ABSTRACT
Macroautophagy/autophagy plays a role in unconventional secretion of leaderless cytosolic proteins. Whether and how secretory autophagy diverges from conventional degradative autophagy is unclear. We have shown that the prototypical secretory autophagy cargo IL1B/IL-1β (interleukin 1 β) is recognized by TRIM16, and that this first to be identified secretory autophagy receptor interacts with the R-SNARE SEC22B to jointly deliver cargo to the MAP1LC3B-II-positive sequestration membranes. Cargo secretion is unaffected by knockdowns of STX17, a SNARE catalyzing autophagosome-lysosome fusion as a prelude to cargo degradation. Instead, SEC22B in combination with plasma membrane syntaxins completes cargo secretion. Thus, secretory autophagy diverges from degradative autophagy by using specialized receptors and a dedicated SNARE machinery to bypass fusion with lysosomes.
KEYWORDS: ferritin, galectins, inflammasome, IL1B, SNAREs, TRIMs, unconventional secretion
The principal morphological feature of autophagy is the formation of a double-membrane organelle called an autophagosome that encloses cytosolic cargo and typically delivers it to lysosomes for degradation. Unconventionally secreted cytosolic proteins are characterized by the absence of leader peptides; thus, they do not enter the lumen of the ER and do not follow the secretory pathway reserved for conventionally secreted proteins that typically go through the ER and the Golgi apparatus, and are secreted by exocytosis of post-Golgi vesicles. An archetypal example of unconventionally secreted proteins is the proinflammatory cytokine IL1B, which has been reported in 1990 as being secreted from mammalian cells despite the absence of a leader peptide. Recently, autophagy as a process has been implicated in the secretion of IL1B in mammalian cells and of the yeast protein Acb1, a homolog of Dictyostelium discoideum AcbA that stimulates encapsulation of prespore cells in the slime mold.
Our previous work has shown that IL1B secretion is dependent on autophagy in primary murine bone marrow-derived macrophages. Others have confirmed utilization of the autophagy apparatus for IL1B secretion. How IL1B is recognized for delivery to autophagic organelles en route for secretion and how it is protected from degradation has hitherto remained unknown. Our present work has identified the first specific receptor for secretory autophagy and defined the SNARE apparatus that bypasses autophagosomal maturation but instead leads to secretion of the cargo at the plasma membrane.
Diverse lysosome-damaging agents, such as silica, alum, monosodium urate (MSU), and Leu-Leu-O-Me (LLOMe), activate inflammasome, which in turn processes pro-IL1B into mature IL1B, induce autophagy and trigger IL1B secretion. We first established that autophagy factors, e.g. MAP1LC3B and ATG16L1, are required for the efficient secretion of IL1B upon LLOMe treatment. Next, we searched for an IL1B receptor for selective secretory autophagy. The TRIM family proteins (with over 80 members in humans) have been shown to mediate autophagy, act as selective autophagic cargo receptors, and act as organizers of autophagy initiation factors. We screened human TRIMs and found that several TRIMs affect IL1B secretion in response to lysosomal damage, such as TRIM10 and TRIM16. Interestingly, an older report indicates that TRIM16 and IL1B physically interact, albeit the purpose of that interaction has never been addressed. We then hypothesized that TRIM16 serves as a selective cargo receptor for secretory autophagy and that it regulates autophagy-dependent unconventional secretion of IL1B in response to lysosomal damage. Indeed, we found through a series of experiments that TRIM16 is required for the optimal secretion of IL1B triggered by lysosomal damage and starvation.
Recognition of endomembrane damage has been associated with galectins, a family of lectin proteins that “patrol” the cytosol and bind to sugars (galactosides) present only on the exofacial (lumenal) leaflets of the plasma membrane or the membranes delimiting intracellular organelles. We thus tested whether galectins contributed to IL1B secretion and found that knockdown of LGALS8/galectin 8, but not of LGALS3/galectin 3, suppresses IL1B secretion induced by lysosomal damage. Furthermore, LGALS8 associates with TRIM16, enhanced by the presence of ULK1. We thus concluded that detection of lysosomal disruption by a LGALS8-TRIM16 recognition particle is the first step leading up to IL1B secretion. Importantly, IL1B binds directly to TRIM16, which then acts as a secretory autophagy receptor recognizing the cargo (IL1B) and delivers it for secretion through autophagic membranes, as delineated below.
TRIM16 has a “hidden” property unique among all TRIMs, i.e. it contains a domain found in some SNAREs. SNARE proteins mediate membrane fusion, with R-SNAREs located on donor membranes and Qa- Qb and Qc-SNAREs associated with the acceptor membranes, which when combined form an intermolecular 4-helix bundle of their SNARE domains to execute membrane fusion. The TRIM16 domain in question has features seen in longin domains, normally found in certain R-SNAREs, such as SEC22B. SEC22B is known primarily for its role in ER-to-Golgi trafficking but has also been used as an incidental marker cofractionating with MAP1LC3B-positive membranes. We found SEC22B and TRIM16 in common protein complexes and when membranes from Trim16 knockout cells are subjected to differential centrifugation, IL1B is no longer focused on MAP1LC3B-II membranes and is nonspecifically distributed. Thus, TRIM16 delivers IL1B to autophagic membranes.
How does IL1B escape degradation expected after fusion of autophagosomes with lysosomes, hitherto considered to be the inevitable itinerary for autophagosomes? A knockdown of STX17 (syntaxin 17; a Qa-SNARE inserted into nascent autophagic organelles to enable fusion between autophagosomes and lysosomes) does not affect IL1B secretion, suggesting that IL1B-containing autophagic profiles do not fuse with lysosomes en route for secretion. We next considered the possibility that autophagic profiles harboring IL1B, and positive for MAP1LC3B and the R-SNARE SEC22B, escape fusion with lysosomes altogether. We then screened plasma membrane Q-SNAREs, searching for those that might be involved in fusion processes during IL1B secretion. Individual knockdowns of the Qa-SNAREs STX3 (syntaxin 3) and STX4 (syntaxin 4) reduce IL1B secretion, whereas a combined knockdown of STX3 and STX4 abrogates IL1B secretion. Knockdowns of Qbc SNAREs SNAP23 and SNAP29 also reduce IL1B secretion. Thus, SNARE complexes involved in secretion of IL1B at the plasma membrane may consist of the R-SNARE SEC22B on autophagic intermediates destined for secretion, Qa STX3 (or alternatively STX4) on the plasma membrane, and Qbc SNAREs contributing 2 additional SNARE domains for completion of the 4-helix bundle. We propose that these SNARE combinations represent the underpinnings for the unconventional secretory pathway that delivers IL1B to the extracellular milieu where it performs its proinflammatory function. Moreover, we have shown that the secretory autophagy pathway described here is also involved in secretion of other leaderless cytosolic proteins, such as ferritin.
In conclusion, our report has defined the intracellular trafficking pathway for secretory autophagy (Fig. 1), delineated the molecular interactions enabling this pathway, identified the first cargo receptor for secretory autophagy, and established that autophagy is not always terminated in lysosomal fusion. Further studies will define other cargo and receptors using this unconventional secretory pathway and determine the physiologic scope and impact of secretory autophagy in mammalian signaling, tissue homeostasis, and human disease.
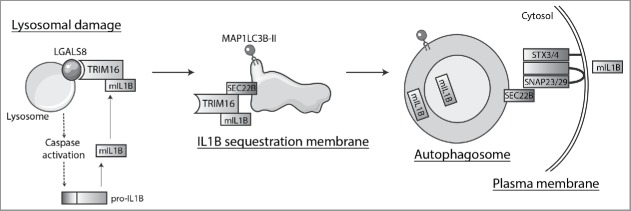
Figure 1.
Secretory autophagy pathway. Lysosomal damage induces inflammasome activation that leads to CASP1/caspase 1-dependent processing of cytosolic pro-IL1B into mature IL1B (mIL1B). At the same time, lysosomal damage is recognized by LGALS8, which detects β-galactosides on the exposed lumenal leaflet of lysosomal membrane and activates the LGALS8-TRIM16 complex. TRIM16 binds secretory autophagy cargo, e.g. mIL1B and potentially others, although other specific receptors may act independently or in combination with TRIM16. TRIM16 is in complexes with SEC22B and transfers the cargo to the autophagy-related MAP1LC3B-II-positive membrane carriers. SEC22B, now acting as an R-SNARE on the delimiting membrane facing the cytosol, carries out fusion at the plasma membrane in conjunction with the Qbc-SNARE SNAP23 (or SNAP29) and one of the plasma membrane Qa-SNAREs STX3 or STX4 (STX3/4), thus delivering mIL1B to the extracellular milieu where it exerts its biological functions.
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.