Abstract
The cardiovascular, respiratory, and anesthetic effects of medetomidine-ketamine (20 μg/kg bodyweight [BW] and 10 mg/kg BW) (MK group) or dexmedetomidine-ketamine (10 μg/kg BW and 10 mg/kg BW) (DK group) were studied in golden-headed lion tamarins. Heart rate decreased after administration of both combinations; this reduction was statistically greater in the DK group than in the MK group after 15 and 45 minutes. Systolic arterial pressure decreased in a similar way in both groups, except at 15 minutes, when systolic arterial pressure was significantly lower in the DK group. Diastolic arterial pressure, mean arterial pressure, respiratory rate, and rectal temperature were progressively reduced in all groups. Sedation time was significantly shorter and anesthesia time was significantly longer in the DK group compared with MK group. Anesthetic quality and analgesia scores were significantly greater at 5 and 15 minutes in the DK group compared with the MK group. The administration of dexmedetomidine-ketamine is as safe and effective as the administration of medetomidine-ketamine in tamarins.
Abstract
Résumé — Comparaison des anesthésies à la médétomidine-kétamine et à la dexmédétomidine-kétamine chez le tamarin à tête dorée. Les effets cardiovasculaires, respiratoires et anesthésiques de la médétomidine-kétamine (20 μg/kg de poids corporel (PC) et 10 mg/kg PC) (groupe MK) ou de la dexmédétomidine — kétamine (10 μg/kg/PC et 10 mg/kg PC) (groupe DK) ont été étudiés chez le tamarin à tête dorée. La fréquence cardiaque a diminué après l’administration des 2 associations; cette diminution était statistiquement plus importante chez le groupe DK que chez le groupe MK après 15 et 45 minutes. La pression artérielle systolique a diminué de façon similaire chez les 2 groupes, sauf à 15 minutes, alors que la pression artérielle systolique était significativement plus basse chez le groupe DK. La pression artérielle diastolique, la pression artérielle moyenne, la fréquence respiratoire et la température rectale diminuaient progressivement chez tous les groupes. Le temps de sédation était significativement plus court, le temps d’anesthésie était significativement plus long chez le groupe DK comparé au groupe MK. La qualité de l’anesthésie et les cotations analgésiques étaient significativement plus grandes à 5 et 15 minutes chez le groupe DK en comparaison avec le groupe MK. L’administration de dexmétomidinekétamine est aussi sécuritaire et efficace que l’administration de médétomidine-kétamine chez le tamarin.
(Traduit par Docteur André Blouin)
Introduction
The golden-headed lion tamarin (Leontopithecus chrysomelas), one of 4 species of lion tamarins, is considered an endangered species (1). Conservation programs for tamarins have been successful in the establishment of new conservation areas and community lishment involvement in the conservation of these species (1,2).
Chemical immobilization is often required for research and veterinary care of lion tamarins. Ketamine is the drug of choice for primates, because it rapidly induces dissociative anesthesia accompanied by analgesia (3). Ketamine administration is characterized by an increase in heart rate, cardiac output, and blood pressure, and minimal respiratory depression (4,5). Concurrent use of α2-adrenoceptor agonists, such as xylazine, reduces the amount of ketamine needed, as well as minimizing the excitement, increased muscle tone, and salivation associated with ketamine administration in nonhuman primates (4,5).
Medetomidine is a potent, selective, and specific α2-adrenoceptor agonist that contains equal parts of 2 optical enantiomers, dexmedetomidine and levome-detomidine. It has been widely used for sedation in animals (6,7). Combinations of medetomidine with ketamine have become very popular for use in wild animals (3). The individual disadvantages of medetomidine and ketamine are counterbalanced when used in combination (3). Medetomidine compensates for the poor muscle relaxant and analgesic effects of ketamine, while the cardiac stimulating properties of ketamine partially compensate the medetomidine-induced bradycardia (8,9).
The pharmacological effects of medetomidine are stereospecific and due almost entirely to its dextroisomer, dexmedetomidine, which constitutes half of the amount of the racemate and, when administered at half the dose, induces similar effects as medetomidine (10,11). Dexmedetomidine has undergone extensive clinical studies in humans (12), cats, and dogs (10,11,13), indicating its potential use in clinical practice.
A limited amount of information is available about the physiological effects of anesthetic protocols in tamarins or in new-world primates (3,4). To the author’s knowledge, there are no reports of the use of dexmedetomidine-ketamine or medetomidine-ketamine combinations in callitrichids or any other small primates.
The present study was undertaken to compare the anesthetic effects and cardiorespiratory variables of dexmedetomidine-ketamine or medetomidine-ketamine administered, IM, in golden-headed lion tamarins.
Materials and methods
Six adult golden-headed lion tamarins, 3 males and 3 females, housed in pairs in outdoor cages, were studied (mean bodyweight [BW] ± standard deviation [0.498, s = 0.054 kg]). Food and water were withheld for 4 h before chemical restraint. All procedures were carried out during daytime. This project was approved by the local institutional animal care and use committee.
A randomized block design was applied. Each animal underwent immobilization on 2 successive occasions. At least 4 wk were allowed between interventions. The intent was to administer 10 μg/kg BW dexmedetomidine and 10 mg/kg BW ketamine (DK group) or 20 μg/kg BW medetomidine and 10 mg/kg BW ketamine (MK group). Doses were calculated on the basis of BW obtained from each tamarin’s record. After immobilization, each animal was reweighed and the actual anesthetic doses used were then calculated. The animals were caught by netting and the selected anesthetic combination was delivered as a single IM injection by use of a hand syringe. Dexmedetomidine (Precedex; Abbott Laboratories, North Carolina, USA) and ketamine (Vetaset, Fort Dodge, Iowa, USA) in group DK and medetomidine (Domitor; Pfizer Animal Health, Exton, Pennsylvania, USA) and ketamine in group MK were mixed with 0.9% saline in a syringe to a total volume of 0.20 mL/kg. Time to initial sedative effect and lateral recumbency were defined and recorded as time from injection to ataxia and from injection to nonarousable recumbency, respectively. Anesthesia time was considered as the time interval between complete immobilization and the first attempt made by the animal to lift its head a few centimeters above the ground. Standing time was defined as the time interval between the end of anesthesia and the animal standing without assistance for longer than 10 s, and walking time was defined as time from standing until the animal could walk or climb a short distance.
After immobilization, heart rate (HR) and rhythm were displayed continuously by using a lead II electrocardiogram (ECG) (ECGPC; Tecnologia Eletrônica Brasileira, São Paulo, Brazil). Respiratory rate was counted over a period of 1 min. Hemoglobin oxygen saturation was measured by using a pulse oximeter (DX 2010; Dixtal Biomedica, Manaus, Brazil) with the probe placed on the tamarin’s hand. Rectal temperature was measured with a digital thermometer (Digital Clinical Thermometer; Omrom, China). Systolic, diastolic, and mean arterial blood pressures were recorded by using a noninvasive oscillometric device (DX 2010; Dixtal Biomedica, Manaus, Brazil). A neonatal cuff (Medical Accessories, Trenton, New Jersey, USA) was placed over the femoral artery in the animal’s right hind limb, with the cuff width approximating 40% of the thigh circumference. Animals were kept over a regulated heating-pad (Colchonete térmico; Brasmed, São Paulo, Brazil) during immobilization.
Sedation, analgesia (response to interdigital pad pinch), muscle relaxation, and auditory response (a handclap close to the animal’s ears) were subjectively evaluated during the duration of anesthesia by using a previously described scoring system (14).
All variables were recorded within 5 min of administration and at 15-min intervals. Induction, anesthesia, standing, and walking times, and doses used in each group were compared between groups by use of Student’s t-test. Data were analyzed by using repeated measures analysis of variance (ANOVA), followed by a Tukey’s test to evaluate changes within each group and to compare physiologic variables between groups. Sedation, analgesia, muscle relaxation, and auditory response were compared by using a Kruskal-Wallis repeated measures ANOVA (SAS, 1996; SAS, Cary, North Carolina, USA). Values of P < 0.05 were considered statistically significant.
Results
Actual doses administered were 20.7, s = 1.4 μg/kg BW of medetomidine and 10.2, s = 0.8 mg/kg BW of ketamine in the MK group, and 10.4, s = 0.4 μg/kg BW of dexmedetomidine and 10.03, s = 0.9 mg/kg BW of ketamine in the DK group. There were no significant differences between estimated doses and actual doses administered in each protocol, or between ketamine doses in both groups.
Induction of anesthesia was very smooth in both groups. Marked salivation did not occur after anesthetic administration. Mean time to initial sedation was significantly shorter in the DK group (0.9, s = 0.3 min) than in the MK group (1.8, s = 1.2 min), but there was no statistically significant difference in mean recumbency time between MK (1.1, s = 0.3 min) and DK (1.0, s = 0.5 min) groups. Anesthetic duration in the DK group (67.8, s = 13.3 min) was longer than in the MK group (44.6, s = 17.3 min). Standing time was not statistically different between treatment groups (11.1, s = 10.0 and 14.1, s = 8.7 min for DK and MK, respectively). Walking time when tamarins were given DK (16.0, s = 3.4 min) was significantly longer than when tamarins were given MK (8.6, s = 5.9 min).
Heart rate progressively decreased 15 min after drug administration in both groups; the HR in the DK group was significantly lower than that in the MK group at 15 and 45 min post drug administration (Table 1). The ECG showed no signs of arrhythmia with either drug combination. In the DK group, systolic arterial pressure was significantly lower after 15 min of anesthesia when compared with that of the MK group; however, systolic arterial pressure progressively decreased in both groups beyond 15 min. Diastolic and mean arterial pressure decreased significantly at 30 min in both groups, but the decrease was not statistically significant between groups (Table 1).
Table 1.
Mean (standard deviation [s]) of physiological variables in golden-headed lion tamarins (n = 6) undergoing medetomidine/ketamine (MK) or dexmedetomidine/ketamine (DK) anesthesia.
| Time (minutes) | ||||||
|---|---|---|---|---|---|---|
| Variable | Group | 5 | 15 | 30 | 45 | 60 |
| HR | MK | 178.3, s = 13.7 | 143.8, s = 22.8a | 120.0, s = 17.3 | 113.2, s = 15.4a | |
| (beats minute−1) | DK | 168.7, s = 10.2 | 121.2, s = 15.3b | 116.8, s = 10.5 | 100.3, s = 13.7b | 98.00, s = 10.2 |
| SAP | MK | 116.8, s = 4.7 | 110.1, s = 10.7a | 102.5, s = 10,7 | 104.7, s = 12.7 | |
| (mm Hg) | DK | 118.7, s = 9.4 | 99.8, s = 13.5b | 98.4, s = 12.7 | 98.9, s = 10.1 | 100.0, s = 16.4 |
| DAP | MK | 75.5, s = 4.4 | 66.5, s = 6.5 | 59.5, s = 4.6 | 60.2, s = 4.2 | |
| (mm Hg) | DK | 72.5, s = 7.6 | 64.0, s = 8.0 | 50.5, s = 8.3 | 52.6, s = 6.7 | 58.2, s = 9.5 |
| MAP | MK | 86.8, s = 5.3 | 77.8, s = 7.4 | 73.5, s = 5.5 | 72.7, s = 2.6 | |
| (mm Hg) | DK | 92.7, s = 9.5 | 79.3, s = 8.4 | 70.9, s = 7.5 | 72.4, s = 4.6 | 100.5, s = 9.2 |
| RR | MK | 49.3, s = 8.2 | 40.0, s = 9.1 | 27.0, s = 6.8 | 27.0, s = 6.8 | |
| (breaths minute−1) | DK | 42.7, s = 9.6 | 40.3, s = 10.4 | 33.6, s = 9.5 | 30.8, s = 7.2 | 30.3, s = 12.4 |
| SpO2 | MK | 94.0, s = 1.5 | 96.1, s = 1.7 | 94.2, s = 2.9 | 95.7, s = 2.9 | |
| (%) | DK | 96.0, s = 0.8 | 98.2, s = 1.2 | 92.3, s = 1.9 | 96.7, s = 2.4 | 98.3, s = 2.8 |
| RT | MK | 38.5, s = 1.2 | 37.2, s = 0.9 | 36.0, s = 1.6 | 35.3, s = 1.5 | |
| (°C) | DK | 38.8, s = 1.7 | 37.4, s = 1.8 | 36.1, s = 1.2 | 35.1, s = 1.2 | 35.1, s = 1.0 |
Time — minutes after drug administration; HR — heart rate; SAP — systolic arterial pressure; DAP — diastolic arterial pressure; MAP — mean arterial pressure; RR — respiratory rate; SpO2 — hemoglobin saturation; RT — rectal temperature
Mean values between groups with different alphabetic superscripts are significantly different (P < 0.05)
Respiratory rate decreased significantly in a similar way in both groups 30 min after administration of the drug combinations. Despite this reduction, hemoglobin oxygen saturation did not vary during the anesthetic period in both groups (Table 1).
When compared with tamarins given MK, tamarins given DK demonstrated significantly lower sedation, muscle relaxation, analgesia, and auditory response scores during the first 15 min after drug administration. However, after 15 min, there were no significant differences in the subjectively scored indicators between the 2 treatment groups (Figures 1 and 2).
Figure 1.
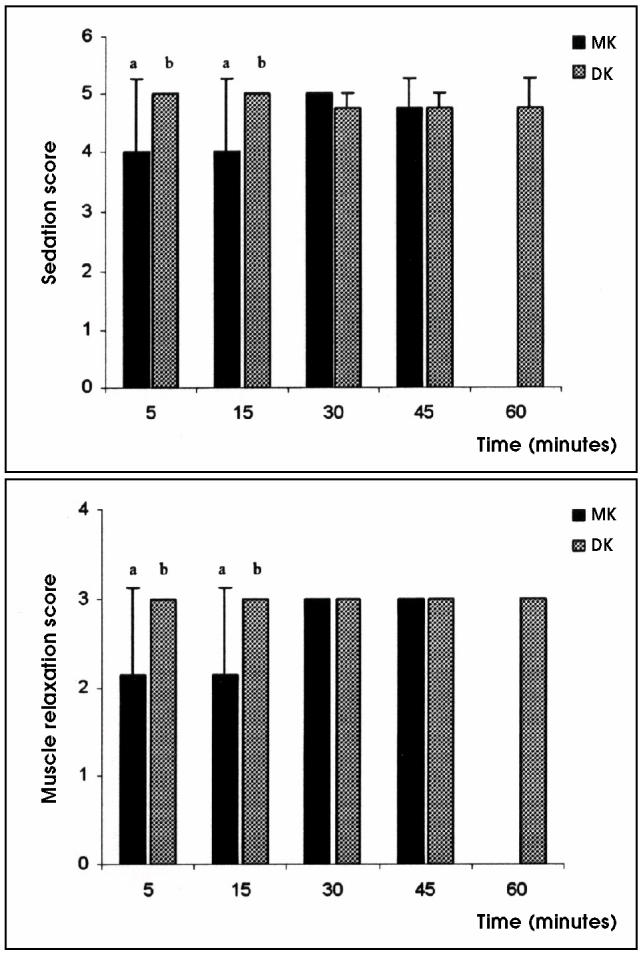
Mean sedation and muscle relaxation scores in golden-headed lion tamarins undergoing medetomidine/ketamine (MK) or dexmedetomidine/ketamine (DK) anesthesia. Mean values between groups with different alphabetic superscripts are significantly different (P < 0.05).
Figure 2.
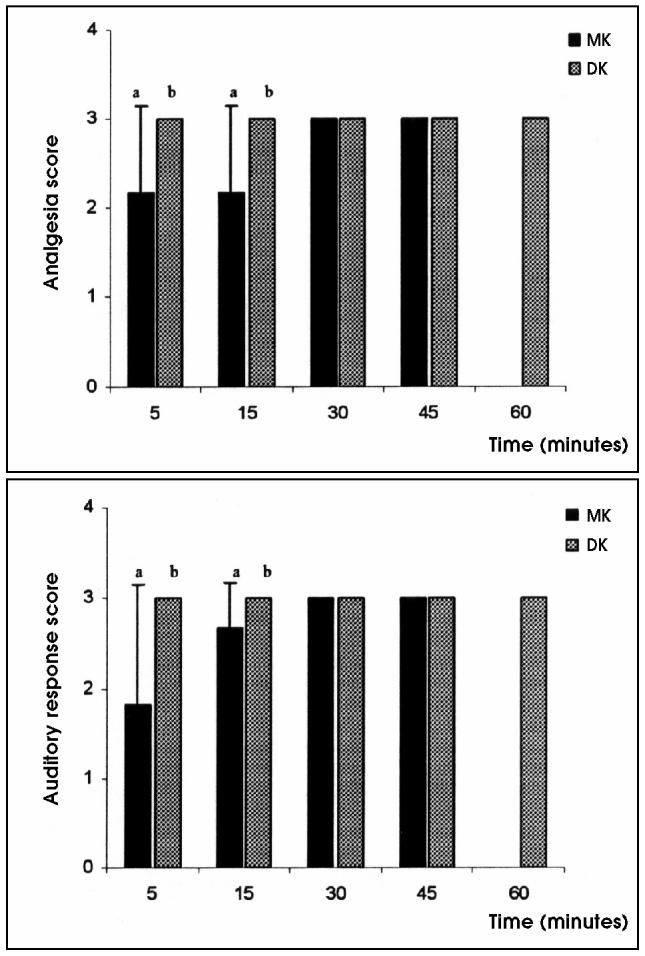
Mean analgesia and auditory response scores in golden-headed lion tamarins undergoing medetomidine/ketamine (MK) or dexmedetomidine/ketamine (DK) anesthesia. Mean values between groups with different alphabetic superscripts are significantly different (P < 0.05).
Discussion
Studies have demonstrated that levomedetomidine, an inactive ingredient in medetomidine, may act as a weak partial α2-adrenoceptor agonist or as an inverse α2-adrenoceptor agonist; therefore, levomedetomidine and dexmedetomidine may have opposite sedative and analgesic effects (13,15). In this study with tamarins, dexmedetomidine-ketamine administration resulted in higher sedation, muscle relaxation, and auditory response scores than did medetomidine-ketamine administration for the first 15 min following administration. Previous studies in dogs and cats have demonstrated that the sedative effects caused by the administration of dexmedetomidine did not differ from those following corresponding doses of medetomidine, over time (10,11,13). So, in tamarins, dexmedetomidine may have a longer lasting sedative effect than those of racemic medetomidine; however, further studies are needed to confirm the clinical advantage, if any, of dexmedetomidine over medetomidine in nonhuman primates.
In this study, concurrent use of dexmedetomidine with ketamine induced a longer lasting anesthetic effect than did the administration of medetomidine-ketamine, which is in agreement with results in cats and dogs (10,11). Interdigital pad pinch reflex may not be the most reliable method of assessing analgesia, because it is spinally mediated and may not involve pain perception (16); however, it is widely used and considered valid when α2-adrenoceptor agonist-induced analgesia is evaluated (11), as the analgesic properties of these agents are also mainly spinally mediated (7). In dogs, administration of high doses of levomedetomidine reduced the analgesic effect caused by the administration of dexmedetomidine (13), which suggests that the presence of the levoisomer may have some interaction with the dextro form, perhaps causing antagonism or activation of central α1-adrenoceptors (11,13).
The administration of dexmedetomidine and medetomidine at different doses demonstrated that the onset of sedation and the time to recumbency for these agents were not dose dependent in cats, but depended on the affinity for the receptors and their intrinsic activity (10). It is possible that in tamarins the higher affinity of dexmedetomidine for α2-adrenoceptors resulted in a shorter time to sedation in the DK group, when compared with the MK group, and the prolonged anesthesia time in the DK group (17).
Baseline values for physiological parameters in wild species are nearly impossible to obtain, and even in such small animals as tamarins, physical restraint is not effective for this purpose (3). It is particularly important to consider the stress of the physical restraint when comparing our findings with those observed in dogs or cats, as the tamarins in this study were probably highly stressed before drug administration, as a result of capture. Normal resting values have been obtained in wild animals through the use of attached or implanted measuring devices (18), however, there are no reports of physiological parameters for tamarins or any other callitrichids. Our intent was to evaluate the cardiovascular effects of DK on tamarins and to compare them with those of MK, an anesthetic protocol widely used in primates. In fact, whether these 2 anesthetic protocols could cause important undesirable effects remains unclear, as there were no baseline values for the animals of this study. However, monitoring of physiological parameters after drug administration enables trends during anesthesia to be evaluated, and similar effects of the same drugs in different species might be expected, although this is not true in every situation (19).
α2-Adrenoceptors agonists induce bradycardia through an increase in arterial pressure and partially by inhibition of central sympathetic activity (8,20). A stable heart rate following administration of α2-adrenoceptors agonists and dissociative anesthetics (5,8), such as medetomidine-ketamine or dexmedetomidine-ketamine, was not observed in this study, which is in agreement with studies involving baboons (Papio cynocephalus) given ketamine and ) xylazine, IM, (5) and cats given medetomidine and ketamine (9). In cats and dogs, significant differences between the effects of the corresponding doses of dexmedetomidine and medetomidine in heart rate were not observed (10,11), indicating that the effects of medetomidine are due almost exclusively to the dextroisomer, dexmedetomidine. The significant decrease in heart rate observed at 15 and 45 min of anesthesia in the tamarins given DK could have resulted from differences in degree of sedation and analgesia, since sympathetic tone could have been affected differently as a result of different degrees of consciousness.
Sinus arrhythmias and 1st and 2nd degree atrioventricular blocks have been observed in some dogs treated with medetomidine and dexmedetomidine (6,7,11), but in tamarins, these α2-adrenoceptor agonists did not produce changes in electric conductivity of the myocardium.
Biphasic response, with an initial increase followed by a decrease in arterial pressure, is a characteristic of α2-adrenoceptor agonists (6,7,17). Studies have shown that higher doses of medetomidine resulted in an increase in blood pressure due to the drug’s peripheral vasoconstrictive action (8,21). In dogs and cats, medetomidine or dexmedetomidine produced similar dose-dependent depression of arterial pressure, which could explain the findings of this present study. The reduction in arterial pressure observed in the tamarins was most likely related to the utilization of lower doses of α2-adrenoceptor agonists, in which case stimulation of central adrenoceptors and arterial hypotension predominates over peripheral vasoconstrictive effects (20), as has been observed previously in dogs and cats (9).
Dexmedetomidine and medetomidine tend to maintain the respiratory rate near to basal values in cats and dogs (10,13), though a slight decrease has been observed after IM administration in dogs (11). In tamarins, the respiratory rate decreased significantly in all treatment groups. Decreases in respiratory rate induced by the administration of α2-adrenoceptor agonists and ketamine is often observed (8,21), primarily influenced by the dose of the dissociative anesthetic used (9). Despite the decrease in respiratory rate in all treatment groups, hemoglobin oxygen saturation values were not statistically different between groups over time, suggesting that, in tamarins, both α2-adrenoceptor agonists may be relatively safe for use as a sedative, as these animals were breathing room air.
Decrease in body temperature can occur in any species during prolonged anesthesia, but it affects smaller species, such as tamarins, to a greater extent (3,4). The hypothermia reported after the administration of α2-adrenoceptor agonists could be related to the decrease in muscular activity (17) or to a direct effect on noradrenergic hypothalamic mechanisms implicated in thermoregulation (7). Core temperatures less than 32°C may be life-threatening, but none of the tamarins in this study developed a temperature lower than 34°C; however, body temperature needs to be externally supported and closely monitored.
This study demonstrated that both DK and MK produced good immobilization in golden-headed lion tamarins, with a rapid and smooth onset of action, and that DK is as safe and effective as MK. CVJ
References
- 1.Nowak RM, ed. Walker’s Mammals of the World. 5th ed. Baltimore: John Hopkins Univ Pr, 1991:435–444.
- 2.Valladares-Pádua C. Biology and conservation: family Callitrichidae. In: Fowler ME, Cubas ZS, eds. Biology, Medicine, and Surgery of South American Wild Animals. Ames: Iowa State Univ Pr, 2001: 259–261.
- 3.Bush M. Methods of capture, handling, and anesthesia. In: Kleinman DG, Allen ME, Thompson KV, Lumpkin S, eds. Wild Animals in Captivity — Principles and Techniques. Chigaco: Univ Chicago Pr, 1996:37–38.
- 4.Wallach JD, Boever WJ. Immobilizing agents for primates. In: Wallach JD, Boever WJ, eds. Diseases of Exotic Animals — Medical and Surgical Management. 1st ed. Philadelphia: WB Saunders, 1983:19.
- 5.White GL, Cummings JF. Comparison of ketamine and ketamine-xylazine in the baboon. Vet Med Small Anim Clin. 1969;74:392–396. [PubMed] [Google Scholar]
- 6.Vainio O, Vaha-Vahe T, Palmu L. Sedative and analgesic effects of medetomidine in dogs. J Vet Pharmacol Ther. 1989;12:225–231. doi: 10.1111/j.1365-2885.1989.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 7.Virtanen R. Pharmacologic profiles of medetomidine and its antagonist atipamezole. Acta Vet Scand. 1989;85:29–37. [PubMed] [Google Scholar]
- 8.Jalanka HH, Röken BO. The use of medetomidine, medetomidine-ketamine combinations, and atipamezole in nondomestic mammals: a review. J Zoo Wildl Med. 1990;21:259–238. [Google Scholar]
- 9.Verstegen J, Fargetton X, Ectors F. Medetomidine-ketamine anaesthesia in cats. Acta Vet Scand. 1989;85:117–123. [PubMed] [Google Scholar]
- 10.Ansah OB, Raekallio M, Vainio O. Comparison of three doses of dexmedetomidine with medetomidine in cats following intramuscular administration. J Vet Pharmacol Ther. 1998;21:380–387. doi: 10.1046/j.1365-2885.1998.00155.x. [DOI] [PubMed] [Google Scholar]
- 11.Kuusela E, Raekallio M, Anttila M. Clinical effects and pharmacokinetics of medetomidine and its enatiomers in dogs. J Vet cokinetics Pharmacol Ther. 2000;23:15–20. doi: 10.1046/j.1365-2885.2000.00245.x. [DOI] [PubMed] [Google Scholar]
- 12.Hayashi Y, Maze M. Alpha2 adrenoceptor agonists and anaesthesia. Br J Anaesth. 1993;71:108–118. doi: 10.1093/bja/71.1.108. [DOI] [PubMed] [Google Scholar]
- 13.Kuusela E, Vainio O, Kaistinen A. Sedative, analgesic, and cardiovascular effects of levomedetomidine alone and in combinations with dexmedetomidine in dogs. Am J Vet Res. 2001;62:616–621. doi: 10.2460/ajvr.2001.62.616. [DOI] [PubMed] [Google Scholar]
- 14.Selmi AL, Barbudo-Selmi GR, Moreira CF, et al. Evaluation of sedative and cardiorespiratory effects of romifidine and romifidine-butorphanol in cats. J Am Vet Med Assoc. 2002;221:506–510. doi: 10.2460/javma.2002.221.506. [DOI] [PubMed] [Google Scholar]
- 15.Kuusela E, Raekallio M, Vaisanen M, Mykkanen K, Ropponen H, Vainio O. Comparison of medetomidine and dexmedetomidine as premedicants in dogs undergoing propofol-isoflurane anesthesia. Am J Vet Res. 2001;62:1073–1080. doi: 10.2460/ajvr.2001.62.1073. [DOI] [PubMed] [Google Scholar]
- 16.Kitchell RL. Problems in defining pain and the peripheral mechanisms of pain. J Am Vet Med Assoc. 1987;191:1195–1199. [PubMed] [Google Scholar]
- 17.Savola JM, Virtanen R. Central α2-adrenoceptors are highly stereoselective for dexmedetomidine, the dextro enantiomer of medetomidine. Eur J Pharmacol. 1991;195:193–199. doi: 10.1016/0014-2999(91)90535-x. [DOI] [PubMed] [Google Scholar]
- 18.Duarte DP, Silva VL, Jaguaribe AM, Gilmore DP, Da Costa CP. Circadian rhythms in blood pressure in free-ranging three-toed sloths (Bradypus variegatus) Braz J Med Biol Res. 2003;36:273–278. doi: 10.1590/s0100-879x2003000200016. [DOI] [PubMed] [Google Scholar]
- 19.Selmi AL, Mendes GM, Figueiredo JP, et al. Chemical restraint of peccaries with tiletamine/zolazepam and xylazine or tiletamine/zolazepam and butorphanol. Vet Anaesth Analg. 2003;30:24–29. doi: 10.1046/j.1467-2995.2003.00087.x. [DOI] [PubMed] [Google Scholar]
- 20.Klein LV, Klide AM. Central α2-adrenergic and benzodiazepine -agonists and their antagonists. J Zoo Wildl Med. 1989;20:138–153. [Google Scholar]
- 21.Jalanka H. The use of medetomidine, medetomidine-ketamine combinations and atipamezole at Helsinki Zoo — a review of 240 cases. Acta Vet Scand. 1989;85:193–197. [PubMed] [Google Scholar]


