Abstract
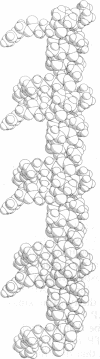
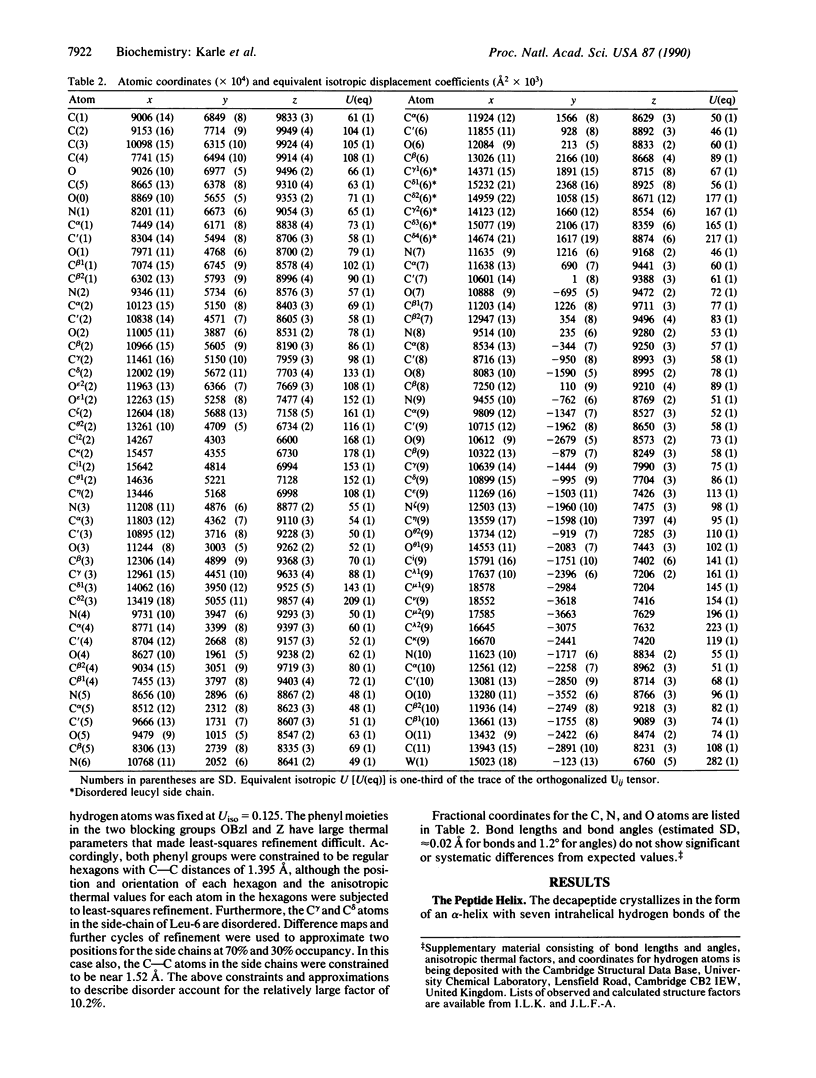
The crystal structure of the decapeptide Boc-Aib-Glu(OBzl)-Leu-Aib-Ala-Leu-Aib-Ala-Lys(Z)-Aib-OMe (where Aib is alpha-aminoisobutyryl, Boc is t-butoxycarbonyl, OBzl is benzyl ester, and Z is benzyloxycarbonyl) illustrates a parallel zipper arrangement of interacting helical peptide columns. Head-to-tail NH...OC hydrogen bonding extends the alpha-helices formed by the decapeptide into long columns in the crystal. An additional NH...OC hydrogen bond in the head-to-tail region, between the extended side chains of Glu(OBzl), residue 2 in one molecule, and Lys(Z), residue 9 in another molecule, forms a "double tooth" on the side of the column. These double teeth are repeated regularly on the helical columns with spaces of six residues between them (approximately 10 A). The double teeth on a pair of parallel columns (all carbonyl groups pointed in the same direction) interdigitate in a zipper motif. All contacts in the zipper portion are of the van der Waals type. The peptide, with formula C66H103N11O17.H2O, crystallizes in space group P2(1)2(1)2(1) with a = 10.677(4) A, b = 16.452(6) A, and c = 43.779(13) A; overall agreement R = 10.2% for 3527 observed reflections (magnitude of /F0/ greater than 3 sigma); resolution 0.9 A.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen C., Parry D. A. Alpha-helical coiled coils and bundles: how to design an alpha-helical protein. Proteins. 1990;7(1):1–15. doi: 10.1002/prot.340070102. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Balaram P. Structural characteristics of alpha-helical peptide molecules containing Aib residues. Biochemistry. 1990 Jul 24;29(29):6747–6756. doi: 10.1021/bi00481a001. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J. L., Uma K., Balaram P. Helix aggregation in peptide crystals: occurrence of either all parallel or antiparallel packing motifs for alpha-helices in polymorphs of Boc-Aib-Ala-Leu-Ala-Leu-Aib-Leu-Ala-Leu-Aib-OMe. Biopolymers. 1990 Dec;29(14):1835–1845. doi: 10.1002/bip.360291414. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J. L., Uma K., Balaram P. Modular design of synthetic protein mimics. Characterization of the helical conformation of a 13-residue peptide in crystals. Biochemistry. 1989 Aug 8;28(16):6696–6701. doi: 10.1021/bi00442a024. [DOI] [PubMed] [Google Scholar]
- Karle I. L., Flippen-Anderson J., Uma K., Balaram P. Aqueous channels within apolar peptide aggregates: solvated helix of the alpha-aminoisobutyric acid (Aib)-containing peptide Boc-(Aib-Ala-Leu)3-Aib-OMe.2H2O.CH3OH in crystals. Proc Natl Acad Sci U S A. 1988 Jan;85(2):299–303. doi: 10.1073/pnas.85.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landschulz W. H., Johnson P. F., McKnight S. L. The leucine zipper: a hypothetical structure common to a new class of DNA binding proteins. Science. 1988 Jun 24;240(4860):1759–1764. doi: 10.1126/science.3289117. [DOI] [PubMed] [Google Scholar]
- Mathew M. K., Balaram P. Alamethicin and related membrane channel forming polypeptides. Mol Cell Biochem. 1983;50(1):47–64. doi: 10.1007/BF00225279. [DOI] [PubMed] [Google Scholar]
- O'Shea E. K., Rutkowski R., Kim P. S. Evidence that the leucine zipper is a coiled coil. Science. 1989 Jan 27;243(4890):538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- Prasad B. V., Balaram P. The stereochemistry of peptides containing alpha-aminoisobutyric acid. CRC Crit Rev Biochem. 1984;16(4):307–348. doi: 10.3109/10409238409108718. [DOI] [PubMed] [Google Scholar]
- Vinson C. R., Sigler P. B., McKnight S. L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989 Nov 17;246(4932):911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]