Abstract
An estimated 16 million people in the United States have coronary artery disease (CAD), and approximately 325,000 people die annually from cardiac arrest. About two-thirds of unexpected cardiac deaths occur without prior recognition of cardiac disease. A vast majority of these deaths are attributable to the rupture of ‘Vulnerable atherosclerotic plaques’. Clinically, plaque vulnerability is typically assessed through imaging techniques, and ruptured plaques leading to acute myocardial infarction are treated through angioplasty or stenting. Despite significant advances, it is clear that current imaging methods are insufficiently capable for elucidating plaque composition—which is a key determinant of vulnerability. Further, the exciting improvement in the treatment of CAD afforded by stenting procedures has been buffered by significant undesirable host-implant effects, including restenosis and late thrombosis. Nanotechnology has led to some potential solutions to these problems by yielding constructs that interface with plaque cellular components at an unprecedented size scale. By leveraging the innate ability of macrophages to phagocytose nanoparticles, contrast agents can now be targeted to plaque inflammatory activity. Improvements in nano-patterning procedures have now led to increased ability to regenerate tissue isotropy directly on stents, enabling gradual regeneration of normal, physiologic vascular structures. Advancements in immunoassay technologies promise lower costs for biomarker measurements, and in the near future, may enable the addition of routine blood testing to the clinician’s toolbox—decreasing the costs of atherosclerosis-related medical care. These are merely three examples among many stories of how nanotechnology continues to promise advances in the diagnosis and treatment of vulnerable atherosclerotic plaques.
INTRODUCTION
Cardiovascular disease continues to be the leading cause of death in the United States, accounting for 1 of every 2.9 deaths.1 The development of atherosclerotic plaques is responsible for many of these events, and is characterized by a cascade of events including the accumulation of lipids in arterial walls, oxidation of the lipids, and recruitment of inflammatory cells into these lipid-rich regions.2 Under the influence of chronic lipid deposition and oxidation, inflammatory activity, and resulting abnormal blood flow and mechanical loading conditions in the region, these plaques may suddenly rupture and lead to a potentially lethal acute event such as a stroke or a myocardial infarction. Therefore, identifying and locating plaques at particular risk of rupture (vulnerable plaques) may potentially provide the basis for therapeutic interventions in the prevention of sudden cardiac death and myocardial infarction.
Despite advances in diagnostic tools, many patients at increased risk for acute coronary events (vulnerable patients) are not identified, as they do not present with any symptoms prior to an acute coronary event.3 At present, there is no consensus regarding the appropriate treatment of a coronary plaque believed to be at high risk for rupture. Stent implantation, currently used to treat symptomatic obstructive CAD and ruptured plaques in acute myocardial infarctions, may represent a potential therapeutic option for treatment of vulnerable plaques in the future. However, subjecting an asymptomatic patient to the procedural and long-term risks of stenting, including a renarrowing or sudden thrombosis within the stent, is not justified by scientific evidence at this time.4,5 It is clear that novel cellular and molecular imaging tools are required in order to better assess a patient’s risk of potentially life-threatening acute plaque rupture. At the same time, for the mechanical treatment of asymptomatic vulnerable plaques to become a feasible approach, improvements in treatment technologies are required in order to reduce the likelihood of undesirable host responses to implanted materials and unanticipated adverse events, as well as the restoration of physiological vascular function.
CHARACTERISTICS OF VULNERABLE ATHEROSCLEROTIC PLAQUES: MORPHOLOGICAL, MECHANICAL, AND MATERIAL PROPERTIES
From an engineering standpoint, an accurate morphological, mechanical, and material description of vulnerable atherosclerotic plaques is necessary in order to better design treatment tools for their management. It is important to note that each of these categories is interconnected, as changes in morphology and composition of plaques will undoubtedly affect changes in mechanical and material properties of plaques, and vice versa.6,7
While the morphological determinants of plaque vulnerability remain difficult to pinpoint conclusively, autopsy studies have suggested that vulnerable plaques are very likely to exhibit some of the following five features: (1) active inflammatory activity, (2) thin fibrous caps and large lipid cores, (3) endothelial erosion and thrombosis, (4) fissured or ruptured caps, and (5) luminal stenosis (>90%).3,8
The multiple roles of inflammatory activity in influencing plaque vulnerability are particularly important to note. Under normal conditions, low-density lipoprotein (LDL) particles in the bloodstream and in the arterial wall may become oxidized, producing oxidized LDL (oxLDL), which is rapidly cleared by macrophages in the liver.9,10 Early in lesion formation, however, the balance between the entry of LDL into the vascular wall and its efflux becomes disturbed, leading to increased retention of LDL at focal points in the vascular wall. Combined with normal oxidation processes, elevated local levels of oxLDL produces an environment that is chemoattractive to monocytes and T-cells, among others.10
The influx of macrophages into an atherosclerotic lesion leads to some major repercussions. Macrophages within a lesion express various scavenger receptors that facilitate endocytosis of lipids and other interstitial debris. This activity eventually results in transformation of macrophages into foam cells, and their further activation leads to apoptosis—usually triggered by high local concentrations of oxLDL, TNF-α, Fas ligand, intracellular accumulation of free cholesterol, or hypoxia—contributing to the gradual expansion of the necrotic core.11,12 The production of matrix metalloproteinase (MMP)-8 and -9 by monocytes/macrophages in response to pathological stimuli leads to degradation and destabilization of the local extracellular matrix, increasing the likelihood of plaque rupture.13–17 For these reasons, atherosclerosis is commonly thought of as an inflammatory disease, and this classification continues to guide burgeoning work in this area.18
Mechanically, the formation of a plaque is characterized by intimal thickening, leading to a dramatic increase in intimal stiffness and elastic modulus, relative to normal arteries.7,19–24 (Figure 1(a)). Conversely, the media and adventitia of the diseased blood vessels become abnormally softer than their corresponding layers in normal arteries. Therefore, changes in the layer-by-layer modulus of pathological arteries show an opposite pattern to normal arteries where the modulus gradually increases from intima to adventitia. This change in the layer-by-layer modulus of arterial wall can be explained by a theory with respect to progressive arterial remodeling from intimal thickening to outward expansion20 in response to pathological stimuli (Figure 1(b)). In normal arteries, the intima provides an elastic layer of path for blood flow, which is supported by the media, while the adventitia supports the structural integrity of vessel wall. Therefore, the modulus increases gradually from intima to adventitia. Under pathological situations, the formation of lesion with fibrous caps contributes to a dramatic increase in the stiffness of the intima, resulting in a significant increase in the modulus of intima. Then the artery expands outward to increase the lumen for restoration of proper blood flow, which was decreased substantially by intimal thickening. Expansion beyond the limits required for maintenance of tissue integrity often results in rupture of the outer layer.
FIGURE 1.
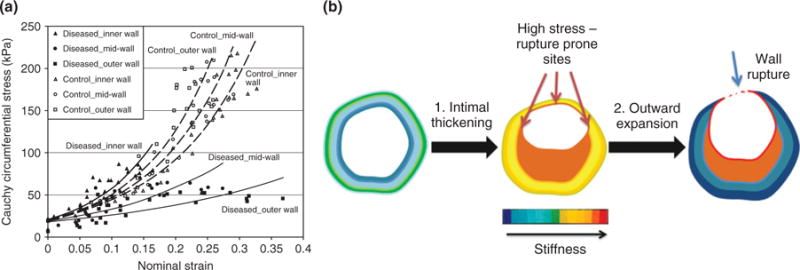
Changes in mechanical properties of the vascular wall as a result of pathological vascular remodeling. (a) Ex vivo stress—strain curves of porcine plaque-laden versus healthy vascular tissue. All samples were taken from the left anterior descending coronary artery in juvenile pigs, with atherosclerosis induced via standard balloon angioplasty injury. Control samples were obtained from pigs without induced injury. (Reprinted with permission from Ref 24. Copyright 2003 ASME Publications.) (b) Schematic of changes in layer-by-layer stiffness during progression of pathological vascular remodeling. Pathological changes that decrease the lumen of a remodeling artery include intimal thickening and constrictive geometric remodeling of the wall, leading to a significant increase in the stiffness of inner layer, while vessel wall rupture that is resulted from artery expansion to increase the lumen for restoration of proper blood flow leads to decreased stiffness of middle and outer layers.
Pathological observations and experimental studies indicate that MMP expression is increased in high stress sites of such arteries.25 At the same time, rupture of the vessel wall with plaque destabilization occurs in arteries that have undergone outward remodeling, suggesting that degradation of matrix by MMPs may eventually lead to weakening and destabilization of the media and adventia, as indicated by decreases in modulus of middle and outer layers. In addition, the development of these properties is partially influenced by the luminal shear stresses. In particular, stenotic regions experience increased shear stresses, resulting in changes in endothelial gene expression and integrin-ECM binding affinity.6 Further, regions of lower shear stress may form downstream of stenotic regions, resulting in upregulation of VCAM-1, C-reactive protein (CRP), and IL-6 by endothelial cells. The expression of VCAM-1 and IL-6 increases the recruitment of inflammatory cells into the local interstitium, and IL-6 can further regulate the production of MMPs.26
From a materials standpoint, local porosity and compliance are the most important parameters. Plaque endothelium is not fenestrated, but is generally known to exhibit higher permeability to nanoparticles than healthy endothelium.27 Further, endothelial cells are also known to be capable of transcytosis, providing an alternative route for translocating circulating nanoparticles to the interstitium.28 Local compliance is governed primarily by the variations in mechanical properties across the different tissue layers in the vasculature, including the pathologic structures such as the necrotic core, as well as the percentage composition of each of these structures. Mechanical properties can be heterogeneous even within the same plaque, leading to unpredictable stretch-relaxation behaviors under dynamic, pulsatile blood flow conditions.29 It is well understood that when two adjacent tissue components exhibit a significant level of compliance mismatch, such blood flow conditions can rapidly develop fractures in the interface between these components can escalate into deadly aneurysms.30
Taken altogether, vulnerable plaques can be identified by abnormal local inflammatory and proteolytic activity, thrombosis, heterogeneity of mechanical properties in the local vascular wall, and an increase in interstitial perfusion.
A CLINICAL PERSPECTIVE: DIAGNOSIS AND IMAGING OF VULNERABLE PLAQUES TODAY
With the recognition that clinically silent, nonobstructive (<70% luminal stenosis) CAD may present a great risk of acute myocardial infarction and sudden cardiac death,31–34 cardiologists are increasingly interested in identifying vulnerable plaques. However, in clinical practice, both noninvasive testing and invasive imaging are designed to identify obstructive coronary artery disease, which can provide important prognostic information and also guide decisions regarding coronary revascularization. The use of currently available imaging modalities used in clinical practice to identify coronary plaques with morphologic features consistent with a vulnerable plaque phenotype is thus somewhat limited. However, as interest in the identification and treatment of vulnerable plaques increases, there have been significant recent advances in intra-coronary imaging of inflammatory plaques, which are summarized below.
The imaging modalities are divided into non-invasive and invasive techniques. While noninvasive techniques are more applicable to the asymptomatic patient with nonobstructive coronary disease, they are generally limited by cardiac movement and the tortuous nature of coronary arteries. Because of direct visualization of the coronary arteries, invasive techniques allow improved resolution but involve increased risk to the patient and are not suitable for screening of asymptomatic patients.
Noninvasive Imaging
Cardiac applications of computed tomography (CT) have progressed rapidly with technologic advances. Electron-beam CT was first used to predict the likelihood of coronary events by identifying coronary calcification which correlates with calcific plaque burden. With the development of fast multidetector CT (MDCT), the coronary artery lumen as well as the vessel wall can be assessed. MDCT has the capacity to examine plaque composition by using density measurements but is limited by blooming artifact and overlap of CT attenuation values between lipid-rich and fibrous noncalcified plaque.35 The recent introduction of dual source CT has improved temporal resolution and accurately identified lipid plaques compared to intravascular ultrasound (IVUS) without excluding calcified plaques.36 On the basis of these capabilities, CT can accurately determine overall plaque burden and characterize arterial remodeling. CT still has significant limitations related to radiation exposure, resolution, and soft tissue contrast; but it holds great promise as techniques continue to improve.
Magnetic resonance imaging (MRI) provides excellent soft tissue contrast and does not expose the patient to ionizing radiation. MRI has been extensively studied in the imaging of inflammatory plaques in the carotid arteries and has shown strong correlations with histology in the identification of the lipid core and fibrous cap.37 Unfortunately, low spatial resolution has hindered its applicability to coronary artery imaging. Gadolinium-based contrast agents have been used to extend MRI capabilities to include smaller blood vessels, but because of concerns with renal toxicity, alternative materials such as superparamagnetic iron oxides have received much interest.28,38–42 Additionally, nanoparticulate contrast agents are advantageous because they are avidly recognized and uptaken by macrophages regardless of surface chemistry, and vulnerable plaques tend to exhibit high macrophage contents.28,43–51 Finally, another disadvantage of MRI is that many patients are not compatible with this procedure because of metallic medical implants including pacemakers and implantable cardiac defibrillators.
Positron emission tomography (PET) and single photon emission computed tomography (SPECT) require radioactive tracers, allow less spatial resolution than MRI, and expose the patient to ionizing radiation, but are more sensitive than MRI for detection of plaque biomarkers.52 Because the disadvantages of MRI fall into the advantages of PET/SPECT, and vice versa, multimodal contrast agents that can be detected through multiple imaging platforms are of much interest. For example, Nahrendorf et al. synthesized an iron oxide nanoparticle labeled with 64Cu PET tracer to enable bimodal imaging of the contrast agent via PET/MRI.48 Following in vivo imaging studies, plaque material was digested and analyzed by flow cytometry, and indicated that the nanoparticles were predominantly uptaken by macrophages.
Imaging with transcutaneous ultrasound has several advantages, including its safety, cost, and wide availability. With the use of contrast agents, ultrasound can detect the presence of intraplaque neovascularization. As inflammatory plaques enlarge, they require their own blood supply and develop a mature network of arterioles (vasa vasorum) on the adventitial surface of the vessel.53 Contrast-enhanced ultrasound has the ability to detect the presence of vasa vasorum.54 Currently, this application is limited to superficial vessels such as carotid arteries.
Molecular imaging using radionuclides has potential for adding functional information about inflammatory coronary plaques to anatomical information obtained from other imaging modalities. While promising, thus far the data is still limited55–57 and not ready for clinical application.
Invasive Imaging Techniques
Coronary angiography is the most common invasive technique used, but only assesses the vascular lumen, causing deficiencies in identifying mild plaques, evaluating vessel size, and assessing plaque characteristics.
Intravascular ultrasound (IVUS) has traditionally been utilized to assess the anatomical characteristics of plaque and disease burden often underestimated by angiography because of positive remodeling. Although the resolution of IVUS (100–250 μm) does not allow for identification of the thin fibrous cap (estimated 23 ± 19 μm by histology of ruptured plaques),58 the presence of the necrotic core as identified by large echolucent areas or attenuation of the echo signal in noncalcified areas may have clinical relevance.59–61 However, gray-scale IVUS is limited in its ability to characterize plaque morphology. The addition of integrated backscatter analysis to grayscale IVUS, called radiofrequency-IVUS (RF-IVUS) can provide improved characterization of the individual components of atherosclerotic plaque including fibrotic tissue, fibrofatty tissue, necrotic core, and dense calcium.62–65 A prospective study of patients with high-risk coronary artery disease recently demonstrated that RF-IVUS can identify coronary plaques with a morphologic phenotype (TCFA) that are at increased risk for subsequent cardiovascular events.66
Optical coherence tomography (OCT) measures back-reflected infrared light and provides the highest resolution (5–20 μm) of all the invasive imaging techniques.67,68 Because of its excellent resolution, histopathological correlations have demonstrated sensitivities of 87–92% and specificities of 94–100% in identification of various plaques.67 Although imaging acquisition procedures are improving, its clinical application is currently limited because of signal attenuation by blood necessitating prolonged, proximal occlusions to screen long arterial segments. Intracoronary optical frequency domain imaging (OFDI), a next-generation OCT-derived imaging method, overcomes some of these limitations, with a substantially increased speed of image acquisition when compared with first-generation time-domain OCT.69 However, both OCT and OFDI have a somewhat limited penetration and are thus unable to adequately image beyond the inner fibrous cap and lipid core in larger arteries.
Intravascular MRI depicts components of atherothrombotic plaque including lipid, fibrous tissue, calcium, and thrombus formation.70,71 There is potential for combination with cellular and molecular targeting.72 In vivo MRI catheters have had limited human testing,73 and real time endovascular MRI imaging continues to advance experimentally,74 but intravascular MRI is not a clinical reality yet.
Optical imaging is commonly used in the lab to verify the reliability of plaque biomarkers because of its widespread availability, ease of use, and the lack of ionizing radiation exposure to the user. However, the application of this technique to in vivo imaging in humans is difficult because of tissue autofluorescence and optical attenuation and scattering—effects that are less marked in the near-infrared window of 650–900 nm.75,76 Therefore, the success of optical imaging approaches for human applications will require the design of probes that exhibit absorbance or excitation and emission spectra that occur within this 300 nm window. Indeed, near-infrared spectroscopy (NIRS) analyzes the chemical composition of plaque components based on the amount of absorption at different wavelengths. Further, near-infrared spectroscopy (NIRS) catheters enable assessment of intravascular chemical composition, and have been successfully employed to identify lipid-core plaques in human patients.77,78 The ability of NIRS to identify the chemical composition of plaque components has also been validated with histologic correlations79–81 and is currently being evaluated in multiple trials including as a combined imaging technique with IVUS. Because NIRS is unable to elucidate tissue structures, the combination of NIRS with IVUS enables correlation of plaque composition with tissue structure on one platform, and has been recently demonstrated in humans.82
Angioscopy and thermography also deserve mention but have failed to show clinical utility in the identification of vulnerable plaques despite initial promising results.
IMAGING VULNERABLE PLAQUES WITH NANOTECHNOLOGY
One of the main challenges in the prevention of myocardial infarctions and strokes involves the identification of plaques at highest risk of rupture that are in most need of prophylactic therapeutic intervention. As discussed earlier, plaque composition appears to be the primary determinant of plaque instability.3,8 As a result, the primary molecular imaging targets for the identification of vulnerable plaques includes markers of inflammation, thrombosis, apoptosis, or angiogenesis.83,84 Advances in noninvasive imaging approaches are revolutionizing the ability to spatially pinpoint the locations of these vulnerable plaques in the clinic. Nanotechnology has led to the creation of several nanoparticle platforms that can be applied as contrast agents for a range of noninvasive imaging modalities.
Recently, a library of nanoparticle platforms has been developed for molecular imaging of plaque biomarkers (Table 1). This table is not meant to be an exhaustive list, but rather, highlights key developments in this area that have been used to image vulnerable plaques in vivo or ex vivo. Below, we highlight novel approaches for imaging cell receptors and cell types, as well as protease activity in vulnerable plaques. A word of caution must be added when interpreting the vast collection of data in this area, as an ideal animal model that exhibits intrinsic plaque destabilization and rupture remains undeveloped. The apoE−/− mouse—likely the most widely used small animal model of atherosclerosis—is known to develop atherosclerotic lesions spontaneously, but generally require extrinsic triggers or exogenously administered means in order to trigger plaque rupture.85 The lack of a robust animal model that develops atherosclerotic lesions closely mimicking plaque destabilization and rupture in human patients remains one of the largest barriers in the translation of these promising results from the bench to the bedside.
TABLE 1.
Nanoparticle-Mediated In Vivo/Ex Vivo Imaging of Atherosclerosis Related Biomarkers
| Molecular/ Cellular Target |
Nanoparticle Type | Targeting Motif | Imaging Modality | Animal Models1 | References |
|---|---|---|---|---|---|
| Cathepsins (nonspecific activity probe) | Polyethylene glycol-poly-lysine | Degradable peptide | FMT, CT | apoE−/− mice | 86 |
| Dextran receptors (macrophages) | Au-coated iron oxide nanoroses | Dextran surface coating | Hyperspectral microscopy (3d post-injection, harvested aorta) | New Zealand white rabbits, cholesterol-fed + balloon injury | 87 |
| E-selectin | Iron oxide | Anti-human CD62E F(ab’)2 | T2-weighted MRI | Athymic female ν/ν mice | 88 and 89 |
| Fibrin | Lipid-stabilized perfluorocarbon emulsion | Anti-fibrin Mab | T1-weighted MRI | Dog, induced thrombus in open circulation | 90 |
| Fibrin | Synthetic phospholipid micelle | CREKA peptide | Fluorescence imaging | apoE−/− mice | 91 |
| HDL receptors / transporters | Modified HDL mimic with Au, iron oxide, or quantum dots | Apo-A1 | Multimodal; CT, MRI, Fluorescence | apoE−/− mice | 92–95 |
| Macrophages | Sinerem (AMAG Pharmaceuticals, Lexington, MA) | Nonspecific binding | T2-weighted MRI | human | 46 and 96 |
| Macrophages | Iron oxide (dextran coated) | Nonspecific binding | T2-weighted MRI | New Zealand white rabbits, cholesterol-fed + balloon injury | 97 |
| Macrophages | Au, mPEG-stabilized | Nonspecific binding | Intravascular photoacoustic/intravascular ultrasound | Excised rabbit aortas; cholesterol-fed (ex vivo) | 98 |
| Macrophages | Poloxamer-stabilized iodine | Nonspecific binding | CT | New Zealand white rabbits, cholesterol-fed + balloon injury | 99 and 100 |
| MMP-2 | Iron oxide | MMP-2-degradable peptide | T2-weighted MRI | Nude mice/HT-1080 tumor cell injection | 101 |
| MMP-9 | Iron oxide | MMP-9-degradable peptide | T2-weighted MRI | None | 102 |
| Malondialdehydelysine, oxidized phospholipids | Gd-oriron oxide-containing micelles | Antibodies against oxidation-specific epitopes | MRI, fluorescence | apoE−/− mice | 103 and 104 |
| Phosphatidylserine (apoptotic/necrotic cells) | Gd- and rhodamine-tagged micelles | Annexin A5 | T1-weighted MRI, Fluorescence | apoE−/− mice | 105 |
| Reactive oxygen species | Peroxalate polymer nanoparticles | Peroxide-sensitive peroxalate linkages | Chemiluminescence | C57Bl/6 mice | 106 |
| SR-A (macrophage scavenger receptor A) | Gd-containing micelles | Anti-murine CD204 IgG | T1-weighted MRI, Fluorescence | apoE−/− mice | 107 and 108 |
| SR-B (macrophage scavenger receptor B) | Gd-containing micelles | Anti-human CD36 IgG | T1-weighted MRI, Fluorescence | human (ex vivo) | 47 |
| VCAM-1 | Iron oxide (dextran-coated) | Peptide, Anti-VCAM-1 Ab | T2-weighted MRI, Fluorescence | apoE−/− mice, C57Bl/6 mice | 109–111 |
| αVβ3 integrins | Lipid-stabilized perfluorocarbon emulsion | RGD peptide | T1-weighted MRI | New Zealand white rabbits, cholesterol-fed | 112 |
All models noted are in vivo unless otherwise noted.
Imaging Cell Receptors in Atherosclerosis
As described above, atherosclerosis is generally classified as an inflammatory disease, and as a result, a range of inflammatory biomarkers have been implicated in plaque destabilization. These biomarkers include the adhesion molecules ICAM-1, VCAM-1, and E-selectin, as well as macrophage markers such as CD40, CD68, dextran receptors, Mac-3, and scavenger receptors MSR-A/MSR-B.12,48,113–117 These biomarkers have been targeted in a variety of studies involving nanoparticle-mediated molecular imaging (Table 1).
Work in the Fayad and Tsimikas groups has produced a library of synthetic micelles and lipoprotein mimics for imaging of some of these biomarkers, including oxidation-specific epitopes47,92–94,103,104,107,118 (Figure 2). Imaging oxLDL is of particular interest because it allows for the identification of oxidized epitopes in the vascular wall, and when used in tandem with measurements of circulating oxLDL—a well-developed paradigm for assessing the risk of coronary events—demonstrates a biomarker and an imaging application based on the same epitope.103,104,119 For imaging applications, contrast agents are incorporated into the nanoparticles in two ways. In the first method, phospholipids are covalently modified with gadolinium chelates or fluorescent tags, and then used in preparation of antibody-displaying micelles.92,103 As a result, contrast agents are displayed on the nanoparticle surface. In a second method, contrast agents are incorporated into the core of the micelles. This is possible because a variety of contrast agent nanocrystals, including quantum dots, iron oxides, and gold nanoparticles are synthesized in large quantities and at high monodispersity in organic solvents, but remain capped by hydrophobic surfactants.120–122 Therefore, inclusion of such nanocrystals in micellization processes encapsulates them with phospholipids.93,95 After micellization, membrane proteins such as ApoA-I can be incorporated into the phospholipid monolayer in a single buffer change step, producing mimics of high-density lipoprotein.95
FIGURE 2.
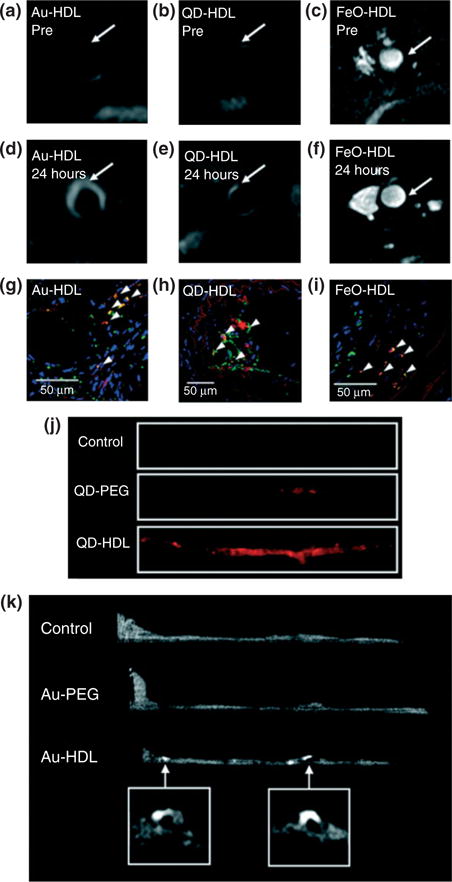
Multimodality imaging of atherosclerosis using nanocrystals encapsulated in high density lipoprotein. T1-weighted MR images of the aorta of apoE KO mice pre- (a and b) and 24 h postinjection (d and e) with Au-HDL or QD-HDL. Arrows indicate areas enhanced in the post images. (c and f) T2*-weighted images of an apoE KO mouse pre- and 24 h postinjection with FeO-HDL. (g–i) Confocal microscopy images of aortic sections of mice injected with nanocrystal HDL. Red is nanocrystal HDL, macrophages are green, and nuclei are blue. Yellow indicates colocalization of nanocrystal HDL with macrophages and is indicated by arrowheads. (j) Fluorescence image of aortas of mice injected with QD-HDL, QDPEG, and saline. (k) Ex vivo sagittal CT images of the aortas of mice injected with Au-HDL, Au-PEG, and saline. (Reprinted with permission from Ref 95. Copyright 2008 American Chemical Society)
Multimodal contrast agents can be constructed through the marriage of the two approaches described above.95 Alternatively, dextranated iron oxide nanoparticles can be surface-modified with contrast agents such as the PET tracer 64Cu, in order to enable PET/MRI imaging of plaque biomarkers.48 Both of these platforms have been further functionalized with antibodies and peptide mimics for molecular imaging.103,108,109,123
The Johnston group recently demonstrated a ‘nanorose’ platform for NIR fluorescence and photothermal optical coherence tomography (OCT) imaging of macrophages in a rabbit model of atherosclerosis.87,124 To synthesize the contrast agent, 2 nm monolayers of Au were grown on iron oxide nanoparticles with dextrose as a reducing agent, yielding dextran-coated iron oxide/gold nanoclusters. The close proximity of the singlets in the cluster enhances NIR absorbance of the system.87 Further, because of the thinness of the Au layers, iron oxide particles are placed in close proximity to one another, leading to higher R2 relaxivities with clustered particles versus with dispersed particles.125 The dextran coating facilitates uptake of the nanoroses by macrophages, enabling inflammatory cell-laden plaques to be easily imaged.117
Imaging Protease Activity in Atherosclerosis
Increased activity of extracellular proteases such as MMP-2, -8, and -9; and cathepsins B, K, L, and S has been implicated in plaque destabilization and rupture.16,17,21,126–130 As a result, probes for imaging proteases activities are expected to improve the detection of vulnerable plaques in the clinic.
The Weissleder group pioneered NIR fluorescence beacons for imaging protease activity in plaques, where polymers are modified with NIR fluorophores in such a way that the dyes exhibit self-quenching in the absence of protease activity.127,131,132 Following protease degradation of linkers holding the fluorophores in proximity to one another, release of the fluorophores results in the disappearance of the self-quenching effect and an increase in NIR emission, which has been used to specifically image active cathepsin B, D, and K and MMP-2 and -9.127,131,133,134
However, it is worthwhile to note that a drawback behind these approaches involves the use of organic fluorophores, which in general, exhibit much lower quantum yields and higher photobleaching effects versus fluorescent nanoparticles such as quantum dots. While these novel ‘smart’ probes have led to promising results from mouse models (Table 1), their successful application in the clinic will necessitate highly sensitive fluorescence detectors and brighter excitation sources, counteracting the significantly higher optical attenuation and scattering effects expected.135
As an alternative to using environmentally activatable organic dyes, Chang et al. presented a protease-activatable fluorescence probe based on quantum dot-Au nanoparticle complexes.136 In this scheme, Au colloid was attached to CdSe/CdS QDs via MMP-1-degradable peptide linkers. At close proximity (<10nm), the gold colloid absorbs quantum dot emission via a FRET mechanism. However, the presence of MMP-1 results in release of the quenching Au colloid from the QD surface, and ‘activation’ of QD emission. Recent work by the same group has led to NIR-emitting quantum dots that are likely more suitable for clinical applications because of their optical properties.137 Other approaches have also been followed in order to obtain NIR-emitting QDs, but because of the presence of cadmium in most of these structures, cytotoxicity remains a primary concern and roadblock to potential applications of this technology in humans.135,138–143
While a majority of approaches to actively image plaque biomarkers utilize one targeting ligand (peptide, protein, or antibody) to target the biomarker directly, a promising new approach developed in the Giorgio and Bhatia groups can potentially enable specific imaging of plaques that exhibit two separate characteristics.101,144,145 Originally designed for tumor imaging, this approach is termed ‘proximity-activated targeting’ (PAT), where nanoparticle surfaces are functionalized with two distinct molecular ligands necessary for pathologically specific targeting. Specifically, quantum dots were surface functionalized to include a polyethylene glycol (PEG) chain interrupted by the peptide target of matrix metalloprotease-7 (MMP-7) and a second, shorter structure that terminated in folic acid. The construct was targeted to breast cancer metastases through the folate ligand that was initially concealed by the peptide-bridged PEG (Figure 3(a)), which remained biologically inactive until exposure to MMP-7 (Figure 3(b) and (c)). MMP-7 degradation of the peptide bridge uncoupled the passivating PEG structure from the construct, revealing the folate ligand only in the proximity of the tumor144,146 (Figure 3(d)). The modular approach used in the construction of these contrast agents enables other nanoparticles to be used in the core, such as iron oxide, for MRI.101 Other proteases and receptors can also be targeted using this approach simply by changing the cleavable peptide unit and the peptide targeting unit used in the final construct.
FIGURE 3.
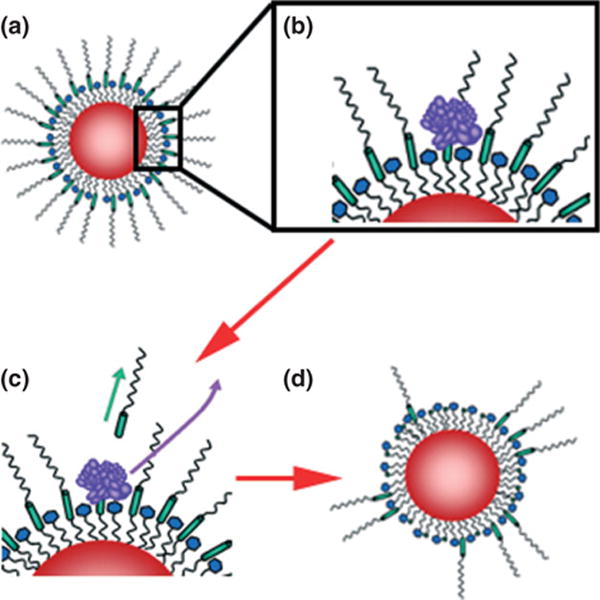
Dual-ligand, protease-activatable quantum dots for imaging applications. In ‘Proximity Activated Targeting’ (PAT) the targeting ligand (blue) is initially concealed (a) until proteolytic activity through tumor-secreted MMPs (purple) cleaves a peptide bridge (green) within long chain PEGs. Subsequent diffusion of the MMP and cleavage fragments (c) reveals the ligand-targeted construct only in the proximity of the tumor (d).
Advantages of Nanoparticulate Contrast Agents in Vulnerable Plaque Imaging
Nanoparticles provide an array of advantages in plaque imaging versus their small molecular probe counterparts, including circulation half-life, site selectivity, and modular design.
Because most nanoparticulate contrast agents for plaque imaging applications exhibit diameters above 10 nm, they are generally too large to be filtered through the glomeruli in the kidneys, where pores exhibit cutoff diameters of 8 nm or less.147 Small molecule contrast agents are easily filtered through the kidneys, and are rarely reabsorbed, leading to much smaller circulation half-lives than nanoparticles. Circulating nanoparticles tend to be cleared through the reticuloendothelial system (RES), also known as the mononuclear phagocyte system (MPS), which includes phagocytic cells residing in the spleen and the liver, among other organs.148,149 The kinetics of nanoparticle clearance through this route can be significantly slowed through the appropriate use of polymeric surface coatings such as poly(ethylene glycol) (PEG) or poly(hydroxypropyl methacrylate) (HPMA), which reduce opsonization of nanoparticles.150,151
Site selectivity can be mediated to some extent by the enhanced permeation and retention (EPR) effect in a similar fashion as seen in cancer, as vulnerable plaques tend to exhibit multiple fissures in the fibrous cap, as well as impaired endothelial function.3,152,153 Although the EPR effect has been conclusively demonstrated in induced tumors in animal models, to our knowledge, only one observation of this effect has been reported in humans to date.154 Further, the larger size of nanoparticles versus small molecular probes provides a platform for multivalent coupling of ligands for active cell targeting, such as antibodies and peptides, which improves affinity of the nanoparticles for their molecular targets.155
Finally, nanoparticulate contrast agents are known for their modular design, in that the contrast agents, targeting agents, stabilizing polymers, and other functionalities can be easily tuned during synthesis. Several schemes have already been described earlier in this review. The simplicity in generating functional nanoparticulate contrast agents has also led to the facile synthesis of multimodal contrast agents.48,86,87,95,124
Ongoing advances in nanoparticle synthesis and processing processes have led to materials with novel properties appropriate for applications in this area, including iron oxide nanoparticles with R1 relaxation and biocompatible silicon-based quantum dots.156,157 ‘Smart’ environmentally sensitive dendrimer-based nanobeacons have been synthesized by the Matrisian group, which emit NIR fluorescence only in the presence of proteases of interest.158,159 This platform can also be loaded with small molecule drugs, producing a ‘theranostic’ platform capable of site-specific therapy and imaging. For all of these reasons, nanotechnology will continue to be a significant player in the imaging of vulnerable plaques.
THE IN VITRO DIAGNOSTICS REVOLUTION: APPLICATIONS OF NANOTECHNOLOGY IN BLOOD TESTING AND BIOMARKER DETECTION
Rapid, accurate, noninvasive measurement of circulating, plasma-based biomarkers potentially provides powerful information for diagnosis and risk stratification of patients with cardiovascular diseases. A few such biomarkers that are already being measured in the clinic for this purpose include C-reactive protein (CRP), lipoprotein (a), myeloperoxidase, oxLDL, troponin I, and MMP-9, among others, as reviewed elsewhere.160,161 However, it is clear that no single biomarker can lead to conclusive risk assessment. Rather, assessment of a small library of biomarkers is necessary to accurately diagnose a patient’s susceptibility to potentially lethal acute cardiac events.161
A challenge is that current methods to measure concentrations of circulating biomarkers in the clinic generally require expensive and impractical equipment, long sample handling and preparation times, significant reagent usage, and moreover, can only assay one analyte at a time. Nanotechnology has led to the development of several platforms that hold promise in tackling all of these challenges.
For example, nanomaterials functionalized with antibodies specific for a biomarker of interest have been used in novel experimental immunoassays.162–168 These novel immunoassays are sensitive enough to detect analytes at femtomolar concentrations, primarily because of the significantly higher biosensing surface area offered by immobilization of the sensing elements—the antibodies—to nanostructured surfaces. Further, most of such nanoparticle-based biosensing platforms also possess physical properties that change based on the aggregation state of the nanoparticles. Specifically in the presence of analytes of interest, the nanoparticles aggregate, producing significant changes in optical, magnetic, or fluorescent properties of the system. In some cases, detection is possible even in unfractionated blood, thereby greatly reducing sample processing time.166,169,170
Microfluidics technology has also miniaturized immunoassays, in terms of sample and reagent volumes as well as equipment, enabling multiplexed antigen detection in sub-microliter volumes at unprecedentedly low costs.171–173 Smaller, microfluidics-sized versions of the highly technical equipment usually required for nanoparticle-based immunoassays in the laboratory have also been recently been constructed, including flow cytometers,174,175 spectrophotometers,176 magnetic relaxometers,177,178 and dynamic light scattering (DLS) analyzers,179,180 among others. Integrating nanotechnology-enabled immunoassay schemes with microfluidics holds promise in revolutionizing clinical detection of libraries of circulating biomarkers that collectively report the existence of vulnerable plaques.
Soman et al. described a method for flow cytometric detection of blood-borne analytes based on agglomeration of antibody-functionalized quantum dots.163,181 As nanoparticle agglomerates scatter light more effectively than individual, dispersed nanoparticles,182 the light scattering instrumentation equipped on standard flow cytometers are capable of detecting the presence of analytes based on the appearance of high-scattering species following as little as 60 min incubation time. By producing a library of quantum dots—each population displaying antibodies for a different target—and identifiable by a different emission wavelength range, analytes can be ‘bar-coded’, enabling multiplexed detection of a library of analytes through fluorescent emission versus light scattering plots.181 The authors detected vascular endothelial growth factor A and angiopoietin-2 in saline, down to a femtomolar sensitivity limit.181 However, detection capabilities in whole, unfractionated blood were not investigated.
Hirsch et al. demonstrated detection of analytes in whole blood using antibody-functionalized silica-gold core-shell nanoshells.162,166 These nano-shells absorb at wavelengths in the near-infrared (NIR) window of 700–1000 nm—where optical attenuation and scattering effects because of blood components are minimized79 (Figure 4). After rabbit IgG was mixed into whole human blood, nanoshells agglomerated, resulting in a red-shift in plasmon resonance of the system within 15–30 min. This effect is also accompanied by a decrease in peak extinction of the system.166 A light, portable, single-wavelength LED-based ‘spectrophotometer’ was constructed to enable translation of the nanoshell immunoassay to low-resource, point-of-care applications.183
FIGURE 4.
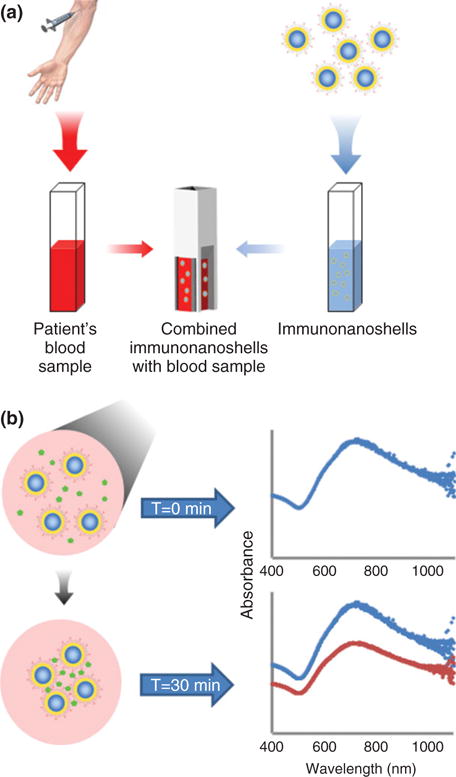
Nanoshell immunoassay. (a) In concept, the nanoshell immunoassay is designed to enable rapid and accurate analyte concentration measurements in whole blood, while minimizing dependency on long sample handling and instrumentation typified by standard immunoassays.166 (b) Absorbance spectra of nanoshells prior to versus 30 min after analyte addition. Peak absorbance is estimated at 725 nm. A decrease in material extinction coefficient is observed as a result of clustering of the nanoshells.
Ibraimi et al. developed a ~5 min immunoassay that works in a similar fashion as these methods, involving the use of polyclonal anti-CRP-functionalized silica microparticles and monoclonal anti-CRP-functionalized superparamagnetic iron oxide nanoparticles.170 The silica microparticles generally sediment within 5 min even in the absence of agglomeration with CRP. However, when CRP is present, iron oxide nanoparticles become incorporated into the precipitate, increasing its magnetic permeability. A portable magnetic permeability detector was used to quantify concentration of CRP in as little as 4 μL of whole blood, and the sensitivity and accuracy of the method was comparable to several commercially available reference methods.170
Magnetic nanoparticle-based immunoassays can also be carried out with optical detectors, as the general principle of analyte-mediated nanoparticle agglomeration results in increased light scattering properties and extinction in the system.182 In such setups, the nanoparticle surface simply provides a vehicle for analyte immobilization, while the magnetic properties enable facile handling and isolation of the particle-analyte complexes.
Recently, this scheme was combined with a total internal reflection (TIR) detector to enable quantitative detection of troponin I in human serum and plasma down to sub-picomolar concentrations.184 In this work, Bruls et al. preloaded a microfluidic chip with anti-troponin I-functionalized magnetic nanoparticles, and used a magnetic field to capture the nanoparticles during sample loading. Magnetic actuation of the nanoparticles in the presence of the sample enables quick mixing and formation of nanoparticle-analyte complexes within 4 min. The TIR sensor surface is also pre-functionalized with anti-troponin antibodies, enabling high-affinity interactions between the complexes and the surfaces. Application of a magnetic field removes excess nanoparticles prior to optical detection of analyte.184
The McDevitt group developed microfabricated chips consisting of arrays of singly confined microparticles, each functionalized with a different antibody for an analyte of interest.172,185,186 The design of the immunoassay is not very different from that of a sandwich ELISA, except in this case, porous agarose microparticles serve as a scaffold for attachment of capture antibodies. After flowing sample through the beads, application of fluorophore-conjugated detection antibodies and rinsing resulted in quantitative readout of analyte concentrations.172 This approach has been used to screen human patients for biomarkers of acute myocardial infarction through measurements of CRP, myoglobin, and myeloperoxidase in salivary samples.185
Buch and Rishpon coated screen-printed carbon electrodes with a film of multiwalled carbon nanotubes (MWCNTs), forming a surface for quantification of CRP in human serum by electrochemical immunoassay.187 Like the immunoassay above, this immunoassay was not very different from a sandwich ELISA in idea. The difference is that quantification of CRP concentration is achieved by monitoring the electrochemical potential at the electrodes, which varies based on the oxidation of the HRP substrate TMB. Therefore, the MWCNT film was further modified with anti-CRP as a capture antibody, while an HRP-conjugate anti-CRP was used as a detection antibody. Application of human serum, followed by wash steps, then the detection antibody and then TMB substrate, quantified CRP down to 0.5 ngmL−1, compared to 10 ng mL−1 when the MWCNT film is not present.187 This difference in detection limit is attributed to the enhanced electrochemical reactivity of peroxides at CNT-modified electrodes, as reviewed elsewhere.188 The authors reported assay time to be around 10 min.
Given the predominance of costly, time-consuming ELISAs for measuring circulating bio-marker levels in the clinic today, the advantages offered by these novel nanotechnology-based immunoassays will make biomarker-based blood diagnostics much more accessible to the public. Further, the rapid assay times offered by these new approaches will enable their routine use, multiple times daily, to track the prognosis of the highest risk patients and enable strategic therapeutic intervention.
A CLINICAL PERSPECTIVE: FUTURE DIRECTIONS FOR THE TREATMENT OF VULNERABLE ATHEROSCLEROTIC PLAQUES
The development of drug eluting stents has revolutionized the treatment of obstructive CAD (Table 2). The initial alternative to open heart surgery for mechanical revascularization was percutaneous transluminal coronary angioplasty with balloon expansion. However, because of problems with acute vessel closure and restenosis,189–191 the coronary stent was developed with bare metal providing scaffold to prevent closure and recoil.192 There was rapid adoption of percutaneous coronary interventions (PCI) using bare metal stents (BMS), and target-lesion revascularizations (TLR) were reduced from 25–35% with balloon angioplasty to 10–15% with BMS.193 Drug eluting stents (DES), developed to prevent the in-stent neointimal hyperplasia leading to restenosis in BMS,194–196 resulted in a further reduction in TLR to 4–6%.197–199 However, initial enthusiasm for DES was dampened in 2006 with evidence of a small, but significant, increase in stent thrombosis.200–202
TABLE 2.
Technical Specifications of Representative Clinically Approved Drug-Eluting Stents in the United States and Europe
| Coating | Stent | Drug | Polymer | Stent Platform | Strut/coating Thickness (μm) | Approval |
|---|---|---|---|---|---|---|
| Durable polymer | CYPHER | Sirolimus | Polyethylene co-vinyl acetate and poly-n-butyl methacrylate | SS | 140/12.6 | FDA/CE |
| TAXUS Express | Paclitaxel | Poly(styrene-b-isobutylene-b-styrene) | SS | 132/16 | FDA/CE | |
| TAXUS Liberte’ | Paclitaxel | Poly(styrene-b-isobutylene-b-styrene) | SS | 97/16 | FDA/CE | |
| Endeavor | Zotarolimus | Phosphorylcholine | CoCr | 91/4.1 | FDA/CE | |
| Xience V | Everolimus | Polyvinylidene fluoride co-hexafluoropropylene and poly-n-butyl methacrylate | CoCr | 81/7.6 | FDA/CE | |
| Endeavor RESOLUTE | Zotarolimus | C10/C19/polyvinyl pyrrolidone | CoCr | 91/4.1 | CE | |
| Promus element | Everolimus | Polyvinylidene fluoride co-hexafluoropropylene and poly-n-butyl methacrylate | PtCr | 81/6 | CE | |
| Biodegradable polymer | NOBORI | Biolimus A9 | Abluminal poly-L-Lactide | SS | 112/101 | CE |
| Axxess | Biolimus A9 | Abluminal poly-L-Lactide | Nitinol | 152/151 | CE | |
| BioMatrix | Biolimus A9 | Abluminal poly-L-Lactide | SS | 112/101 | CE | |
| XTENT | Biolimus A9 | Abluminal poly-L-Lactide | CoCr | NA | CE | |
| Polymer free | Yukon Choice | Sirolimus | None | SS | 87 | CE |
| Amazonia Pax | Paclitaxel | None | CoCr | 73/51 | CE | |
| Bioabsorbable/biodegradable stents | Igaki-Tamai | None | None | Poly-lactic acid | 170/NA | CE |
| ABSORB | Everolimus | Poly-D,L-lactide | Poly-lactic acid | 150/6 | CE |
FDA, Food and Drug Administration; CE, Conformité Européene; SS, stainless steel; CoCr, cobalt chromium; PtCr, platinum chromium.
Only abluminal.
The basic components of a DES are the stent platform, a polymer that elutes the drug from the stent surface, and the drug. CYPHER and TAXUS, the first generation drug eluting stents, have stainless steel platforms and elute the antiproliferative agents sirolimus (an immunosuppressant) and paclitaxel (an antineoplastic agent), respectively. The drugs are released by diffusion from nonbiodegradable polymers. Although similar in many respects, sirolimus-eluting stents have superior restenosis rates compared to the paclitaxel-eluting stents because of multiple differences including underlying stent design, polymeric coating, mechanism of drug action, drug-release kinetics, and drug distribution across the vessel wall.203
As concerns of stent thrombosis in first generation DES were raised, one of the major considerations was the impaired endothelization by antiproliferative drugs resulting in blood exposure to a thrombogenic stent strut. Stent thrombosis can also be precipitated by patient-related factors (including diabetes, malignancy, renal failure, poor response to antiplatelet therapy), inadequate duration of antiplatelet therapy, lesion characteristics, and procedural factors (inadequate stent expansion, incomplete stent apposition). Also although greatly reduced, in-stent restenosis (ISR) still occurs in DES albeit with a different mechanism from ISR in BMS.204
In order to address some of these concerns, second generation DES (Endeavor, XIENCE) were developed. The cobalt chromium stent platform of the second generation DES allows thinner struts which may enhance endothelial coverage, reduce restenosis and improve deliverability.205–207 Drugs from the limus family similar to sirolimus are used as the antiproliferative agents. Also because of mounting evidence of the negative role of the polymers used in first generation DES, manufacturers utilized alternative biocompatible polymers which cause less inflammation. Although the new polymers are different from the first-generation DES polymers, second-generation DES in the United States do not employ bioabsorbable polymers. Early comparisons of first and second generation DES have been conflicting, but suggest that second-generation DES are at least as effective, and in the SPIRIT IV trial, the XIENCE stent was demonstrated to be superior to the TAXUS stent.208
Given the continued shortcomings of currently available DES, there are multiple other stent innovations currently under investigation. Some technologies have focused on addressing the negative effects of polymers with the development of biodegradable polymeric DES or nonpolymeric DES. Early results comparing these technologies, available commercially in Europe, are most promising for the nonpolymeric DES.209,210 Because of inherent limitations of DES, biodegradable and bioabsorbable stents are also under development and undergoing preclinical and clinical trials.211–215 These stents have the potential advantage of degrading or eroding completely over time, allowing for short-term drug delivery to the vascular wall, while avoiding long-term exposure of the stent to pro-inflammatory components in the bloodstream.215 While preliminary results appear promising, some of the polymeric materials used in these stents, including poly-L-lactic acid and poly-D,L-lactide, may be degraded and eroded into small particles that can be phagocytosed.214,215 The phagocytosis of these particles have been previously shown to trigger superoxide production by the phagocytes.216 Despite this possibility, the investigators of the ABSORB stent have not reported any significant inflammatory responses to the implant within 2 years of implantation, and only time will tell if these responses will arise later on in the lifetime of the stent.215
Currently, these stent technologies are only applicable to the treatment of obstructive CAD, and they have not been rigorously evaluated for the treatment of nonobstructive vulnerable plaque. As little is known about the lesion-specific risk of future cardiovascular events in high-risk plaques, implantation of a coronary stent in a clinically silent lesion—in order to prevent a future event—is not justified at this time. The recently completed PROSPECT study (Providing Regional Observations to Study Predictors of Events in the Coronary Tree) demonstrated that the majority of initially asymptomatic, unstented plaques that led to major coronary events within three years featured thin fibrous caps, minimal luminal areas of ≤4mm2,and ≥70% plaque burden.66 There are now ongoing pilot studies utilizing stents specially designed for use in nonobstructive coronary plaques which may provide insight into the feasibility of such an approach.217 Perhaps with continued advancement of stent technology, stents to safely and effectively treat vulnerable plaques will become a reality.
NANO APPROACHES IN THE DESIGN OF NEXT-GENERATION STENTS
Arterial stenting has been one of the most rapidly adopted treatments for coronary and peripheral artery disease.218 However, unforeseen problems with DES have necessitated further investigation and advancement of stent technology. One of the primary concerns is the significant increase in the occurrence of late stent thrombosis months, even years after implantation of DES as compared to BMS. It is believed that the degradation products of DES, primarily large, poorly biocompatible polymer fragments, are the main contributor to thrombosis and undesired inflammatory events.219,220 Furthermore, the antiproliferative agents used in DES, when delivered through the current mechanism, can be too effective and prevent the necessary long-term formation of new endothelium.221
Drug-Bearing Nanofilms and Nanoparticles
There are many techniques and materials in nanotechnology that may be utilized to address the current problems with DES. The implementation of these nanoscale tools has given rise to the next generation of DES that possesses a larger degree of material complexity and leverage stent coatings with nanoscale modifications designed to enhance biocompatibility. Nanoscale components made from biodegradable polymers for the next generation of DES improve biocompatibility by releasing degradable products that are more biocompatible and less inflammatory than the first-generation DES.222
One novel approach utilizes a chitosan film, containing drug loaded poly(D,L-lactic-co-glycolic acid) (PLGA) nanoparticles, as a metal stent coating material.222 These materials have multiple advantageous properties not present in the first generation of DES. First, PLGA nanoparticles can be made easily, rapidly, and in a scalable fashion using oil-in-water emulsification, which can be done in a single stage synthesis.223 Also, nanoparticles are tunable to control the time period and kinetics of local drug release.224,225 The presence of two drug loadable vehicles (the nanoparticles and the film itself) provides the opportunity to utilize two drugs that may exhibit different hydrophobicity (which might normally be incompatible in a single device) and tailor the release kinetics of both of these drugs, primarily by altering the degradation rate of chitosan film. The use of a biomolecule-based drug delivery vehicle—a chitosan film—reduces the likelihood of release of large polymer fragments as the film degrades. The nanometer dimensions of the polymeric particles is specifically advantageous for reducing immune responses that can be often triggered by large polymer fragments.222
Another next-generation stent design utilizes an inorganic drug eluting coating material comprised of carbon nanoparticles embedded in a thin film of glassy, polymeric carbon.226 The nanoparticles and carbon matrix create a bioinert, porous coating with favorable elasto-mechanical properties. The porosity of the surface increases the surface area for adsorption of drugs into the construct. The drug release from the carbon matrix can then be specifically tailored by altering the pore size in the coating. This particular study found that paclitaxel loaded carbon—carbon nanoparticle coated stents shows lower mean injury, better endothelialization, and less restenosis than paclitaxel loaded PLGA coated stents, indicating improved biocompatibility for the inorganic coating.226
Other stent modification approaches rely on the mechanical and geometric properties of the surface coating to improve re-endothelialization. Using an extracellular matrix-mimicking nanofibrous matrix of nitric oxide-releasing peptide amphiphiles, selective recruitment of endothelial cells was demonstrated, in competition with smooth muscle cells.227 By utilizing nanofibers 8–10 nm in width and several microns in length, the coating was also capable of significantly decreasing platelet adhesion as compared to a collagen or stainless steel surface.227,228
New stent designs have also incorporated complex arrangements of multiple types of nanoparticles to impart increased functionality and biocompatibility. One method of DES fabrication under investigation is the formation of a multifunctional stent coating created by the layer-by-layer assembly of bioinert biomolecule-based nanoparticles. The nanoparticle layers are formed by spontaneous self-assembly because of alternating electrostatic charges.229 Using the scalable layer-by-layer assembly method, it is possible to achieve more than 80% stent surface coverage with nanoparticles. A previous study has shown that it is possible to coat a metal stent surface with many different populations of nanoparticles, each of which can be loaded with a different drug. This multifunctional coat can then provide simultaneous, sustained release kinetics of several drugs and can be controlled by altering nanoparticle composition, density, and size, as well as encapsulated drug concentration.230 The same study also has demonstrated that image contrast agents can be packaged in the same fashion as drugs on a stent surface, resulting in enhancing the quality of imaging by micro computed tomography (using gold nanoparticles) and X-ray (using barium sulfate).
Other studies have produced radio-opaque polymer stents by incorporating poly(ethylene glycol) (PEG) and iodine into the stent, producing X-ray scattering stents capable of decreasing fibrinogen adsorption levels and reducing platelet adhesion to the stent.231 Furthermore, it has been shown that this technique produces stent images without producing blooming artifacts observed with metal stents.232
These complex, multifunctional designs represent a significant advancement in the utilization of nanotechnology for drug eluting stents.
Nanopatterned Surfaces on Bare Metal Stents (BMS)
Many of the negative effects caused by drug eluting stents, such as late stent thrombosis, are because of by-products of stent coating degradation and poor in-stent endothelialization.233,234 Occurrence of late stent thrombosis is significantly increased by the use of DES compared to BMS.200–202 Therefore, as an alternative to DES, advanced BMS attempt to improve stent endothelialization by nanostructuring the stent surface. Introduction of nanoscale surface alterations through patterning or roughening have been shown to improve endothelial cell proliferation and morphology on polymeric and metal materials.235–238
Nanopatterned BMS are designed to prevent late stent thrombosis while improving endothelial cell proliferation and retarding smooth muscle cell migration and proliferation. This strategy promotes stent endothelialization and is a promising strategy for preventing restenosis and thrombosis.239 One group modified a titanium stent surface, creating nanostructured posts with the minimum feature size of 40 × 40 × 10 nm3 (l × w × h).240 They performed a co-culture of rat aorta endothelial cells (RAECs) and rat aorta smooth muscle cells (RASMCs) on the fabricated submicron scale and nanoscale roughed surfaces, with a flat Ti surface serving as a control. RAEC proliferation is increased on roughed Ti surfaces relative to a flat surface even after 1 day and RASMC proliferation is depressed after 5 days. The roughed surfaces exhibited more hydrophilicity as compared to the flat surfaces, improving adsorption of important extracellular matrix proteins such as fibronectin and vitronectin,241 in addition to increasing RAEC elastin and collagen synthesis after 1 and 2 weeks. Mechanical spreading of RAECs is presumed to increase on roughened surfaces and these morphological changes may account for increased extracellular matrix protein production.240
Another group modified the surface of a MP35N alloy (35% Co, 35% Ni, 20% Cr, 10% Mo) stent surface with nanopillars ranging 100–300 nm in diameter and 0.5–5 μm in length.242 After 2 and 7 days, the textured surfaces showed 50–60% improved bovine aortic endothelial cell adhesion versus a flat stent surface. More importantly, the morphology of the cells on the textured stent surface was comparable to the control cultures and showed the presence of peri-junctional cortical bands of actin filaments indicating physiologically normal arrangement of endothelium. The flat stent surface resulted in poorly organized cellular aggregates exhibiting poorly pronounced cellular junctions and a large amount of poorly organized extracellular matrix protein, indicating the possibility of restenosis242 (Figure 5).
FIGURE 5.
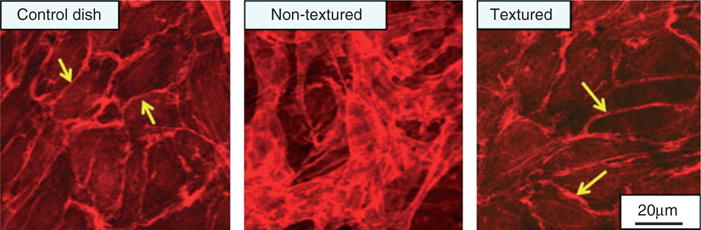
Bovine aortic endothelial cell adhesion and function on nanotextured versus conventional MP35N surfaces. Actin staining on a control dish (plastic), non-nanotextured and nanotextured MP35N surfaces. The presence of peri-junctional cortical bands of filamentous actin are clearly visible (arrows) on both the control and textured surfaces, but are not as pronounced on the nontextured surface, showing multiple layers of possibly aggregated cells. (Reprinted with permission from Ref 242. Copyright 2008 Elsevier)
In addition to the stimulatory mechanical cues presented by the nanopatterned stent surface, the spaces between the nanopillars can potentially be used as depots for drug storage and controlled release as shown with other systems.243 Drug release kinetics can be controlled by altering the geometric properties of the pillars. BMS modification schemes have also been applied to other stent alloy surfaces such as Ni:Ti alloys and nanoporation of stent surfaces has also been investigated as mechanism of producing surface roughness and drug storage.244,245
Nanotechnology based improvements are currently trending toward complex combinatorial approaches designed to inhibit immune response to the implant and prevent restenosis while simultaneously promoting controlled endothelialization using highly biocompatible or bioinert components. To that end, a strong candidate for the next generation of vascular stents may use a surface modified, nanoroughed BMS surface which in turn contains multiple types of drug eluting and/or contrast enhancing nanoparticles made from biomolecules. This approach would leverage the strengths of the patterned BMS surface while also allowing for localized, sustained drug delivery with highly controllable release kinetics. The implementation of a biomolecular NP drug delivery system would also mitigate the inflammatory and prothrombotic effects of large polymer fragments.
CONCLUSION
Nanotechnology is expected to trigger a revolution in the diagnosis and management of vulnerable plaques. By providing large surface areas for ligand attachment, nanoparticle-based immunoassays and contrast agents are extending the boundaries of sensitivity and detection possible in today’s clinic. Further, improvements in nanoparticle design and synthesis continue to produce novel multifunctional platforms, enabling next-generation medical technologies such as multimodal imaging with the same contrast agent, thera-nostic platforms capable of site-specific imaging and therapeutic delivery, multiplexed immunoassays for detecting multiple analytes at once in a single sample, as well as environmentally sensitive ‘smart’ biosensing. Rapid, low-reagent volume, simple immunoassay setups suggested by the most recent marriages between nanotechnology and microfluidics indicate that routine blood biomarker testing may soon take a more central role in the diagnosis and risk stratification of atherosclerotic patients.
In the cardiac catheterization lab, stenting will continue to be a major means for CAD, and nanotechnology provides a number of unique potential solutions to the most common reasons for stent failure. A long-term goal of the stenting industry is to encourage regeneration of functional endothelium over the stent. Nanopatterning and nanostructuring of stent surfaces creates structures that interact directly with cells on a very relevant size scale, and early studies show promise for this approach to encourage re-endothelialization. Improvements in DES controlled release technologies have also been facilitated through advances in nanoparticle and nanofilm research.
Given the body of promising in vivo data in animal models, clinical studies will soon come for these new nanoparticulate diagnostics, contrast agents, controlled-release devices, and stent designs. Next-generation approaches to diagnose and manage vulnerable atherosclerotic plaques are likely to feature nano-based design elements. It is clear that such advances require the concerted efforts of researchers in biomaterials, vascular biology, chemistry, micro-fabrication, and more. These novel approaches are injecting new life into a field, and suggest that a future is coming when vulnerable plaques are detected early and treated accordingly with host-‘friendly’ stents.
Acknowledgments
S.S.Y., R.A.O., and T.D.G. would like to acknowledge financial support from a Vanderbilt University Discovery Grant (4-48-999-9132) and the Department of Defense Congressionally Directed Medical Research Program (#W81XWH-08-1-0502). H.J.S. acknowledges financial support through the National Institutes of Health (#1R21HL091465) and the National Science Foundation (NSF/DMR BMAT 1006558). S.S.Y. acknowledges his former senior design team members at Rice University—Stacy Cheng, Kai Chu, Shuvro De, Natalia Vasco, & Eva Wang—for providing artwork for Figure 4.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Insull W., Jr The pathology of atherosclerosis: plaque development and plaque responses to medical treatment. Am J Med. 2009;122:S3–S14. doi: 10.1016/j.amjmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 3.Naghavi M, Falk E. Asymptomatic Atherosclerosis. New York, NY: Humana Press; 2010. From vulnerable plaque to vulnerable patient; pp. 13–38. [Google Scholar]
- 4.Sousa JE, Serruys PW, Costa MA. New frontiers in cardiology: drug-eluting stents: part I. Circulation. 2003;107:2274–2279. doi: 10.1161/01.CIR.0000069330.41022.90. [DOI] [PubMed] [Google Scholar]
- 5.Finn AV, Nakazawa G, Joner M, Kolodgie FD, Mont EK, Gold HK, Virmani R. Vascular responses to drug eluting stents: importance of delayed healing. Arterioscler Thromb Vasc Biol. 2007;27:1500–1510. doi: 10.1161/ATVBAHA.107.144220. [DOI] [PubMed] [Google Scholar]
- 6.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res. 2002;91:769–775. doi: 10.1161/01.res.0000038487.19924.18. [DOI] [PubMed] [Google Scholar]
- 7.Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions. Cardiovasc Res. 1999;41:369–375. doi: 10.1016/s0008-6363(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 8.Saam T, Hatsukami TS, Takaya N, Chu B, Under-hill H, Kerwin WS, Cai J, Ferguson MS, Yuan C. The vulnerable, or high-risk, atherosclerotic plaque: non-invasive MR imaging for characterization and assessment. Radiology. 2007;244:64–77. doi: 10.1148/radiol.2441051769. [DOI] [PubMed] [Google Scholar]
- 9.Van Berkel TJ, De Rijke YB, Kruijt JK. Different fate in vivo of oxidatively modified low density lipoprotein and acetylated low density lipoprotein in rats. Recognition by various scavenger receptors on Kupffer and endothelial liver cells. J Biol Chem. 1991;266:2282–2289. [PubMed] [Google Scholar]
- 10.Williams KJ, Tabas I. The response-to-retention hypothesis of early atherogenesis. Arterioscler Thromb Vasc Biol. 1995;15:551–561. doi: 10.1161/01.atv.15.5.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabas I. Consequences and therapeutic implications of macrophage apoptosis in atherosclerosis: the importance of lesion stage and phagocytic efficiency. Arterioscler Thromb Vasc Biol. 2005;25:2255–2264. doi: 10.1161/01.ATV.0000184783.04864.9f. [DOI] [PubMed] [Google Scholar]
- 12.Pluddemann A, Neyen C, Gordon S. Macrophage scavenger receptors and host-derived ligands. Methods. 2007;43:207–217. doi: 10.1016/j.ymeth.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 13.Herman MP, Sukhova GK, Libby P, Gerdes N, Tang N, Horton DB, Kilbride M, Breitbart RE, Chun M, Schonbeck U. Expression of neutrophil collagenase (matrix metalloproteinase-8) in human atheroma: a novel collagenolytic pathway suggested by transcriptional profiling. Circulation. 2001;104:1899–1904. doi: 10.1161/hc4101.097419. [DOI] [PubMed] [Google Scholar]
- 14.Johnson C, Sung HJ, Lessner SM, Fini ME, Galis ZS. Matrix metalloproteinase-9 is required for adequate angiogenic revascularization of ischemic tissues: potential role in capillary branching. Circ Res. 2004;94:262–268. doi: 10.1161/01.RES.0000111527.42357.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gough PJ, Gomez IG, Wille PT, Raines EW. Macro-phage expression of active MMP-9 induces acute plaque disruption in apoE-deficient mice. J Clin Invest. 2006;116:59–69. doi: 10.1172/JCI25074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sluijter JP, Pulskens WP, Schoneveld AH, Velema E, Strijder CF, Moll F, de Vries JP, Verheijen J, Hanemaaijer R, de Kleijn DP, et al. Matrix metal-loproteinase 2 is associated with stable and matrix metalloproteinases 8 and 9 with vulnerable carotid atherosclerotic lesions: a study in human endarterectomy specimen pointing to a role for different extracellular matrix metalloproteinase inducer glycosylation forms. Stroke. 2006;37:235–239. doi: 10.1161/01.STR.0000196986.50059.e0. [DOI] [PubMed] [Google Scholar]
- 17.de Nooijer R, Verkleij CJ, von der Thusen JH, Jukema JW, van der Wall EE, van Berkel TJ, Baker AH, Biessen EA. Lesional overexpression of matrix metalloproteinase-9 promotes intraplaque hemorrhage in advanced lesions but not at earlier stages of atherogenesis. Arterioscler Thromb Vasc Biol. 2006;26:340–346. doi: 10.1161/01.ATV.0000197795.56960.64. [DOI] [PubMed] [Google Scholar]
- 18.Libby P. Inflammation in atherosclerosis. Nature. 2002;420:868–874. doi: 10.1038/nature01323. [DOI] [PubMed] [Google Scholar]
- 19.Finet G, Ohayon J, Rioufol G. Biomechanical interaction between cap thickness, lipid core composition and blood pressure in vulnerable coronary plaque: impact on stability or instability. Coron Artery Dis. 2004;15:13–20. doi: 10.1097/00019501-200402000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 21.Galis ZS, Sukhova GK, Lark MW, Libby P. Increased expression of matrix metalloproteinases and matrix degrading activity in vulnerable regions of human atherosclerotic plaques. J Clin Invest. 1994;94:2493–2503. doi: 10.1172/JCI117619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee RT, Grodzinsky AJ, Frank EH, Kamm RD, Schoen FJ. Structure-dependent dynamic mechanical behavior of fibrous caps from human atherosclerotic plaques. Circulation. 1991;83:1764–1770. doi: 10.1161/01.cir.83.5.1764. [DOI] [PubMed] [Google Scholar]
- 23.Beaussier H, Masson I, Collin C, Bozec E, Laloux B, Calvet D, Zidi M, Boutouyrie P, Laurent S. Carotid plaque, arterial stiffness gradient, and remodeling in hypertension. Hypertension. 2008;52:729–736. doi: 10.1161/HYPERTENSIONAHA.108.115972. [DOI] [PubMed] [Google Scholar]
- 24.Tajaddini A, Kilpatrick DL, Vince DG. A novel experimental method to estimate stress-strain behavior of intact coronary arteries using intravascular ultrasound (IVUS) J Biomech Eng. 2003;125:120–123. doi: 10.1115/1.1536929. [DOI] [PubMed] [Google Scholar]
- 25.Kim YS, Galis ZS, Rachev A, Han HC, Vito RP. Matrix metalloproteinase-2 and -9 are associated with high stresses predicted using a nonlinear heterogeneous model of arteries. J Biomech Eng. 2009;131:011009. doi: 10.1115/1.3005163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng C, Tempel D, van Haperen R, van der Baan A, Grosveld F, Daemen MJ, Krams R, de Crom R. Atherosclerotic lesion size and vulnerability are determined by patterns of fluid shear stress. Circulation. 2006;113:2744–2753. doi: 10.1161/CIRCULATIONAHA.105.590018. [DOI] [PubMed] [Google Scholar]
- 27.Douma K, Prinzen L, Slaaf DW, Reutelingsperger CP, Biessen EA, Hackeng TM, Post MJ, van Zand-voort MA. Nanoparticles for optical molecular imaging of atherosclerosis. Small. 2009;5:544–557. doi: 10.1002/smll.200801079. [DOI] [PubMed] [Google Scholar]
- 28.Trivedi RA, Mallawarachi C, UK-I JM, Graves MJ, Horsley J, Goddard MJ, Brown A, Wang L, Kirk-patrick PJ, Brown J, et al. Identifying inflamed carotid plaques using in vivo USPIO-enhanced MR imaging to label plaque macrophages. Arterioscler Thromb Vasc Biol. 2006;26:1601–1606. doi: 10.1161/01.ATV.0000222920.59760.df. [DOI] [PubMed] [Google Scholar]
- 29.Holzapfel GA, Sommer G, Regitnig P. Anisotropic mechanical properties of tissue components in human atherosclerotic plaques. J Biomech Eng. 2004;126:657–665. doi: 10.1115/1.1800557. [DOI] [PubMed] [Google Scholar]
- 30.Richardson PD. Biomechanics of plaque rupture: progress, problems, and new frontiers. Ann Biomed Eng. 2002;30:524–536. doi: 10.1114/1.1482781. [DOI] [PubMed] [Google Scholar]
- 31.Little WC, Constantinescu M, Applegate RJ, Kutcher MA, Burrows MT, Kahl FR, Santamore WP. Can coronary angiography predict the site of a subsequent myocardial infarction in patients with mild-to-moderate coronary artery disease? Circulation. 1988;78:1157–1166. doi: 10.1161/01.cir.78.5.1157. [DOI] [PubMed] [Google Scholar]
- 32.Giroud D, Li JM, Urban P, Meier B, Rutishauer W. Relation of the site of acute myocardial infarction to the most severe coronary arterial stenosis at prior angiography. Am J Cardiol. 1992;69:729–732. doi: 10.1016/0002-9149(92)90495-k. [DOI] [PubMed] [Google Scholar]
- 33.Cutlip DE, Chhabra AG, Baim DS, Chauhan MS, Marulkar S, Massaro J, Bakhai A, Cohen DJ, Kuntz RE, Ho KK. Beyond restenosis: five-year clinical outcomes from second-generation coronary stent trials. Circulation. 2004;110:1226–1230. doi: 10.1161/01.CIR.0000140721.27004.4B. [DOI] [PubMed] [Google Scholar]
- 34.Glaser R, Selzer F, Faxon DP, Laskey WK, Cohen HA, Slater J, Detre KM, Wilensky RL. Clinical progression of incidental, asymptomatic lesions discovered during culprit vessel coronary intervention. Circulation. 2005;111:143–149. doi: 10.1161/01.CIR.0000150335.01285.12. [DOI] [PubMed] [Google Scholar]
- 35.de Weert TT, Ouhlous M, Meijering E, Zondervan PE, Hendriks JM, van Sambeek MR, Dippel DW, van der Lugt A. In vivo characterization and quantification of atherosclerotic carotid plaque components with multidetector computed tomography and histopathological correlation. Arterioscler Thromb Vasc Biol. 2006;26:2366–2372. doi: 10.1161/01.ATV.0000240518.90124.57. [DOI] [PubMed] [Google Scholar]
- 36.Das M, Braunschweig T, Muhlenbruch G, Mahnken AH, Krings T, Langer S, Koeppel T, Jacobs M, Gunther RW, Mommertz G. Carotid plaque analysis: comparison of dual-source computed tomography (CT) findings and histopathological correlation. Eur J Vasc Endovasc Surg. 2009;38:14–19. doi: 10.1016/j.ejvs.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 37.Cai J, Hatsukami TS, Ferguson MS, Kerwin WS, Saam T, Chu B, Takaya N, Polissar NL, Yuan C. In vivo quantitative measurement of intact fibrous cap and lipid-rich necrotic core size in atherosclerotic carotid plaque: comparison of high-resolution, contrast-enhanced magnetic resonance imaging and histology. Circulation. 2005;112:3437–3444. doi: 10.1161/CIRCULATIONAHA.104.528174. [DOI] [PubMed] [Google Scholar]
- 38.Beduneau A, Ma Z, Grotepas CB, Kabanov A, Rabinow BE, Gong N, Mosley RL, Dou H, Boska MD, Gendelman HE. Facilitated monocyte-macrophage uptake and tissue distribution of superparmagnetic iron-oxide nanoparticles. PLoS One. 2009;4:e4343. doi: 10.1371/journal.pone.0004343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Briley-Saebo KC, Johansson LO, Hustvedt SO, Haldorsen AG, Bjornerud A, Fayad ZA, Ahlstrom HK. Clearance of iron oxide particles in rat liver—effect of hydrated particle size and coating material on liver metabolism. Invest Radiol. 2006;41:560–571. doi: 10.1097/01.rli.0000221321.90261.09. [DOI] [PubMed] [Google Scholar]
- 40.Briley-Saebo KC, Mani V, Hyafil F, Cornily JC, Fayad ZA. Fractionated feridex and positive contrast: in vivo MR imaging of atherosclerosis. Magn Reson Med. 2008;59:721–730. doi: 10.1002/mrm.21541. [DOI] [PubMed] [Google Scholar]
- 41.Tang TY, Muller KH, Graves MJ, Li ZY, Walsh SR, Young V, Sadat U, Howarth SP, Gillard JH. Iron oxide particles for atheroma imaging. Arterioscler Thromb Vasc Biol. 2009;29:1001–1008. doi: 10.1161/ATVBAHA.108.165514. [DOI] [PubMed] [Google Scholar]
- 42.Thomsen HS. Gadolinium-based contrast media may be nephrotoxic even at approved doses. Eur Radiol. 2004;14:1654–1656. doi: 10.1007/s00330-004-2379-0. [DOI] [PubMed] [Google Scholar]
- 43.Bastus NG, Sanchez-Tillo E, Pujals S, Farrera C, Lopez C, Giralt E, Celada A, Lloberas J, Puntes V. Homogeneous conjugation of peptides onto gold nanoparticles enhances macrophage response. ACS Nano. 2009;3:1335–1344. doi: 10.1021/nn8008273. [DOI] [PubMed] [Google Scholar]
- 44.Simberg D, Park JH, Karmali PP, Zhang WM, Merkulov S, McCrae K, Bhatia SN, Sailor M, Ruoslahti E. Differential proteomics analysis of the surface heterogeneity of dextran iron oxide nanoparticles and the implications for their in vivo clearance. Biomaterials. 2009;30:3926–3933. doi: 10.1016/j.biomaterials.2009.03.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fujimoto S, Hartung D, Ohshima S, Edwards DS, Zhou J, Yalamanchili P, Azure M, Fujimoto A, Isobe S, Matsumoto Y, et al. Molecular imaging of matrix metalloproteinase in atherosclerotic lesions: resolution with dietary modification and statin therapy. J Am Coll Cardiol. 2008;52:1847–1857. doi: 10.1016/j.jacc.2008.08.048. [DOI] [PubMed] [Google Scholar]
- 46.Kooi ME, Cappendijk VC, Cleutjens KB, Kessels AG, Kitslaar PJ, Borgers M, Frederik PM, Daemen MJ, van Engelshoven JM. Accumulation of ultrasmall superparamagnetic particles of iron oxide in human atherosclerotic plaques can be detected by in vivo magnetic resonance imaging. Circulation. 2003;107:2453–2458. doi: 10.1161/01.CIR.0000068315.98705.CC. [DOI] [PubMed] [Google Scholar]
- 47.Lipinski MJ, Frias JC, Amirbekian V, Briley-Saebo KC, Mani V, Samber D, Abbate A, Aguinaldo JG, Massey D, Fuster V, et al. Macrophage-specific lipid-based nanoparticles improve cardiac magnetic resonance detection and characterization of human atherosclerosis. JACC Cardiovasc Imaging. 2009;2:637–647. doi: 10.1016/j.jcmg.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nahrendorf M, Zhang H, Hembrador S, Panizzi P, Sosnovik DE, Aikawa E, Libby P, Swirski FK, Weissleder R. Nanoparticle PET-CT imaging of macrophages in inflammatory atherosclerosis. Circulation. 2008;117:379–387. doi: 10.1161/CIRCULATIONAHA.107.741181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trivedi RA, UK-I JM, Graves MJ, Kirkpatrick PJ, Gillard JH. Noninvasive imaging of carotid plaque inflammation. Neurology. 2004;63:187–188. doi: 10.1212/01.wnl.0000132962.12841.1d. [DOI] [PubMed] [Google Scholar]
- 50.Waldo SW, Li Y, Buono C, Zhao B, Billings EM, Chang J, Kruth HS. Heterogeneity of human macrophages in culture and in atherosclerotic plaques. Am J Pathol. 2008;172:1112–1126. doi: 10.2353/ajpath.2008.070513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raynal I, Prigent P, Peyramaure S, Najid A, Rebuzzi C, Corot C. Macrophage endocytosis of super-paramagnetic iron oxide nanoparticles: mechanisms and comparison of ferumoxides and ferumoxtran-10. Invest Radiol. 2004;39:56–63. doi: 10.1097/01.rli.0000101027.57021.28. [DOI] [PubMed] [Google Scholar]
- 52.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 53.Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, Badimon JJ, O’Connor WN. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation. 2004;110:2032–2038. doi: 10.1161/01.CIR.0000143233.87854.23. [DOI] [PubMed] [Google Scholar]
- 54.Giannarelli C, Ibanez B, Cimmino G, Garcia Ruiz JM, Faita F, Bianchini E, Zafar MU, Fuster V, Garcia MJ, Badimon JJ. Contrast-enhanced ultrasound imaging detects intraplaque neovascularization in an experimental model of atherosclerosis. JACC Cardiovasc Imaging. 2010;3:1256–1264. doi: 10.1016/j.jcmg.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Manca C, Parenti G, Bellina R, Boni G, Grosso M, Bernini W, Palombo C, Paterni M, Pelosi G, Lanza M, et al. In-111 platelet scintigraphy for the noninvasive detection of carotid plaque thrombosis. Stroke. 2001;32:719–727. doi: 10.1161/01.str.32.3.719. [DOI] [PubMed] [Google Scholar]
- 56.Annovazzi A, Bonanno E, Arca M, D’Alessandria C, Marcoccia A, Spagnoli LG, Violi F, Scopinaro F, De Toma G, Signore A. 99mTc-interleukin-2 scintigraphy for the in vivo imaging of vulnerable atherosclerotic plaques. Eur J Nucl Med Mol Imaging. 2006;33:117–126. doi: 10.1007/s00259-005-1899-4. [DOI] [PubMed] [Google Scholar]
- 57.Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, et al. In vivo 18F-fluorode-oxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818–1824. doi: 10.1016/j.jacc.2006.05.076. [DOI] [PubMed] [Google Scholar]
- 58.Burke AP, Farb A, Malcom GT, Liang YH, Smialek J, Virmani R. Coronary risk factors and plaque morphology in men with coronary disease who died suddenly. N Engl J Med. 1997;336:1276–1282. doi: 10.1056/NEJM199705013361802. [DOI] [PubMed] [Google Scholar]
- 59.Yamagishi M, Terashima M, Awano K, Kijima M, Nakatani S, Daikoku S, Ito K, Yasumura Y, Miyatake K. Morphology of vulnerable coronary plaque: insights from follow-up of patients examined by intravascular ultrasound before an acute coronary syndrome. J Am Coll Cardiol. 2000;35:106–111. doi: 10.1016/s0735-1097(99)00533-1. [DOI] [PubMed] [Google Scholar]
- 60.Bayturan O, Tuzcu EM, Nicholls SJ, Balog C, Lavoie A, Uno K, Crowe TD, Magyar WA, Wolski K, Kapadia S, et al. Attenuated plaque at nonculprit lesions in patients enrolled in intravascular ultrasound atherosclerosis progression trials. JACC Cardiovasc Interv. 2009;2:672–678. doi: 10.1016/j.jcin.2009.05.007. [DOI] [PubMed] [Google Scholar]
- 61.Lee SY, Mintz GS, Kim SY, Hong YJ, Kim SW, Okabe T, Pichard AD, Satler LF, Kent KM, Suddath WO, et al. Attenuated plaquedetected byintravascular ultrasound: clinical, angiographic, and morphologic features and post-percutaneous coronary intervention complications in patients with acute coronary syndromes. JACC Cardiovasc Interv. 2009;2:65–72. doi: 10.1016/j.jcin.2008.08.022. [DOI] [PubMed] [Google Scholar]
- 62.Nair A, Kuban BD, Tuzcu EM, Schoenhagen P, Nissen SE, Vince DG. Coronary plaque classification with intravascular ultrasound radiofrequency data analysis. Circulation. 2002;106:2200–2206. doi: 10.1161/01.cir.0000035654.18341.5e. [DOI] [PubMed] [Google Scholar]
- 63.Van Herck J, De Meyer G, Ennekens G, Van Herck P, Herman A, Vrints C. Validation of in vivo plaque characterisation by virtual histology in a rabbit model of atherosclerosis. EuroIntervention. 2009;5:149–156. doi: 10.4244/eijv5i1a23. [DOI] [PubMed] [Google Scholar]
- 64.Rodriguez-Granillo GA, Garcia-Garcia HM, Mc Fadden EP, Valgimigli M, Aoki J, de Feyter P, Serruys PW. In vivo intravascular ultrasound-derived thin-cap fibroatheroma detection using ultrasound radiofrequency data analysis. J Am Coll Cardiol. 2005;46:2038–2042. doi: 10.1016/j.jacc.2005.07.064. [DOI] [PubMed] [Google Scholar]
- 65.Serruys PW. Fourth annual American College of Cardiology international lecture: a journey in the interventional field. J Am Coll Cardiol. 2006;47:1754–1768. doi: 10.1016/j.jacc.2005.12.051. [DOI] [PubMed] [Google Scholar]
- 66.Stone GW, Maehara A, Lansky AJ, de Bruyne B, Cristea E, Mintz GS, Mehran R, McPherson J, Farhat N, Marso SP, et al. A prospective natural-history study of coronary atherosclerosis. New Engl J Med. 2011;364:226–235. doi: 10.1056/NEJMoa1002358. [DOI] [PubMed] [Google Scholar]
- 67.Low AF, Tearney GJ, Bouma BE, Jang IK. Technology Insight: optical coherence tomography-current status and future development. Nat Clin Pract Cardiovasc Med. 2006;3:154–162. doi: 10.1038/ncpcardio0482. quiz 172. [DOI] [PubMed] [Google Scholar]
- 68.Stamper D, Weissman NJ, Brezinski M. Plaque characterization with optical coherence tomography. J Am Coll Cardiol. 2006;47:C69–C79. doi: 10.1016/j.jacc.2005.10.067. [DOI] [PubMed] [Google Scholar]
- 69.Templin C, Meyer M, Muller MF, Djonov V, Hlushchuk R, Dimova I, Flueckiger S, Kronen P, Sidler M, Klein K, et al. Coronary optical frequency domain imaging (OFDI) for in vivo evaluation of stent healing: comparison with light and electron microscopy. Eur Heart J. 2010;31:1792–1801. doi: 10.1093/eurheartj/ehq168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fuster V, Fayad ZA, Moreno PR, Poon M, Corti R, Badimon JJ. Atherothrombosis and high-risk plaque: Part II: approaches by noninvasive computed tomo-graphic/magnetic resonance imaging. J Am Coll Cardiol. 2005;46:1209–1218. doi: 10.1016/j.jacc.2005.03.075. [DOI] [PubMed] [Google Scholar]
- 71.Fuster V, Corti R, Fayad ZA, Schwitter J, Badimon JJ. Integration of vascular biology and magnetic resonance imaging in the understanding of atherothrombosis and acute coronary syndromes. J Thromb Haemost. 2003;1:1410–1421. doi: 10.1046/j.1538-7836.2003.00271.x. [DOI] [PubMed] [Google Scholar]
- 72.Sirol M, Fuster V, Fayad ZA. Plaque imaging and characterization using magnetic resonance imaging: towards molecular assessment. Curr Mol Med. 2006;6:541–548. doi: 10.2174/156652406778018617. [DOI] [PubMed] [Google Scholar]
- 73.Moreno PR. Vulnerable plaque: definition, diagnosis, and treatment. Cardiol Clin. 2010;28:1–30. doi: 10.1016/j.ccl.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 74.Sathyanarayana S, Schar M, Kraitchman DL, Bottomley PA. Towards real-time intravascular endoscopic magnetic resonance imaging. JACC Cardiovasc Imaging. 2010;3:1158–1165. doi: 10.1016/j.jcmg.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Hirsch LR, Stafford RJ, Bankson JA, Sershen SR, Rivera B, Price RE, Hazle JD, Halas NJ, West JL. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci USA. 2003;100:13549–13554. doi: 10.1073/pnas.2232479100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Luker GD, Luker KE. Optical imaging: current applications and future directions. J Nucl Med. 2008;49:1–4. doi: 10.2967/jnumed.107.045799. [DOI] [PubMed] [Google Scholar]
- 77.Goldstein JA, Grines C, Fischell T, Virmani R, Rizik D, Muller J, Dixon SR. Coronary embolization following balloon dilation of lipid-core plaques. JACC Cardiovasc imaging. 2009;2:1420–1424. doi: 10.1016/j.jcmg.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 78.Madden SP, Sum ST, Muller JE. Assessment of coronary stenosis with near infrared spectroscopy. In: Escaned J, Serruys P, editors. Coronary Stenosis Imaging, Structure, and Physiology. Toulouse: PCR Publishing; 2010. [Google Scholar]
- 79.Caplan JD, Waxman S, Nesto RW, Muller JE. Near-infrared spectroscopy for the detection of vulnerable coronary artery plaques. J Am Coll Cardiol. 2006;47:C92–C96. doi: 10.1016/j.jacc.2005.12.045. [DOI] [PubMed] [Google Scholar]
- 80.Gardner CM, Tan H, Hull EL, Lisauskas JB, Sum ST, Meese TM, Jiang C, Madden SP, Caplan JD, Burke AP, et al. Detection of lipid core coronary plaques in autopsy specimens with a novel catheter-based near-infrared spectroscopy system. JACC Cardiovasc Imaging. 2008;1:638–648. doi: 10.1016/j.jcmg.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 81.Moreno PR, Lodder RA, Purushothaman KR, Charash WE, O’Connor WN, Muller JE. Detection of lipid pool, thin fibrous cap, and inflammatory cells in human aortic atherosclerotic plaques by near-infrared spectroscopy. Circulation. 2002;105:923–927. doi: 10.1161/hc0802.104291. [DOI] [PubMed] [Google Scholar]
- 82.Schultz CJ, Serruys PW, van der Ent M, Ligthart J, Mastik F, Garg S, Muller JE, Wilder MA, van de Steen AFW, Regar E. First-in-man clinical use of combined near-infrared spectroscopy and intravascular ultrasound a potential key to predict distal embolization and no-reflow? J Am Coll Cardiol. 2010;56:314–314. doi: 10.1016/j.jacc.2009.10.090. [DOI] [PubMed] [Google Scholar]
- 83.Libby P, Aikawa M. Stabilization of atherosclerotic plaques: new mechanisms and clinical targets. Nat Med. 2002;8:1257–1262. doi: 10.1038/nm1102-1257. [DOI] [PubMed] [Google Scholar]
- 84.Jaffer FA, Libby P, Weissleder R. Molecular and cellular imaging of atherosclerosis: emerging applications. J Am Coll Cardiol. 2006;47:1328–1338. doi: 10.1016/j.jacc.2006.01.029. [DOI] [PubMed] [Google Scholar]
- 85.Ni M, Chen WQ, Zhang Y. Animal models and potential mechanisms of plaque destabilisation and disruption. Heart. 2009;95:1393–1398. doi: 10.1136/hrt.2008.143461. [DOI] [PubMed] [Google Scholar]
- 86.Nahrendorf M, Waterman P, Thurber G, Groves K, Rajopadhye M, Panizzi P, Marinelli B, Aikawa E, Pittet MJ, Swirski FK, et al. Hybrid in vivo FMT-CT imaging of protease activity in atherosclerosis with customized nanosensors. Arterioscler Thromb Vasc Biol. 2009;29:1444–1451. doi: 10.1161/ATVBAHA.109.193086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ma LL, Feldman MD, Tam JM, Paranjape AS, Cheruku KK, Larson TA, Tam JO, Ingram DR, Paramita V, Villard JW, et al. Small multifunctional nanoclusters (nanoroses) for targeted cellular imaging and therapy. ACS Nano. 2009;3:2686–2696. doi: 10.1021/nn900440e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kang HW, Josephson L, Petrovsky A, Weissleder R, Bogdanov A., Jr Magnetic resonance imaging of inducible E-selectin expression in human endothelial cell culture. Bioconjug Chem. 2002;13:122–127. doi: 10.1021/bc0155521. [DOI] [PubMed] [Google Scholar]
- 89.Kang HW, Torres D, Wald L, Weissleder R, Bogdanov AA., Jr Targeted imaging of human endothelial-specific marker in a model of adoptive cell transfer. Lab Invest. 2006;86:599–609. doi: 10.1038/labinvest.3700421. [DOI] [PubMed] [Google Scholar]
- 90.Flacke S, Fischer S, Scott MJ, Fuhrhop RJ, Allen JS, McLean M, Winter P, Sicard GA, Gaffney PJ, Wickline SA, et al. Novel MRI contrast agent for molecular imaging of fibrin: implications for detecting vulnerable plaques. Circulation. 2001;104:1280–1285. doi: 10.1161/hc3601.094303. [DOI] [PubMed] [Google Scholar]
- 91.Peters D, Kastantin M, Kotamraju VR, Karmali PP, Gujraty K, Tirrell M, Ruoslahti E. Targeting atherosclerosis by using modular, multifunctional micelles. Proc Natl Acad Sci U S A. 2009;106:9815–9819. doi: 10.1073/pnas.0903369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cormode DP, Briley-Saebo KC, Mulder WJ, Aguinaldo JG, Barazza A, Ma Y, Fisher EA, Fayad ZA. An ApoA-I mimetic peptide high-density-lipoprotein-based MRI contrast agent for atherosclerotic plaque composition detection. Small. 2008;4:1437–1444. doi: 10.1002/smll.200701285. [DOI] [PubMed] [Google Scholar]
- 93.Cormode DP, Roessl E, Thran A, Skajaa T, Gordon RE, Schlomka JP, Fuster V, Fisher EA, Mulder WJ, Proksa R, et al. Atherosclerotic plaque composition: analysis with multicolor CT and targeted gold nanoparticles. Radiology. 2010;256:774–782. doi: 10.1148/radiol.10092473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Frias JC, Williams KJ, Fisher EA, Fayad ZA. Recombinant HDL-like nanoparticles: a specific contrast agent for MRI of atherosclerotic plaques. J Am Chem Soc. 2004;126:16316–16317. doi: 10.1021/ja044911a. [DOI] [PubMed] [Google Scholar]
- 95.Cormode DP, Skajaa T, van Schooneveld MM, Koole R, Jarzyna P, Lobatto ME, Calcagno C, Barazza A, Gordon RE, Zanzonico P, et al. Nanocrystal core high-density lipoproteins: a multimodality contrast agent platform. Nano Lett. 2008;8:3715–3723. doi: 10.1021/nl801958b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tang TY, Howarth SP, Miller SR, Graves MJ, Patterson AJ, UK-I JM, Li ZY, Walsh SR, Brown AP, Kirkpatrick PJ, et al. The ATHEROMA (Atorvastatin Therapy: Effects on Reduction of Macrophage Activity) Study. Evaluation using ultrasmall superpara-magnetic iron oxide-enhanced magnetic resonance imaging in carotid disease. J Am Coll Cardiol. 2009;53:2039–2050. doi: 10.1016/j.jacc.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 97.Morishige K, Kacher DF, Libby P, Josephson L, Ganz P, Weissleder R, Aikawa M. High-resolution magnetic resonance imaging enhanced with superpara-magnetic nanoparticles measures macrophage burden in atherosclerosis. Circulation. 2010;122:1707–1715. doi: 10.1161/CIRCULATIONAHA.109.891804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang B, Yantsen E, Larson T, Karpiouk AB, Sethuraman S, Su JL, Sokolov K, Emelianov SY. Plasmonic intravascular photoacoustic imaging for detection of macrophages in atherosclerotic plaques. Nano Lett. 2009;9:2212–2217. doi: 10.1021/nl801852e. [DOI] [PubMed] [Google Scholar]
- 99.Hyafil F, Cornily JC, Feig JE, Gordon R, Vucic E, Amirbekian V, Fisher EA, Fuster V, Feldman LJ, Fayad ZA. Noninvasive detection of macrophages using a nanoparticulate contrast agent for computed tomography. Nat Med. 2007;13:636–641. doi: 10.1038/nm1571. [DOI] [PubMed] [Google Scholar]
- 100.Hyafil F, Cornily JC, Rudd JH, Machac J, Feldman LJ, Fayad ZA. Quantification of inflammation within rabbit atherosclerotic plaques using the macrophage-specific CT contrast agent N1177: a comparison with 18F-FDG PET/CT and histology. J Nucl Med. 2009;50:959–965. doi: 10.2967/jnumed.108.060749. [DOI] [PubMed] [Google Scholar]
- 101.Harris TJ, von Maltzahn G, Lord ME, Park JH, Agrawal A, Min DH, Sailor MJ, Bhatia SN. Protease-triggered unveiling of bioactive nanoparticles. Small. 2008;4:1307–1312. doi: 10.1002/smll.200701319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schellenberger E, Rudloff F, Warmuth C, Taupitz M, Hamm B, Schnorr J. Protease-specific nanosensors for magnetic resonance imaging. Bioconjug Chem. 2008;19:2440–2445. doi: 10.1021/bc800330k. [DOI] [PubMed] [Google Scholar]
- 103.Briley-Saebo KC, Shaw PX, Mulder WJ, Choi SH, Vucic E, Aguinaldo JG, Witztum JL, Fuster V, Tsimikas S, Fayad ZA. Targeted molecular probes for imaging atherosclerotic lesions with magnetic resonance using antibodies that recognize oxidation-specific epitopes. Circulation. 2008;117:3206–3215. doi: 10.1161/CIRCULATIONAHA.107.757120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Briley-Saebo KC, Cho YS, Shaw PX, Ryu SK, Mani V, Dickson S, Izadmehr E, Green S, Fayad ZA, Tsimikas S. Targeted iron oxide particles for in vivo magnetic resonance detection of atherosclerotic lesions with antibodies directed to oxidation-specific epitopes. J Am Coll Cardiol. 2011;57:337–347. doi: 10.1016/j.jacc.2010.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Tilborg GA, Vucic E, Strijkers GJ, Cormode DP, Mani V, Skajaa T, Reutelingsperger CP, Fayad ZA, Mulder WJ, Nicolay K. Annexin A5-functionalized bimodal nanoparticles for MRI and fluorescence imaging of atherosclerotic plaques. Bioconjug Chem. 2010;21:1794–1803. doi: 10.1021/bc100091q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lee D, Khaja S, Velasquez-Castano JC, Dasari M, Sun C, Petros J, Taylor WR, Murthy N. In vivo imaging of hydrogen peroxide with chemiluminescent nanoparticles. Nat Mater. 2007;6:765–769. doi: 10.1038/nmat1983. [DOI] [PubMed] [Google Scholar]
- 107.Lipinski MJ, Amirbekian V, Frias JC, Aguinaldo JG, Mani V, Briley-Saebo KC, Fuster V, Fallon JT, Fisher EA, Fayad ZA. MRI to detect atherosclerosis with gadolinium-containing immunomicelles targeting the macrophage scavenger receptor. Magn Reson Med. 2006;56:601–610. doi: 10.1002/mrm.20995. [DOI] [PubMed] [Google Scholar]
- 108.Amirbekian V, Lipinski MJ, Briley-Saebo KC, Amirbekian S, Aguinaldo JG, Weinreb DB, Vucic E, Frias JC, Hyafil F, Mani V, et al. Detecting and assessing macrophages in vivo to evaluate atherosclerosis non-invasively using molecular MRI. Proc Natl Acad Sci USA. 2007;104:961–966. doi: 10.1073/pnas.0606281104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nahrendorf M, Jaffer FA, Kelly KA, Sosnovik DE, Aikawa E, Libby P, Weissleder R. Noninvasive vascular cell adhesion molecule-1 imaging identifies inflammatory activation of cells in atherosclerosis. Circulation. 2006;114:1504–1511. doi: 10.1161/CIRCULATIONAHA.106.646380. [DOI] [PubMed] [Google Scholar]
- 110.Tsourkas A, Shinde-Patil VR, Kelly KA, Patel P, Wolley A, Allport JR, Weissleder R. In vivo imaging of activated endothelium using an anti-VCAM-1 magne-tooptical probe. Bioconjug Chem. 2005;16:576–581. doi: 10.1021/bc050002e. [DOI] [PubMed] [Google Scholar]
- 111.Kelly KA, Allport JR, Tsourkas A, Shinde-Patil VR, Josephson L, Weissleder R. Detection of vascular adhesion molecule-1 expression using a novel multimodal nanoparticle. Circ Res. 2005;96:327–336. doi: 10.1161/01.RES.0000155722.17881.dd. [DOI] [PubMed] [Google Scholar]
- 112.Winter PM, Morawski AM, Caruthers SD, Fuhrhop RW, Zhang H, Williams TA, Allen JS, Lacy EK, Robertson JD, Lanza GM, et al. Molecular imaging of angiogenesis in early-stage atherosclerosis with α(v)β3-integrin-targeted nanoparticles. Circulation. 2003;108:2270–2274. doi: 10.1161/01.CIR.0000093185.16083.95. [DOI] [PubMed] [Google Scholar]
- 113.Tomokiyo R, Jinnouchi K, Honda M, Wada Y, Hanada N, Hiraoka T, Suzuki H, Kodama T, Takahashi K, Takeya M. Production, characterization, and interspecies reactivities of monoclonal antibodies against human class A macrophage scavenger receptors. Atherosclerosis. 2002;161:123–132. doi: 10.1016/s0021-9150(01)00624-4. [DOI] [PubMed] [Google Scholar]
- 114.Guray U, Erbay AR, Guray Y, Yilmaz MB, Boyaci AA, Sasmaz H, Korkmaz S, Kutuk E. Levels of soluble adhesion molecules in various clinical presentations of coronaryatherosclerosis. Int J Cardiol. 2004;96:235–240. doi: 10.1016/j.ijcard.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 115.de Boer OJ, van der Wal AC, Teeling P, Becker AE. Leucocyte recruitment in rupture prone regions of lipid-rich plaques: a prominent role for neovascularization? Cardiovasc Res. 1999;41:443–449. doi: 10.1016/s0008-6363(98)00255-7. [DOI] [PubMed] [Google Scholar]
- 116.Brown MS, Goldstein JL. Lipoprotein metabolism in the macrophage: implications for cholesterol deposition in atherosclerosis. Annu Rev Biochem. 1983;52:223–261. doi: 10.1146/annurev.bi.52.070183.001255. [DOI] [PubMed] [Google Scholar]
- 117.Nagaoka K, Takahara K, Tanaka K, Yoshida H, Steinman RM, Saitoh S, Akashi-Takamura S, Miyake K, Kang YS, Park CG, et al. Association of SIGNR1 with TLR4-MD-2 enhances signal transduction by recognition of LPS in gram-negative bacteria. Int Immunol. 2005;17:827–836. doi: 10.1093/intimm/dxh264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Mulder WJ, Strijkers GJ, Briley-Saboe KC, Frias JC, Aguinaldo JG, Vucic E, Amirbekian V, Tang C, Chin PT, Nicolay K, et al. Molecular imaging of macrophages in atherosclerotic plaques using bimodal PEG-micelles. Magn Reson Med. 2007;58:1164–1170. doi: 10.1002/mrm.21315. [DOI] [PubMed] [Google Scholar]
- 119.Tsimikas S, Mallat Z, Talmud PJ, Kastelein JJP, Wareham NJ, Sandhu MS, Miller ER, Benessiano J, Tedgui A, Witztum JL, et al. Oxidation-Specific Biomarkers, Lipoprotein(a), and Risk of Fatal and Nonfatal Coronary Events. J Am Coll Cardiol. 2010;56:946–955. doi: 10.1016/j.jacc.2010.04.048. [DOI] [PubMed] [Google Scholar]
- 120.Woo K, Hong J, Choi S, Lee HW, Ahn JP, Kim CS, Lee SW. Easy synthesis and magnetic properties of iron oxide nanoparticles. Chem Mater. 2004;16:2814–2818. [Google Scholar]
- 121.Dubertret B, Skourides P, Norris DJ, Noireaux V, Brivanlou AH, Libchaber A. In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science. 2002;298:1759–1762. doi: 10.1126/science.1077194. [DOI] [PubMed] [Google Scholar]
- 122.Park J, Joo J, Kwon SG, Jang Y, Hyeon T. Synthesis of monodisperse spherical nanocrystals. Angew Chem Int Ed. 2007;46:4630–4660. doi: 10.1002/anie.200603148. [DOI] [PubMed] [Google Scholar]
- 123.Kelly KA, Nahrendorf M, Yu AM, Reynolds F, Weissleder R. In vivo phage display selection yields atherosclerotic plaque targeted peptides for imaging. Mol Imaging Biol. 2006;8:201–207. doi: 10.1007/s11307-006-0043-6. [DOI] [PubMed] [Google Scholar]
- 124.Paranjape AS, Kuranov R, Baranov S, Ma LL, Villard JW, Wang T, Sokolov KV, Feldman MD, Johnston KP, Milner TE. Depth resolved photothermal OCT detection of macrophages in tissue using nanorose. Biomed Opt Exp. 2010;1:2–16. doi: 10.1364/BOE.1.000002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yu SS, Scherer RL, Ortega RA, Bell CS, O’Neil CP, Hubbell JA, Giorgio TD. Enzymatic- and temperature-sensitive controlled release of ultrasmall superpara-magnetic iron oxides (USPIOs) J Nanobiotechnol. 2011;9:7. doi: 10.1186/1477-3155-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Beumer JH, Boisdron-Celle M, Clarke W, Courtney JB, Egorin MJ, Gamelin E, Harney RL, Hammett-Stabler C, Lepp S, Li Y, et al. Multicenter evaluation of a novel nanoparticle immunoassay for 5-fluorouracil on the Olympus AU400 analyzer. Ther Drug Monit. 2009;31:688–694. doi: 10.1519/JSC.0b013e3181b866d0. [DOI] [PubMed] [Google Scholar]
- 127.Jaffer FA, Kim DE, Quinti L, Tung CH, Aikawa E, Pande AN, Kohler RH, Shi GP, Libby P, Weissleder R. Optical visualization of cathepsin K activity in atherosclerosis with a novel, protease-activatable fluorescence sensor. Circulation. 2007;115:2292–2298. doi: 10.1161/CIRCULATIONAHA.106.660340. [DOI] [PubMed] [Google Scholar]
- 128.Sukhova GK, Shi GP, Simon DI, Chapman HA, Libby P. Expression of the elastolytic cathepsins S and K in human atheroma and regulation of their production in smooth muscle cells. J Clin Invest. 1998;102:576–583. doi: 10.1172/JCI181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rodgers KJ, Watkins DJ, Miller AL, Chan PY, Karanam S, Brissette WH, Long CJ, Jackson CL. Destabilizing role of cathepsin S in murine atherosclerotic plaques. Arterioscler Thromb Vasc Biol. 2006;26:851–856. doi: 10.1161/01.ATV.0000203526.75772.4b. [DOI] [PubMed] [Google Scholar]
- 130.Li W, Yuan XM. Increased expression and translocation of lysosomal cathepsins contribute to macrophage apoptosis in atherogenesis. Ann N Y Acad Sci. 2004;1030:427–433. doi: 10.1196/annals.1329.053. [DOI] [PubMed] [Google Scholar]
- 131.Tung CH, Bredow S, Mahmood U, Weissleder R. Preparation of a cathepsin D sensitive near-infrared fluorescence probe for imaging. Bioconjug Chem. 1999;10:892–896. doi: 10.1021/bc990052h. [DOI] [PubMed] [Google Scholar]
- 132.Lakowicz JR. Principles of Fluorescence Spectroscopy. 3rd. New York: Springer; 2006. [Google Scholar]
- 133.Deguchi JO, Aikawa M, Tung CH, Aikawa E, Kim DE, Ntziachristos V, Weissleder R, Libby P. Inflammation in atherosclerosis: visualizing matrix metallo-proteinase action in macrophages in vivo. Circulation. 2006;114:55–62. doi: 10.1161/CIRCULATIONAHA.106.619056. [DOI] [PubMed] [Google Scholar]
- 134.Chen J, Tung CH, Mahmood U, Ntziachristos V, Gyurko R, Fishman MC, Huang PL, Weissleder R. In vivo imaging of proteolytic activity in atherosclerosis. Circulation. 2002;105:2766–2771. doi: 10.1161/01.cir.0000017860.20619.23. [DOI] [PubMed] [Google Scholar]
- 135.Altinoglu EI, Adair JH. Near infrared imaging with nanoparticles. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:461–477. doi: 10.1002/wnan.77. [DOI] [PubMed] [Google Scholar]
- 136.Chang E, Miller JS, Sun J, Yu WW, Colvin VL, Drezek R, West JL. Protease-activated quantum dot probes. Biochem Biophys Res Commun. 2005;334:1317–1321. doi: 10.1016/j.bbrc.2005.07.028. [DOI] [PubMed] [Google Scholar]
- 137.Sun J, Fu K, Zhu M-Q, Bickford L, Post E, Drezek R. Near-infrared quantum dot contrast agents for fluorescence tissue imaging: a phantom study. Curr Nanosci. 2009;5:160–166. [Google Scholar]
- 138.Smith AM, Nie S. Bright and compact alloyed quantum dots with broadly tunable near-infrared absorption and fluorescence spectra through mercury cation exchange. J Am Chem Soc. 2010;133:24–26. doi: 10.1021/ja108482a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma Q, Su X. Near-infrared quantum dots: synthesis, functionalization and analytical applications. Analyst. 2010;135:1867–1877. doi: 10.1039/c0an00233j. [DOI] [PubMed] [Google Scholar]
- 140.Kim S, Lim YT, Soltesz EG, De Grand AM, Lee J, Nakayama A, Parker JA, Mihaljevic T, Laurence RG, Dor DM, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–97. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Bailey RE, Strausburg JB, Nie S. A new class of far-red and near-infrared biological labels based on alloyed semiconductor quantum dots. J Nanosci Nanotechnol. 2004;4:569–574. doi: 10.1166/jnn.2004.004. [DOI] [PubMed] [Google Scholar]
- 142.Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WC. In vivo quantum-dot toxicity assessment. Small. 2010;6:138–144. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- 143.Chang E, Thekkek N, Yu WW, Colvin VL, Drezek R. Evaluation of quantum dot cytotoxicity based on intra-cellular uptake. Small. 2006;2:1412–1417. doi: 10.1002/smll.200600218. [DOI] [PubMed] [Google Scholar]
- 144.Sewell SL, Giorgio TD. Synthesis and enzymatic cleavage of dual-ligand quantum dots. Mater Sci Eng C Mater Biol Appl. 2009;29:1428–1432. [Google Scholar]
- 145.Smith R, Sewell SL, Giorgio TD. Proximity-activated nanoparticles: in vitro performance of specific structural modification by enzymatic cleavage. Int J Nanomed. 2008;3:95–103. doi: 10.2147/ijn.s2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sewell SL, Giorgio TD. Surface functionalized nano-particles for proximity-activated detection and imaging of breast cancer. Cancer Res. 2009;69:348s. [Google Scholar]
- 147.Guyton AC, Hall JE. Textbook of Medical Physiology. 11th. Philadelphia: Elsevier Saunders; 2006. [Google Scholar]
- 148.Brannon-Peppas L, Blanchette JO. Nanoparticle and targeted systems for cancer therapy. Adv Drug Deliv Rev. 2004;56:1649–1659. doi: 10.1016/j.addr.2004.02.014. [DOI] [PubMed] [Google Scholar]
- 149.Storm G, Belliot SO, Daemen T, Lasic DD. Surface modification of nanoparticles to oppose uptake by the mononuclear phagocyte system. Adv Drug Delivery Rev. 1995;17:31–48. [Google Scholar]
- 150.Li SD, Huang L. Nanoparticles evading the reticuloendothelial system: role of the supported bilayer. Biochim Biophys Acta. 2009;1788:2259–2266. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Kirkland-York S, Zhang Y, Smith AE, York AW, Huang F, McCormick CL. Tailored design of Au nanoparticle-siRNA carriers utilizing reversible addition-fragmentation chain transfer polymers. Biomacromolecules. 2010;11:1052–1059. doi: 10.1021/bm100020x. [DOI] [PubMed] [Google Scholar]
- 152.Wickline SA, Neubauer AM, Winter PM, Caruthers SD, Lanza GM. Molecular imaging and therapy of atherosclerosis with targeted nanoparticles. J Magn Reson Imaging. 2007;25:667–680. doi: 10.1002/jmri.20866. [DOI] [PubMed] [Google Scholar]
- 153.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Contr Rel. 2000;65:271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 154.Davis ME, Zuckerman JE, Choi CH, Seligson D, Tolcher A, Alabi CA, Yen Y, Heidel JD, Ribas A. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature. 2010;464:1067–1070. doi: 10.1038/nature08956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Weissleder R, Kelly K, Sun EY, Shtatland T, Josephson L. Cell-specific targeting of nanoparticles by multi-valent attachment of small molecules. Nat Biotechnol. 2005;23:1418–1423. doi: 10.1038/nbt1159. [DOI] [PubMed] [Google Scholar]
- 156.Senpan A, Caruthers SD, Rhee I, Mauro NA, Pan D, Hu G, Scott MJ, Fuhrhop RW, Gaffney PJ, Wickline SA, et al. Conquering the dark side: colloidal iron oxide nanoparticles. Acs Nano. 2009;3:3917–3926. doi: 10.1021/nn900819y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Erogbogbo F, Yong K-T, Roy I, Xu G, Prasad PN, Swihart MT. Biocompatible luminescent silicon quantum dots for imaging of cancer cells. Acs Nano. 2008;2:873–878. doi: 10.1021/nn700319z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.McIntyre JO, Scherer RL, Matrisian LM. Near-infrared optical proteolytic beacons for in vivo imaging of matrix metalloproteinase activity. Methods Mol Biol. 2010;622:279–304. doi: 10.1007/978-1-60327-299-5_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Scherer RL, VanSaun MN, McIntyre JO, Matrisian LM. Optical imaging of matrix metalloproteinase-7 activity in vivo using a proteolytic nanobeacon. Mol Imaging. 2008;7:118–131. [PMC free article] [PubMed] [Google Scholar]
- 160.Tsimikas S, Willerson JT, Ridker PM. C-reactive protein and other emerging blood biomarkers to optimize risk stratification of vulnerable patients. J Am Coll Cardiol. 2006;47:C19–C31. doi: 10.1016/j.jacc.2005.10.066. [DOI] [PubMed] [Google Scholar]
- 161.Vasan RS. Biomarkers of cardiovascular disease: molecular basis and practical considerations. Circulation. 2006;113:2335–2362. doi: 10.1161/CIRCULATIONAHA.104.482570. [DOI] [PubMed] [Google Scholar]
- 162.Hirsch LR, Halas NJ, West JL. Whole-blood immunoassay facilitated by gold nanoshell-conjugate antibodies. Methods Mol Biol. 2005;303:101–111. doi: 10.1385/1-59259-901-X:101. [DOI] [PubMed] [Google Scholar]
- 163.Soman CP, Giorgio TD. Quantum dot self-assembly for protein detection with sub-picomolar sensitivity. Langmuir. 2008;24:4399–4404. doi: 10.1021/la704078u. [DOI] [PubMed] [Google Scholar]
- 164.Kim KS, Park JK. Magnetic force-based multiplexed immunoassay using superparamagnetic nanoparticles in microfluidic channel. Lab Chip. 2005;5:657–664. doi: 10.1039/b502225h. [DOI] [PubMed] [Google Scholar]
- 165.Perez JM, Josephson L, O’Loughlin T, Hogemann D, Weissleder R. Magnetic relaxation switches capable of sensing molecular interactions. Nat Biotechnol. 2002;20:816–820. doi: 10.1038/nbt720. [DOI] [PubMed] [Google Scholar]
- 166.Hirsch LR, Jackson JB, Lee A, Halas NJ, West JL. A whole blood immunoassay using gold nanoshells. Anal Chem. 2003;75:2377–2381. doi: 10.1021/ac0262210. [DOI] [PubMed] [Google Scholar]
- 167.Blinka E, Loeffler K, Hu Y, Gopal A, Hoshino K, Lin K, Liu X, Ferrari M, Zhang JX. Enhanced micro-contact printing of proteins on nanoporous silica surface. Nanotechnology. 2010;21:415302. doi: 10.1088/0957-4484/21/41/415302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Agrawal A, Sathe T, Nie S. Single-bead immunoassays using magnetic microparticles and spectral-shifting quantum dots. J Agric Food Chem. 2007;55:3778–3782. doi: 10.1021/jf0635006. [DOI] [PubMed] [Google Scholar]
- 169.Bulte JWM, Modo MMJ, Perez JM, Kaittanis C. Magnetic nanosensors for probing molecular interactions. In: Ferrari M, editor. Nanoparticles in Biomedical Imaging. Vol. 102. New York: Springer; 2008. pp. 183–197. [Google Scholar]
- 170.Ibraimi F, Kriz D, Lu M, Hansson L-O, Kriz K. Rapid one-step whole blood C-reactive protein magnetic permeability immunoassay with monoclonal antibody conjugated nanoparticles as superparamagnetic labels and enhanced sedimentation. Anal Bioanal Chem. 2006;384:651–657. doi: 10.1007/s00216-005-0094-6. [DOI] [PubMed] [Google Scholar]
- 171.Ng AH, Uddayasankar U, Wheeler AR. Immunoassays in microfluidic systems. Anal Bioanal Chem. 2010;397:991–1007. doi: 10.1007/s00216-010-3678-8. [DOI] [PubMed] [Google Scholar]
- 172.Jokerst JV, Raamanathan A, Christodoulides N, Floriano PN, Pollard AA, Simmons GW, Wong J, Gage C, Furmaga WB, Redding SW, et al. Nano-bio-chips for high performance multiplexed protein detection: determinations of cancer biomarkers in serum and saliva using quantum dot bioconjugate labels. Biosens Bio-electron. 2009;24:3622–3629. doi: 10.1016/j.bios.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Sia SK, Whitesides GM. Microfluidic devices fabricated in poly(dimethylsiloxane) for biological studies. Electrophoresis. 2003;24:3563–3576. doi: 10.1002/elps.200305584. [DOI] [PubMed] [Google Scholar]
- 174.McClain MA, Culbertson CT, Jacobson SC, Ramsey JM. Flow cytometry of Escherichia coli on microfluidic devices. Anal Chem. 2001;73:5334–5338. doi: 10.1021/ac010504v. [DOI] [PubMed] [Google Scholar]
- 175.Wlodkowic D, Faley S, Zagnoni M, Wikswo JP, Cooper JM. Microfluidic single-cell array cytometry for the analysis of tumor apoptosis. Anal Chem. 2009;81:5517–5523. doi: 10.1021/ac9008463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Adams ML, Enzelberger M, Quake S, Scherer A. Microfluidic integration on detector arrays for absorption and fluorescence micro-spectrometers. Sensors Actuat A Phys. 2003;104:25–31. [Google Scholar]
- 177.Haun JB, Yoon TJ, Lee H, Weissleder R. Magnetic nanoparticle biosensors. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2010;2:291–304. doi: 10.1002/wnan.84. [DOI] [PubMed] [Google Scholar]
- 178.Lee H, Sun E, Ham D, Weissleder R. Chip-NMR biosensor for detection and molecular analysis of cells. Nat Med. 2008;14:869–874. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Chastek TQ, Beers KL, Amis EJ. Miniaturized dynamic light scattering instrumentation for use in microfluidic applications. Rev Sci Instrum. 2007;78:072201. doi: 10.1063/1.2755569. [DOI] [PubMed] [Google Scholar]
- 180.Chastek TQ, Iida K, Amis EJ, Fasolka MJ, Beers KL. A microfluidic platform for integrated synthesis and dynamic light scattering measurement of block copolymer micelles. Lab Chip. 2008;8:950–957. doi: 10.1039/b718235j. [DOI] [PubMed] [Google Scholar]
- 181.Soman C, Giorgio T. Sensitive and multiplexed detection of proteomic antigens via quantum dot aggregation. Nanomed: Nanotechnol Biol Med. 2009;5:402–409. doi: 10.1016/j.nano.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 182.Wyatt PJ. Light-Scattering and the absolute characterization of macromolecules. Anal Chim Acta. 1993;272:1–40. [Google Scholar]
- 183.Oden M, Mirabal Y, Epstein M, Richards-Kortum R. Engaging undergraduates to solve global health challenges: a new approach based on bioengineering design. Ann Biomed Eng. 2010;38:3031–3041. doi: 10.1007/s10439-010-0036-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Bruls DM, Evers TH, Kahlman JAH, van Lankvelt PJW, Ovsyanko M, Pelssers EGM, Schleipen JJHB, de Theije FK, Verschuren CA, van der Wijk T, et al. Rapid integrated biosensor for multiplexed immunoassays based on actuated magnetic nanoparticles. Lab Chip. 2009;9:3504–3510. doi: 10.1039/b913960e. [DOI] [PubMed] [Google Scholar]
- 185.Floriano PN, Christodoulides N, Miller CS, Ebersole JL, Spertus J, Rose BG, Kinane DF, Novak MJ, Steinhubl S, Acosta S, et al. Use of saliva-based nano-biochip tests for acute myocardial infarction at the point of care: a feasibility study. Clin Chem. 2009;55:1530–1538. doi: 10.1373/clinchem.2008.117713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Lavigne JJ, Savoy S, Clevenger MB, Ritchie JE, McDoniel B, Yoo S-J, Anslyn EV, McDevitt JT, Shear JB, Neikirk D. Solution-based analysis of multiple analytes by a sensor array: toward the development of an “electronic tongue”. J Am Chem Soc. 1998;120:6429–6430. [Google Scholar]
- 187.Buch M, Rishpon J. An electrochemical immunosensor for C-reactive protein based on multi-walled carbon nanotube-modified electrodes. Electroanalysis. 2008;20:2592–2594. [Google Scholar]
- 188.Wang J. Carbon-nanotube based electrochemical biosensors: a review. Electroanalysis. 2005;17:7–14. [Google Scholar]
- 189.Gruntzig AR, Senning A, Siegenthaler WE. Nonoperative dilatation of coronary-artery stenosis: percutaneous transluminal coronary angioplasty. N Engl J Med. 1979;301:61–68. doi: 10.1056/NEJM197907123010201. [DOI] [PubMed] [Google Scholar]
- 190.de Feyter PJ, de Jaegere PP, Serruys PW. Incidence, predictors, and management of acute coronary occlusion after coronary angioplasty. Am Heart J. 1994;127:643–651. doi: 10.1016/0002-8703(94)90675-0. [DOI] [PubMed] [Google Scholar]
- 191.Sigwart U, Urban P, Golf S, Kaufmann U, Imbert C, Fischer A, Kappenberger L. Emergency stenting for acute occlusion after coronary balloon angioplasty. Circulation. 1988;78:1121–1127. doi: 10.1161/01.cir.78.5.1121. [DOI] [PubMed] [Google Scholar]
- 192.Sigwart U, Puel J, Mirkovitch V, Joffre F, Kappen-berger L. Intravascular stents to prevent occlusion and restenosis after transluminal angioplasty. N Engl J Med. 1987;316:701–706. doi: 10.1056/NEJM198703193161201. [DOI] [PubMed] [Google Scholar]
- 193.Moliterno DJ. Healing achilles—sirolimus versus paclitaxel. N Engl J Med. 2005;353:724–727. doi: 10.1056/NEJMe058140. [DOI] [PubMed] [Google Scholar]
- 194.Karas SP, Gravanis MB, Santoian EC, Robinson KA, Anderberg KA, King SB., 3rd Coronary intimal proliferation after balloon injury and stenting in swine: an animal model of restenosis. J Am Coll Cardiol. 1992;20:467–474. doi: 10.1016/0735-1097(92)90119-8. [DOI] [PubMed] [Google Scholar]
- 195.Gordon PC, Gibson CM, Cohen DJ, Carrozza JP, Kuntz RE, Baim DS. Mechanisms of restenosis and redilation within coronary stents—quantitative angiographic assessment. J Am Coll Cardiol. 1993;21:1166–1174. doi: 10.1016/0735-1097(93)90241-r. [DOI] [PubMed] [Google Scholar]
- 196.Hoffmann R, Mintz GS, Dussaillant GR, Popma JJ, Pichard AD, Satler LF, Kent KM, Griffin J, Leon MB. Patterns and mechanisms of in-stent restenosis. a serial intravascular ultrasound study. Circulation. 1996;94:1247–1254. doi: 10.1161/01.cir.94.6.1247. [DOI] [PubMed] [Google Scholar]
- 197.Babapulle MN, Joseph L, Belisle P, Brophy JM, Eisenberg MJ. A hierarchical Bayesian meta-analysis of randomised clinical trials ofdrug-elutingstents. Lancet. 2004;364:583–591. doi: 10.1016/S0140-6736(04)16850-5. [DOI] [PubMed] [Google Scholar]
- 198.Stone GW, Ellis SG, Cox DA, Hermiller J, O’Shaughnessy C, Mann JT, Turco M, Caputo R, Bergin P, Greenberg J, et al. One-year clinical results with the slow-release, polymer-based, paclitaxel-eluting TAXUS stent: the TAXUS-IV trial. Circulation. 2004;109:1942–1947. doi: 10.1161/01.CIR.0000127110.49192.72. [DOI] [PubMed] [Google Scholar]
- 199.Moses JW, Leon MB, Popma JJ, Fitzgerald PJ, Holmes DR, O’Shaughnessy C, Caputo RP, Kereiakes DJ, Williams DO, Teirstein PS, et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- 200.Nordmann AJ, Briel M, Bucher HC. Mortality in randomized controlled trials comparing drug-eluting vs. bare metal stents in coronary artery disease: a meta-analysis. Eur Heart J. 2006;27:2784–2814. doi: 10.1093/eurheartj/ehl282. [DOI] [PubMed] [Google Scholar]
- 201.Camenzind E, Steg PG, Wijns W. Stent thrombosis late after implantation of first-generation drug-eluting stents: a cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. discussion 1455. [DOI] [PubMed] [Google Scholar]
- 202.Lagerqvist B, James SK, Stenestrand U, Lindback J, Nilsson T, Wallentin L. Long-term outcomes with drug-eluting stents versus bare-metal stents in Sweden. N Engl J Med. 2007;356:1009–1019. doi: 10.1056/NEJMoa067722. [DOI] [PubMed] [Google Scholar]
- 203.Kastrati A, Dibra A, Eberle S, Mehilli J, Suarez de Lezo J, Goy JJ, Ulm K, Schomig A. Sirolimus-eluting stents vs paclitaxel-eluting stents in patients with coronary artery disease: meta-analysis of randomized trials. JAMA. 2005;294:819–825. doi: 10.1001/jama.294.7.819. [DOI] [PubMed] [Google Scholar]
- 204.Niccoli G, Montone RA, Ferrante G, Crea F. The evolving role of inflammatory biomarkers in risk assessment after stent implantation. J Am Coll Cardiol. 2010;56:1783–1793. doi: 10.1016/j.jacc.2010.06.045. [DOI] [PubMed] [Google Scholar]
- 205.Kastrati A, Mehilli J, Dirschinger J, Dotzer F, Schuhlen H, Neumann FJ, Fleckenstein M, Pfafferott C, Seyfarth M, Schomig A. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO) trial. Circulation. 2001;103:2816–2821. doi: 10.1161/01.cir.103.23.2816. [DOI] [PubMed] [Google Scholar]
- 206.Pache J, Kastrati A, Mehilli J, Schuhlen H, Dotzer F, Hausleiter J, Fleckenstein M, Neumann FJ, Sattel-berger U, Schmitt C, et al. Intracoronary stenting and angiographic results: strut thickness effect on restenosis outcome (ISAR-STEREO-2) trial. J Am Coll Cardiol. 2003;41:1283–1288. doi: 10.1016/s0735-1097(03)00119-0. [DOI] [PubMed] [Google Scholar]
- 207.Kereiakes DJ, Cox DA, Hermiller JB, Midei MG, Bachinsky WB, Nukta ED, Leon MB, Fink S, Marin L, Lansky AJ. Usefulness of a cobalt chromium coronary stent alloy. Am J Cardiol. 2003;92:463–466. doi: 10.1016/s0002-9149(03)00669-6. [DOI] [PubMed] [Google Scholar]
- 208.Stone GW, Rizvi A, Newman W, Mastali K, Wang JC, Caputo R, Doostzadeh J, Cao S, Simonton CA, Sudhir K, et al. Everolimus-eluting versus paclitaxel-eluting stents in coronary artery disease. New Engl J Med. 2010;362:1663–1674. doi: 10.1056/NEJMoa0910496. [DOI] [PubMed] [Google Scholar]
- 209.Byrne RA, Kufner S, Tiroch K, Massberg S, Laugwitz KL, Birkmeier A, Schulz S, Mehilli J. Randomised trial of three rapamycin-eluting stents with different coating strategies for the reduction of coronary restenosis: 2-year follow-up results. Heart. 2009;95:1489–1494. doi: 10.1136/hrt.2009.172379. [DOI] [PubMed] [Google Scholar]
- 210.Byrne RA, Mehilli J, Iijima R, Schulz S, Pache J, Seyfarth M, Schomig A, Kastrati A. A polymer-free dual drug-eluting stent in patients with coronary artery disease: a randomized trial vs. polymer-based drug-eluting stents. Eur Heart J. 2009;30:923–931. doi: 10.1093/eurheartj/ehp044. [DOI] [PubMed] [Google Scholar]
- 211.Tamai H, Igaki K, Kyo E, Kosuga K, Kawashima A, Matsui S, Komori H, Tsuji T, Motohara S, Uehata H. Initial and 6-month results of biodegradable poly-l-lactic acid coronary stents in humans. Circulation. 2000;102:399–404. doi: 10.1161/01.cir.102.4.399. [DOI] [PubMed] [Google Scholar]
- 212.Tsuji T, Tamai H, Igaki K, Hsu Y-S, Kosuga KMO, Nakamura T, Fujita S. Four-year follow-up of the biodegradable stent (IGAKI-TAMAI Stent) Circ J. 2004;68:135. [Google Scholar]
- 213.Erbel R, Di Mario C, Bartunek J, Bonnier J, de Bruyne B, Eberli FR, Erne P, Haude M, Heublein B, Horrigan M, et al. Temporary scaffolding of coronary arteries with bioabsorbable magnesium stents: a prospective, non-randomised multicentre trial. Lancet. 2007;369:1869–1875. doi: 10.1016/S0140-6736(07)60853-8. [DOI] [PubMed] [Google Scholar]
- 214.Ormiston JA, Serruys PW, Regar E, Dudek D, Thuesen L, Webster MW, Onuma Y, Garcia-Garcia HM, McGreevy R, Veldhof S. A bioabsorbable everolimus-eluting coronary stent system for patients with single de-novo coronary artery lesions (ABSORB): a prospective open-label trial. Lancet. 2008;371:899–907. doi: 10.1016/S0140-6736(08)60415-8. [DOI] [PubMed] [Google Scholar]
- 215.Serruys PW, Ormiston JA, Onuma Y, Regar E, Gonzalo N, Garcia-Garcia HM, Nieman K, Bruining N, Dorange C, Miquel-Hebert K, et al. A bioabsorbable everolimus-eluting coronary stent system (ABSORB): 2-year outcomes and results from multiple imaging methods. Lancet. 2009;373:897–910. doi: 10.1016/S0140-6736(09)60325-1. [DOI] [PubMed] [Google Scholar]
- 216.Jiang W-W, Su S-H, Eberhart RC, Tang L. Phagocyte responses to degradable polymers. J Biomed Mater Res A. 2007;82A:492–497. doi: 10.1002/jbm.a.31175. [DOI] [PubMed] [Google Scholar]
- 217.Ramcharitar S, Gonzalo N, van Geuns RJ, Garcia-Garcia HM, Wykrzykowska JJ, Ligthart JMR, Regar E, Serruys PW. First case of stenting of a vulnerable plaque in the SECRITT I trial—the dawn of a new era? Nat Rev Cardiol. 2009;6:374–378. doi: 10.1038/nrcardio.2009.34. [DOI] [PubMed] [Google Scholar]
- 218.O’Brien B, Carroll W. The evolution of cardiovascular stent materials and surfaces in response to clinical drivers: A review. Acta Biomater. 2009;5:945–958. doi: 10.1016/j.actbio.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 219.Virmani R, Guagliumi G, Farb A, Musumeci G, Grieco N, Motta T, Mihalcsik L, Tespili M, Valsecchi O, Kolodgie FD. Localized hypersensitivity and late coronary thrombosis secondary to a sirolimus-eluting stent should we be cautious? Circulation. 2004;109:701–705. doi: 10.1161/01.CIR.0000116202.41966.D4. [DOI] [PubMed] [Google Scholar]
- 220.Kuchulakanti PK, Chu WW, Torguson R, Ohlmann P, Rha SW, Clavijo LC, Kim SW, Bui A, Gevorkian N, Xue ZY, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents. Circulation. 2006;113:1108–1113. doi: 10.1161/CIRCULATIONAHA.105.600155. [DOI] [PubMed] [Google Scholar]
- 221.Joner M, Finn AV, Farb A, Mont EK, Kolodgie FD, Ladich E, Kutys R, Skorija K, Gold HK, Virmani R. Pathology of drug-eluting stents in humans—delayed healing and late thrombotic risk. J Am Coll Cardiol. 2006;48:193–202. doi: 10.1016/j.jacc.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 222.Tada DB, Singh S, Nagesha D, Jost E, Levy CO, Gultepe E, Cormack R, Makrigiorgos GM, Sridhar S. Chitosan film containing Poly(D,L-Lactic-Co-glycolic acid) nanoparticles: a platform for localized dual-drug release. Pharma Res. 2010;27:1738–1745. doi: 10.1007/s11095-010-0176-9. [DOI] [PubMed] [Google Scholar]
- 223.Jayagopal A, Sussman EM, Shastri VP. Functionalized solid lipid nanoparticles for transendothelial delivery. IEEE Trans Nanobiosci. 2008;7:28–34. doi: 10.1109/TNB.2008.2000147. [DOI] [PubMed] [Google Scholar]
- 224.Jin C, Bai L, Wu H, Song W, Guo GZ, Dou KF. Cyto-toxicity of paclitaxel incorporated in PLGA nanoparticles on hypoxic human tumor cells. Pharma Res. 2009;26:1776–1784. doi: 10.1007/s11095-009-9889-z. [DOI] [PubMed] [Google Scholar]
- 225.Ranganath SH, Kee I, Krantz WB, Chow PKH, Wang CH. Hydrogel matrix entrapping PLGA-paclitaxel microspheres: drug delivery with near zero-order release and implantability advantages for malignant brain tumour chemotherapy. Pharma Res. 2009;26:2101–2114. doi: 10.1007/s11095-009-9922-2. [DOI] [PubMed] [Google Scholar]
- 226.Bhargava B, Reddy NK, Karthikeyan G, Raju R, Mishra S, Singh S, Waksman R, Virmani R, Somaraju B. A novel paclitaxel-eluting porous carboncarbon nanoparticle coated, nonpolymeric cobalt-chromium stent: evaluation in a porcine model. Cathet Cardiovasc Intervent. 2006;67:698–702. doi: 10.1002/ccd.20698. [DOI] [PubMed] [Google Scholar]
- 227.Kushwaha M, Anderson JM, Bosworth CA, Andukuri A, Minor WP, Lancaster JR, Anderson PG, Brott BC, Jun HW. A nitric oxide releasing, self assembled peptide amphiphile matrix that mimics native endothelium for coating implantable cardiovascular devices. Biomaterials. 2010;31:1502–1508. doi: 10.1016/j.biomaterials.2009.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Andukuri A, Minor WP, Kushwaha M, Anderson JM, Jun HW. Effect of endothelium mimicking self-assembled nanomatrices on cell adhesion and spreading of human endothelial cells and smooth muscle cells. Nanomed Nanotechnol Biol Med. 2010;6:289–297. doi: 10.1016/j.nano.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Decher G. Fuzzy nanoassemblies: toward layered polymeric multicomposites. Science. 1997;277:1232–1237. [Google Scholar]
- 230.Soike T, Streff AK, Guan CX, Ortega R, Tantawy M, Pino C, Shastri VP. Engineering a material surface for drug delivery and imaging using layer-by-layer assembly of functionalized nanoparticles. Adv Mater. 2010;22:1992. doi: 10.1002/adma.200903069. [DOI] [PubMed] [Google Scholar]
- 231.Weber N, Pesnell A, Bolikal D, Zeltinger J, Kohn J. Viscoelastic properties of fibrinogen adsorbed to the surface of biomaterials used in blood-contacting medical devices. Langmuir. 2007;23:3298–3304. doi: 10.1021/la060500r. [DOI] [PubMed] [Google Scholar]
- 232.Otsuka M, Tanimoto S, Sianos G, Kukreja N, Weustink AC, Serruys PW, De Feyter PJ. “Radio-lucent” and “radio-opaque” coronary stents characterized by multislice computed tomography. Int J Cardiol. 2009;132:E8–E10. doi: 10.1016/j.ijcard.2007.07.089. [DOI] [PubMed] [Google Scholar]
- 233.Luscher TF, Steffel J, Eberli FR, Joner M, Nakazawa G, Tanner FC, Virmani R. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- 234.Daemen J, Wenaweser P, Tsuchida K, Abrecht L, Vaina S, Morger C, Kukreja N, Juni P, Sianos G, Hellige G, et al. Early and late coronary stent thrombosis of sirolimus-eluting and paclitaxel-eluting stents in routine clinical practice: data from a large two-institutional cohort study. Lancet. 2007;369:667–678. doi: 10.1016/S0140-6736(07)60314-6. [DOI] [PubMed] [Google Scholar]
- 235.Peng L, Eltgroth ML, LaTempa TJ, Grimes CA, Desai TA. The effect of TiO2 nanotubes on endothelial function and smooth muscle proliferation. Biomaterials. 2009;30:1268–1272. doi: 10.1016/j.biomaterials.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 236.Lu J, Rao MP, MacDonald NC, Khang D, Webster TJ. Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 2008;4:192–201. doi: 10.1016/j.actbio.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 237.Miller DC, Thapa A, Haberstroh KM, Webster TJ. Endothelial and vascular smooth muscle cell function on poly(lactic-co-glycolic acid) with nanostructured surface features. Biomaterials. 2004;25:53–61. doi: 10.1016/s0142-9612(03)00471-x. [DOI] [PubMed] [Google Scholar]
- 238.Fine E, Zhang L, Fenniri H, Webster TJ. Enhanced endothelial cell functions on rosette nanotube-coated titanium vascular stents. Int J Nanomed. 2009;4:91–97. doi: 10.2147/ijn.s5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Kipshidze N, Dangas G, Tsapenko M, Moses J, Leon MB, Kutryk M, Serruys P. Role of the endothelium in modulating neointimal formation: vasculoprotective approaches to attenuate restenosis after percutaneous coronaryinterventions. J Am Coll Cardiol. 2004;44:733–739. doi: 10.1016/j.jacc.2004.04.048. [DOI] [PubMed] [Google Scholar]
- 240.Lu J, Khang D, Webster TJ. Greater endothelial cell responses on submicron and nanometer rough titanium surfaces. J Biomed Mater Res A. 2010;94A:1042–1049. doi: 10.1002/jbm.a.32778. [DOI] [PubMed] [Google Scholar]
- 241.Khang D, Kim SY, Liu-Snyder P, Palmore GTR, Durbin SM, Webster TJ. Enhanced fibronectin adsorption on carbon nanotube/poly(carbonate) urethane: independent role of surface nano-roughness and associated surface energy. Biomaterials. 2007;28:4756–4768. doi: 10.1016/j.biomaterials.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 242.Loya MC, Brammer KS, Choi C, Chen LH, Jin SH. Plasma-induced nanopillars on bare metal coronary stent surface for enhanced endothelialization. Acta Biomater. 2010;6:4589–4595. doi: 10.1016/j.actbio.2010.07.007. [DOI] [PubMed] [Google Scholar]
- 243.Brammer KS, Choi C, Oh S, Cobb CJ, Connelly LS, Loya M, Kong SD, Jin S. Antibiofouling, sustained antibiotic release by Si nanowire templates. Nano Lett. 2009;9:3570–3574. doi: 10.1021/nl901769m. [DOI] [PubMed] [Google Scholar]
- 244.Samaroo HD, Lu J, Webster TJ. Enhanced endothelial cell density on NiTi surfaces with sub-macron to nanometer roughness. Int J Nanomed. 2008;3:75–82. doi: 10.2147/ijn.s2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Wieneke H, Dirsch O, Sawitowski T, Gu YL, Brauer H, Dahmen U, Fischer A, Wnendt S, Erbel R. Synergistic effects of a novel nanoporous stent coating and tacrolimus on intima proliferation in rabbits. Cathet Cardiovasc Intervent. 2003;60:399–407. doi: 10.1002/ccd.10664. [DOI] [PubMed] [Google Scholar]
FURTHER READING
- Buxton DB. Current status of nanotechnology approaches for cardiovascular disease: a personal perspective. WIREs Nanomed Nanobiotechnol. 2009;1:149–155. doi: 10.1002/wnan.8. [DOI] [PubMed] [Google Scholar]
- Jarzyna PA, Gianella A, Skajaa T, Knudsen G, Deddens LH, Cormode DP, Fayad ZA, Mulder WJM. Multifunctional imaging nanoprobes. WIREs Nanomed Nanobiotechnol. 2010;2:138–150. doi: 10.1002/wnan.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puskas JE, Muñoz-Robledo LG, Hoerr RA, Foley J, Schmidt SP, Evancho-Chapman M, Dong J, Frethem C, Haugstad G. Drug eluting stent coatings. WIREs Nanomed Nanobiotechnol. 2009;1:451–462. doi: 10.1002/wnan.38. [DOI] [PubMed] [Google Scholar]


