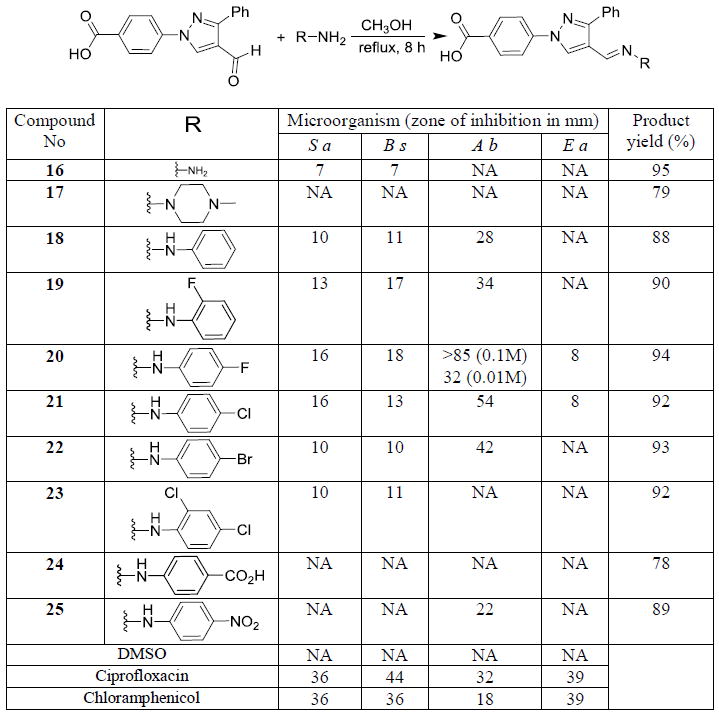
Synthesis and antimicrobial studies of hydrazone derivatives, Gram-positive bacteria: S. aureus (S a) and B. subtilis (B s), Gram-negative bacteria: P. aeruginosa (P a) & E. coli (E c), and NA = No activity.
Synthesized hydrazones were evaluated against Gram-positive and Gram-negative bacteria. The simplest hydrazone derivative (16) showed moderate activity against both the Gram-positive bacteria (S. aureus & B. subtilis) in zone of inhibition studies. N-Methyl piperazine derived hydrazone (17) did not show any activity against the tested microorganisms. N-Phenyl derived hydrazone (18) showed moderate activity against tested Gram-positive bacteria. To our surprise, this hydrazone (18) also exhibited potent activity, 28 mm size zone of inhibition, against a Gram-negative bacterium, A. baumannii. Addition of a fluoro substituent (19) at the ortho position increased the activity of the resultant molecule. Positioning the fluoro group at the para carbon (20) increased the activity exponentially. At 0.1M concentration, 10 μL of the solution of the compound completely cleared the 85 mm Petri dish plate. After tenfold dilution, 0.01 M concentration, it showed 32 mm zone of inhibition. Based on our literature search, this molecule (20) is the most potent pyrazole derivative as anti-A. baumannii agent. This hydrazone derivative (20) also showed moderate activity against E. aerogenes in addition to showing good activity against Gram-positive bacteria. Corresponding chloro substituted compound (21) showed up to 54 mm zone of inhibition against A. baumannii and almost the same activity against other bacteria. Bromo substituted hydrazone derivative (22) exhibited good activity against A. baumannii and moderate activity against Gram-positive bacteria. Dichloro substitution (23) completely eliminated the activity against A. baumanni but showed moderate activity against Gram-positive bacteria. Strong electron withdrawing groups such as CO2H and NO2 completely ceased the activity of the resultant hydrazone derivatives (24 & 25). We found a clear structure activity relationship (SAR) of the synthesized compounds for the growth inhibition against the A. baumanni. Moderate electron withdrawing groups (halogens) showed good activity compared to other functional groups. Among the halogens, greater the electronegativity, better the activity of the molecule. Thus the fluoro substituted hydrazone showed the highest activity against the microorganisms, particularly against the A. baumannii. The positive controls, chloramphenicol and ciprofloxacin have shown 22 mm and 37 mm zone of inhibition respectively. Thus, these synthesized novel hydrazone derivatives have shown better activity than that of positive controls.
After finding lead molecules in the hydrazone series, we were encouraged to synthesize N-acylhydrazones to test against different bacteria (Scheme 3). We synthesized semicarbazone (26), and methyl hydrazinocarboxylate (27) derivatives by reacting with the corresponding hydrazide in refluxing methanol. Similarly N-acyl and N-benzoyl hydrazones (28 & 29) were synthesized and the products were isolated by filtration followed by recrystallization in acetonitrile to get the pure products. None of these compounds showed any activity except the simple benzoyl substituted hydrazone in zone of inhibition studies. Hydroxy substituted N-benzoyl hydrazones (30 & 31) were also synthesized but these products did not show any appreciable growth inhibition of bacteria. 4-Methoxy (32) and 2-fluoro (33) substituted N-benzoyl derivatives showed moderate activity against Gram-positive bacteria. Surprisingly, 4-fluoro substituted N-benzoyl hydrazone (34) did not show any activity in contrast to the corresponding hydrazone derivative (20). Synthesis and antimicrobial studies of isoniazid derivatives (35 & 36) also did not give any positive result. 2-Hydroxy naphthoic acid derived hydrazone (37) also did not show any activity. Although most of the molecules in this series did not show any activity, hydrophobic N-benzoyl hydrazones exhibited moderate activity against Gram-positive bacteria (Scheme 3).
Scheme 3.
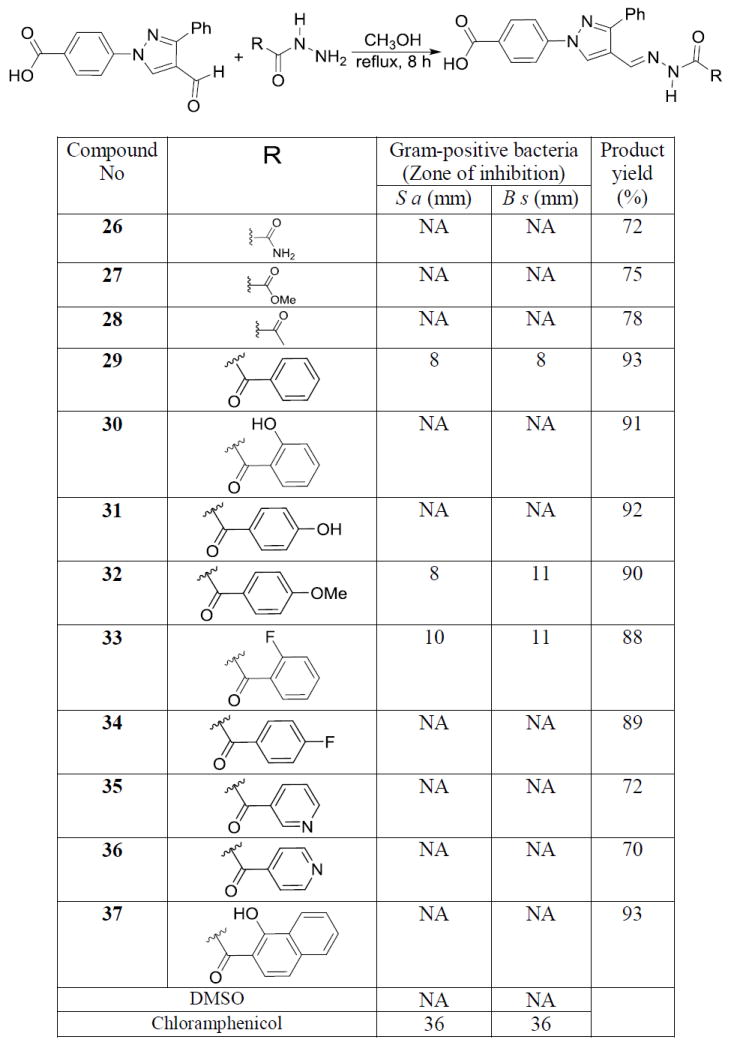
Synthesis and antimicrobial data of semicarbazones derivatives
Sulfonylhydrazones are well known to show a wide range pharmacological properties such as antiviral,[21] antifungal,[22] antitumor,[23] anti-inflamatory,[24] among other activities. We synthesized a thiosemicarbazide derivative (38) and two sulfonylhydrazones (39 & 40). The sulfonylhydrazones showed moderate activity against Gram-positive bacteria. These two molecules provided leads to synthesize more sulfonamide derivatives as potent anti-Gram-positive bacterial agents.
Compounds showing activity against Gram-positive bacteria were tested against methicillin resistant S. aureus (MRSA) for MIC studies. Seven of these compounds have shown promising activity against this drug resistant bacteria. Methyl substitution along with the chloro and bromo substituted N-arylamine derivatives (3 & 5) have shown activity up to 16 μg/mL concentration in MIC studies. Trifluoromethyl substituted N-arylamines (8, 9, 10, & 11) have shown better activity in zone of inhibition studies, but these molecules are less potent in MIC studies than the methyl substituted compounds. Dichloro substituted N-arylamine-derived pyrazole showed activity up to 16 μg/mL concentration against MRSA. All four molecules have shown activity at 32 μg/mL concentration. Hydrazone derivatives (20, 21, & 22) showing activity against A. baumannii in disk diffusion assay were also subjected to MIC studies. Fluoro and bromo substituted compounds (20 & 22) have shown activity against A. baumannii in 8 μg/mL concentration. Surprisingly, the chloro derivative (21) which was less active in zone of inhibition studies showed better activity (4 μg/mL) in MIC studies against A. baumannii (Table 1). The positive control, chloramphenicol, inhibited the growth of A. baumannii at 16 μg/mL in MIC studies. Thus, our potent compound (21) is four times more potent than the approved drug, chloramphenicol (positive control).
Table 1.
MIC values for active compounds: S. aureus ATCC 43300 (MRSA), A. baumannii ATCC 19606 (Type strain)
| Compounds | MRSA MIC (μg/mL) |
|---|---|
| 3 | 16 |
| 5 | 16 |
| 8 | 32 |
| 9 | 32 |
| 10 | 32 |
| 11 | 32 |
| 14 | 16 |
| A. baumannii | |
| 20 | 8 |
| 21 | 4 |
| 22 | 8 |
| Chloramphenicol | 16 |
| Ciprofloxacin | 1.56 |
To predict the mode of action of potent molecules, we carried out the docking studies of compounds (20, 21, & 22) with several druggable proteins of Gram-negative bacteria. We found the best affinity (~ -9.8 kcal/mol) of compound (21) against LpxC enzyme. These three potent molecules show similar interactions with enzyme except the chlorine atom of 21, which forms a halogen bond-like interaction with phenyl ring phenyl alanine (PHE 182 & PHE 155) of LpxC enzyme (Figure 2). This additional interaction of 21 with the LpxC protein could be the reason of its potent activity against A. baumannii in MIC studies.
Figure 2.
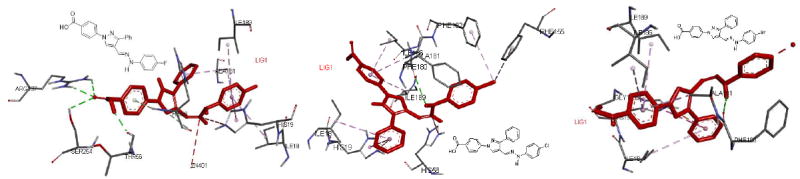
Docking of compounds 20, 21, & 22 in the active site of LpxC enzyme (PDB ID: 5DRO).
To study the druglike properties of the active compounds, all ten compounds were evaluated for cytotoxic properties for 50% cytotoxic concentration (CC50) values against healthy human (HEK293) cells. N-Aryl derivatives (3, 5, & 14) without trifluoromethyl showed significant cytotoxicity against HEK293 cells, and N-aryl amines (8, 9, 10, & 11) with trifluoromethyl as a substituent did not show significant cytotoxicity against this healthy cell line up to 32 μg/mL concentration. This finding is very significant to design N-arylamines as noncytotoxic antibacterial agents. Hydrazone derivatives (20, 21, & 22) did not show any noticeable cytotoxicity against HEK293 cell lines (Table 2). Therefore, these three hydrazone derivatives have given an excellent starting point to design noncytotoxic anti-Gram-negative bacterial agents.
Table 2.
CC50 values of potent compounds against HEK293 cells
| Compounds | HEK293, ATCC CRL-1573 CC50 (μg/mL) |
|---|---|
| 3 | 20 |
| 5 | 17.9 |
| 8 | >32 |
| 9 | >32 |
| 10 | >32 |
| 11 | >32 |
| 14 | 18 |
| 20 | >32 |
| 21 | >32 |
| 22 | >32 |
We reported an efficient synthesis of new 1,3-dipenyl pyrazole derived N-aryl amines, hydrazones, semicarbazones, and sulfahydrazones from readily available reagents and starting materials. From these compounds, we found seven molecules possessing promising anti-MRSA activity and three molecules that demonstrated potent anti-Acinetobacter baumannii activities in MIC studies. Hydrazone derivatives have shown potent activity without any toxic effect on healthy mammalian cells (HEK293). Many variables in the lead molecules and ease of synthesis will help us to optimize the activity and antimicrobial potential of these novel agents.
Figure 1.
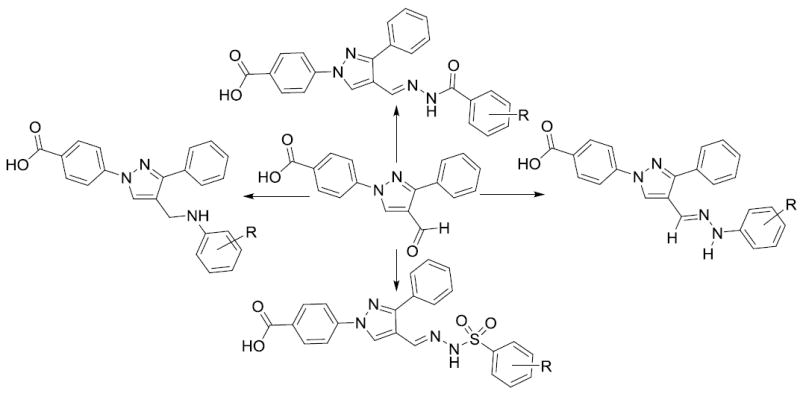
Synthesized Pyrazole derivatives as antibacterial agents
Scheme 4.
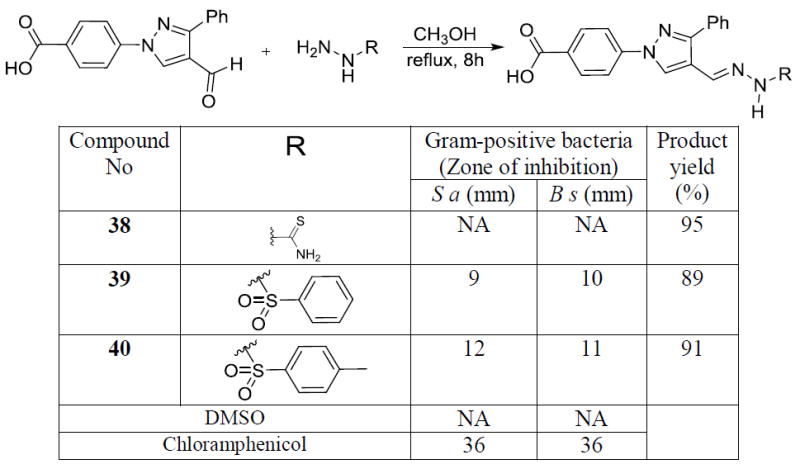
Synthesis and antimicrobial data of thiosemicarbazone sulfonyl hydrazone
Acknowledgments
This work was supported by College of Science and Mathematics Arkansas State University, Jonesboro. Arkansas Statewide MS facility, Grant Number P30 GM103450 from the National Institute of General Medical Sciences of the National Institutes of Health (NIH) for recording mass spectrometry. This publication was made possible by the Arkansas INBRE program, supported by grant funding from the National Institutes of Health (NIH) National Institute of General Medical Sciences (NIGMS) (P20 GM103429) (formerly P20RR016460). Arkansas Biosciences Institute - Arkansas State University (ABI-A-state) start-up fund helped to complete this project. Anti-MRSA screening was performed by CO-ADD (The Community for Antimicrobial Drug Discovery), funded by the Welcome Trust (UK) and the University of Queensland (Australia).
Footnotes
Conflict of Interest:
The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.CDC. Antibiotic / Antimicrobial Resistance. Centers for Disease Control and Prevention; Atlanta: 2016. [Google Scholar]
- 2.CDC. Healthcare-associated Infections (HAIs) Centers for Disease Control and Prevention; Atlanta: 2016. [Google Scholar]
- 3.CDC, Acinetobacter in Healthcare Settings. Acinetobacter in Healthcare Settings. CDC; Atlanta: 2016. [Google Scholar]
- 4.Huang XZ, Chahine MA, Frye JG, Cash DM, Lesho EP, Craft DW, Lindler LE, Nikolich MP. Molecular analysis of imipenem-resistant Acinetobacter baumannii isolated from US service members wounded in Iraq, 2003-2008. Epidemiol Infect. 2012;140:2302–2307. doi: 10.1017/S0950268811002871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Scott P, Deye G, Srinivasan A, Murray C, Moran K, Hulten E, Fishbain J, Craft D, Riddell S, Lindler L, Mancuso J, Milstrey E, Bautista CT, Patel J, Ewell A, Hamilton T, Gaddy C, Tenney M, Christopher G, Petersen K, Endy T, Petruccelli B. An outbreak of multidrug-resistant Acinetobacter baumannii-calcoaceticus complex infection in the US military health care system associated with military operations in Iraq. Clin Infect Dis. 2007;44:1577–1584. doi: 10.1086/518170. [DOI] [PubMed] [Google Scholar]
- 6.Wencewicz TA, Miller MJ. Biscatecholate-monohydroxamate mixed ligand siderophore-carbacephalosporin conjugates are selective sideromycin antibiotics that target Acinetobacter baumannii. J Med Chem. 2013;56:4044–4052. doi: 10.1021/jm400265k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Su SC, Vaneechoutte M, Dijkshoorn L, Wei YF, Chen YL, Chang TC. Identification of non-fermenting Gram-negative bacteria of clinical importance by an oligonucleotide array. J Med Microbiol. 2009;58:596–605. doi: 10.1099/jmm.0.004606-0. [DOI] [PubMed] [Google Scholar]
- 8.Tommasi R, Brown DG, Walkup GK, Manchester JI, Miller AA. ESKAPEing the labyrinth of antibacterial discovery. Nat Rev Drug Discov. 2015;14:529–542. doi: 10.1038/nrd4572. [DOI] [PubMed] [Google Scholar]
- 9.Privileged scaffolds in medicinal chemistry : design, synthesis, evaluation. Royal Society of Chemistry; Cambridge, England: 2016. [Google Scholar]
- 10.Kucukguzel SG, Senkardes S. Recent advances in bioactive pyrazoles. Eur J Med Chem. 2015;97:786–815. doi: 10.1016/j.ejmech.2014.11.059. [DOI] [PubMed] [Google Scholar]
- 11.Keri RS, Chand K, Ramakrishnappa T, Nagaraja BM. Recent progress on pyrazole scaffold-based antimycobacterial agents. Arch Pharm (Weinheim) 2015;348:299–314. doi: 10.1002/ardp.201400452. [DOI] [PubMed] [Google Scholar]
- 12.Fulp A, Zhang Y, Bortoff K, Seltzman H, Snyder R, Wiethe R, Amato G, Maitra R. Pyrazole antagonists of the CB1 receptor with reduced brain penetration. Bioorg Med Chem. 2016;24:1063–1070. doi: 10.1016/j.bmc.2016.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nakai T, Perl NR, Barden TC, Carvalho A, Fretzen A, Germano P, Im GY, Jin H, Kim C, Lee TW, Long K, Moore J, Rohde JM, Sarno R, Segal C, Solberg EO, Tobin J, Zimmer DP, Currie MG. Discovery of IWP-051, a Novel Orally Bioavailable sGC Stimulator with Once-Daily Dosing Potential in Humans. ACS Med Chem Lett. 2016;7:465–469. doi: 10.1021/acsmedchemlett.5b00479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khan MF, Alam MM, Verma G, Akhtar W, Akhter M, Shaquiquzzaman M. The therapeutic voyage of pyrazole and its analogs: A review. Eur J Med Chem. 2016;120:170–201. doi: 10.1016/j.ejmech.2016.04.077. [DOI] [PubMed] [Google Scholar]
- 15.Chen LW, Wang PF, Tang DJ, Tao XX, Man RJ, Qiu HY, Wang ZC, Xu C, Zhu HL. Metronidazole containing pyrazole derivatives potently inhibit tyrosyl-tRNA synthetase: design, synthesis, and biological evaluation. Chem Biol Drug Des. 2016;88:592–598. doi: 10.1111/cbdd.12793. [DOI] [PubMed] [Google Scholar]
- 16.Hafez HN, El-Gazzar AR, Al-Hussain SA. Novel pyrazole derivatives with oxa/thiadiazolyl, pyrazolyl moieties and pyrazolo[4,3-d]-pyrimidine derivatives as potential antimicrobial and anticancer agents. Bioorg Med Chem Lett. 2016;26:2428–2433. doi: 10.1016/j.bmcl.2016.03.117. [DOI] [PubMed] [Google Scholar]
- 17.Nayak N, Ramprasad J, Dalimba U. New INH-pyrazole analogs: Design, synthesis and evaluation of antitubercular and antibacterial activity. Bioorganic & Medicinal Chemistry Letters. 2015;25:5540–5545. doi: 10.1016/j.bmcl.2015.10.057. [DOI] [PubMed] [Google Scholar]
- 18.Brider J, Rowe T, Gibler DJ, Gottsponer A, Delancey E, Branscum MD, Ontko A, Gilmore D, Alam MA. Synthesis and antimicrobial studies of azomethine and N-arylamine derivatives of 4-(4-formyl-3-phenyl-1H-pyrazol-1-yl)benzoic acid as potent anti-methicillin-resistant Staphylococcus aureus agents. Medicinal Chemistry Research. 2016:1–7. [Google Scholar]
- 19.Zhao M, Kamada T, Takeuchi A, Nishioka H, Kuroda T, Takeuchi Y. Structure-activity relationship of indoloquinoline analogs anti-MRSA. Bioorg Med Chem Lett. 2015;25:5551–5554. doi: 10.1016/j.bmcl.2015.10.058. [DOI] [PubMed] [Google Scholar]
- 20.Khan SA, Yusuf M. Synthesis, spectral studies and in vitro antibacterial activity of steroidal thiosemicarbazone and their palladium (Pd (II)) complexes. European Journal of Medicinal Chemistry. 2009;44:2270–2274. doi: 10.1016/j.ejmech.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Tian B, He M, Tang S, Hewlett I, Tan Z, Li J, Jin Y, Yang M. Synthesis and antiviral activities of novel acylhydrazone derivatives targeting HIV-1 capsid protein. Bioorg Med Chem Lett. 2009;19:2162–2167. doi: 10.1016/j.bmcl.2009.02.116. [DOI] [PubMed] [Google Scholar]
- 22.Backes GL, Neumann DM, Jursic BS. Synthesis and antifungal activity of substituted salicylaldehyde hydrazones, hydrazides and sulfohydrazides. Bioorg Med Chem. 2014;22:4629–4636. doi: 10.1016/j.bmc.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Lawson M, Hamze A, Peyrat JF, Bignon J, Dubois J, Brion JD, Alami M. An efficient coupling of N-tosylhydrazones with 2-halopyridines: synthesis of 2-alpha-styrylpyridines endowed with antitumor activity. Org Biomol Chem. 2013;11:3664–3673. doi: 10.1039/c3ob40263k. [DOI] [PubMed] [Google Scholar]
- 24.Dollinger H, Mack J, Martyres D, Jung B, Nickolaus P. Preparation of pteridines as PDE4 phosphodiesterase inhibitors for the treatment of inflammatory diseases. Boehringer Ingelheim International G.m.b.H; Germany: 2006. p. 21. [Google Scholar]


