Abstract
Value-based care, which balances high-quality care with the most efficient use of resources, has been considered the next frontier in cancer care and a means to maintain health system sustainability. Created to promote value-based care, Choosing Wisely Canada—modelled after Choosing Wisely in the United States—is a national clinician-driven campaign to identify unnecessary or harmful services that are frequently used in Canada. As part of the campaign, national medical societies have developed recommendations for tests and treatments that clinicians and patients should question. Here, we present baseline indicator findings about current practice patterns associated with 7 cancer-related recommendations from Choosing Wisely Canada and about the effects of those practices on patients and the health care system.
Indicator findings point to substantial variations in cancer system performance between Canadian jurisdictions, most notably for breast cancer screening practices, treatment practices for men with low-risk localized prostate cancer, and radiation therapy practices for early-stage breast cancer and bone metastases. Extrapolating indicator findings to the entire country, it was estimated that 740,000 breast and cervical cancer screening tests were performed outside of the recommended age ranges, and within 1 year of diagnosis, 17,000 patients received treatments that could be low-value. A 15% reduction in the use of the 7 screening and treatment practices examined could lead to multiple benefits for patients and the health care system: 9000 false-positive results and 3000 treatments and related side effects could be avoided, and 4500 hours of linear accelerator capacity could be freed up each year.
Interjurisdictional performance variations suggest potential differences in clinical practice patterns in the planning and delivery of cancer control services, and in some cases, in disease management outcomes. Although the cancer screening and treatment practices described might be unnecessary for some patients, it is important to realize that they could, in fact, be necessary for other patients. Further research into appropriate rates of use could help to determine how much cancer care represents overuse of practices that are not supported by evidence or underuse of practices that are supported by evidence.
Keywords: Value-based care, appropriateness of care, cancer screening, surgery, chemotherapy, radiation therapy
INTRODUCTION
Value-based care, which balances high-quality care with the most efficient use of resources, has been considered the next frontier in cancer care and a means to maintain health system sustainability1–3. Although perspectives about how outcomes and resources should be prioritized and measured differ, a desire for high-value care unites patients, clinicians, the health care system, and society.
Ensuring value-based care is becoming even more important, given that, in Canada, estimates suggest that the average annual number of new cancer cases will increase by 79% from 2003–2007 to 2028–20324. The increase in new cancer cases is driven primarily by Canada’s aging and growing population. Between 2003–2007 and 2028–2032, Canadians 65 years of age or older will come to represent close to one quarter of the population, and estimates place the growth of the Canadian population at 29% (that is, 9.5 million more residents), putting considerable strain on limited health care resources4.
Created to promote value-based care, Choosing Wisely Canada—modelled after Choosing Wisely in the United States—is a national campaign to identify unnecessary or harmful services that are frequently used in Canada5. This clinician-driven campaign is intended to facilitate conversations between clinicians and patients about unnecessary tests and treatments, and helps both groups to make effective choices about care. As part of the campaign, national medical societies such as the Canadian Society of Surgical Oncology, the Canadian Association of Medical Oncologists, the Canadian Association of Radiation Oncology, the Canadian Medical Association, and the College of Family Physicians of Canada have developed cancer-related recommendations about tests and treatments that clinicians and patients should question6–8.
Here, we present baseline findings about current practice patterns associated with 7 cancer-related recommendations from Choosing Wisely Canada and the effects of those practices on patients and the health care system.
METHODS
Choosing Wisely Canada describes 15 cancer-related practices that should be questioned by patients and clinicians. We were able to measure current practice patterns associated with 7 of those 15 cancer control practices (Table i). The other 8 practices were not included because of data limitations or lack of available data.
TABLE I.
Choosing Wisely Canada cancer-related recommendations and associated indicators
| Specialty | Recommendation | Rationale | Proxy indicator |
|---|---|---|---|
| Family medicine | Do not routinely use screening mammography for average-risk women 40–49 years of age. | For women 40–49 years of age, the benefits of screening mammography (that is, on mortality) are low, and the risk of a false positive is higher than it is for older women7,9,10. | Self-reported breast cancer screening mammography performed on average-risk women 40–49 years of age |
| Family medicine | Do not screen women with Pap smears if under 21 years of age or over 69 years of age. | Compared with screening beginning at 21 years of age, screening women younger than 21 does not contribute to additional reductions in incidence and mortality and can lead to greater harm than benefit. Screening after age 69 also shows little to no benefit11,12. | Self-reported cervical cancer screening outside the recommended age range of 21–69 years |
| Oncology | Do not routinely use extensive locoregional therapy in most cancer situations in which there is metastatic disease and minimal symptoms attributable to the primary tumour. | Generally, for patients with metastatic disease from solid-organ malignancies and a relatively asymptomatic primary tumour, systemic therapy is the priority treatment13–15. In many such cases, locoregional treatments such as surgery or radiation therapy do not yield material improvements in outcome (for example, survival) and are associated with significant morbidity in patients with metastatic disease16–19. | Colorectal resections for patients with stage IV colorectal cancer Radiation therapy to the primary cancer site for patients with stage IV rectal cancer Mastectomy or lumpectomy for patients with stage IV breast cancer |
| Oncology | Do not initiate management in patients with low-risk prostate cancer without first discussing active surveillance. | Many prostate cancers are slow-growing and will not cause morbidity or death in a man’s lifetime if left untreated. To mitigate the risks associated with overdiagnosis and consequent overtreatment, active surveillance is recommended for many men with low- risk localized prostate cancer20. | Treatment for patients with low-risk localized prostate cancer |
| Oncology | Do not initiate whole-breast radiation therapy in 25 fractions as part of breast-conservation therapy in women 50 or more years of age with early-stage invasive breast cancer without considering shorter treatment schedules. | Compared with conventionally fractionated schedules (that is, 45–50 Gy in 25–28 fractions over 5 weeks, with or without a subsequent radiation boost to the primary site), shorter courses of radiation (for example, 42.5 Gy in 16 fractions delivered over 3 weeks, with or without a boost) have been suggested to provide equivalent tumour control and cosmetic outcomes21–23. | Conventional fractionation (that is, 25 fractions) after breast-conserving surgery for women with stage I or II breast cancer |
| Oncology | Do not recommend more than a single fraction of palliative radiation for an uncomplicated painful bone metastasis. | Compared with multi-fraction regimens, single-fraction radiation to a previously unirradiated, uncomplicated peripheral bone metastasis has been suggested to offer equivalent pain relief and morbidity (but a higher incidence of re-treatment at a later date)24,25. | Multiple fractions (>1) of palliative radiation therapy for bone metastasis in cancer patients |
| Oncology | Avoid chemotherapy and instead focus on symptom relief and palliative care in patients with advanced cancer unlikely to benefit from chemotherapy. | In general, cancer-directed therapies are not likely to be effective in patients with advanced metastatic tumours who are markedly debilitated by their cancer. Providing symptom control and palliative care aimed at improving quality of life should therefore be the priority26. | Start of a new chemotherapy regimen in the last 30 days of life |
Data about breast and cervical cancer screening practices were obtained from the Canadian Community Health Survey. Data about treatment practices were obtained from provincial cancer registries and associated databases. Standardized specifications and methodologies were used to ensure comparability across jurisdictions.
The effects of the practices were calculated using data from several sources: the Canadian Community Health Survey (number of Pap tests or mammograms in each jurisdiction), provincial cancer registries (number of treatments provided in each jurisdiction), Statistics Canada’s socioeconomic databases (number of cancer cases in each age group in Canada and by jurisdiction), the published literature (false-positive rates, linear accelerator time used per fraction), and OncoSim, a Web-based microsimulation economic forecasting model.
For the detailed calculation methods used for both the indicators and the effects, see the Technical Appendix in Quality and Sustainability: A System Performance Spotlight Report at http://systemperformance.ca.
RESULTS
-
■ Do not routinely use screening mammography for average-risk women 40–49 years of age.
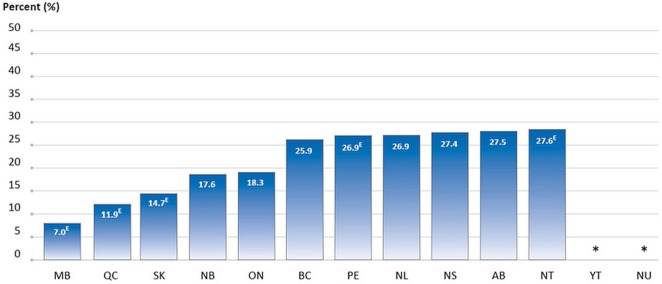
The percentage of screening mammograms performed in the past year for women 40–49 years of age ranged from a low of 7.0% to a high of 27.6% (2008–2012 data), depending on the province or territory (13 jurisdictions submitted data; Figure 1).
-
■ Do not screen women with Pap smears if under 21 years of age or over 69 years of age.
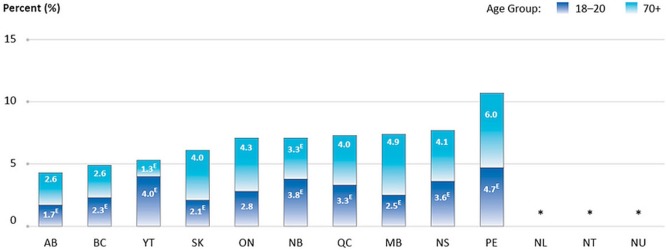
Of all Pap tests performed in the 3 years preceding the question being asked (2008–2012 data), between 4.3% and 10.7% were performed for women outside the recommended age range of 21–69 years, depending on the province or territory (13 jurisdictions submitted data, Figure 2).
-
■ Do not routinely use extensive locoregional therapy in most cancer situations in which there is metastatic disease and minimal symptoms attributable to the primary tumour.
The percentage of patients with stage iv colorectal cancer who underwent colorectal resection in 2013 ranged from 32.1% to 58.3%, depending on the province (5 provinces submitted data, Figure 3). The percentage of patients with stage iv rectal cancer who received radiation therapy to their primary cancer site ranged from 15.2% to 39.0%, depending on the province (6 provinces submitted data, Figure 3). The percentage of patients with stage iv breast cancer who received a mastectomy or lumpectomy ranged from 20.0% to 48.0%, depending on the province (5 provinces submitted data, Figure 3).
-
■ Do not initiate management in patients with low-risk prostate cancer without first discussing active surveillance.
The percentage of men with low-risk localized prostate cancer who received treatment (surgery, radiation therapy, or both) ranged from 8.6% to 46.6%, depending on the province (6 provinces submitted data, Figure 4).
-
■ Do not initiate whole-breast radiation therapy in 25 fractions as part of breast-conservation therapy in women 50 or more years of age with early-stage invasive breast cancer without considering shorter treatment schedules.
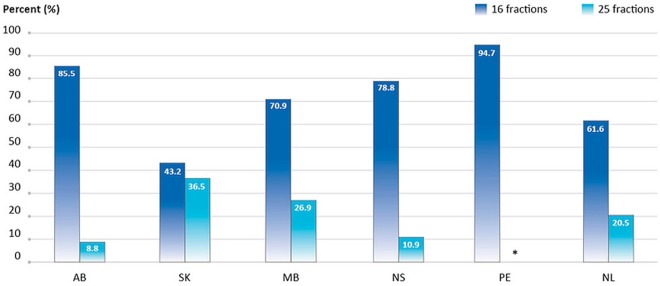
The percentage of women 50 or more years of age diagnosed in 2013 with stage i or ii breast cancer who received 25 fractions as part of breast-conservation therapy ranged from 8.8% to 36.5%, depending on the province (6 provinces submitted data, but data from Prince Edward Island was suppressed because of small numbers; Figure 5). In contrast, between 43.2% and 94.7% of women 50 or more years of age received 16 fractions as part of breast-conservation therapy (Figure 5).
-
■ Do not recommend more than a single fraction of palliative radiation for an uncomplicated painful bone metastasis.
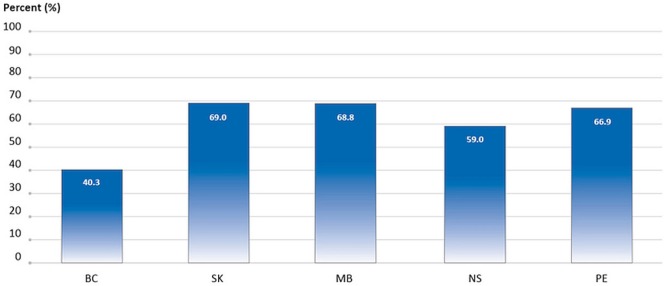
The percentage of cancer patients who received more than 1 fraction of radiation to bone in 2013 ranged from 40.3% to 69.0%, depending on the province (5 provinces submitted data, Figure 6).
-
■ Avoid chemotherapy, and instead focus on symptom relief and palliative care in patients with advanced cancer unlikely to benefit from chemotherapy.
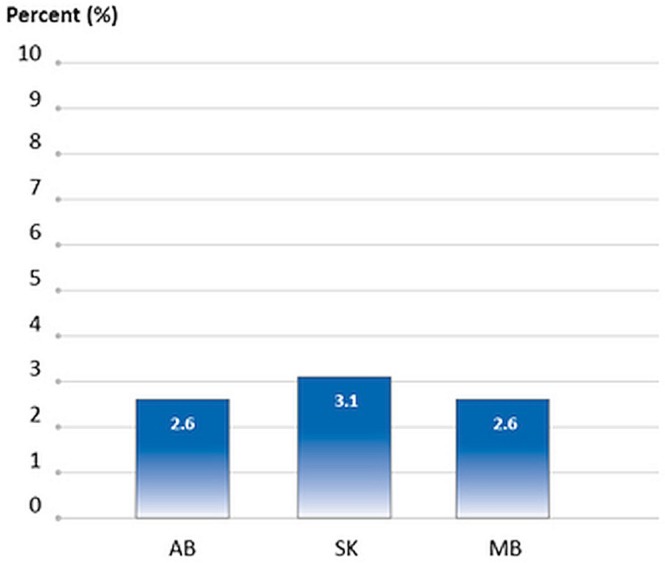
For cancer patients who died in 2012 and 2013, between 2.6% and 3.1% started a new chemotherapy regimen in their last 30 days of life, depending on the province (3 provinces submitted data, Figure 7).
FIGURE 1.
Percentage of all screening mammograms in the preceding year that were reported for women 40–49 years of age, by province or territory, 2008–2012 reporting years combined. A woman was deemed to have undergone screening mammography if the reason for the test was one of family history of breast cancer, regular check-up or routine screening, age, or current use of hormone replacement therapy. Women 40 years of age and older were included in the denominator for this indication. All jurisdictions provided data for 2008 and 2012. Screening data were optional during 2009–2011, and the jurisdictions providing data for those years were AB, NB, NS, NL, NT (2009); AB, NB, NS, NL, NT (2010); and AB, ON, NL, NU (2011). Data for YT and NU are suppressed because of small numbers. EThese data should be interpreted with caution because of large variability in the estimates. Data source: Statistics Canada, Canadian Community Health Survey.
FIGURE 2.
Percentage of all Pap tests performed in the preceding 3 years that were reported for women outside the recommended age range of 21–69 years, by province or territory, 2008–2012 reporting years combined. All jurisdictions provided data for 2008 and 2012. Screening data were optional during 2009–2011, and the jurisdictions providing data for those years were NS, PE, YT, NU (2009); NS, PE, YT, NU (2010); and ON, NU (2011). Data for NL, NT, and NU are suppressed because of small numbers. EThese data should be interpreted with caution because of large variability in the estimates. Data source: Statistics Canada, Canadian Community Health Survey.
FIGURE 3.
Percentage of patients with stage IV cancer receiving treatment to the primary site, by province and disease site, 2013 diagnosis year. The data include surgery to the primary site for colorectal and breast cancer within 1 year of diagnosis, and radiation therapy to the primary site for rectal cancer within 1 year of diagnosis. A horizontal bar indicates unavailable data; an asterisk indicates data suppressed because of small numbers. Data source: Provincial cancer agencies and programs.
FIGURE 4.
Percentage of men with low-risk prostate cancer who received various types of treatment, by province, 2013 diagnosis year. In MB and PE, “Surgery only,” “RT only,” and “Surgery with adjuvant RT” were combined because of small numbers. In NS, “RT only” and “Surgery with adjuvant RT” were combined because of small numbers. RT = radiation therapy. Data source: Provincial cancer agencies and programs.
FIGURE 5.
Percentage of patients 50 or more years of age with stage I or II breast cancer receiving 16 compared with 25 fractions of radiation therapy after breast-conserving surgery, by province, 2013 diagnosis year. Data include female patients only and exclude boosts. In PE, data for 25 fractions were suppressed because of small numbers. In MB, data reflect the number of planned fractions rather than the number of fractions actually delivered. Data source: Provincial cancer agencies and programs.
FIGURE 6.
Percentage of cancer patients receiving more than 1 fraction of palliative radiation therapy to bone, by province, 2013 treatment year. In MB, data reflect the number of planned fractions rather than the number of fractions actually delivered. Data source: Provincial cancer agencies and programs.
FIGURE 7.
Percentage of cancer patients receiving chemotherapy in the last 30 days of life, by province, 2012 and 2013 death years combined. In AB, SK, and MB, oral chemotherapy is included. In MB, data for oral chemotherapy were not complete in the cancer registry, but have been included if available. In AB, all data reflect patients who started a new chemotherapy regimen within 30 days of death. In SK, death information was not available for all of 2013, and so their 2013 data cover January to July; all data reflect patients who started a new chemotherapy regimen within 30 days of death, as indicated by a new chemotherapy order. In MB, only 2012 data are shown, because cause-of-death information was not available for 2013. In addition, their chemotherapy data are recorded only once annually, and so only patients who started their first cycle of chemotherapy within 30 days of death are included. Data source: Provincial cancer agencies and programs.
Effect on Patients and the Health Care System
Extrapolating the indicator findings to the entire country, approximately 740,000 breast and cervical cancer screening tests were performed outside of the recommended age ranges, and within 1 year of diagnosis, 17,000 cancer patients received treatments that could be low-value. A 15% reduction in the use of the 7 screening and treatment practices examined could lead to multiple benefits for patients and the health care system:
■ 9000 false-positive results could be avoided each year.
■ 3000 treatments and treatment-related side effects could be avoided each year.
■ 4500 hours of linear accelerator capacity could be freed up each year.
DISCUSSION AND CONCLUSIONS
Here, we presented baseline findings about the practice patterns currently associated with 7 Choosing Wisely Canada recommendations. Findings suggest that some high-quality, sustainable cancer control practices are already in place. For example, cervical cancer screening outside the recommended age range is minimal, and use of aggressive end-of-life care—chemotherapy in the last month of life—is low. Indicator findings also point to substantial variations in cancer control practices across the country. Some of the more notable areas with substantial interjurisdictional variations are breast cancer screening practices, treatment practices for men with low-risk localized prostate cancer, and radiation therapy practices for early-stage breast cancer and bone metastases. Those variations have implications for quality of life and, in some cases, could result in differences in disease management outcomes. Reporting on those indicators at the system level allows for the identification of interjurisdictional gaps in performance and of Canadian benchmarks representing leading practices that can be adopted across the country. Follow-up work is being done to understand the causes of practice variation for some indicators and to promote adherence to recommended practice.
In extrapolating indicator findings to the entire country, it was estimated that 740,000 breast and cervical cancer screening tests were performed outside the recommended age ranges and that, within 1 year of diagnosis, 17,000 patients received treatments that could be low-value. The recommendations related to those indicators were created by national medical societies and provide information about when tests and treatments might not be appropriate. Although the cancer screening and treatment practices described might be unnecessary for some patients, it is important to realize that they could, in fact, be necessary for other patients. The Choosing Wisely campaign therefore encourages clinicians and patients to discuss the evidence-based recommendations so that informed decisions can be made about the most appropriate care based on each patient’s individual situation. Further research into appropriate rates of use could help to determine the proportion of cancer care that represents overuse of practices that are not supported by evidence or underuse of practices that are supported by evidence.
More information about the system performance initiative and its reports and indicators can be found at http://systemperformance.ca.
Limitations
The present analysis has several limitations. Because of data limitations or a lack of available data, all provinces and territories are not represented in all indicators. Findings might therefore not be generalizable to provinces that are not represented. Depending on the indicator, between 3 and 13 jurisdictions are included. Screening indicators are based on self-reported survey data. Treatment indicators are derived from administrative databases maintained at the provincial level. Patient and provider preferences are important considerations when assessing appropriateness of care; those data were not available. Lastly, the practices described are necessary and beneficial for some patients and therefore Choosing Wisely Canada suggests that decisions about care should be guided by each person’s individual situation. However, substantial variations in screening and treatment practices might suggest interpretive differences between clinicians and differences in clinical practice between provinces.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Goulart BHL. Value: the next frontier in cancer care. Oncologist. 2016;21:651–3. doi: 10.1634/theoncologist.2016-0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter ME. What is value in health care? N Engl J Med. 2010;363:2477–81. doi: 10.1056/NEJMp1011024. [DOI] [PubMed] [Google Scholar]
- 3.Porter ME. A strategy for health care reform—toward a value-based system. N Engl J Med. 2009;361:109–12. doi: 10.1056/NEJMp0904131. [DOI] [PubMed] [Google Scholar]
- 4.Canadian Cancer Society’s Advisory Committee on Cancer Statistics . Canadian Cancer Statistics 2015. Toronto, ON: Canadian Cancer Society; 2015. [Google Scholar]
- 5.Choosing Wisely Canada . What Is CWC? [Web page] Toronto, ON: Canadian Medical Association; n.d. [Available at: http://www.choosingwiselycanada.org/about/what-is-cwc; cited 26 January 2017] [Google Scholar]
- 6.Choosing Wisely Canada . Oncology: Ten Things Physicians and Patients Should Question [Web page] Toronto, ON: Canadian Medical Association; 2014. [Available at: http://www.choosingwiselycanada.org/recommendations/oncology; cited 26 January 2017] [Google Scholar]
- 7.Choosing Wisely Canada . Family Medicine: Eleven Things Physicians and Patients Should Question [Web page] Toronto, ON: Canadian Medical Association; 2014. [Available at: http://www.choosingwiselycanada.org/recommendations/family-medicine; cited 26 January 2017] [Google Scholar]
- 8.Mitera G, Earle C, Latosinsky S, et al. Choosing Wisely Canada cancer list: ten low-value or harmful practices that should be avoided in cancer care. J Oncol Pract. 2015;11:e296–303. doi: 10.1200/JOP.2015.004325. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization (who) WHO Position Paper on Mammography Screening. Geneva, Switzerland: WHO; 2014. [PubMed] [Google Scholar]
- 10.Tonelli M, Gorber SC, Joffres M, et al. on behalf of the Canadian Task Force on Preventive Health Care Recommendations on screening for breast cancer in average-risk women aged 40–74 years. CMAJ. 2011;183:1991–2001. doi: 10.1503/cmaj.110334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson J, Tsakonas E, Conner Gorber S, et al. on behalf of the Canadian Task Force on Preventive Health Care Recommendations on screening for cervical cancer. CMAJ. 2013;185:35–45. doi: 10.1503/cmaj.113-2136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moyer VA, on behalf of the U.S. Preventive Services Task Force Screening for cervical cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;156:880–91. W312. doi: 10.7326/0003-4819-156-12-201206190-00424. [Erratum in: Ann Intern Med 2013;158:852] [DOI] [PubMed] [Google Scholar]
- 13.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Rectal Cancer. Fort Washington, PA: NCCN; 2017. Ver. 3.2017. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/rectal.pdf (free registration required); cited 7 May 2017] [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Colon Cancer. Fort Washington, PA: NCCN; 2017. Ver. 2.2017. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/colon.pdf (free registration required); cited 7 May 2017] [DOI] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Fort Washington, PA: NCCN; 2017. Ver. 2.2017. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (free registration required); cited 7 May 2017] [Google Scholar]
- 16.Badwe R, Parmar V, Hawaldar R, et al. Surgical removal of primary tumor and axillary lymph nodes in women with metastatic breast cancer at first presentation: a randomized controlled trial [abstract S2-02] Cancer Res. 2013;73(suppl) doi: 10.1158/0008-5472.SABCS13-S2-02. [DOI] [Google Scholar]
- 17.Kleespies A, Fuessl KE, Seeliger H, et al. Determinants of morbidity and survival after elective non-curative resection of stage iv colon and rectal cancer. Int J Colorectal Dis. 2009;24:1097–109. doi: 10.1007/s00384-009-0734-y. [DOI] [PubMed] [Google Scholar]
- 18.Nitzkorski JR, Farma JM, Watson JC, et al. Outcome and natural history of patients with stage iv colorectal cancer receiving chemotherapy without primary tumor resection. Ann Surg Oncol. 2012;19:379–83. doi: 10.1245/s10434-011-2028-1. [DOI] [PubMed] [Google Scholar]
- 19.Cirocchi R, Trastulli S, Abraha I, et al. Non-resection versus resection for an asymptomatic primary tumour in patients with unresectable stage iv colorectal cancer. Cochrane Database Syst Rev. 2012:CD008997. doi: 10.1002/14651858.CD008997.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Comprehensive Cancer Network (nccn) NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Fort Washington, PA: NCCN; 2017. Ver. 2.2017. [Current version available online at: https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf (free registration required); cited 7 May 2017] [Google Scholar]
- 21.Smith BD, Bentzen SM, Correa CR, et al. Fractionation for whole breast irradiation: an American Society for Radiation Oncology (astro) evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;81:59–68. doi: 10.1016/j.ijrobp.2010.04.042. [DOI] [PubMed] [Google Scholar]
- 22.Holloway CL, Panet-Raymond V, Olivotto I. Hypofractionation should be the new “standard” for radiation therapy after breast conserving surgery. Breast. 2010;19:163–7. doi: 10.1016/j.breast.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 23.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–20. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 24.Chow E, Harris K, Fan G, Tsao M, Sze WM. Palliative radiotherapy trials for bone metastases: a systematic review. J Clin Oncol. 2007;25:1423–36. doi: 10.1200/JCO.2006.09.5281. [DOI] [PubMed] [Google Scholar]
- 25.Lutz S, Berk L, Chang E, et al. on behalf of the American Society for Radiation Oncology (astro) Palliative radiotherapy for bone metastases: an astro evidence-based guideline. Int J Radiat Oncol Biol Phys. 2011;79:965–76. doi: 10.1016/j.ijrobp.2010.11.026. [DOI] [PubMed] [Google Scholar]
- 26.Peppercorn JM, Smith TJ, Helft PR, et al. on behalf of the American Society of Clinical Oncology American Society of Clinical Oncology statement: toward individualized care for patients with advanced cancer. J Clin Oncol. 2011;29:755–60. doi: 10.1200/JCO.2010.33.1744. [DOI] [PubMed] [Google Scholar]