Abstract
Background
Personal health information, including diagnoses and hospital admissions, is routinely collected in administrative databases. Patients enrolling on clinical trials consent to separate collection and storage of their personal health information. We evaluated patient preferences for linking long-term data from administrative databases with clinical trials.
Methods
Adults with cancer attending outpatient clinics at 3 Ontario hospitals were surveyed about their willingness, when faced with the hypothetical scenario of participating in a clinical trial, to provide potentially identifying information such as initials and date of birth to facilitate long-term research access to normally deidentified publicly collected databases.
Results
Of 569 patients surveyed, 335 (59%) were women, 452 (79%) were white, 385 (68%) had a post-secondary education, and 386 (68%) had never participated in a clinical trial. Median age in the group was 59 years. Most participants (93%, cohort 1) would allow long-term access to their information and allow personal information to be used to match clinical trial with administrative data. At the time of clinical trial closure, two thirds of participants (68%, cohort 2) preferred to make additional clinical information available through linkage with administrative databases, and 8 (9%) preferred to have no further information made available to researchers. No significant differences were found in the subset of patients who were part of a clinical trial and those who had never participated (p = 0.65).
Interpretation
Almost all patients would allow a clinical trial research team to access their confidential information, providing a more comprehensive assessment of an intervention’s long-term risks and benefits.
Keywords: Survey, clinical trials, consent, data linkage
INTRODUCTION
Clinical trials are the “gold standard” by which new health care interventions are tested in a rigorous scientific manner, providing data for regulatory and funding bodies to determine which drugs and devices should be made available to the general population for the betterment of their health. David Sackett, widely regarded as the father of evidence-based medicine, argued in 1980 that “strategies that improve the efficiency of randomized trials and still protect their validity ... ought to be a high priority for clinicians and methodologists alike”1. In the current era, with its dazzling array of emerging drugs in association with necessary fiscal constraint, the need to identify more cost-effective approaches to clinical research has become even more pressing so as to ensure that the most effective agents are made available to patients in a timely manner. Use of data linkage to improve the efficiency and comprehensiveness of its clinical trials is a key strategic priority for the Canadian Cancer Trials Group, Canada’s academic cooperative clinical trial network that conducts clinical trials across cancer types and therapeutic modalities.
Duplication of workload, resulting in potentially avoidable costs, is inherent in the current paradigm for conducting clinical trials. The cost of bringing a drug to market is estimated to be between $161 million and $2 billion2–4. In all phases of clinical trials, administrative staff costs and site monitoring costs contribute 20%–43% of costs as reported by the U.S. Department of Health and Human Services2. Several countries, including Scotland5, have established safe processes for the routine use of clinical data in research, with the goal of expediting advances in health care and becoming hubs for scientific investment and financial reward6. Scandinavian registry-based trials have made meaningful contributions to the scientific literature in cancer, cardiovascular disease, and other fields7–9. Canada’s universal health care system, with its rich administrative and registry databases alongside its world-class expertise in clinical trials10,11, places it in a prime position to develop a harmonized approach to clinical research. Such an approach offers more than cost savings. The longitudinal nature of administrative data offers higher data quantity and quality, particularly in regard to the long-term follow-up required to fully understand the benefits and late toxicities of a given intervention12,13.
Linkage of clinical trial data collected for the purposes of research with administrative databases created in the context of health care delivery requires meticulous consideration of ethics and regulatory principles, clear processes to ensure that patient privacy is upheld, and genuine inclusion of patients and the public as partners. When reviewing research proposals, research ethics boards are obliged to consider “the public interest in conducting the research and the public interest in protecting the privacy of the individuals whose personal health information is being disclosed”14.
The objective of the present study was to establish a baseline for patient opinion and interest regarding the collection and use of personal health information in research, and specifically to determine
■ patient willingness to allow research access to publicly collected databases for long-term clinical trial follow-up, and
■ patient willingness for secure storage of potentially identifiable information within a clinical trial to facilitate data linkage.
METHODS
A self-administered questionnaire was designed to evaluate the willingness of cancer patients to allow research access to Ontario registry and administrative databases in the setting of hypothetical clinical trial participation. Eligible patients were those 18 years of age and older with an established malignant diagnosis and no significant psychological impairment, who were able to communicate in English. Research ethics board approval was obtained from the 3 participating Ontario centres: the Princess Margaret Cancer Centre in Toronto, the Stronach Regional Cancer Centre in Newmarket, and the Cancer Centre of Southeastern Ontario in Kingston. Individual patients attending outpatient cancer clinics were approached by trained research staff, invited to participate, and given an informed consent form.
Consenting participants heard a brief scripted overview of routine health data collection procedures in Ontario and collection procedures for clinical trials, with opportunities to ask questions. The subsequent self-administered questionnaire presented a theoretical scenario of being treated on a clinical trial. Participants were asked to state their willingness to allow secure storage of potentially identifiable information at a central research coordinating centre and linkage of their Ontario administrative data (such as from the Ontario Health Insurance Plan) with their clinical trial data, based on the aforementioned hypothetical scenario (see Table i). Each patient’s stated preferences were completed on a tablet computer or paper, depending on the preferences of the site and the patient. Lastly, individual study participants were presented with a brief questionnaire designed to collect their basic sociodemographic characteristics, including age, sex, ethnicity, education level, marital status, occupation, and use of Webbased social networks. Research assistants were available to assist patients in completing the questionnaires, and they documented any comments made by the participants and any sections of the questionnaire that posed particular difficulty or caused confusion.
TABLE I.
Patient preference for research access questionnaire
| A clinical trial is a study designed to evaluate the ability of new therapies or drugs to improve the outcomes of a disease. Participation in a clinical trial typically requires collection of information which may include details of
Usually when the clinical trial closes and the study period ends, additional data is rarely collected on patients. However, we know that there may be important consequences of treatment of any sort, long term; these considerations include long term side effects, disease outcomes, and costs to the health care system. Currently there is controversy as to whether information that is already routinely collected in Ontario can be used to help researchers understand long term consequences of clinical trial treatments. For example, doctor visits and procedures paid for by OHIP, discharges from hospital, and deaths are all collected in databases for government use, and are not available to researchers who are interested in the long term effects of treatments. |
| PART A | ||||||||||
|
| ||||||||||
| We would like to introduce a hypothetical scenario that is not related to your current medical treatments. In this hypothetical scenario you have already agreed to participate in a clinical trial evaluating a new treatment. We are interested to find out if in this hypothetical scenario, you would be willing to allow researchers to confidentially use the information about you which is stored in Ontario administrative databases. Potential benefits of this approach may include reducing the cost of research, possibly reducing the number of clinic visits for patients after treatment is complete, and gathering long term outcomes that would otherwise not be collected. Based on this hypothetical scenario, we would like to ask you several questions to discover your personal preferences on this subject. | ||||||||||
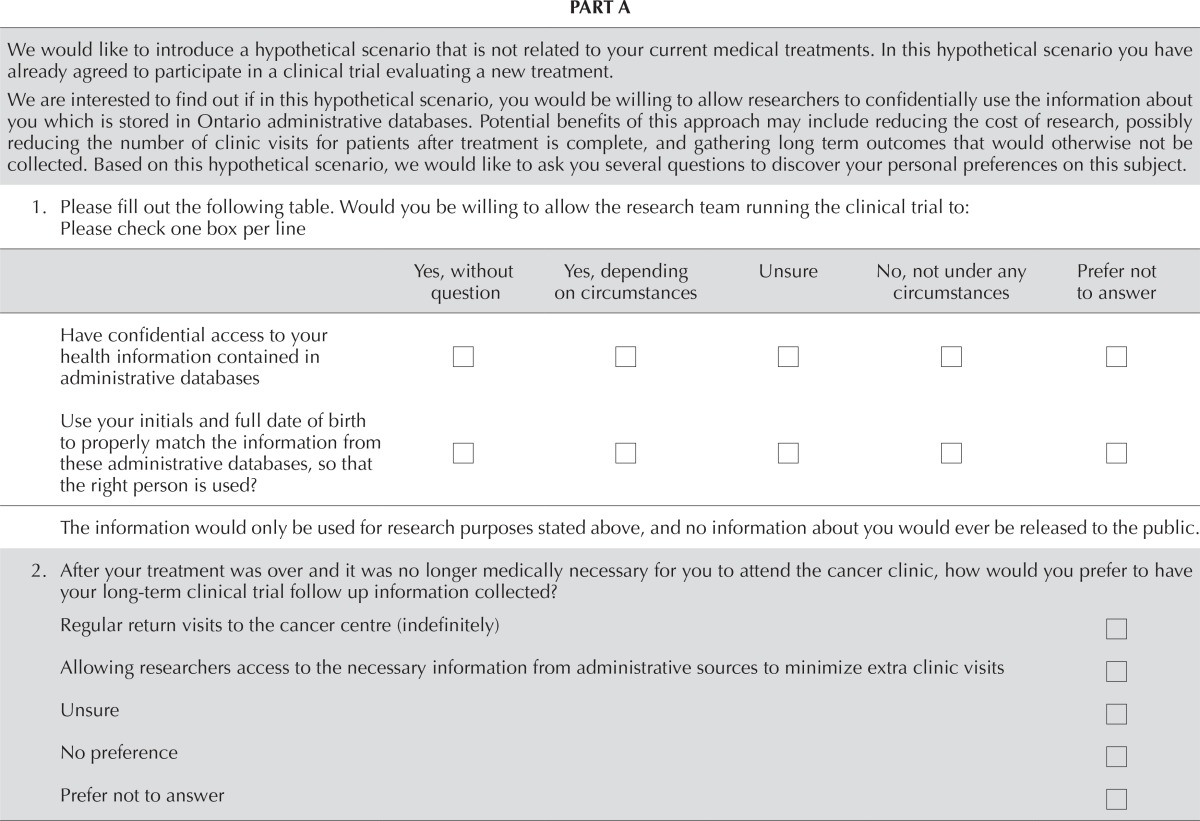
| 1. Please fill out the following table. Would you be willing to allow the research team running the clinical trial to: Please check one box per line | ||||||||||
|
| ||||||||||
| Yes, without question | Yes, depending on circumstances | Unsure | No, not under any circumstances | Prefer not to answer | ||||||
|
| ||||||||||
| Have confidential access to your health information contained in administrative databases | □ | □ | □ | □ | □ | |||||
| Use your initials and full date of birth to properly match the information from these administrative databases, so that the right person is used? | □ | □ | □ | □ | □ | |||||
|
| ||||||||||
| The information would only be used for research purposes stated above, and no information about you would ever be released to the public. | ||||||||||
| 2. After your treatment was over and it was no longer medically necessary for you to attend the cancer clinic, how would you prefer to have your long-term clinical trial follow up information collected? | ||||||||||
| Regular return visits to the cancer centre (indefinitely) | □ | |||||||||
| Allowing researchers access to the necessary information from administrative sources to minimize extra clinic visits | □ | |||||||||
| Unsure | □ | |||||||||
| No preference | □ | |||||||||
| Prefer not to answer | □ | |||||||||
|
| ||||||||||
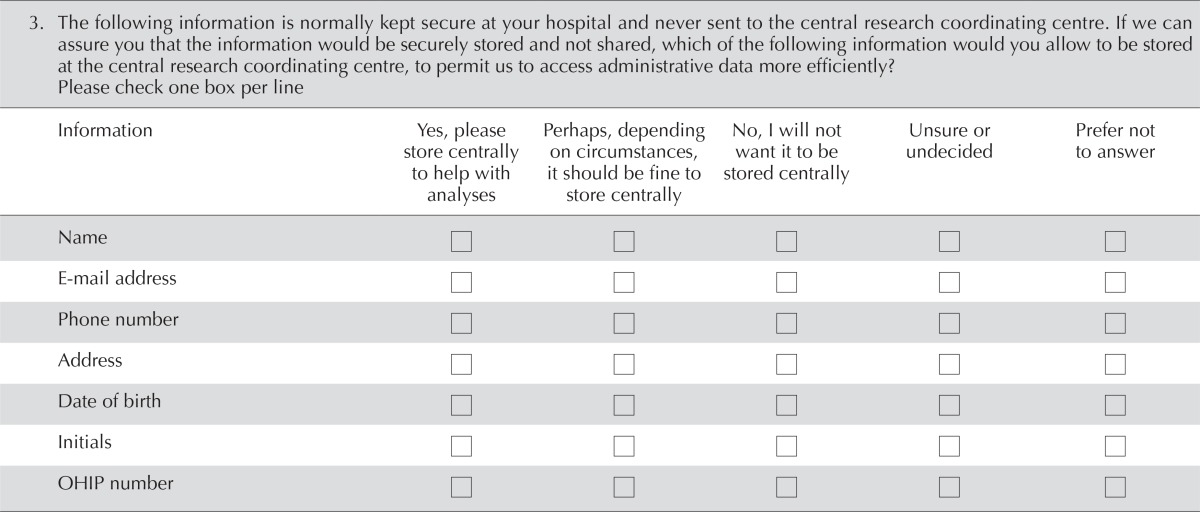
| 3. The following information is normally kept secure at your hospital and never sent to the central research coordinating centre. If we can assure you that the information would be securely stored and not shared, which of the following information would you allow to be stored at the central research coordinating centre, to permit us to access administrative data more efficiently? Please check one box per line | ||||||||||
|
| ||||||||||
| Information | Yes, please store centrally to help with analyses | Perhaps, depending on circumstances, it should be fine to store centrally | No, I will not want it to be stored centrally | Unsure or undecided | Prefer not to answer | |||||
|
| ||||||||||
| Name | □ | □ | □ | □ | □ | |||||
| E-mail address | □ | □ | □ | □ | □ | |||||
| Phone number | □ | □ | □ | □ | □ | |||||
| Address | □ | □ | □ | □ | □ | |||||
| Date of birth | □ | □ | □ | □ | □ | |||||
| Initials | □ | □ | □ | □ | □ | |||||
| OHIP number | □ | □ | □ | □ | □ | |||||
| PART B | |||||
|
| |||||
| Please provide us with some general Information about yourself. | |||||
1. In which month and year were you born?
| |||||
| 2. What is your gender? □ Male □ Female | |||||
| 3. Ethnicity □ White/Caucasian □ Hispanic/Latino (Central and South America) □ Black/African Canadian □ Arab/Middle Eastern □ Caribbean □ First Nations/Aboriginal/Inuit □ South Asian (East Indian, Pakistani, Sri Lankan) □ East Asian/SE Asian (Chinese, Japanese, Filipino, Vietnamese, Malaysian etc.) □ Prefer not to answer □ Other: ________________________ | |||||
| 4. Maritalstatus □ Single □ Married/living with partner □ Widowed □ Separated/divorced □ Prefer not to answer | |||||
| 5. Highest level of education completed □ Professional degree, Masters, PhD □ University, college degree □ Vocational, technical, diploma, certificate □ High school graduate □ Some high school □ Elementary school □ Prefer not to answer | |||||
| 6. What is your current employment status? □ Employed □ Unemployed □ Self-employed □ On leave □ Never employed □ Retired □ Full/part-time student | |||||
| 7. Have you ever participated in a clinical trial of cancer treatment? □ Yes □ No □ Unsure | |||||
| 8. Which of these social media do you use? □ Facebook □ Twitter □ LinkedIn □ Personal blog □ None □ Other: ___________________ | |||||
|
| |||||
| Thank you for completing this questionnaire! | |||||
Upon completion of the interview, a research assistant accessed the individual’s medical records and collected information about the patient’s cancer type, date of diagnosis, treatment received, and prior clinical trial participation. Results were recorded in a secure, password-protected database.
Descriptive statistics (mean, median, range, frequency, and percentage) were used to describe each sociodemographic and medical variable. Comparisons of variables between cohorts were performed using Student t-tests and nonparametric tests (chi-square and Mann–Whitney U), as appropriate. Subgroup analyses evaluated site differences for the sociodemographic and medical variables, and the primary outcomes of willingness and preferences for how health information should be used. Finally, multivariable logistic regression was used to assess the relations between the 3 sites and patient willingness concerning linking and sharing personal health information, while accounting for differences in sociodemographic and medical data. All analyses were conducted using the SAS software application (version 9.2: SAS Institute, Cary, NC, U.S.A.).
RESULTS
Between August 2014 and May 2015, 569 eligible patients completed the survey at the 3 Ontario cancer centres, covering both academic and community oncology practices. A total of 177 patients who were approached declined to participate (163 in Toronto, 10 in Kingston, 4 in Newmarket). Table ii presents the characteristics of the study participants. As a measure of electronic literacy in the sample, 47% made use of some form of social media: 40% used Facebook, 19% used LinkedIn, 29% used Twitter, 3% had a personal blog, and 6% used other forms of social media. The original survey was administered to the greater proportion of the patients (cohort 1), but because of a potential misinterpretation related to one particular question, that question was rephrased and additional cancer patients were surveyed primarily on it (cohort 2).
TABLE II.
Characteristics of the study participants
| Characteristic | Cohort | |
|---|---|---|
|
| ||
| 1 | 2 | |
| Eligible participants (n) | 483 | 86 |
| Age (years) | ||
| Mean | 59 | 58 |
| Range | 20–93 | 19–88 |
| Sex [n (%) women] | 285 (59) | 50 (58) |
| Currently working [n (%)] | 238 (49) | 29 (34) |
| Ethnicity [n (%)] | ||
| White | 393 (81) | 59 (69) |
| Asian | 49 (10) | 19 (22) |
| Other or prefer not to answer | 36 (7) | 7 (8) |
| Missing | 5 (1) | 1 (1) |
| Highest education [n (%)] | ||
| University. college. professional | 293 (61) | 53 (62) |
| Vocational. technical. diploma | 36 (7) | 3 (3) |
| Elementary. high school | 141 (29) | 28 (33) |
| Prefer not to answer | 0 | 1 (1) |
| Missing | 13 (3) | 1 (1) |
| Marital status [n (%)] | ||
| Married or living with partner | 348 (72) | 60 (70) |
| Single, separated, divorced, widowed | 124 (26) | 24 (28) |
| Prefer not to answer | 0 | 1 (1) |
| Missing | 11 (2) | 1 (1) |
| Participated in clinical trial [n (%)] | 155 (32) | 28 (33) |
| Treating centre [n (%)] | ||
| Princess Margaret Cancer Centre | 353 (73) | 86 (100) |
| South Lake Regional Cancer Centre | 30 (6) | 0 |
| Cancer Centre of South Eastern Ontario | 100 (21) | 0 |
| Cancer type [n (%)] | ||
| Breast | 97 (20) | 12 (14) |
| Gastrointestinal | 78 (16) | 8 (9) |
| Genitourinary | 72 (15) | 11 (13) |
| Thoracic | 67 (14) | 3 (3) |
| Hematologic | 58 (12) | 15 (17) |
| Head and neck | 55 (11) | 18 (21) |
| Gynecologic | 52 (11) | 11 (13) |
| Other | 4 (1) | 8 (9) |
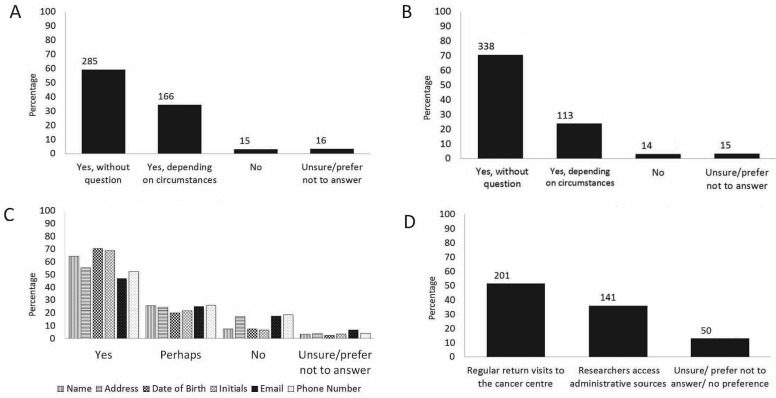
Figure 1 presents the responses of the 483 eligible patients defined as cohort 1. A wide variety of malignancies were represented, including breast (n = 97), gastrointestinal (n = 78), genitourinary (n = 72), thoracic (n = 67), and hematologic (n = 58). One third of the group had previously participated in a clinical trial. Most participants (n = 451, 93%) were willing to allow the central research team running a clinical trial to access their health information contained in administrative databases. A preponderance of them were also willing to confidentially provide personal identifiers, including date of birth (n = 436, 90%), initials (n = 433, 90%), name (n = 432, 89%), address (n = 380, 79%), telephone number (n = 373, 77%), Ontario Health Insurance Plan number (n = 370, 77%), and e-mail address (n = 322, 67%) to facilitate data linkage and conduct of the clinical trial. However, when asked what their preference would be for long-term clinical trial follow-up, more preferred regular return visits to the cancer centre (n = 201, 42%) than data collection from administrative sources (n = 141, 29%), even when clinic visits were no longer medically required. However, 91 patients (19%) did not answer that question.
FIGURE 1.
Responses of eligible participants in cohort 1 to selected survey questions. (A) Would you be willing to allow the research team running the clinical trials confidential access to your health information contained in administrative databases? [n]. (B) Would you be willing to allow the research team running the clinical trials to use your initials and full date of birth to match the information from administrative databases? [n]. (C) What information would you allow to be stored securely at the central research coordinating centre? (D) After your treatment was over and it was no longer necessary for you to attend the cancer clinic, how would you prefer to have your long-term follow-up information collected? [n].
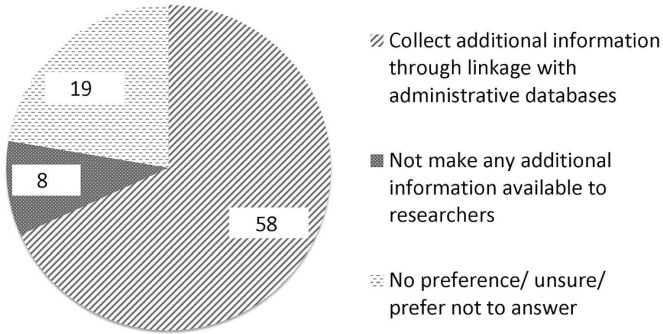
Informal comments from a number of patients, documented by research assistants, revealed that the preference for regular return visits was meant to ensure that the participant received long-term follow-up by a physician for their cancer, which was not the intent of that particular question. Subsequently, 86 eligible patients (cohort 2), all from the Princess Margaret Cancer Centre, were therefore asked to complete a separate clarification question. At the time of clinical trial closure, in the absence of an option to return to the cancer clinic, two thirds of those patients (n = 58, 68%) preferred to make additional clinical information available by linkage with administrative databases; only 8 (9%) preferred to have no further information made available to researchers. The remainder were uncertain or had no preference (Figure 2).
FIGURE 2.
Responses of eligible participants in cohort 2 to the question “After entering a clinical trial, individuals are followed up for a period of time. At some point the trial closes and no additional information is collected. What would be your preference after trial closure?” (n).
Documentation from the research assistants responsible for obtaining informed consent and answering questions for the patients revealed that a number of patients were at first confused by current practice. Those who had previously participated in a clinical trial provided all their identifying information to the research team at their centre; a number did not realize it was not made available to the study sponsor at the central research coordinating centre. Several patients also appeared unaware of the routine collection of their health information in administrative and registry databases in Ontario.
Results were similar across all the sociodemographic subgroups studied. No significant differences were found in the subset of patients who were part of a clinical trial and those who had never participated (p = 0.65). Older patients were even more likely than younger patients to allow access to data (p = 0.02). Differences in preferences were observed between the study sites. Logistic regression analysis revealed that, compared with patients at the Cancer Centre of Southeastern Ontario in Kingston, those at the Princess Margaret Cancer Centre in Toronto were less likely to prefer data sharing and linkage (p < 0.001; odds ratio: 0.275; 95% Wald confidence interval: 0.146 to 0.515). When comparing Kingston and Newmarket (Stronach Regional Cancer Centre), no significant difference was observed (p = 0.655; odds ratio: 0.783; 95% Wald confidence interval: 0.267 to 2.292). Nonetheless, in all centres, more than half the patients favoured data sharing and linkage.
INTERPRETATION
Our survey of 569 eligible cancer patients attending outpatient clinics in Ontario demonstrates that most participants support data linkage for clinical trial purposes and would be willing to confidentially provide personal identifying information to facilitate linkage. In addition, the study revealed a lack of knowledge and understanding on the part of patients, even those who had participated in clinical trials, about provincial processes in place to routinely collect and store their health care data and about the overall conduct of multicentre clinical trials. Increased willingness for data linkage was reported by participants attending outpatient cancer clinics in Newmarket and Kingston compared with those attending clinics in Toronto. Although confounding factors are likely contributing to the observed difference, it might be hypothesized that cultural differences between large cities and smaller communities could alter an individual’s level of concern about issues of privacy and confidentiality.
Data sharing is increasingly recommended to expedite health care advances in a cost-effective manner6. The report Sharing Clinical Trial Data from the U.S. National Academy of Medicine15 emphasizes that responsible sharing of clinical trial data is in the public interest, maximizing contributions made by clinical trial participants to scientific knowledge that benefits future patients and society as a whole. The report’s first recommendation is that stakeholders in clinical trials foster a culture in which data sharing is the expected norm and that they commit to responsible strategies aimed at maximizing the benefits, minimizing the risks, and overcoming the challenges for all parties. Guiding principles of data sharing include respecting individual participants and increasing public trust15.
In Canada, realization of the importance of responsible data sharing across research modalities and provinces for the public good is increasing. More than 300,000 Canadians have already enrolled themselves on the Canadian Partnership for Tomorrow Project population health research platform, consenting to collection of their future health information from administrative databases for research purposes (http://www.partnershipfortomorrow.ca). Through the Canadian Primary Care Sentinel Surveillance Network, data contained in electronic records for more than 1,000,000 patients in 8 provinces and territories are available for public health research (http://cpcssn.ca). The Network has a clear policy in place to ensure the protection of personal health information and patient privacy; individual patient consent is not required. Senior representatives from organizations across Canada are working together to develop a Pan-Canadian Real-World Health Data Network that will make multi-province studies viable, with the goal of designing a research data infrastructure that is useful and transformative for Canadian researchers and policymakers (http://www.prhdn.ca).
Yet, within clinical trials in many parts of the world, including Canada, such linkage does not routinely occur. Indeed, there has been a perceived barrier to requesting, from clinical trial participants, identifying information that would be essential to enable linkage. A special session at the Clinical Trials Ontario 2015 conference focused on opportunities and challenges in linking data for clinical research, including the Canadian Randomized Registry Trial Initiative that is in development16. To minimize costs, a team of researchers from across Canada—including physicians from multiple specialties, bioethicists, and health service researchers—has prioritized improving the national climate for registry trials to evaluate short- and long-term outcomes.
The results of the survey described here provide insight into the preferences of a subset of Ontario cancer patients in the context of hypothetical clinical trial participation. Other disease types, research settings, and geographic locations were not included. The study design did not allow for the ordering of patient preferences. Nonetheless, the survey responses provide an important baseline of patient opinion that is supportive of secure data linkage. Under the Canadian Personal Health Information Protection Act, 200414 [chapter 2, schedule A, section 44(3) (c)], research ethics boards are required, when reviewing proposed research, to consider the public interest both in conducting the research and in protecting the privacy of the individuals whose personal health information is being disclosed. Findings from our study demonstrate patient willingness to provide personal health information for the conduct of research—a willingness that might help research ethics boards and institutions during their decision-making process. Additionally, results could help to guide researchers and data custodians in their development of future clinical trials and a more harmonized research infrastructure.
Within the Canadian Cancer Trials Group, formal collaborations with Canadian holders of administrative and registry data are in development, crucially with support and input from the Canadian Cancer Trials Group Lay Representative Committee. This engaged and capable group of patient advocates, with personal experience of cancer and an understanding of clinical research processes, provides a critical link between researchers and the public, as together we seek new ways of working.
Clinical trials, a vital part of clinical research, must adapt to an evolving environment to survive and thrive. Although reduced funding threatens the viability of academic clinical trials, that challenge presents an opportunity to make use of Canada’s rich data resources for public benefit. By optimizing data collection procedures already in place (typically used to conduct health services research), clinical trials would be enhanced with longitudinal data, providing a greater understanding of an intervention’s long-term risks and benefits. Simultaneously, there is a great potential to reduce the workload and costs associated with clinical trials, which would be key to testing a large and expanding pool of therapeutic interventions with reduced financial resources. Patients are supportive of secure data linkage, although some have a limited understanding of current research practices and administrative data collection processes. In collaboration with all stakeholders, it is vital to engage the public in conversation to ensure that research is designed to benefit them and is conducted in accordance with their wishes and the law.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Sackett DL. The competing objectives of randomized trials. N Engl J Med. 1980;303:1059–60. doi: 10.1056/NEJM198010303031812. [DOI] [PubMed] [Google Scholar]
- 2.Sertkaya A, Birkenbach A, Berlind A, Eyraud J, on behalf of the Eastern Research Group . Examination of Clinical Trial Costs and Barriers for Drug Development [report to the U.S. Department of Health and Human Services, Assistant Secretary of Planning and Evaluation] Lexington, MA: Eastern Research Group; 2014. [Google Scholar]
- 3.Goozner M. The $800 Million Pill: The Truth Behind the Cost of New Drugs. Berkeley, CA: University of California Press; 2004. [Google Scholar]
- 4.Light DW, Lexchin JR. Pharmaceutical research and development: what do we get for all that money? BMJ. 2012;345:e4348. doi: 10.1136/bmj.e4348. [DOI] [PubMed] [Google Scholar]
- 5.Hagger-Johnson G. Opportunities for longitudinal data linkage in Scotland. Scott Med J. 2016;61:136–45. doi: 10.1177/0036933015575214. [DOI] [PubMed] [Google Scholar]
- 6.Joined-Up Data for Better Decisions: Guiding Principles for Data Linkage. Edinburgh, Scotland: The Scottish Government; 2015. [Google Scholar]
- 7.Nystrom L, Andersson I, Bjurstam N, Frisell J, Nordenskjold B, Rutqvist LE. Long-term effects of mammography screening: updated overview of the Swedish randomised trials. Lancet. 2002;359:909–19. doi: 10.1016/S0140-6736(02)08020-0. [DOI] [PubMed] [Google Scholar]
- 8.Jensen LO, Thayssen P, Maeng M, et al. Randomized comparison of a sirolimus-eluting Orsiro stent with a biolimus-eluting Nobori stent in patients treated with percutaneous coronary intervention: rationale and study design of the Scandinavian Organization for Randomized Trials with Clinical Outcome vii trial. Am Heart J. 2015;170:210–15. doi: 10.1016/j.ahj.2015.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Rubin KH, Holmberg T, Rothmann MJ, et al. The risk-stratified osteoporosis strategy evaluation study (rose): a randomized prospective population-based study. Design and baseline characteristics. Calcif Tissue Int. 2015;96:167–79. doi: 10.1007/s00223-014-9950-8. [DOI] [PubMed] [Google Scholar]
- 10.Adams J. Benchmarking international research. Nature. 1998;396:615–18. doi: 10.1038/25219. [DOI] [PubMed] [Google Scholar]
- 11.Yusuf S, Cairns J. The perilous state of independent randomized clinical trials and related applied research in Canada. CMAJ. 2012;184:1997–2002. doi: 10.1503/cmaj.110598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robles SC, Marrett LD, Clarke EA, Risch HA. An application of capture–recapture methods to the estimation of completeness of cancer registration. J Clin Epidemiol. 1988;41:495–501. doi: 10.1016/0895-4356(88)90052-2. [DOI] [PubMed] [Google Scholar]
- 13.Williams JI, Young W. A summary of studies on the quality of health care administrative databases in Canada. In: Goel V, Williams JI, Anderson GM, Blackstien-Hirsch P, Fooks C, Naylor CD, editors. The ICES Practice Atlas: Patterns of Health Care in Ontario. 2nd ed. Ottawa, ON: Canadian Medical Association; 1996. pp. 339–45. [Google Scholar]
- 14.Ontario . Canadian Personal Health Information Protection Act, 2004. Toronto, ON: Government of Ontario; 2004. Chapter 3, part IV. [Google Scholar]
- 15.United States, The National Academies, National Academy of Medicine . Sharing Clinical Trial Data: Maximizing Benefits, Minimizing Risk. Washington, DC: The National Academies Press; 2015. [PubMed] [Google Scholar]
- 16.Clinical Trials Ontario . Clinical Trials Conference: Conference Digest 2015. Toronto, ON: Clinical Trials Ontario; 2015. [Available online at: http://www.ctontario.ca/cms/media/CTO-2015-Clinical-Trials-Conference-Digest1.pdf; cited 14 May 2017] [Google Scholar]