Abstract
The purpose of part 1 is to provide an overview of published literature (1980–2002) on chronic wasting disease (CWD) to inform Canadian readers about the disease and to explain Canadian regulatory approaches to the surveillance and control of CWD. Much of the scientific information is drawn from American publications obtained from internet searches in PubMed and Medline databases. The following keywords were used: chronic wasting disease, prion, diagnosis, transmissible spongiform encephalopathies, CWD and deer, CWD and elk, and CWD and environment. The article also presents information from Canadian publications and unpublished observations, Canadian Food Inspection Agency (CFIA) documents, and both government and nongovernment internet Web sites. The article highlights some different features of CWD in Canada, as compared with the situation in the United States, and mentions public health implications of the disease. It also describes the basis for development of Canada’s surveillance and control program. Part 2 will detail the activities and results of the surveillance and control program during 2000 to 2002 and discuss factors that will influence the feasibility of eradicating CWD. Chronic wasting disease appears to have been introduced into Canada through the importation of infected farmed elk from the United States in the late 1980s and early 1990s, at a time when little was known about the disease. Since then, eradication efforts in Canada have led to the control of the spread of CWD in the farmed elk industry. Still, management of this disease, especially in free-ranging cervids, is a challenge.
Abstract
Résumé — Maladie débilitante chronique au Canada : première partie. Le but de cette première partie est de fournir une vue d’ensemble de la littérature publiée (1980–2002) sur la maladie débilitante chronique (MDC) afin de renseigner le lecteur canadien sur la maladie et d’expliquer la démarche canadienne concernant les réglementations destinées à la surveillance et au contrôle de la MDC.
Une grande partie de l’information scientifique provient de publications américaines obtenus par recherche sur Internet dans les banques de données PubMed et Medline. Les mots clés suivants ont été utilisés : maladie débilitante chronique, prion, diagnostic, encéphalopathies spongiformes transmissibles, MDC et cerf, MDC et wapiti et MDC et environnement. L’article présente également de l’information provenant de publications canadiennes ainsi que des observations non publiées, des documents de l’Agence canadienne d’inspection des aliments ainsi que des sites Web Internet gouvernementaux et non gouvernementaux. L’article souligne certains aspects différents de la MDC au Canada par rapport à la situation aux États-Unis et mentionne les implications de la maladie sur la vernementaux Canada par rapport à la situation aux États-Unis et mentionne les implications de la maladie sur la santé publique. Il décrit également les bases d’un programme canadien de surveillance et de contrôle. La partie 2 racontera en détail les activités et les résultats du programme de surveillance et de contrôle entre 2000 et 2002 et discutera des facteurs qui influenceront la faisabilité de l’éradication de la MDC. La maladie débilitante chronique semble avoir été introduite au Canada par l’importation de wapitis d’élevage infectés provenant des États-Unis à la fin des années 1980 et au début des années 1990 à d’élevage infectés provenant des États-Unis à la fin des années 1980 et au début des années 1990 à un moment où peu de choses étaient connues sur la maladie. Depuis lors, les efforts d’éradication au Canada ont conduit au contrôle de la dissémination de la MDC dans l’industrie du wapiti d’élevage. Depuis, la gestion de cette maladie, particulièrement chez les cervidés en liberté, constitue un défi.
(Traduit par Docteur André Blouin)
Introduction
Chronic wasting disease (CWD) is a fatal prion disease affecting mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus), and Rocky Mountain elk (Cervus elaphus nelsoni) (1). Although CWD has been diagnosed only in these 3 species to date, it is likely that subspecies of these cervids, such as red deer (Cervus elaphus), also are susceptible to infection (1,2). This disease was first recognized clinically in the 1960s in captive cervids held at research facilities first in Colorado and later in Wyoming (1). In 1978, CWD was recognized as a transmissible spongiform encephalopathy (TSE) (3). It is the only TSE known to affect free-living species (2). While CWD was first diagnosed in wild elk in 1981, surveillance data and epidemiological modelling suggest that CWD may have been present in some free-ranging deer populations for 2 decades or more before being detected (1,4).
This is the first of 2 articles prepared by the Canadian Food Inspection Agency (CFIA) on chronic wasting disease (CWD). The purpose of these articles is to inform Canadian readers about CWD, a prion disease of cervids that has been reported from several US states and the provinces of Saskatchewan and Alberta, and to explain Canada’s surveillance and control program for CWD. This first article does not present the results of original scientific research. Rather, it provides an overview of relevant literature (1980–2002) to inform Canadian readers about the disease and to explain Canadian regulatory approaches to the surveillance and control of CWD.
Literature review
Articles were obtained through searches on Medline and PubMed databases accessed between 1994 and November 2002. Key words used were as follows: chronic wasting disease, prion, diagnosis, transmissible spongiform encephalopathies, CWD and deer, CWD and elk, and CWD and environment. Articles published between 1980 and 2002 were included in the review. Other articles were collected from conferences, Canadian Food Inspection Agency (CFIA) publications, and provincial government and international agencies’ publications through Web sites. Unpublished observations were provided by Canadian veterinarians who have worked with the cervid industry and provincial veterinary services since 2000 with the goal of eradicating CWD from farmed cervids and protecting wildlife from the disease.
Host range
There is no evidence that CWD has infected species other than cervids under natural conditions. Other non-domestic ruminants (moose, pronghorn sheep, mountain goats) that have been in contact with CWD-infected deer and elk, or have resided in premises where CWD has occurred, have not developed the disease (1). Also, domestic livestock are not known to be naturally susceptible to the disease. A limited number of cattle, sheep, and goats have resided in research facilities together with CWD-affected animals for prolonged periods of time without developing the disease (1). Many species — domestic ferrets, mice, domestic goats, sheep, mink, squirrel monkeys, and domestic cattle — are experimentally susceptible to developing CWD through intracerebral inoculation (1). A study by Hamir et al (5) reported that experimental transmission of CWD to cattle was possible. However, intracerebral inoculation is an unnatural route of transmission and these findings have little bearing on the potential for cattle, or other species, to become infected under natural conditions (1,5).
Geographic distribution
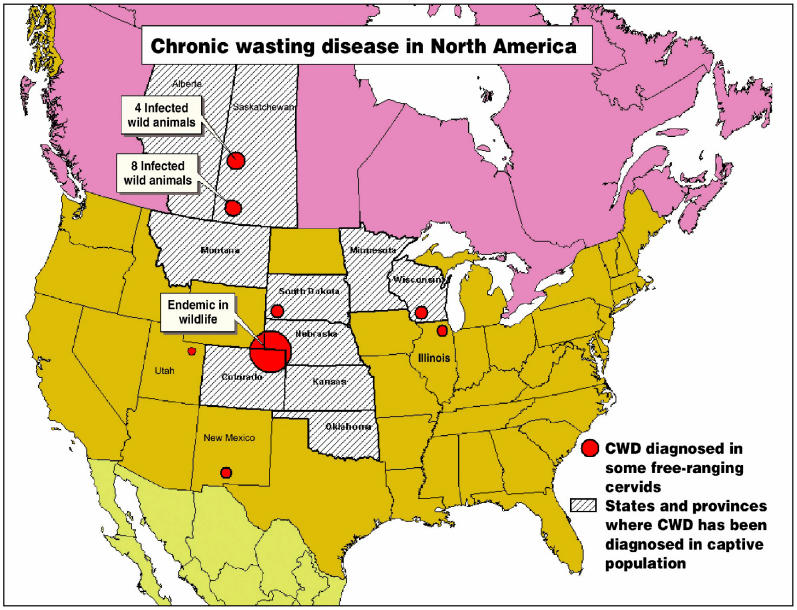
Williams (1,2) has described 2 contemporary epidemics (Figure 1). One epidemic is in free-ranging cervids, the other in farmed cervids (1,2). Chronic wasting disease is considered endemic in free-ranging deer and elk in the area comprising northeastern Colorado, southeastern Wyoming, and the southwest corner of the panhandle of Nebraska (1). The disease has also been identified in these free-ranging species in southwestern South Dakota; northwestern Nebraska; and, more recently, in south-central Wisconsin, northwestern Colorado, southern New Mexico (2), Illinois, and Utah. Since 1996, infection in farmed cervids has been reported in Colorado, Wyoming, Kansas, Nebraska, South Dakota, Oklahoma, Wisconsin, Montana, and Minnesota (1).
Figure 1.
Distribution of chronic wasting disease (CWD) in North America.
In Canada, CWD has been reported in farmed cervids in only 2 provinces, Saskatchewan and Alberta, with 40 (95%) of the 42 infected farms being located in Saskatchewan. The first case in farmed elk was diagnosed in 1996 in Saskatchewan. The first case in farmed white-tailed deer was diagnosed in Alberta in November 2002. Testing of 13 947 wild cervids between 1996 and 2002 (11 055 in Saskatchewan and 2892 in Alberta) has found only 12 infected wild deer, in the province of Saskatchewan. Limited testing in other provinces has not detected any CWD-infected animals to date.
Chronic wasting disease has been diagnosed outside North America only once (6). In 1994 and 1997, a total of 125 elk were exported to South Korea from a Saskatchewan farm, later known to be infected with CWD; 1 of these elk was confirmed as being positive for CWD in 2001.
Etiology
There are several theories about the origin and causal agent of CWD. The characteristics of the agent are poorly understood, but it is presumed to be a prion (1,2). It may have arisen from a spontaneous conformational alteration of the normal prion (PrP-c) to a resistant form (PrP-CWD) occurring in mule deer, with subsequent transmission to other deer and elk; a cervid-adapted strain of scrapie; a genetic form of TSE arising in deer, with subsequent natural transmission; or infection with a new strain of prion (1,2). Polymorphism in the PrP gene of elk seems to be associated with variations in susceptibility to CWD, and O’Rourke (7) found that Rocky Mountain elk that were homozygous for the PrP codon 132-Met (M/M) (M/M) were overrepresented in cases of CWD infection in free-ranging and farmed elk when compared with respective control groups. More studies are needed to evaluate the interaction of PrP polymorphism and CWD and the gene’s effect on susceptibility, transmission, and pathogenesis of the disease.
Origin
The time and place of emergence of CWD in North America cannot be determined with certainty (1). In the United States where, based on observations and epidemiological modelling, Miller et al (4) found an epicenter of high prevalence in wild deer north of Fort Collins, Colorado. If this is the origin of CWD in free-ranging cervids, CWD might have spread in the area via natural migration and movement of mule deer (4) to produce the current endemic area described by American researchers (1,2). However, Williams (1) indicates that the current epidemics in free-ranging and farmed cervids appear to be essentially independent entities with minimal geographic overlap; if these epidemics share a common origin, it may date back several decades. Therefore, the origin of CWD in farmed cervids in the United States is still unknown.
While CWD appears to have been introduced into Canadian farmed elk through the importation of infected animals from a farm in South Dakota, later found to be infected with CWD, the origin of CWD in free-ranging Canadian cervids is unknown. Possible explanations include extension from infected farms to free-ranging cervids via accidental contact between farmed and wild cervids, or spontaneous emergence of a sporadic form of CWD in wild cervids.
Transmission
The mode of transmission of CWD is not fully under-stood (8). In contrast to bovine spongiform encephalopathy (BSE), CWD is not a food-borne disease associated with ruminant meat-and-bone meal (1,2). Canadian farmed cervids are normally raised in pastoral husbandry systems and are not fed protein concentrate rations (9). Moreover, Canada (and the USA) banned the feeding of ruminant-derived protein to ruminants in 1997. Oral exposure is the most plausible route of infection under natural conditions. This theory is supported by the fact that PrP-CWD is observed in the lymphoid tissue of the alimentary tract early in the incubation period (10), which suggests that infected cervids may shed PrP-CWD in excretions such as feces and saliva (2). Epidemic modelling suggests that shedding of PrP-CWD starts before the onset of clinical signs (2). This shedding appears to be progressive through the course of the disease (1).
According to unpublished Canadian observations and consistent with US research findings (4,11,12), lateral transmission is significant in the epidemiology of CWD. It appears that cervids are exposed via ingestion of PrP-CWD from sources in the environment, direct contact with animals that are excreting the agent, or both (1). Increased population density as a consequence of keeping animals in captivity or artificial feeding of wildlife may increase the likelihood of direct and indirect transmission between individuals (1,2). In a heavily infected research facility in the United States, more than 90% of resident mule deer either died or were euthanized due to clinical CWD over a 2-year period (13). Also in the United States, CWD was the primary cause of adult mortality in 2 captive elk herds (5 [71%] of 7; 4 [23%] of 23) and a prevalence of 59% was detected by immunohistochemistry (IHC) at slaughter in a group of 17 elk from a CWD-affected farm (1). In Canada, there are several instances where the introduction of 1 infected animal apparently resulted in lateral transmission of infection to several other animals in the herd (unpublished observations).
Apparent cases of maternal transmission are thought to result from lateral transmission between infected dams and their offspring rather than from true vertical transmission (11). Direct transmission between animals probably requires more than transient exposure (2). As well, the duration of exposure and the amount of agent present in the environment will probably determine the level of infection in captive herds. Therefore, herds kept in highly contaminated areas for a sufficient period of time should develop high infection levels. In Canada, the prevalence of infection was < 5% in 65% of infected herds in Saskatchewan, only 4 (9.5%) of 42 infected herds had high prevalence of CWD (range 14% to 33%) (unpublished observations). These data, coupled with the fact that 91% of infected animals did not show clinical signs of CWD, led us to assume that CWD was detected early in most of these infected herds. It is clear that early detection and elimination are the key to the control of CWD (11).
Pathogenesis
Sigurdson et al (8) detected PrP-CWD in the myenteric plexus, the vagosympathetic trunk, and the intermediolateral cell column of the spinal cord. Their findings suggest that prion trafficking may occur by centripetal or centrifugal nerve transport (8). The vagus nerve could serve as a major transit route for the PrP-CWD, as occurs with scrapie, another TSE. Other significant transit routes (blood, sensory, or cranial nerves) cannot be excluded at this time (8). Resistant prion protein (PrP-CWD) was also found in endocrine glands, suggesting the possibility of transport via peripheral nerves (8). Although there is no inflammatory response to infection, the immune system plays a role in the pathogenesis of peripheral infection with TSE agents. Immune cells can be the first site of replication of these agents (13). In the case of scrapie, for example, infectivity is also associated with follicular dendritic cells in secondary lymphoid structures, which are critical in the process of neuroinvasion (13,14).
Under experimental conditions in the United States, incubation periods for CWD were 15 to 23 mo in mule deer and 12 to 34 mo in elk (1,2). The youngest animal diagnosed with naturally occurring CWD was 17 mo of age (2). It is not known when the animal was actually infected; therefore, it is not possible to make any assumptions on the minimum incubation period under natural conditions (2). In Canada, cervids under 12 mo of age have been identified as having preclinical infection by means of IHC conducted during depopulation and testing of CWD infected herds (unpublished observations). Adults and young animals appear to be equally susceptible to CWD (11) and, based on field observations in Canada, male and female elk also appear to be equally susceptible. The clinical course may last for a few days to a year. The maximum disease course is not known, but in experimentally infected animals, it can exceed 25 mo in mule deer and 34 mo in elk (1,2). The duration is less certain in naturally occurring cases (2).
Clinical signs
As in the United States, CWD in deer and elk in Canada is characterized by loss of body condition and behavioral changes. However, these changes are often subtle, especially in the early stage of the disease. Williams (2) suggested that clinical signs are milder and the clinical course more prolonged in elk than in deer, but too few deer have been identified with CWD to confirm if this hypothesis holds true in Canada.
According to the CFIA Manual of Procedures for the control of CWD, a clinical diagnosis of CWD should be considered in cervids over 16 mo of age having a compatible epidemiological or clinical history and displaying any of the following signs: excess salivation, unusual behavior (including decreased interaction with other animals, listlessness, depression, and aggressive or violent behavior), neurological signs (including paralysis, difficulty swallowing, fine head tremors, ataxia, polydipsia/polyuria, proprioception deficiencies, or recumbency), weight loss, retention of winter hair coat, and pneumonia. Diagnosis of CWD cannot be based solely on clinical signs; laboratory confirmation must be obtained.
Laboratory diagnosis
Presumptive diagnosis of CWD is based upon the appearance of clinical signs referable to the upper central nervous system (CNS) and secondary complications; it was confirmed by microscopic examination (3,15–17). In a majority of clinical cases, the diagnosis of CWD is confirmed with the presence of widespread spongiform changes in the brain, but, in some cases, the changes may be minimal or focal. In addition, minor differences occur in the intensity and anatomic location of the lesions between affected elk and deer, and among individual animals (15,16). Microscopic diagnosis is also dependent on satisfactory preservation of tissues, and even mildly autolyzed brain is unsuitable for histologic confirmation. Therefore, screening by microscopic examination alone may not be definitive. Similar to other TSEs, the accumulation of an abnormal isoform (PrP-CWD) of a host protein (PrP-C) in the CNS and peripheral lymphoid tissues of CWD-affected cervids is a cardinal feature of the lesions of CWD (18). Detection of PrP-CWD in tissue sections by IHC is a well-established diagnostic technique that is regularly used (19–21) to complement microscopic examination in both Canada and the United States. The dorsal motor nuclei of the vagus nerve (DMNV) at the level of the obex of the medulla are the primary and earliest targets in the brain, and PrP-CWD accumulation in this anatomical site precedes lesion development (20–22). Hence, the obex of the medulla is the most significant part of the brain to be examined for diagnosing CWD, so it should be submitted from every suspected or exposed cervid. The number of positively stained neurons can be very low in the early stages of the disease, so IHC examination by well-trained pathologists remains the most reliable means for diagnosis of CWD.
Since 2000, American and Canadian laboratories have actively collaborated in developing and applying diagnostic protocols for CWD. In the early stages of the Canadian CWD program, diagnoses were commonly cross-checked in 2 laboratories (1 in Canada; 1 in the USA) before confirming infection. As a result of this close collaboration, Canadian and US diagnosticians now use identical criteria in the diagnosis of CWD.
There are variations in the pathogenesis of the disease in deer and elk, and the approach to diagnostic testing must be adjusted for these differences (8,15). In deer, CWD is typically associated with abundant deposits of PrP-CWD in easily accessible lymphoid tissue, such as the tonsil and retropharyngeal lymph nodes, which are useful for early detection by IHC (20,21,23,24). Although early accumulation of PrP-CWD in lymphoid tissue has been observed in the preclinical stage of infection in elk, a substantial proportion of elk with brains that test positive do not have detectable PrP-CWD in either tonsil or retropharyngeal nodes (personal communication, Terry Spraker, Colorado State University, Fort Collins, Colorado, USA). Because of this inconsistency, IHC diagnosis of CWD in elk necessitates examination of the DMNV of the medulla, as well as tonsil or retropharyngeal lymph nodes.
The CFIA, the United States Department of Agriculture — Agricultural Research Service (USDA-ARS, Pullman, Washington), the Colorado State University, and the University of Wyoming have worked collaboratively on a highly specific monoclonal antibody-based immunohistochemical assay (F99/97-IHC) for the detection of PrP-CWD (20). This improved, standardized assay is highly specific because antibody binding can be shown to be associated with particular regions of lymphoid tissue or brain, and minor artifacts can be distinguished from the PrP-CWD specific immunostaining. Additional diagnostic tests include immunoblotting for detection of PrP-CWD in brain and lymphoid tissue, and fibril detection in detergent-treated brain homogenate by transmission electron microscopy (12). Several rapid tests, originally validated for the diagnosis of BSE by the European Commission, are being evaluated for the diagnosis of CWD in the surveillance of slaughtered and hunter-harvested cervids.
The advent of tonsil and third eyelid biopsy has enabled the detection of preclinical infection in scrapie-affected sheep (25,26). Tonsil biopsies can be used to diagnose CWD in live deer (23,24); although the procedure is minimally invasive, it requires general anesthesia and would not, therefore, be practical under field conditions in Canada. In the examination of tissue biopsies to date, most third eyelid of mule deer and elk contained no germinal centers, although diffused lymphoid cells were present (personal communication, Katherine O’Rourke, Agriculture Research Service, USDA). Therefore, third eyelid testing does not seem to be an option for testing at this time. For farmed cervids, the development of a blood- or urine-based live animal test with high prognostic value would improve the efficiency of diagnosis and facilitate effective surveillance.
The environment as a source of CWD infectivity
In the United States, there is evidence that the environment can be a source of infection for cervids. According to Williams and Miller (1), proper management of environmental contamination is important in the control of CWD. In an outbreak of CWD at a research facility in Wyoming that was handled by the destruction of all elk and deer in the facility but without disinfection and removal of soil, CWD reoccurred 5 y after the detection of the original infection, even though healthy cervids had not been reintroduced until 1 y after the depopulation (12).
Transmissible spongiform encephalopathy agents exhibit an unusual resistance to conventional methods of chemical and physical decontamination. They are not significantly affected by disinfectants like formalin and ethylene oxide, and infectivity persists after autoclaving at conventional times and temperatures (121°C for 15 min) (27). They are also extremely resistant to high doses of ionizing and ultraviolet irradiation, and some residual activity has been shown to survive for long periods in the environment (27). Brown et al (28) reported a loss of 2 to 3 log units of the initial infectivity but still detected residual infectivity in soil 3 y after the burial of a brain homogenate from a hamster inoculated with the scrapie agent. Some dispersion and dilution of PrP-res in soil due to wind and water erosion and movement by worms or other animate forces would have been expected. Abnormal prion proteins (PrP-res) bind to proteins and carbohydrates in carcasses; with decay, the bound PrP-res are released and disperse, binding to other particulate material in soil (29). It is thought that the movement of PrP-res in leachate is not significant, as the abnormal prions tend to bind to solid matter and settled sediments (29). Removal of soil from areas that have been heavily contaminated by infected animals may help to decrease the PrP-res contamination of the environment and the risk of reinfection of healthy cervids used to restock after depopulation of infected herds.
Management practices on the distribution of CWD in Canada
Cervids farmed in Canada include elk (Cervus elaphus), fallow deer (Dama dama), mule deer, red deer, reindeer (Rangifer tarandus), and white-tailed deer. Elk are the species most commonly farmed, and the farms produce antler velvet, venison, trophy animals, and breeding stock. Antler velvet is a commercially important product that is primarily exported to markets in Asia, while venison and trophies are utilized mainly in North America. White-tailed deer are the second most commonly farmed cervids in Alberta and Saskatchewan. In 2001, 43% of elk herds were located in the province of Alberta and 36% in Saskatchewan (30). Also in 2001, 55% of white-tailed deer herds were located in Alberta and 22% in Saskatchewan (30). Elk are typically raised on pasture seeded with either alfalfa or a combination of alfalfa and a perennial grass, and they are fed supplemental forage and grain during the winter months. Fencing, constructed according to provincial government requirements, helps to prevent contact between farmed and wild cervids.
In 1987, when the provinces of Alberta and Saskatchewan first allowed game farming, the elk industry grew very rapidly and the domestic demand for breeding elk could not be met. As a result, herds were built upon the importation of some 2000 elk (695 in Saskatchewan and 1318 in Alberta) from the United States. Manitoba did not allow elk farming until 1997, and in British Columbia, elk farming remains prohibited.
In Alberta, white-tailed deer were captured from the wild until 1990, when authorities first limited and then eliminated this practice. Provincial regulations restricted the capture or importation of white-tailed deer in Canada or from the United States onto game farms, primarily due to concerns about the translocation of the meningeal worms (Parelaphostrongylus tenuis). The number of white-tailed deer on farms increased during the 1990s via natural increase and the limited addition of deer from the wild (orphan fawns). In addition, deer entered farms from 1 Alberta game park (personal communication, Margo Pybus, Alberta Fish and Wildlife). The province of Saskatchewan has never allowed the trapping of white-tailed deer from the wild, instead deer were introduced from farms in other provinces, including surplus animals from game parks and zoos (personal communication, Ron Lind, Saskatchewan Fish and Wildlife Branch).
Fallow deer and red deer are more commonly farmed in the eastern provinces, where elk farms are rare. Thus, the authors believe that the farmed elk populations of Saskatchewan and Alberta have been reasonably well separated from farmed cervids in other provinces. The finding of CWD in farmed elk in Saskatchewan and Alberta, and in farmed white-tailed deer in Alberta, does not imply that the disease occurs in farmed elk or white-tailed deer in other provinces. More surveillance will be carried out to accurately define the situation with CWD throughout Canada.
In the late 1980s and early 1990s, federal and provincial jurisdictions introduced animal health measures to control diseases such as bovine tuberculosis (TB) and the meningeal parasite, Paraelaphostrongylus tenuis (31). An outbreak of TB was diagnosed in an elk herd in Alberta in December 1990. The province had not reported a case of TB in cattle since 1986 and the farmed bovine and bison sector was and remains TB-free. At the time, the outbreak was thought to have been associated with the importation of elk from the United States prior to September 1988 (31). From 1990 until 1993, 16 TB-infected herds in Alberta were depopulated to protect the TB-free status of Alberta’s cattle and to eradicate TB from farmed elk (31). Alberta provincial records of 1993 indicate that 300 elk with US ear tags remained alive after this depopulation (personal communication, Chuck Huedepohl, Agriculture, Food and Rural Development, Alberta). The destruction of some 77% of elk imported from the United States into Alberta may have helped to limit the exposure of elk farms in Alberta to infection with CWD if, as hypothesized in this paper, CWD was introduced via the importation of infected elk from the United States.
Other control measures introduced in the late 1980s and early 1990s had some effect in reducing the movement of farmed cervids within western Canada and may have helped to minimize the dispersion of animals infected with CWD. In September 1988, the province of Alberta prohibited the importation of ungulates from other provinces and from the USA (32). In 1990, the CFIA introduced both national requirements for movement permits and individual identification for farmed cervids. These requirements were to provide the means of tracing farmed cervid movements that enabled CFIA to carry out the CWD control program introduced in 2000. In 1998, the province of Manitoba placed a ban on the importation of farmed elk. The federal government banned the importation of live cervids from the USA from 1990 to May 1999 to prevent the entry of TB. The authors consider that this measure would have effectively prevented the introduction of new cases of CWD via imported elk. While provincial government control measures may have limited the spread of CWD between provinces, there were no veterinary controls to prevent CWD from spreading within Saskatchewan during the early part of the 1990s.
In Canada, the 1st case of CWD in a farmed cervid (elk) was diagnosed in 1996 at the Saskatchewan provincial diagnostic laboratory. In the same year, the herd was humanely destroyed; all animals were tested for CWD and found to be negative. A 2nd case was diagnosed in farmed elk in 1998 by the CFIA. That year, the mother and siblings of the infected elk were humanely destroyed and subsequently found to be negative for CWD. That herd was monitored for additional evidence of CWD; it was humanely destroyed after a 2nd case of CWD was found in the herd. The herd that was the original source of the infected elk found in 1996 and 1998 was investigated and was placed in quarantine in 2000. Since May 1999, the CFIA has permitted the importation of cervids from the United States into Canada, subject to measures to prevent the entry of CWD and other diseases of concern.
The reporting of CWD in 2000 resulted in the closure of some export markets for antler velvet, which had serious consequences on the farmed cervid industry. Live elk and elk semen were exported to many countries including the United States, New Zealand, Russia, and Korea. The closure of export markets for live elk, elk semen, and antler velvet (in or after 2000) has caused significant reductions in prices paid for velvet and live animals. For example, the price per pound of unprocessed velvet dropped from Cnd $60 in 2000 to Cnd $22 in 2002 (30). Although other factors did play a role in this reduction in price, CWD has definitely been costly to the cervid industry.
Management and control
No treatment or vaccine is currently available for CWD. In Canada, management of CWD in captive cervids is based on classical disease control principles. These include quarantine and depopulation of CWD-infected herds; testing of all depopulated adult animals (generally those over 12 mo of age); tracing of all animals in contact (trace-in and trace-out) with the infected herd; humane destruction and testing of traced animals that have left the herd within 36 mo prior to the detection of infection; and the tracing and surveillance of all animals that left the herd between 36 and 60 mo prior to the detection of infection. Surveillance should be applied to all dead and culled cervids on farms to maximize the probability of detecting this disease.
Williams and Miller (1) indicate that the following factors impede management of CWD: the limited ability to diagnose infection in live cervids, the long incubation period, the subtle early clinical signs, and the possible persistence of PrP-CWD in the environment (1). Effective removal of all sources of infectivity may be difficult in highly contaminated environments, since there are currently no tests available to confirm or rule out the presence of PrP-CWD in the environment. Control programs need to include provisions for such contaminated facilities. These may include the prohibition of restocking with susceptible species, as is the case in Canada.
The presence of CWD in free-ranging cervids also represents serious management problems (1). Surveillance is costly, but more importantly, the effects of CWD on population dynamics of deer and elk are still unknown. Williams and Miller (2) suggest that wildlife management and animal health agencies act to limit the distribution and occurrence of CWD in free-ranging and farmed cervids.
Public health
Health Canada is in agreement with the current thinking of the World Health Organization (WHO) regarding the lack of evidence that CWD in cervids can be transmitted to humans via consumption of cervid products. While CWD is a TSE, scientific observations to date suggest that CWD is more like scrapie than BSE. Scrapie affects sheep and goats in Canada and most other countries of the world. The disease has been recognized for over 200 y (33); it has never been associated with disease in humans handling or slaughtering sheep or goats, or consuming products of these animals. In vitro studies demonstrated that the abnormal PrP-CWD could convert, albeit inefficiently, the human prion (PrPC) to the abnormal isoform (34). The results of this study demonstrated that a barrier at the molecular level should limit the susceptibility of certain noncervid species (human, bovine, ovine) to CWD (34).
In the absence of definitive information, public health agencies recommend that the public avoid contact with the CWD agent. The public is advised not to consume high risk materials, such as brain, spinal cord, and offal; to wear latex or rubber gloves when handling or dressing deer or elk from CWD — endemic areas; and to thoroughly wash knives and other implements used to process cervid carcasses (2).
While human consumption of cervid products, including venison and antler velvet, have not been associated with adverse health consequences, scientific information on the potential risks is limited. The WHO recommends that humans not eat any part or product of any animal with evidence of CWD or other TSE (35). While the distribution of PrP-CWD in infected cervids is not completely understood, obviously, it would be prudent not to consume nervous tissues and organs associated with the intestinal tract. In limited studies conducted to date, it appears that the risk of CWD-specific PrP contamination of velvet may be very low. A velvet sample, collected from an elk 3 mo prior to developing clinical CWD, showed no detectable CWD-specific PrP accumulation by routine immunoblotting and IHC methods. The affected animal had severe brain lesions and extensive disease-specific staining in the brain and peripheral lymphoid tissue (unpublished observations). Although CWD has not been shown to present a human health risk, Health Canada’s policy is that TSE-infected or exposed animals or their products must not enter either the food chain or animal feed (36).
The provinces of Manitoba, Saskatchewan, and Alberta require the testing of all slaughtered adult cervids. All carcasses tested for CWD are held at the slaughterhouse until the test results have been obtained. The head, spinal cord, and offal may be disposed of by deep burial or incineration without first obtaining test results. The provincial governments of Manitoba, Alberta, and Saskatchewan issue velvet identification tags so that this product can be traced in the event that CWD is diagnosed after harvest. In Ontario, Québec, and the Maritimes, the Canadian Cervid Council (CCC) issues these tags. Mandatory testing of slaughter animals is not in place in these provinces. Herd certification programs that require surveillance testing are currently being implemented in all provinces in Canada.
Conclusion
In the year 2000, when the CFIA first considered undertaking an eradication program for CWD, key scientific information on the epidemiology, prevalence, and geographic distribution of CWD was lacking, and it was not known if this would be feasible. However, consultation between the CFIA and provincial and industry partners led to a broad consensus on the need to try to eradicate this disease. This was driven by concerns of the risks this TSE presented for the health of wildlife; the sustainability of cervid farming, if the disease was allowed to spread unchecked; and the possible implications for human health.
Acknowledgments
The authors thank the following people for their contributions and comments: Dr. Brian Peart, Dr. George Luterbach, Dr. Carolyn Inch, Dr. Wayne Lees, and Mr. Vic D’Angiolo of the Canadian Food Inspection Agency; Mr. Serge Buy of the Canadian Cervid Council; and Dr. Trent Bollinger of the Canadian Cooperative Wildlife Health Centre Department of Veterinary Pathology, Western College of Veterinary Medicine, Saskatoon, Saskatchewan. Significant contributions of provincial government agencies and departments to the epidemiology and diagnostics are greatly appreciated by the CFIA. CVJ
References
- 1.Williams ES, Miller MW. Chronic wasting disease in deer and elk in North America. Rev Sci Tech Off Int Epiz. 2002;21:305–316. doi: 10.20506/rst.21.2.1340. [DOI] [PubMed] [Google Scholar]
- 2.Williams ES, Miller MW, Kreeger TJ, Kahn RH, Thorne ET. Chronic wasting disease of deer and elk: a review with recommendations for management. J Wildl Manage. 2002;66:551–563. [Google Scholar]
- 3.Williams E, Young S. Chronic wasting disease of captive mule deer: a spongiform encephalopathy. J Wildl Dis. 1980;16:89–98. doi: 10.7589/0090-3558-16.1.89. [DOI] [PubMed] [Google Scholar]
- 4.Miller MW, Williams ES, McCarty CW, et al. Epizootiology of chronic wasting disease in free-ranging cervids in Colorado and Wyoming. J Wildl Dis. 2000;36:676–690. doi: 10.7589/0090-3558-36.4.676. [DOI] [PubMed] [Google Scholar]
- 5.Hamir AN, Cutlip RC, Miller JM, et al. Preliminary findings on the experimental transmission of chronic wasting disease agent of mule deer to cattle. J Vet Diagn Invest. 2001;13:91–96. doi: 10.1177/104063870101300121. [DOI] [PubMed] [Google Scholar]
- 6.Sohn HJ, Kim JH, Choi KS, et al. A case of chronic wasting disease in an elk imported to Korea from Canada. J Vet Med Sci. 2002;64:855–858. doi: 10.1292/jvms.64.855. [DOI] [PubMed] [Google Scholar]
- 7.O’Rourke KI, Besser TE, Miller MW, et al. PrP genotypes of captive and free-ranging Rocky Mountain elk (Cervus elaphus nelsoni) with chronic wasting disease. J Gen Virol. 1999;80:2765–2769. doi: 10.1099/0022-1317-80-10-2765. [DOI] [PubMed] [Google Scholar]
- 8.Sigurdson CJ, Spraker TR, Miller MW, Oesch B, Hoover EA. PrPCWD in the myenteric plexus, vagosympathetic trunk and endocrine glands of deer with chronic wasting disease. J Gen Virol. 2001;82:2327–2334. doi: 10.1099/0022-1317-82-10-2327. [DOI] [PubMed] [Google Scholar]
- 9.Nixdorf R, Dobbs S, Grimsrud M, Pylypchuk M. Elk production, economic and production information for Saskatchewan producers. Saskatchewan Agriculture and Food, November 2000. Http://www.agr.gov.sk.ca Last accessed November 2002.
- 10.Sigurdson CJ, Williams ES, Miller MW, Spraker TR, O’Rourke KI, Hoover A. Oral transmission and early lymphoid tropism of chronic wasting disease PrPres in mule deer fawns (Odocoileus hemionus) J Gen Virol. 1999;80:2757–2764. doi: 10.1099/0022-1317-80-10-2757. [DOI] [PubMed] [Google Scholar]
- 11.Miller MW, Wild MA, Williams ES. Epidemiology of chronic disease in captive Rocky Mountain elk. J Wildl Dis. 1998;34:532–538. doi: 10.7589/0090-3558-34.3.532. [DOI] [PubMed] [Google Scholar]
- 12.Wolfe LL, Conner MM, Baker TH, et al. Evaluation of antemortem sampling to estimate chronic wasting disease prevalence in free ranging mule deer. J Wildl Manage. 2002;66:564–573. [Google Scholar]
- 13.Mabbott NA, Bruce ME. The immunobiology of TSE diseases. J Gen Virol. 2001;82:2307–2318. doi: 10.1099/0022-1317-82-10-2307. [DOI] [PubMed] [Google Scholar]
- 14.Dormont D. Prion diseases: pathogenesis and public health concerns. FEBS Lett. 2002;529:17–21. doi: 10.1016/s0014-5793(02)03268-4. [DOI] [PubMed] [Google Scholar]
- 15.Spraker TR, Miller MW, Williams ES, et al. Spongiform encephalopathy in free-ranging mule deer (Odocoileus hemionus), white-tailed deer (Odocoileus virginianus) and Rocky Mountain elk (Cervus elaphus nelsoni) in northcentral Colorado. J Wildl Dis. 1997;33:1–6. doi: 10.7589/0090-3558-33.1.1. [DOI] [PubMed] [Google Scholar]
- 16.Williams ES, Young S. Neuropathology of chronic wasting disease of mule deer (Odocoileus hemionus) and elk (Cervus elaphus nelsoni) Vet Pathol. 1993;30:36–45. doi: 10.1177/030098589303000105. [DOI] [PubMed] [Google Scholar]
- 17.Williams ES, Young S. Spongiform encephalopathy of Rocky Mountain Elk. J Wildl Dis. 1982;18:465–471. doi: 10.7589/0090-3558-18.4.465. [DOI] [PubMed] [Google Scholar]
- 18.Prusiner SB. Molecular biology of prion diseases. Science. 1991;252:1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- 19.Guiroy DC, Williams ES, Yanagihara R, Gajdusek DC. Immunolocalization of scrapie amyloid (PrP27-30) in chronic wasting disease of Rocky Mountain elk and hybrids of captive mule deer and white-tailed deer. Neurosci Lett. 1991;126:195–198. doi: 10.1016/0304-3940(91)90552-5. [DOI] [PubMed] [Google Scholar]
- 20.Spraker TR, Zink RR, Cummings BA, Wild MA, Miller MW, O’Rourke KJ. Comparison of histological lesions and immunohistochemical staining of proteinase resistant prion protein in a histochemical naturally-occurring spongiform encephalopathy of free-ranging mule deer (Odocoileus hemionus) with those of chronic wasting ) disease of captive mule deer. Vet Pathol. 2002;39:110–119. doi: 10.1354/vp.39-1-110. [DOI] [PubMed] [Google Scholar]
- 21.Spraker TR, O’Rourke KI, Balachandran A, et al. Validation of monoclonal antibody F99/97.6. 1 for immunohistochemical staining of brain and tonsil in mule deer (Odocoileus hemionus) with ) chronic wasting disease. J Vet Diagn Invest. 2002;14:3–7. doi: 10.1177/104063870201400102. [DOI] [PubMed] [Google Scholar]
- 22.Peters J, Miller JM, Jenny AL, Peterson TL, Carmichael KP. Immunohistochemical diagnosis of chronic wasting disease in preclinically affected elk from a captive herd. J Vet Diagn Invest. 2000;12:579–582. doi: 10.1177/104063870001200618. [DOI] [PubMed] [Google Scholar]
- 23.Wild MA, Spraker TR, Sigurdson CJ, O’Rourke KI, Miller MW. Preclinical diagnosis of chronic wasting disease in captive mule deer (Odocoileus hemionus) and white-tailed deer (Odocoileus virginianus) using tonsillar biopsy. J Gen Virol. 2002;83:2629–2634. doi: 10.1099/0022-1317-83-10-2629. [DOI] [PubMed] [Google Scholar]
- 24.Williams ES, Young S. Spongiform encephalopathies in cervidae. Rev Sci Tech Off Int Epiz. 1992;11:551–567. doi: 10.20506/rst.11.2.611. [DOI] [PubMed] [Google Scholar]
- 25.Schreuder BEC, van Keulan LJM, Vromans MEW, Langveld JPM, Smits MA. Tonsillar biopsy and PrP Sc detection in the preclinical diagnosis of scrapie. Vet Rec. 1998;142:564–568. doi: 10.1136/vr.142.21.564. [DOI] [PubMed] [Google Scholar]
- 26.O’Rourke KI, Baszler TV, Parish SM, Knowles DP. Preclinical detection of PrP-Sc in nictitating membrane lymphoid tissue of sheep. Vet Rec. 1998;142:489–491. doi: 10.1136/vr.142.18.489. [DOI] [PubMed] [Google Scholar]
- 27.United Kingdom Department of Health Advisory Committee on Dangerous Pathogens, Spongiform Encephalopathy Advisory Committee. Transmissible Encephalopathy Agents — Part 1, Background and introduction. 1998. http://www.archive.officialdocuments.co.uk/document/doh/spongifm/part-1.htm Last accessed documents.May 2003.
- 28.Brown PL, Gajdusek D. Survival of scrapie virus after 3 years’ interment. Lancet. 1991;337:269–270. doi: 10.1016/0140-6736(91)90873-n. [DOI] [PubMed] [Google Scholar]
- 29.Gale P, Young G, Stanfield G, Oakes D. Development of a risk assessment for BSE in the aquatic environment. J Appl Microbiol. 1998;84:467–477. doi: 10.1046/j.1365-2672.1998.00495.x. [DOI] [PubMed] [Google Scholar]
- 30.Nixdorf R. Specialized Livestock Inventory and Prices Update. Saskatchewan Agriculture, Food and Rural Revitalization, Livestock Development Branch, October 25, 2002. Contact Rnixdorf@agr.gov.sk.ca
- 31.Nation PN, Fanning EA, Hopf HB, Church TL. Observations on animals and human health during the outbreak of Mycobacterium bovis in game farm wapiti in Alberta. Can Vet J. 1999;40:113–117. [PMC free article] [PubMed] [Google Scholar]
- 32.The Deer and Elk Manual. Province of Alberta 2002. http://www.agric.gov.ab.ca/livestock/elk/derelk9.html Last acessed November 2002.
- 33.Parry HB. Scrapie Disease in Sheep. New York, Acad Pr, 1983: 31–51.
- 34.Raymond GJ, Bossers A, Raymond LD, et al. Evidence of a molecular barrier limiting susceptibility of humans, cattle and sheep to chronic wasting disease. EMBO. 2000;19:4425–4430. doi: 10.1093/emboj/19.17.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.World Health Organisation (WHO). WHO Consultation on Public Health and Animal Transmissible Spongiform Encephalopathies: Epidemiology, Risk and Research Requirements. Geneva, Switzerland, December 1999. Http://www.who.int/emc Last accessed November 2002.
- 36.Le Maguer M. Risk Management of Transmissible Spongiform Encephalopathies. Science Advisory Board, Health Canada. February 13–14, 2001. Http://www.hc-sc.gc.ca/sab-ccs/feb2001_bse_tse_slides_e.pdf Last accessed May 2003.