Abstract
The objective is to investigate the influence of PM2.5 exposure on peripheral blood lymphocyte subsets in pregnant mice and the antagonism of quercetin on adverse effects induced by PM2.5 exposure. Pregnant mice were randomly divided into control group, PM2.5 model group and 3 quercetin intervention groups. Dams in all groups except the control group were exposed to PM2.5 suspension by intratracheal instillation on gestational day (GD) 3, 6, 9, 12 and 15. Meanwhile, each dam was given 0.15% carboxymethylcellulose sodium (CMCS) (control group & PM2.5 model group) and different doses of quercetin (quercetin intervention groups) by gavage once a day from GD0 to GD17. The percentage of lymphocyte subsets, Biomarkers of systemic inflammation injuries (IL-2, IL-6, IL-8 & TNF-α) and oxidative stress indicators (CAT, GSH & HO-1) in peripheral blood of the dams were analyzed. The number of T cells increased, accompanied by increased level of IL-2, IL-6, IL-8 and HO-1 due to PM2.5 exposure. Less CD4+ and CD8+ T cells were counted in 100 mg/kg quercetin intervention group, compared with PM2.5 model group. Quercetin may inhibit cytokine production, especially in IL-6 and IL-8 and may upgrade the level of HO-1. Our findings indicate that PM2.5 could significantly influence the distribution of T-lymphocyte subsets, activate inflammatory reaction and elevate oxidative stress level in peripheral blood of pregnant mice. Certain dose of quercetin administration during pregnancy may protect the dams against the adverse effects through various ways.
Keywords: PM2.5, pregnancy, quercetin, lymphocyte subset, inflammation, oxidative stress
1. Introduction
Fine particulate matter 2.5 (PM2.5, aerodynamic diameter ≤ 2.5 μm) is associated with diseases such as type 1 diabetes [1] and asthma [2]. It is also one of the leading risk factors for premature mortality [3]. As a carrier, PM2.5 may absorb various potentially harmful molecules such as organic molecules, transition metals, reactive gases, microbial components, and minerals [4]. In addition, the composition and concentration of PM2.5 mixture varies dramatically in different regions and seasons. For example, concentrations of its components, including chlorine, zinc, and bromide, have been found to be higher during winter.
Pregnancy is a complex, sophisticated physiological process. The potential impact of environmental stimuli on maternal immune function is directly related to fetal development [5]. Delius demonstrated that PM2.5 could influence macrophage activation state and the possible enhancement of T cell proliferation [6]. The research carried out by Herr et al. showed that the increase of CD3+ and CD4+ lymphocytes percentages in cord blood was significantly associated with PM2.5 exposure during early gestation, and PM2.5 in ambient air may influence fetal immune development via shifts in cord blood lymphocytes distributions [7].
It is reported that PM2.5 may cause many kinds of diseases, while the mechanisms still remain unclear. High oxidative stress [8] and inflammatory response [9], two biologically linked processes [10], are considered to be potential mechanisms based on previous studies. PM exposure is a known risk factor for the release of inflammatory factors [10,11] and the occurrence of systemic oxidative stress in humans [12,13]. Especially during the pregnancy period increased levels of oxidative stress can be expected [14]. One study showed that particulate air pollution exposure in early life plays a role in increasing systemic oxidative stress, at the level of the mitochondria, both in mother and fetus [15]. Many phytochemicals in foods have the extremely important function of anti-oxidation and anti-inflammation. Quercetin, a herbal flavonoid, may be derived from a variety of plants, such as apple, onion, tea, strawberries, and broccoli. It has been identified to possess various biological activities, such as anti-oxidation [16], antimicrobial activity [17], wound-healing [18], anti-cancer activity [19], and immune-modulatory activity [20]. Zhong et al. found that higher flavonoid intake could attenuate adverse cardiac autonomic effects induced by PM2.5 exposure in elderly men [21]. At the molecular level, quercetin inhibits oxidant stress through inducing heme oxygenase-1 (HO-1) and reduces oxidative damage via modulating the expression of antioxidant genes [22].
As far as we know now, few studies have investigated the changes of lymphocyte subsets during pregnancy with PM2.5 exposure, and even less attention has been paid to the antagonism function of quercetin against the adverse effects caused by PM2.5 on pregnant females. The objective of this study is to clarify the effect of quercetin intake on maternal immunity responses induced by PM2.5 exposure.
2. Materials and Methods
2.1. Preparation of PM2.5 and Chemicals
The samples of PM2.5 were collected by a particulate sampler (TH-150C, Wuhan Tianhong Instruments Co. Ltd., Wuhan, China) in residential area of Beijing, China, from 1 December 2014 to 20 February 2015. Filters were cut into 1–2 cm2 squares. The filter squares were agitated in ultrapure water with an ultrasonic shaker for 20 min 3 times. The solution was filtered through 8 layers of gauze and centrifuged at 12,000 rpm for 20 min. The sediment was collected by a vacuum freeze drier (FDU-1100, Tokyo Rikakikai Co. Ltd., Tokyo, Japan). The dry PM2.5 powder was diluted in sterile phosphate-buffered saline (PBS) (0.01 M, pH 7.4) at a concentration of 15 mg/mL and kept at −20 °C before experiments. An extra control sample from unexposed filters was processed identically. Morphology of PM2.5 particles was observed with a scanning electron microscope (SEM) (JSM-5600LV, Jeol Ltd., Tokyo, Japan).
Quercetin (Sigma products, purity ≥ 95.0%) was respectively dissolved in 0.15% CMCS at a concentration of 10, 20, and 40 mg/mL. IL-2, IL-6, IL-8, TNF-α, and HO-1 enzyme-linked immunosorbent assay (ELISA) kit were purchased from Freemore (Beijing, China). FITC-anti-CD3, PE/Cy7-anti-CD8, Brilliant Violet 421-anti-CD4, PE-anti-CD5, APC-anti-CD19, and red blood cell lysis buffer were purchased from BioLegend (San Diego, CA, USA).
2.2. Animals and Treatment
Specific pathogen-free (SPF) 8-week-old ICR mice were provided by the Department of Laboratory Animal Science of Peking University (Beijing, China, SCXK-2012-0001). The animals were quarantined for 7 days after shipping and were maintained in a temperature- and humidity-controlled animal facility with a 12-h/12-h light/dark cycle (lights on 7:00 a.m.). Mice were provided with basic mouse chow and distilled water ad libitum until pregnancy was confirmed. After the quarantine period, female mice were mated with healthy male mice overnight and were checked for vaginal plugs the next morning at 7:00 a.m. The presence of a vaginal plug signified Gestational Day 0 (GD 0).
The plug-positive females were randomly divided into five groups (ten dams/group): a control group (Group A), a PM2.5 model group (Group B), and three quercetin intervention groups (Groups C, D, and E). All dams were individually housed and provided with commercial pregnancy forage for mice and sterile distill water ad libitum until sacrificed. At 9:00–11:00 a.m. on GD 3, 6, 9, 12, and 15, dams were anesthetized with 3% isoflurane after body weight recording and received intratracheal instillation. When we conducted intratracheal instillation, the angle of body restraint we chose was 45 (supine head up), as reported in another paper [23]. Dams in Groups B, C, D, and E received PM2.5 samples (15.0 mg/kg), and dams in Group A were treated with same amount of suspension from extracts of unexposed filters. In addition, dams in Groups C, D, and E received 50 mg/kg, 100 mg/kg, or 200 mg/kg quercetin, respectively, at a volume of 0.005 mL/g per day via oral gavage from GD 0 to GD 17. Dams in Groups A and B received an oral gavage of 0.15% CMCS in the same period. Gavage was conducted at 2–4 p.m. every day. All dams were sacrificed by cervical dislocation on GD 18. The use of animals in this research was conducted in compliance with the Guidelines for Animal Research of Peking University (number of animal experimental ethical investigational tab: LA2015111).
The treatment of the dams are listed in Table 1.
Table 1.
Animal treatment.
| Group | Intervention | N | PM2.5 (mg/kg) | Quercetin (mg/kg) |
|---|---|---|---|---|
| A | Normal control | 10 | - | - |
| B | PM2.5 model control | 10 | 15 | - |
| C | low-dosage quercetin | 10 | 15 | quercetin (50) |
| D | middle-dosage quercetin | 10 | 15 | quercetin (100) |
| E | high-dosage quercetin | 10 | 15 | quercetin (200) |
Body weight and food consumption were recorded on GD 0, 3, 6, 9, 12, 15, and 18, and the food utilization rates were calculated from the following equation: food utilization rate = weight gain/food consumption × 100%.
2.3. Biochemical Analysis of the Maternal Serum
Blood samples were collected from the orbital sinus by removing eyeballs under deep anesthesia. After clotting at room temperature, the blood samples were centrifuged at 3000 rpm for 15 min, then the serum was transferred to new tubes and preserved at −80 °C until analysis. The contents of catalase (CAT) and glutathione (GSH) activity in serum were assayed with a commercial colorimetric assay kit (Nanjing Jiancheng Bioengineering Institute, Jiangsu, China). Interleukin 2 (IL-2), interleukin 6 (IL-6), interleukin 8 (IL-8), tumor necrosis factor α (TNF-α), and heme oxygenase 1 (HO-1) in serum were assayed by ELISA kits, respectively, according to the manufacturer’s instructions.
2.4. Organ Index and Lung Histology
After blood sampling, all dams were scarified by cervical dislocation. Spleen and thymus of each dam were separated immediately and we noted the weight after blood was wiped off. Organ indexes (Sx) of these two organs were calculated as Sx = weight of experimental organ (mg)/weight of experimental animal (g).
Lungs of each dam were separated immediately and inflated with 10% buffered formalin, fixed overnight, and embedded in paraffin, sectioned at 5 μm, and stained with hematoxylin and eosin (H&E). Lung samples were analyzed blinded to group assignments, and the assessment of histological lung injury was performed by grading, as Table 2 shows [24].
Table 2.
Histological lung injury score criteria.
| A | Pulmonary Inflammation Score Criteria |
| 0 | No lesions |
| 1 | Minimal lymphocytic inflammation restricted to perivascular and peribronchiolar regions |
| 2 | Moderate perivascular and peribronchiolar inflammation with mildly increased numbers of alveolar macrophages, lymphocytes, and eosinophils |
| 3 | Marked perivascular and peribronchiolar inflammation with moderately increased numbers of alveolar macrophages, lymphocytes, eosinophils, and multinucleated giant cells |
| 4 | Severe perivascular and peribronchiolar inflammation with markedly increased numbers of alveolar macrophages, eosinophils, lymphocytes and multinucleated giant cells |
| 5 | Severe perivascular and peribronchiolar inflammation with effacement of alveolar parenchyma and small airways by sheets of inflammatory cells |
| B | Interstitial Congestion and Hyaline Membrane Formation |
| 1 | Normal lung |
| 2 | Moderate (<25% of lung section) |
| 3 | Intermediate (25–50% of lung section) |
| 4 | Severe (>50% of lung section) |
| C | Hemorrhage |
| 0 | Absent |
| 1 | Present |
2.5. Flow Cytometric Analysis
Lymphocyte subgroups in peripheral blood were analyzed by a flow cytometer (Gallios, Beckman Coulter, Brea, CA, USA). The blood samples were placed in EDTA containing vacutainer tube before the dams were sacrificed. 50 μL of anticoagulated blood was mixed with anti-mouse mAbs (FITC CD3, PE/Cy7 CD8, Brilliant Violet 421 CD4, PE CD5, and APC CD19) and incubated for 20 min at room temperature in the dark. After first-stage incubation, 1 mL of red blood cell lysis buffer (containing ammonium chloride, potassium carbonate, and EDTA) was added to each blood tube, and was then incubated at room temperature and protected from light for 10 min. Then, the samples were centrifuged at 392× g at 25 °C for 5 min, and the supernatant was discarded. The samples were washed with 3 mL of cell staining buffer before being centrifuged at 392× g at 25 °C for 5 min, and the supernatant was discarded. The cells were resuspended in cell staining buffer and then analyzed on Gallios.
2.6. Statistical Analysis
Values were presented as the mean ± SD. The results were statistically analyzed using SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA). Intergroup differences were analyzed using one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) post-hoc test if equal variance existed, or Tamhane’s T2 post-hoc test if equal variance did not exist. p < 0.05 was regarded as statistically significant.
3. Results
3.1. Morphology of PM2.5 Particles
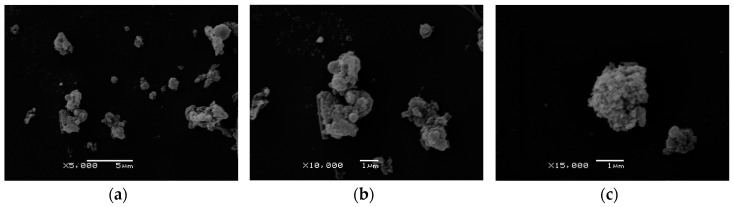
Figure 1 shows the typical SEM picture of PM2.5 suspension: the PM2.5 exhibited circular, elongated, and irregular shapes. Figure 1c shows the typical example of PM2.5 particles with rough surfaces.
Figure 1.
Morphology of PM2.5 particles. (a) Large area image of PM2.5 suspension (5000×); (b) Partial area image of PM2.5 suspension (10,000×); (c) SEM images of particles with rough surface. The scale bars are 5 μm for image (a), 1 μm for image (b), and 1 μm for image (c).
3.2. Body Weight and Food Utilization
A time-dependent increase in the body weight of the dams was observed in each group, without significant differences in the mean body weight among five groups. There was no significant difference in calculated food utilization rate among them either (p > 0.05) (Figure 2).
Figure 2.
Changes of body weight and food utilization rate of dams in different groups. (a) Changes of body weight; (b) Changes of food utilization rate. The food utilization rate was calculated from the following equation: food utilization rate = weight gain/food consumption × 100%. There was no significant difference in body weight and food utilization rate among the five groups (p > 0.05).
3.3. Histological Lung Injury Score
Overall lesion scores indicated that PM2.5 induced apparent pathology changes in the lung. As shown in Figure 3, obvious inflammatory cellular infiltration was observed in the lungs of dams in Groups B and C (p < 0.05). In Groups D and E, inflammatory cell soak was rare. In terms of interstitial congestion and hyaline membrane formation, injury scores in Groups B, D, and E were higher than those in Group A without significant difference (p > 0.05). However, Group C had significantly higher scores compared with Group A (p < 0.05). Hemorrhage occurred commonly in Groups B, C, D, and E (p < 0.05).
Figure 3.
Effects of quercetin on maternal lung structure and injury score with PM2.5 exposure during gestation. (a) Lung sections were stained with hematoxylin and eosin. Original magnification, ×200. Arrows indicate typical areas with inflammatory cell infiltrates. Circles indicate typical areas with interstitial congestion and hyaline membrane formation. Boxes indicate typical area with hemorrhage; (b) Slides were scored by two independent blinded observers for the severity evaluation of lung injury. Histology scores are displayed as mean ± SD. Compared with Group A, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001. The analysis of scores of interstitial congestion and hyaline membrane formation and hemorrhage were conducted with “Tamhane’s T2 test”.
3.4. Organ Coefficient of Spleen and Thymus
As shown in Table 3, there was no significant difference found in the spleen and thymus coefficient among five groups (p > 0.05) (Table 3).
Table 3.
The spleen and thymus indexes (mean ± SD).
| Group | Spleen Index (mg/g) | Thymus Index (mg/g) |
|---|---|---|
| A | 2.09 ± 0.40 | 1.01 ± 0.15 |
| B | 2.04 ± 0.49 | 1.09 ± 0.18 |
| C | 2.21 ± 0.54 | 1.15 ± 0.22 |
| D | 2.18 ± 0.24 | 1.02 ± 0.17 |
| E | 2.18 ± 0.45 | 1.16 ± 0.26 |
3.5. The Lymphocyte Subsets in Peripheral Blood
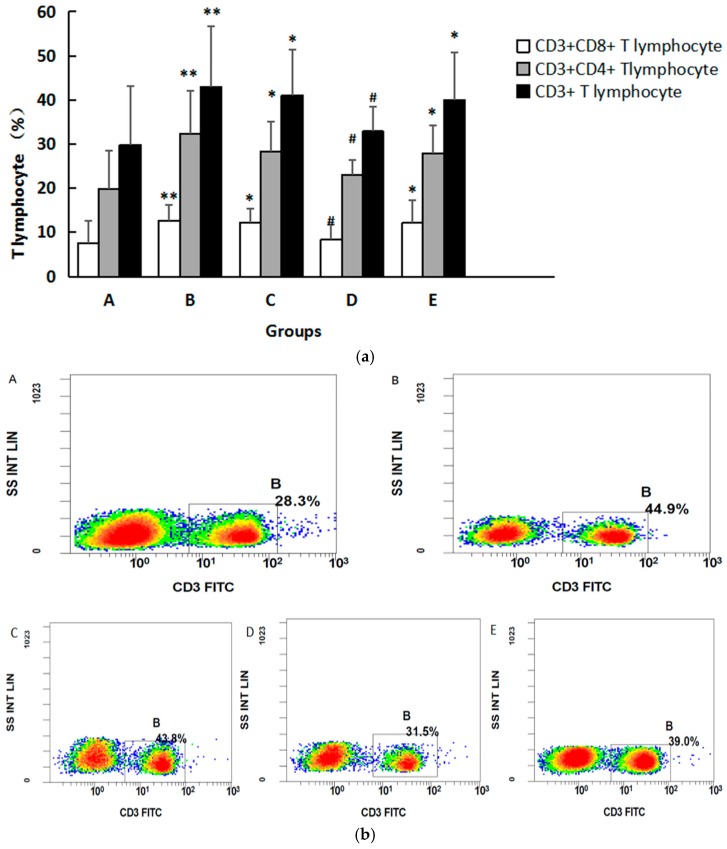
We found that the percentages of CD3+ cells in Groups B, C, and E were significantly higher than those in Group A (p < 0.05). A greater decrease was observed in Group D compared with Group B (p < 0.05). CD3+CD4+ cells in Groups B, C, and E were higher than those in Group A (p < 0.05), and compared with those in Group B there were fewer CD3+CD4+ in Group D (p < 0.05). The percentages of CD3+CD8+ cells in Groups B, C, and E were significantly higher than those in Group A (p < 0.05), and compared with Group B there were fewer CD3+CD8+ cells in Group D (p < 0.05), as shown in Figure 4a. Representative flow plots are shown in Figure 4b–d. There were no significant differences in CD19+, CD19+CD5+, CD19+CD5- B lymphocyte counts among five groups. (See Table 4).
Figure 4.
(a) Changes of T lymphocytes in serum of mice in different groups. The data are expressed as mean ± SD. Compared with Group A, * indicates p < 0.05, ** indicates p < 0.01. Compared with Group B, # indicates p < 0.05; (b) Representative flow plots showing CD3 staining in cells; (c) Representative flow plots showing CD3 and CD4 staining in cells; (d) Representative flow plots showing CD3 and CD8 staining in cells.
Table 4.
The maternal lymphocyte subsets in peripheral blood.
| Group | CD3+ | CD3+CD4+ | CD3+CD8+ | CD19+ | CD19+CD5− | CD19+CD5+ |
|---|---|---|---|---|---|---|
| A | 29.82 ± 13.38 | 19.91 ± 8.60 | 7.56 ± 5.13 | 28.56 ± 14.13 | 97.27 ± 1.71 | 2.73 ± 1.71 |
| B | 42.91 ± 13.70 ** | 32.29 ± 9.87 ** | 12.61 ± 3.62 ** | 31.81 ± 15.67 | 96.98 ± 1.34 | 3.02 ± 1.34 |
| C | 41.09 ± 10.32 * | 28.36 ± 6.70 * | 12.17 ± 3.14 * | 22.12 ± 7.06 | 97.68 ± 1.09 | 2.32 ± 1.09 |
| D | 32.86 ± 5.65 # | 23.03 ± 3.38 # | 8.28 ± 3.43 # | 26.24 ± 7.37 | 97.43 ± 0.57 | 2.58 ± 0.57 |
| E | 39.98 ± 10.71 * | 27.86 ± 6.48 * | 12.09 ± 5.19 * | 23.63 ± 11.55 | 97.49 ± 0.81 | 2.53 ± 0.83 |
Note: compared with Group A, * indicates p < 0.05, ** indicates p < 0.01; compared with Group B, # indicates p < 0.05. The analysis of CD19+ cell was conducted with “Tamhane’s T2 test”.
3.6. Serum Cytokines
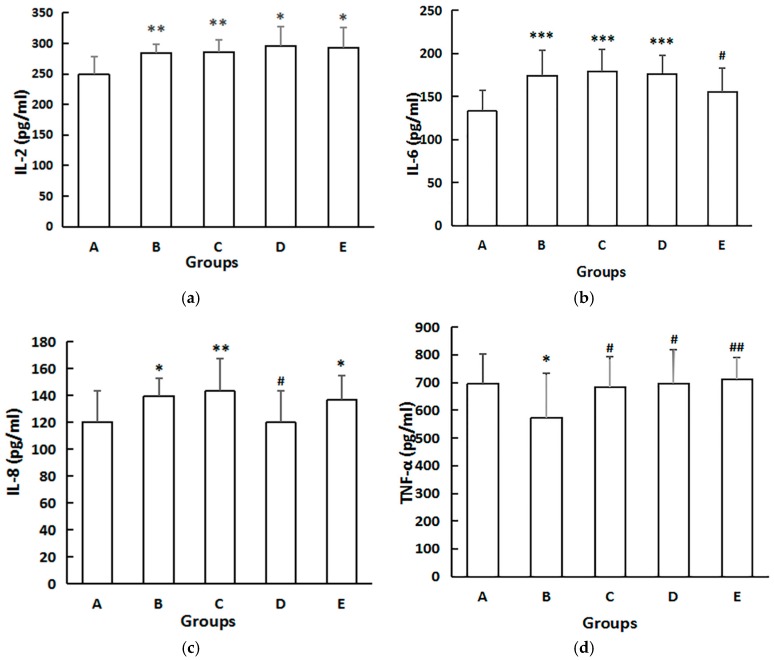
As shown in Figure 5, serum levels of IL-2, IL-6, and IL-8 in the dams were all significantly upregulated (p < 0.01) in Group B, compared with Group A. IL-6 level was lower in Group E than that in Group B. IL-8 levels were considerably lower in Group D compared with those in Group B (p < 0.05). Interestingly, TNF-α levels were considerably lower in Group B compared with those in Group A, and higher in Groups C, D, and E compared with those in Group B (p < 0.05) (Figure 4).
Figure 5.
The effect on dams serum concentrations of cytokines. (a) Effect on serum IL-2; (b) Effect on serum IL-6; (c) Effect on serum IL-8; (d) Effect on serum TNF-α. The serum levels of IL-2, IL-6, IL-8, and TNF-α in serum were detected by ELISA according to the manual of ELISA kits. The data are expressed as mean ± SD of each group. Compared with Group A, * indicates p < 0.05, ** indicates p < 0.01, *** indicates p < 0.001. Compared with Group B, # indicates p < 0.05, ## indicates p < 0.01. The analysis of IL-6 was conducted with “Tamhane’s T2 test”.
3.7. Biomarkers of Systemic Oxidative Injuries
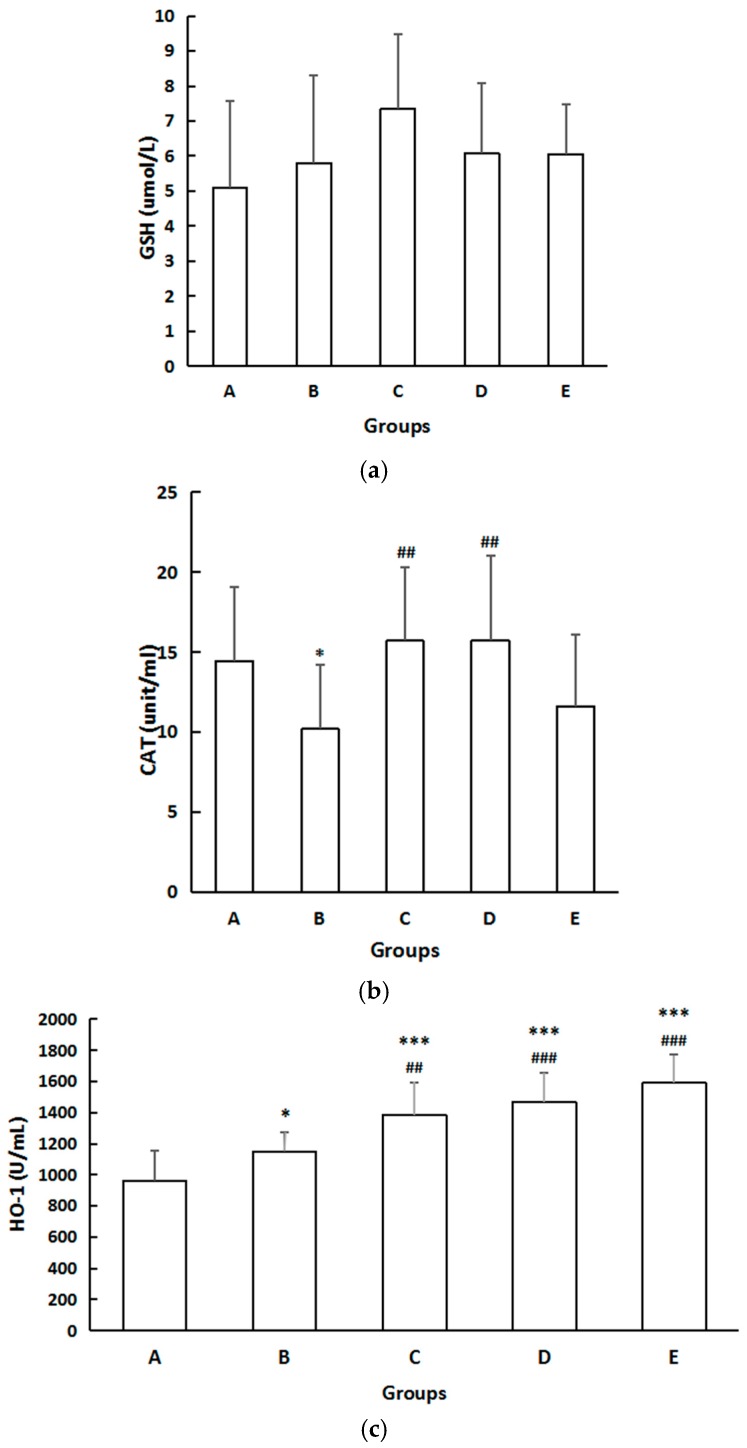
There were no differences in serum GSH among the five groups (Figure 6). However, the CAT level in Group B decreased significantly compared with that in Group A (p < 0.05). Compared with Group B, the serum CAT level increased considerably (p < 0.05) in Groups C and D (Figure 6). Concentration of HO-1 in Group B was higher than that of Group A (p < 0.05), and compared with Group B, serum HO-1 levels were considerably increased (p < 0.05) in Groups C, D, and E (Figure 6).
Figure 6.
(a) The effect on GSH. The data are expressed as mean ± SD of each group. There was no difference in serum GSH among five groups; (b) The effect on CAT. The data are expressed as mean ± SD of each group. Compared with Group A, * indicates p < 0.05. Compared with Group B, ## indicates p < 0.01; (c) The effect on concentration of HO-1. The data are expressed as mean ± SD of each group. Compared with Group A, * indicates p < 0.05, *** indicates p < 0.001. Compared with Group B, ## indicates p < 0.01, ### indicates p < 0.001.
4. Discussion
A growing body of epidemiological evidence indicates that ambient air pollution has adverse effects on pregnant women and fetal development [25,26]. PM2.5 could even attribute 3.2 million premature deaths per year, according to the survey conducted by Global Burden of Disease (GBD) [3]. It is widely known that chemical compositions of PM2.5 can remarkably influence toxicity. According to a previous study, in which PM2.5 collected in the same area, the PM2.5 exhibited high densities of O, Si, C, Fe, Ca, Mg, Al, K, and S [27]. Prior reports have suggested that inhaled particulate matter may potentiate innate immune function [6], while the mechanism of PM2.5 exposure during pregnancy served as a stimulus for serum T cell activation has not been well described. Maternal immune function changes could extend to lactation or even future, resulting in a long-term impact on health for both mother and her offspring [28].
We used animal models to investigate the influence of PM2.5 exposure on maternal immunity, oxidative stress, and inflammation indicators. The intratracheal instillation dosage of PM2.5 was determined on the basis of previous researches [13,29] and our pre-experiment. The results of our present research indicated that PM2.5 exposure during pregnancy had great impact on T-lymphocyte subsets proportion, serum cytokines, and biomarkers of systemic oxidative injuries in maternal peripheral blood.
The activity of T-lymphocyte subsets is an important indicator of immune homeostasis [30]. A report identified three critical phases of immune development during pregnancy: (1) Weeks 8–10: initiation of hematopoiesis; (2) Weeks 10–16: hematopoietic cell migration and progenitor cell expansion; (3) Week 16–birth: colonization of bone marrow and thymus [31]. On our study, dams were exposed to PM2.5 throughout pregnancy. Our research showed that PM2.5 exposure during pregnancy may increase the number of CD3+CD4+ and CD3+CD8+ T lymphocytes, breaking the original homeostasis and activating the immunology response. Elevated levels of serum IL-2, IL-6, and IL-8 were also observed in dams, which indicates the activation of severe systemic inflammatory reaction. Liu et al. have released a similar result that a significant increase of serum IL-6 was examined in dams, who were exposed to PM2.5 on Day 10 and Day 18 during gestation with the dose of 15 mg/kg [29]. The reason of why serum TNF-α in Group B was lower than that of Group A was still unclear, although Aztatzi-Aguilar et al. also observed that TNF-α level of kidney cortices was decreased in the PM2.5 intervention group [32]. Oxidative stress occurred in the dams with PM2.5 exposure, as a decreased level of CAT and an increased level of HO-1 were detected significantly. CAT is an anti-oxidant enzyme that converts hydrogen peroxide to water and oxygen and the decreased level of CAT means decreased anti-oxidant capacity. HO-1 is an enzyme that may catalyze the process of degrading heme to generate CO, biliverdin, and free iron [33], playing an important role in immunoregulation and oxidative stress defense [34,35]. The expression of HO-1 in response to oxidative stress suppresses the release of endogenous proinflammatory ligands from injured cells, thus further promoting the process of relieving inflammation and homeostasis reestablishment [35]. It should be noted that, in the PM2.5 group, there was increased activity of HO-1, which suggested a self-protection effect against oxidative damage.
It is infeasible to solve PM2.5 pollution thoroughly in a short period due to economic and social impact factors, so we hope to boost health against the injury caused by PM2.5 through diet intervention in our daily life. Supported by literature, oxidative damage is regarded as one of the mechanisms by which PM2.5 contributes to adverse effects on the human body, with the definite mechanisms remaining unclear so far. Quercetin, a common flavone widely found in fruits and vegetables, is a powerful antioxidant and free radical scavenger [36]. It can be acquired from a normal diet, whereas its content is not sufficient enough and the intake varies in different groups of people. Quercetin has been reported to show no maternal or fetal toxicity, even with a daily intake of 2000 mg/kg body weight during gestation in rats [37]. Referring to previous research [38], we determined the following three doses of quercetin: 50, 100, and 200 mg/kg.
Our results indicated that quercetin has a protective effect on lymphocyte subgroups, serum cytokines, and oxidative stress changes under PM2.5 exposure. Compared with the model group, the medium-dose quercetin group showed significantly lower percentage of CD3+, CD4+, and CD8+ T-lymphocyte subgroups, further proving that quercetin may improve the immune function in dams. Quercetin also has a pleasurable inhibitory effect on inflammation changes. In quercetin groups, serum levels of IL-6 and IL-8 were close to the control group. We assumed that the release of serum IL-6 and IL-8 were obviously suppressed by quercetin with its anti-inflammatory function. Previous studies have demonstrated that glycosylation of quercetin could enhance the early innate immunity effectively by activating macrophages to secrete TNF-α [20], while the mechanisms involved are largely undefined. This may explain why TNF-α level in quercetin groups were higher than other groups. Quercetin could adjust the oxidative stress state of the body through increasing serum concentration of CAT and HO-1 to reduce the injuries caused by PM2.5. Similarly, it has been reported that quercetin may upregulate HO-1 against endotoxic stress through the involvement of MAPKs [39].
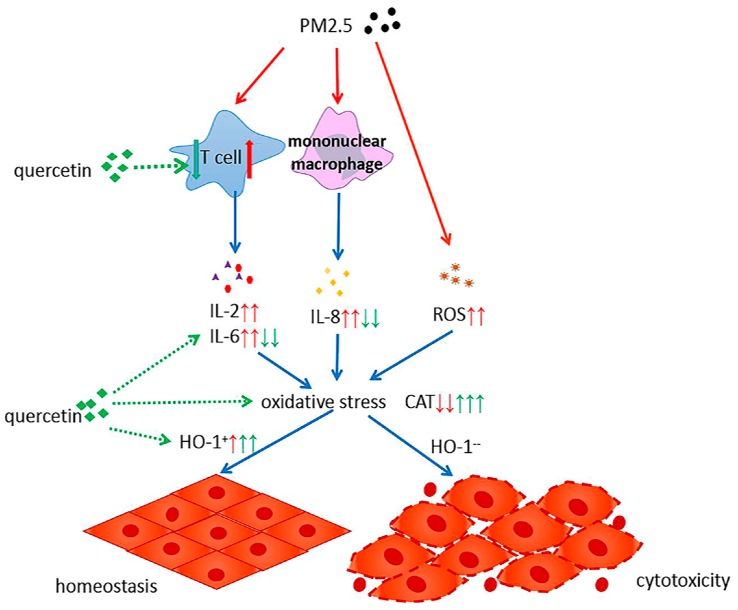
PM2.5 exposure affects the percentage of T-lymphocyte subsets in pregnant mice, with increased inflammatory factors and activated oxidative stress. Our study indicated that quercetin could reduce these adverse effects in multiple ways (Figure 7). In the first place, quercetin may inhibit the proliferation of T lymphocyte. Moreover, quercetin plays an essential role in the process of anti-inflammatory and anti-oxidation to antagonism oxidative stress state caused by PM2.5 exposure. Last but not the least, quercetin may increase the expression of HO-1 to promote body homeostasis reestablishment. The recommended dose of quercetin intake is still a controversial issue [40]. Some studies have shown that high-dose polyphenol intervention could result in negative effects [41,42]. A high dosage of quercetin intake during pregnancy was shown to increase iron storage in the liver by upregulating iron-associated cytokine expression like IL-6 and IL-10 [43]. Our experimental results indicated that the intervention effect of the medium-dose quercetin group was the most apparent, meaning that the protective function of quercetin may be displayed within a proper dose range, and higher dose quercetin intake will not yield further improvement.
Figure 7.
The mechanism of quercetin suppressing the adverse health effects of PM2.5. ↑ and ↓ indicates the effects of PM2.5; ↑ and ↓ indicates the effects of quercetin.
Although the findings of this study could give new insights to the understanding of changes of lymphocyte subgroups, serum cytokines, and oxidative stress under PM2.5 exposure, compared to inhalation, intratracheal instillation could lead to less homogeneous particle distribution. Even though intratracheal instillation is used as an alternative method for studying inhalation exposure, the localization of the test material in the lungs from inhalation and intratracheal instillation differs [23], which may affect the results. In addition, further studies are needed to explore the mechanism of lymphocyte changes under PM exposure.
5. Conclusions
PM2.5 may significantly influence the proportion of T-lymphocyte subsets, and cause inflammation and oxidative damage. Quercetin may partly attenuate these adverse effects in various ways. Quercetin may inhibit the proliferation of T lymphocyte and has a pleasurable inhibitory effect on inflammation changes. In addition, quercetin plays an essential role in the process of anti-oxidation to antagonism oxidative stress state caused by PM2.5 exposure. Taking a proper dose of quercetin as dietary supplements during pregnancy may have beneficial effects on health.
Acknowledgments
We are grateful to Ying Lyv and Dan Xu (Peking Unversity) for their writing assistance.
Abbreviations
| PM2.5 | fine particulate matter |
| PBS | phosphate-buffered saline |
| GD | gestational day |
| CMCS | carboxymethyl cellulose sodium |
| IL-2 | interleukin-2 |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| TNF-α | tumor necrosis factor α |
| CAT | catalase |
| GSH | glutathione |
| HO-1 | heme oxygenase 1 |
| ROS | reactive oxygen species |
| PBS | phosphate-buffered saline |
| SEM | scanning electron microscope |
| H&E | hematoxylin and eosin |
| ELISA | enzyme-linked immunosorbent assay |
| ANOVA | one-way analysis of variance |
Author Contributions
Yajun Xu gave the original idea and was in charge of the whole trial; Wei Liu conceived and designed the experiments; Wei Liu, Aiqin Fan, Minjia Zhang, and Yalin Zhou performed the experiments; Wei Liu and Jinqiu Feng analyzed the data; Minjia Zhang contributed reagents and materials; Wei Liu wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Malmqvist E., Larsson H.E., Jonsson I., Rignell-Hydbom A., Ivarsson S.A., Tinnerberg H., Stroh E., Rittner R., Jakobsson K., Swietlicki E., et al. Maternal exposure to air pollution and type 1 diabetes—Accounting for genetic factors. Environ. Res. 2015;140:268–274. doi: 10.1016/j.envres.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Dales R., Chen L., Frescura A.M., Liu L., Villeneuve P.J. Acute effects of outdoor air pollution on forced expiratory volume in 1 s: A panel study of schoolchildren with asthma. Eur. Respir. J. 2009;34:316–323. doi: 10.1183/09031936.00138908. [DOI] [PubMed] [Google Scholar]
- 3.Lim S.S., Vos T., Flaxman A.D., Danaei G., Shibuya K., Adair-Rohani H., Amann M., Anderson H.R., Andrews K.G., Aryee M., et al. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2224–2260. doi: 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sillanpää M., Hillamo R., Saarikoski S., Frey A., Pennanen A., Makkonen U., Spolnik Z., Grieken R.V., Braniš M., Brunekreef B. Chemical composition and mass closure of particulate matter at six urban sites in Europe. Atmos. Environ. 2006;40:212–223. doi: 10.1016/j.atmosenv.2006.01.063. [DOI] [Google Scholar]
- 5.Bjorksten B. The Window of Opportunity: Pre-Pregnancy to 24 Months of Age. Volume 61. Karger Publishers; Basel, Switzerland: 2008. Environmental influences on the development of the immune system: Consequences for disease outcome; pp. 243–254. [DOI] [PubMed] [Google Scholar]
- 6.Deiuliis J.A., Kampfrath T., Zhong J., Oghumu S., Maiseyeu A., Chen L.C., Sun Q., Satoskar A.R., Rajagopalan S. Pulmonary T cell activation in response to chronic particulate air pollution. Am. J. Physiol. Lung Cell. Mol. Physiol. 2012;302:L399–L409. doi: 10.1152/ajplung.00261.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Herr C.E., Dostal M., Ghosh R., Ashwood P., Lipsett M., Pinkerton K.E., Sram R., Hertz-Picciotto I. Air pollution exposure during critical time periods in gestation and alterations in cord blood lymphocyte distribution: A cohort of livebirths. Environ. Health. 2010;9:46. doi: 10.1186/1476-069X-9-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delfino R.J., Staimer N., Tjoa T., Gillen D.L., Schauer J.J., Shafer M.M. Airway inflammation and oxidative potential of air pollutant particles in a pediatric asthma panel. J. Expo. Sci. Environ. Epidemiol. 2013;23:466–473. doi: 10.1038/jes.2013.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nachman R.M., Mao G., Zhang X., Hong X., Chen Z., Soria C.S., He H., Wang G., Caruso D., Pearson C. Intrauterine Inflammation and Maternal Exposure to Ambient PM2.5 during Preconception and Specific Periods of Pregnancy: The Boston Birth Cohort. Environ. Health Perspect. 2016;124:1608–1615. doi: 10.1289/EHP243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brook R.D., Rajagopalan S., Pope C.R., Brook J.R., Bhatnagar A., Diez-Roux A.V., Holguin F., Hong Y., Luepker R.V., Mittleman M.A. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 11.Wan Q., Cui X., Shao J., Zhou F., Jia Y., Sun X., Zhao X., Chen Y., Diao J., Zhang L. Beijing ambient particle exposure accelerates atherosclerosis in ApoE knockout mice by upregulating visfatin expression. Cell Stress Chaperones. 2014;19:715–724. doi: 10.1007/s12192-014-0499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo B., Shi H., Wang L., Shi Y., Wang C., Yang J., Wan Y., Niu J. Rat lung response to PM2.5 exposure under different cold stresses. Int. J. Environ. Res. Public Health. 2014;11:12915–12926. doi: 10.3390/ijerph111212915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pei Y., Jiang R., Zou Y., Wang Y., Zhang S., Wang G., Zhao J., Song W. Effects of Fine Particulate Matter (PM2.5) on Systemic Oxidative Stress and Cardiac Function in ApoE(−/−) Mice. Int. J. Environ. Res. Public Health. 2016;13:484. doi: 10.3390/ijerph13050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fialova L., Malbohan I., Kalousova M., Soukupova J., Krofta L., Stipek S., Zima T. Oxidative stress and inflammation in pregnancy. Scand. J. Clin. Lab. Investig. 2006;66:121–127. doi: 10.1080/00365510500375230. [DOI] [PubMed] [Google Scholar]
- 15.Grevendonk L., Janssen B.G., Vanpoucke C., Lefebvre W., Hoxha M., Bollati V., Nawrot T.S. Mitochondrial oxidative DNA damage and exposure to particulate air pollution in mother-newborn pairs. Environ. Health-Glob. 2016;15:10. doi: 10.1186/s12940-016-0095-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruse J., Kanitz E., Weitzel J.M., Tuchscherer A., Stefaniak T., Jawor P., Wolffram S., Hammon H.M. Quercetin feeding in newborn dairy calves cannot compensate colostrum deprivation: Study on metabolic, antioxidative and inflammatory traits. PLoS ONE. 2016;11:e146932. doi: 10.1371/journal.pone.0146932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Djouossi M.G., Tamokou J.D., Ngnokam D., Kuiate J.R., Tapondjou L.A., Harakat D., Voutquenne-Nazabadioko L. Antimicrobial and antioxidant flavonoids from the leaves of Oncoba spinosa Forssk. (Salicaceae) BMC Complement. Altern. Med. 2015;15:134. doi: 10.1186/s12906-015-0660-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Upadhyay N.K., Kumar R., Siddiqui M.S., Gupta A. Mechanism of Wound-Healing activity of hippophae rhamnoides l. Leaf extract in experimental burns. Evid. Based Complement. Alternat. Med. 2011;2011:659705. doi: 10.1093/ecam/nep189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mouat M.F., Kolli K., Orlando R., Hargrove J.L., Grider A. The effects of quercetin on SW480 human colon carcinoma cells: A proteomic study. Nutr. J. 2005;4:11. doi: 10.1186/1475-2891-4-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J., Choi J.W., Sohng J.K., Pandey R.P., Park Y.I. The immunostimulating activity of quercetin 3-O-xyloside in murine macrophages via activation of the ASK1/MAPK/NF-kappaB signaling pathway. Int. Immunopharmacol. 2016;31:88–97. doi: 10.1016/j.intimp.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 21.Zhong J., Colicino E., Lin X., Mehta A., Kloog I., Zanobetti A., Byun H.M., Bind M.A., Cantone L., Prada D. Cardiac autonomic dysfunction: Particulate air pollution effects are modulated by epigenetic immunoregulation of toll-like receptor 2 and dietary flavonoid intake. J. Am. Heart Assoc. Cardiovasc. Cerebrovasc. Dis. 2015;4:e1423. doi: 10.1161/JAHA.114.001423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hayashi Y., Matsushima M., Nakamura T., Shibasaki M., Hashimoto N., Imaizumi K., Shimokata K., Hasegawa Y., Kawabe T. Quercetin protects against pulmonary oxidant stress via heme oxygenase-1 induction in lung epithelial cells. Biochem. Biophys. Res. Commun. 2012;417:169–174. doi: 10.1016/j.bbrc.2011.11.078. [DOI] [PubMed] [Google Scholar]
- 23.Hasegawa-Baba Y., Kubota H., Takata A., Miyagawa M. Intratracheal instillation methods and the distribution of administered material in the lung of the rat. J. Toxicol. Pathol. 2014;27:197–204. doi: 10.1293/tox.2014-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rudmann D.G., Preston A.M., Moore M.W., Beck J.M. Susceptibility to Pneumocystis carinii in mice is dependent on simultaneous deletion of IFN-gamma and type 1 and 2 TNF receptor genes. J. Immunol. 1998;161:360–366. [PubMed] [Google Scholar]
- 25.Robledo C.A., Mendola P., Yeung E., Mannisto T., Sundaram R., Liu D., Ying Q., Sherman S., Grantz K.L. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ. Res. 2015;137:316–322. doi: 10.1016/j.envres.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeFranco E., Hall E., Hossain M., Chen A., Haynes E.N., Jones D., Ren S., Lu L., Muglia L. Air pollution and stillbirth risk: Exposure to airborne particulate matter during pregnancy is associated with fetal death. PLoS ONE. 2015;10:e120594. doi: 10.1371/journal.pone.0120594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shi Y., Ji Y., Sun H., Hui F., Hu J., Wu Y., Fang J., Lin H., Wang J., Duan H. Nanoscale characterization of PM2.5 airborne pollutants reveals high adhesiveness and aggregation capability of soot particles. Sci. Rep. 2015;5:11232. doi: 10.1038/srep11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hong Z., Zhengyan Z., Youjun J. Multifactorial analysis of effects of mothers’ autoimmune thyroid disease on their infants’ intellectual development. Chin. J. Pediatr. 2005;43:340–344. [PubMed] [Google Scholar]
- 29.Liu Y., Wang L., Wang F., Li C. Effect of Fine Particulate Matter (PM2.5) on Rat Placenta Pathology and Perinatal Outcomes. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2016;22:3274–3280. doi: 10.12659/MSM.897808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fuentes-Arderiu X., Mestre M. Description of flow cytometry examinations related to human cell differentiation molecules in clinical immunology. Cytom. B Clin. Cytom. 2009;76:291–293. doi: 10.1002/cyto.b.20479. [DOI] [PubMed] [Google Scholar]
- 31.Dietert R.R., Etzel R.A., Chen D., Halonen M., Holladay S.D., Jarabek A.M., Landreth K., Peden D.B., Pinkerton K., Smialowicz R.J. Workshop to identify critical windows of exposure for children’s health: Immune and respiratory systems work group summary. Environ. Health Perspect. 2000;108:483–490. doi: 10.1289/ehp.00108s3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aztatzi-Aguilar O.G., Uribe-Ramírez M., Narváez-Morales J., De Vizcaya-Ruiz A., Barbier O. Early kidney damage induced by subchronic exposure to PM2.5 in rats. Part. Fibre Toxicol. 2016;13:68. doi: 10.1186/s12989-016-0179-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abraham N.G., Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol. Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 34.Paine A., Eiz-Vesper B., Blasczyk R., Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem. Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Soares M.P., Marguti I., Cunha A., Larsen R. Immunoregulatory effects of HO-1: How does it work? Curr. Opin. Pharmacol. 2009;9:482–489. doi: 10.1016/j.coph.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 36.Boots A.W., Haenen G.R., Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Willhite C.C. Teratogenic potential of quercetin in the rat. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 1982;20:75. doi: 10.1016/S0278-6915(82)80012-4. [DOI] [PubMed] [Google Scholar]
- 38.Xu H., Wu Z.H., Xu Y.J. Effects of quercetin in pregnant and lactation period on weight and expression of insulin-like growth factors-1 mRNA of obese female rats offspring. Beijing Da Xue Xue Bao. 2014;46:347–354. [PubMed] [Google Scholar]
- 39.Sun G.Y., Chen Z., Jasmer K.J., Chuang D.Y., Gu Z., Hannink M., Simonyi A. Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS ONE. 2015;10:e141509. doi: 10.1371/journal.pone.0141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mo S.F., Zhou F., Lv Y.Z., Hu Q.H., Zhang D.M., Kong L.D. Hypouricemic action of selected flavonoids in mice: Structure-activity relationships. Biol. Pharm. Bull. 2007;30:1551–1556. doi: 10.1248/bpb.30.1551. [DOI] [PubMed] [Google Scholar]
- 41.Johnson M.K., Loo G. Effects of epigallocatechin gallate and quercetin on oxidative damage to cellular DNA. Mutat. Res. 2000;459:211–218. doi: 10.1016/S0921-8777(99)00074-9. [DOI] [PubMed] [Google Scholar]
- 42.Fan P., Lou H. Effects of polyphenols from grape seeds on oxidative damage to cellular DNA. Mol. Cell. Biochem. 2004;267:67–74. doi: 10.1023/B:MCBI.0000049366.75461.00. [DOI] [PubMed] [Google Scholar]
- 43.Vanhees K., Godschalk R.W., Sanders A., van Waalwijk V.D.S., van Schooten F.J. Maternal quercetin intake during pregnancy results in an adapted iron homeostasis at adulthood. Toxicology. 2011;290:350–358. doi: 10.1016/j.tox.2011.10.017. [DOI] [PubMed] [Google Scholar]