Abstract
The effect of oral treatment with natural or recombinant human interferon alpha (HIA) on inflammatory airway disease in young standardbreds was assessed in a double-blind, randomized clinical trial. A total of 34 horses with nasal discharge, excess mucus in the trachea, and a persistent cough of at least 2 weeks’ duration that interfered with training completed the trial. Horses were rested for 1 week and received oral treatment with either a saline placebo, recombinant human interferon alpha (rHIA; 90 U/horse/day), or natural human interferon alpha (nHIA: 50 U/horse/day) for 5 days. There was a significant decline in nasal discharge and cough scores in all groups and the apparent response rate was similar. However, significantly fewer horses relapsed within 2 weeks once treatment was ceased when interferon rather than placebo was used (P = 0.012). Seventeen of 22 horses treated with rHIA or nHIA were cough-free 4 weeks after treatment, compared with only 4 of 12 after treatment with the placebo. Treatment with oral interferon is a useful adjunct to rest in standardbreds with inflammatory airway disease.
Abstract
Résumé — Traitement à l’interféron de la maladie inflammatoire des voies aériennes chez des jeunes Standardbred. L’effet d’un traitement par voie orale par interféron humain alpha (IHA) naturel ou recombinant sur la maladie inflammatoire des voies aériennes chez de jeunes Standardbred a été étudié dans un essai clinique en double insu chez des sujets répartis au hasard. Un total de 34 chevaux présentant un écoulement nasal, un excès de mucus dans la trachée et une toux persistante depuis au moins 2 semaines, et dont les symptômes gênaient l’entraînement, ont complété l’étude. Les chevaux ont été mis au repos pendant 1 semaine et ont reçu, par voie orale, soit un placebo salin, soit de l’interféron humain recombinant alpha (IHAr : 90 U/cheval/jour) soit de l’interféron humain naturel alpha (IHAn : 50 U/cheval/jour) pendant 5 jours. Il y a eu une diminution significative de l’écoulement nasal et de la cote de toux et quel que fut le groupe expérimental, le taux apparent de réponse était semblable. Cependant, le nombre de chevaux présentant des rechutes dans les 2 semaines suivant la fin du traitement était significativement plus bas dans les groupes interférons que dans le groupe placebo (P = 0,012). Dix-sept des 22 chevaux traités à l’IHAr ou à l’IHAn ne présentaient plus de toux 4 semaines après le traitement contre seulement 4 sur 12 avec le placebo. Le traitement oral à l’interféron constitue un complément au repos chez les Standardbred présentant une maladie inflammatoire des voies aériennes.
(Traduit par Docteur André Blouin)
Introduction
Equine lower respiratory tract diseases include those of infectious etiology (bacterial, parasitic, viral), allergic and inflammatory airway disease, exercise-induced pulmonary hemorrhage, and heaves. The terms inflammatory airway disease (IAD), small airway inflammatory disease, lower respiratory tract inflammation, and reactive airway disease have been variously used to describe a clinical presentation in horses characterized by a persistent cough that is aggravated by, and interferes with, training (1–4). Nasal discharge, decreased race performance, excessive mucus in the nasopharyngeal region and trachea as visualized with bronchoscopy, and evidence of airway inflammation on bronchoalveolar lavage (BAL) generally accompany the cough. Affected horses typically do not display tachypnea or dyspnea, nor do they have abnormal lung sounds on routine auscultation. Bronchoalveolar lavage reveals evidence of inflammation, with an increased total cell count comprised primarily of macrophages, lymphocytes, and neutrophils, often with a relative neutrophilia. There is no evidence of septic inflammation and IAD is generally accepted to reflect a sterile bronchiolitis (1,3). The proposed etiologies for inflammatory airway disease include recurrent pulmonary stress, previous or persistent respiratory viral infections, and allergic pneumonitis, but the pathogenesis is not fully understood (1,2).
On Prince Edward Island (PEI), a clinical syndrome similar to previously reported IAD in horses is observed. It occurs predominantly in 16- to 24-month-old standardbreds in their first training season during the months of November through February. Affected horses generally present with nasal discharge, excess mucus in the trachea on bronchoscopy, and a persistent (at least 2 wk in duration) deep, dry cough. They typically have a history of a previous respiratory disease with clinical signs suggestive of a viral illness (fever, cough, nasal discharge). After a period of partial or complete recovery, horses develop a chronic cough that does not respond well to treatment with antibiotics, systemic corticosteroids, or bronchodilators. The cough may occur at rest, but more commonly it occurs when training is attempted. Affected horses are afebrile and have normal complete blood cell counts and serum biochemical profiles, and varying degrees of nasal discharge. Thoracic auscultation is normal. Bronchoscopy in these horses typically reveals a mucoid or mucopurulent exudate in the pharynx, trachea, and bronchi, without evidence of tracheal inflammation. Bronchoalveolar lavage (BAL) fluid in affected horses contains high total nucleated cell counts, with increased numbers of neutrophils, lymphocytes, and macrophages, compared with normal equine BAL fluid and no evidence of intracellular bacteria. We, therefore, believe this syndrome to be similar IAD (1,2,4), although its occurrence on PEI appears to be limited to horses that are younger than those previously reported.
While some authors have suggested treating IAD with bronchodilator therapy or corticosteroids (3), there are no clinical studies supporting this and we have not found this treatment to be successful in young standardbreds; also, other authors do not suggest the use of this combination (1). Orally administered, low dose, natural human interferon alpha (nHIA; 50 U, q24h for 5 d) has been shown to decrease the total cell counts and other inflammatory markers in BAL fluid of standardbred horses (mean age 3.7 y) with signs of IAD (5,6); however, clinical signs and long-term therapeutic response were not reported. Further, the horses examined were older than the affected population observed on PEI. Nevertheless, based on these studies, we hypothesized that oral human interferon alpha would be of therapeutic benefit in young standardbreds with chronic cough consistent with IAD. We, therefore, conducted a randomized, double-blind, placebo controlled clinical trial to assess the safety and efficacy of natural and recombinant human interferon alpha treatment for IAD in young standardbreds on PEI.
Materials and methods
Animals
This study was approved by the UPEI Animal Care Committee in accordance with the standards of the Canadian Council on Animal Care. Standardbreds, 16- to 24-months-old and in their first training season in Maritime Canada, that presented to the Atlantic Veterinary College (AVC) Equine Ambulatory Service with an initial history of nonproductive cough and nasal discharge of at least 2 weeks’ duration that interfered with training were eligible for initial enrollment. Horses that met the above criteria but had received medication in the 5 d prior to presentation were not considered eligible for inclusion in the study. Owners were informed of the intent of the study and signed a written consent form for the inclusion of their horse in the study, prior to any collection of information or diagnostic procedures.
Baseline data collected included age, sex, vaccination and treatment history, cough duration, and whether the horse was kept in a racetrack or farm environment. Clinical and laboratory evaluation included temperature, pulse, and respiration; a complete blood cell (CBC) count (white blood cell count, red blood cell count, hemoglobin, hematocrit, platelet count, white blood cell differential count) and a serum biochemical profile (sodium, potassium, chloride, calcium, phosphate, magnesium, urea, creatinine, glucose, cholesterol, bilirubin, amylase, alkaline phosphatase, creatinine kinase, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, total protein, albumin, globulin, sorbitol dehydrogenase); a respiratory tract evaluation, including a cough score, a nasal discharge score, and a thoracic auscultation score (Table 1); a bronchoscopic evaluation and score (Table 2); and BAL fluid cytologic examination and bacterial culture. Horses were subsequently excluded from the trial following development or identification of one or more of the following: pyrexia, abnormal findings on routine thoracic auscultation (increased breath sounds, crackles, wheezes, pleural friction rubs, etc.), abnormal findings on CBC count (inflammatory leukogram), and any other concurrent disease requiring medical treatment. Results from BAL fluid analysis that were considered grounds for exclusion from the study after initial enrolment included identification of intracellular bacteria on cytologic examination, with or without isolation of pathogenic bacteria, and eosinophilic inflammation (> 5% eosinophils in BAL cells) consistent with allergic airway disease. A total of 43 horses were initially eligible based on history; however, 2 horses were excluded as they were febrile (1 horse was subsequently diagnosed with a pulmonary abscess and the other had evidence of bacterial bronchitis in the BAL fluid) and 7 additional horses were subsequently excluded because of the presence of intracellular bacteria in the BAL fluid. Although it was proposed that an abnormal routine thoracic auscultation (thoracic auscultation score greater than 1) be a criterion for exclusion, no horses that presented with an appropriate history and were within the eligible population were excluded for this reason. Criteria for withdrawal of horses from the treatment study included the occurrence of an adverse effect, violation of the trial protocol (including administration of any other therapeutic agents during the first 28 d of the study), development of other concurrent disease, or withdrawal of consent. No horses were withdrawn from the trial for these reasons. Therefore, a total of 34 horses completed the trial.
Table 1.
Semi-quantitative evaluation scale for the respiratory system
| Score | Cough | Nasal discharge | Auscultation |
|---|---|---|---|
| 0 | None | None | Normal |
| 1 | Occurs with exercise only Not inducible at rest | Seromucous discharge on exercise | Increased inspiratory sounds following exercise |
| 2 | Cough interferes with training; can be induced at rest by palpation | Seromucous discharge at rest | Increased |
| 3 | Cough at rest; interferes with training; easy to induce | Mucopurulent discharge on exercise | Crackles/wheezes |
| 4 | Moderate to severe cough at rest; cannot train horse | Mucopurulent discharge at rest | Inspiratory and expiratory crackles and wheezes |
Adapted from Moore et al (5)
Table 2.
Bronchoscopy score
| Score | Tracheal exudate | Nasopharyngeal exudate | Pharyngeal lymphoid tissue |
|---|---|---|---|
| 0 | None | None | Few, inactive follicles |
| 1 | Mucus | Seromucous or mucoid | Many inactive follicles, few pink edematous follicles |
| 2 | Mucopurulent | Mucopurulent | Many pink follicles, from wall of pharynx to soft palate |
| 3 | Circumferential tenacious mucopurulent material | Coalescing edematous follicles throughout pharynx |
Adapted from Moore et al (5)
Bronchoalveolar lavage
Horses were sedated with detomidine (Dormosedan; Pfizer Canada, London, Ontario), 0.01 mg/kg body weight (BW), IV, followed by butorphanol (Torbugesic; Ayerst Veterinary Laboratories, Guelph, Ontario), 0.02 mg/kg BW, IV. A BAL catheter (Bivona, Gary, Indiana, USA) was passed through a naris and advanced via the ventral meatus into the trachea until it lodged in a bronchus. Once lodged, the cuff of the BAL catheter was inflated with approximately 6 mL of air. Three 100-mL aliquots of phosphate buffered saline were sequentially infused and aspirated via the BAL catheter. A sample was retained for bacteriological culture (aerobic culture and routine Gram’s method of staining) and the remaining BAL fluid was strained through a single layer of gauze to remove excessive mucus. The samples were then transported to the AVC diagnostic laboratory for immediate processing. Total cell counts were performed with an automated cell counter. Cytologic description and differential nucleated cell counts of 500 consecutive cells were obtained by examination of Wright’s-Giemsa stained, cytocentrifuge preparations. Cell types were reported as percentage and absolute counts.
Study design
This was a placebo-controlled, randomized, double-blind clinical trial of human interferon alpha (HIA) for the treatment of IAD in young standardbreds. Prior to starting the study, a random number generator was used to generate a random order of treatments. Horses were assigned to receive oral treatment for 5 d with either placebo (saline only), 50 U/horse of nHIA (Wellferon; Burroughs Wellcome, Kirkland, Quebec), or 90 U/horse of recombinant human interferon alpha (rHIA) (Intron A; Schering Canada, Pointe-Claire, Quebec). All treatments, including placebo, were prepared in advance by appropriate dilution in a 3-mL volume of sterile normal saline and then freezing at −80°C until use. Neither the owner nor the case veterinarian were aware of the treatment assigned. The general treatment protocol is shown in Table 3. The day that horses were enrolled in the study and initial samples were collected was considered as day 0. Following the results of the CBC count, biochemical analyses, and BAL, treatment with interferon or placebo was started on day 1. The placebo or interferon, thawed just prior to the time of administration, was administered directly into the oral cavity by use of a 3-mL syringe. All treatments were administered by the owners. Due to the small volume administered (3 mL), no loss of drug during administration was expected or reported by the owners. Exercise was limited to walking or turning out until day 7, followed by routine training for the duration of the study. At days 6, 14, 28, 56, 84, and 112 following enrollment, the horses were reevaluated according to the protocol outlined in Table 3. Owners were instructed to contact the veterinarian if there was any recurrence of clinical signs.
Table 3.
Protocol for individual animal assessment and treatment
| Day 0: | Enrollment evaluation and collection of epidemiological data including physical examination, endoscopy, bronchoalveolar lavage, complete blood cell count, serum biochemical profile |
| Day 1: | Start of treatment protocol with interferon or placebo |
| Day 5: | Last treatment |
| Day 6: | Post-treatment physical examination, complete blood cell count, serum biochemical profile |
| Day 14: | Post-treatment complete evaluation, including endoscopy, bronchoalveolar lavage, complete blood cell count, serum biochemical profile |
| Day 28: | Physical examination |
| Day 56: | Physical examination |
| Day 84: | Telephone follow-up or physical examination |
| Day 112: | Physical examination |
Horses were defined as having a positive response if the cough and nasal discharge scores were decreased to 0 or 1. Horses were considered to be cured if they were cough-free (no abnormal coughing noted by the trainer or by the veterinarian on physical examination; cough and nasal discharge scores of 0) at day 28 and had not received re-treatment with oral interferon. Horses were considered to have relapsed if there was a return of cough or nasal discharge (at a level equivalent to a score above 1) that interfered with training between days 14 and 28. These horses were treated in an open label fashion with rHIA. All horses, including those that were retreated, were monitored for 4 mo from the time of enrolment in the study.
Based on our evaluation parameters, an estimate of the expected variance of semi-quantitative scores, a level of significance of 0.05 (Type I error), and a power of 0.80 (Type II error of β = 0.20), we estimated that 12 to 13 horses per group would be required to detect a 50% improvement in evaluation scores. Therefore, our target was 13 horses per group, not anticipating a significant response in the placebo-treated group.
Statistical evaluation
Descriptive statistics were used to characterize the clinical and laboratory findings in the horses at the time of initial presentation. Results are presented as mean ± standard deviation (s) and the range is given where appropriate.
Data presented when groups were compared are reported as the mean ± standard error of the mean (sχ̄). To compare the effects of interferon and placebo on cough, nasal discharge, tracheal exudate, and nasopharyngeal exudate scores, nonparametric tests were used because of the use of a limited range, nonlinear scoring scale, and the small sample size. The use of a limited scoring scale results in a nonnormal distribution of data that is not overcome by a large sample size and the magnitude of the difference between each point on the scale is not constant, so that they do not represent an appropriate parametric scale. The scores at days 6 and 14 were compared with day 0 for each group using the nonparametric Wilcoxon Signed Ranks test. As the nonparametric tests do not allow 2-way comparisons, the Kruskal-Wallis test was used to compare differences between treatment groups at each time point. The use of this statistical approach meant that multiple comparisons were made. The obtained P-values -are reported, but results were only considered statistically significant after correction for multiple comparisons. A maximum of 3 comparisons were made for day 0 samples and, therefore, for an overall P < 0.05, a P-value of less -than 0.017 for any one test was required for it to be considered statistically significant. Only results that were significant after correction for multiple comparisons are shown. Chi-square analysis was used to compare response (day 14) and relapse rates (day 28).
For comparison of BAL data, 4 of a possible 68 samples were uninterpretable as a result of food contamination or inadequate sample, and in an additional 6 samples, total BAL cell counts were not obtained due to laboratory error. This meant that samples on day 0 and day 14 were not available on all horses, precluding the use of repeated measures or paired statistical analysis on the whole data set. To allow maximum use of data, therefore, the interferon groups were combined and treated as a single group for the purposes of statistical analysis. Nevertheless, the data for all the groups is presented. Placebo- and interferon-treated groups were compared by an independent samples t-test at day 0 and day 14, as the primary comparison was between these 2 groups.
All statistical analyses were conducted using a computer statistical analysis program (SPSS for Windows, Release 10.0.5; SPSS, Chicago, Illinois, USA).
Adverse effects were to be reported by descriptive evaluations and not analyzed statistically, as it was anticipated the frequency would be too low for a meaningful statistical comparison. However, no adverse effects were noted.
Results
Thirty-four standardbreds completed the trial and were included in the statistical analysis. All were aged between 16 and 23 mo. There were 18 females, 14 intact males, and 2 geldings. Fourteen horses had been vaccinated (equine rhinopneumonitis-influenza vaccine) and 20 were unvaccinated horses. Twelve of the horses were housed in farm environments and 22 were housed in racetrack stables.
Three horses did not have a known health history, because the horses had been purchased recently by the current owner. All 31 horses with a known history were reported to have experienced a previous episode consistent with a viral respiratory illness (fever, cough, nasal discharge). Nine of the 31 horses were reported to have appeared to recover and then subsequently start to cough. The remainder of the horses intially improved (afebrile, regained appetite and activity level) from the primary event but had a persistent cough and nasal discharge. Treatment for chronic cough had been attempted in 13 of the 34 horses, with no satisfactory response. Nine were treated with 1 or more antibiotics (oxytetracycline, ceftiofur, penicillin, or trimethoprim-sulfadiazine) and 4 were treated with an expectorant combined with a bronchodilator (guaifenesin with aminophylline). The remainder of the horses had not been specifically treated.
At the time of presentation to the AVC Equine Ambulatory Service, the average duration of cough was 45.5 d, s = 32.6 d (range: 14 to 140 d). In some horses, the cough was intermittent, but in most horses, it was a constant cough. No abnormal sounds were detected on routine thoracic auscultation. All but 4 of the horses had nasal discharge on physical examination. Bronchoscopy revealed a mucoid tracheal exudate in all horses and nasopharyngeal exudate in 30 of the study animals. Fifteen horses had visible pharyngeal lymphoid tissue hyperplasia (score of 1).
There were no significant abnormalities on the CBC count or the serum biochemical profiles prior to initiation of therapy (results not shown). Bronchoalveolar lavage results at day 0 were similar among the 3 groups (Table 5). The majority of cells present were macrophages (46%, s = 21%; range: 12% to 76%) and lymphocytes (36%, s = 16%; range: 2% to 59%). The percentage of mast cells was less than 3% in all horses and, apart from 1 horse with 4% eosinophils, the rest had < 1% eosinophils on cytologic examination. The percentage of neutrophils observed ranged from 1% to 82%, with a mean of 14%, s = 21%. Those with highest neutrophil counts had the highest total cell counts. Cytologic examination revealed small to, occasionally, moderate numbers of fungal spores in BAL fluid from 24 out of 34 horses, while small numbers of extracellular bacteria were observed in 6 out of 34 horses. A cyst-like organism containing 8 trophozoites and resembling a Pneumocystis sp. was observed in 10 of the 34 horses. Bacterial culture did not produce any significant pathogen growth in any of the horses. A small number of bacteria were cultured from 16 of the 34 initial BAL samples, but this finding was not associated with the presence of intracellular bacteria on cytologic examination of the fluid and was considered to be nonspecific contamination. The majority of these organisms were identified as Streptococcus zooepidemicus zooepidemicus; 3 Actinobacillus-like organisms were also cultured. There were no differences between the 3 treatment groups in any of these characteristics on day 0.
Table 5.
Effect of oral low-dose human interferon alpha on bronchoalveolar lavage differential cell counts at day 14. Data presented as mean (standard error of the mean). There were no significant differences
| Cell type | Day | Placebo | rHIA | nHIA |
|---|---|---|---|---|
| Neutrophils (%) | 1 | 10.5 (5.2) | 16.7 (6.2) | 14.0 (8.7) |
| 14 | 10.3 (3.4) | 9.75 (3.5) | 8.8 (4.0) | |
| Macrophages (%) | 1 | 51.1 (4.4) | 45.2 (4.4) | 48.9 (5.2) |
| 14 | 48.6 (4.1) | 52.3 (4.2) | 50.9 (3.2) | |
| Lymphocytes (%) | 1 | 37.3 (4.6) | 36.1 (3.4) | 35.8 (5.1) |
| 14 | 39.9 (3.5) | 36.6 (2.7) | 38.9 (3.7) |
rHIA — recombinant human interferon alpha; nHIA — natural human interferon alpha
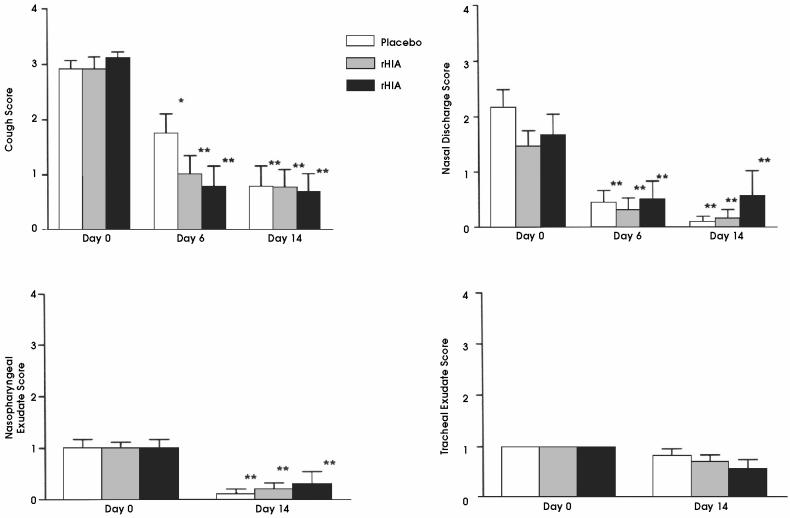
The 43 horses originally considered eligible for the trial were randomly assigned to groups such that, after exclusions, of the remaining 34 horses, 12 were in the placebo group, 13 in the rHIA group, and 9 in the nHIA group. Results of physical examination of the respiratory tract and bronchoscopy are shown in Figure 1. Auscultation scores were always zero and results are not shown. The cough and nasal discharge scores were significantly reduced at day 6 and 14 in all 3 treatment groups, but the treatment groups were not significantly different at any time point. The nasopharyngeal exudate score was also significantly decreased in all groups by day 14. The tracheal exudate scores were low at day 0 and, although some cheal scores were zero at day 14, no statistically significant differences were observed. The appearance of the pharyngeal lymphoid tissue did not change in any of the treatment groups (data not shown).
Figure 1.
Effect of treatment with placebo or oral interferon alpha on cough, nasal discharge, nasopharyngeal exudate, and tracheal exudate score in young standardbreds with inflammatory airway disease receiving 1 week’s rest. Results are presented as mean and standard error of the mean. Significance (*P < 0.017, **P < 0.01, with P < 0.017 considered significant as a result of multiple comparison) refers to day 0 compared with day 6 and day 14 for each treatment group by the Wilcoxon Signed Ranks test. Treatment groups on each day were compared by the Kruskall-Wallis test, but there were no significant differences between the groups on any day.
The positive response rate (a decrease in both the cough and nasal discharge scores to 0 or 1) was similar among the 3 groups at day 6 and day 14 (Figure 2). Of the horses that responded by day 14, all but 2 had nasal discharge scores of 0 and all but 7 had cough scores of 0. Despite the similar initial response rate, relapses (return of a cough or nasal discharge with a score of 2 or above) were significantly more common in the placebo group between day 14 and day 28 (Figure 2; P = 0.012).
Figure 2.
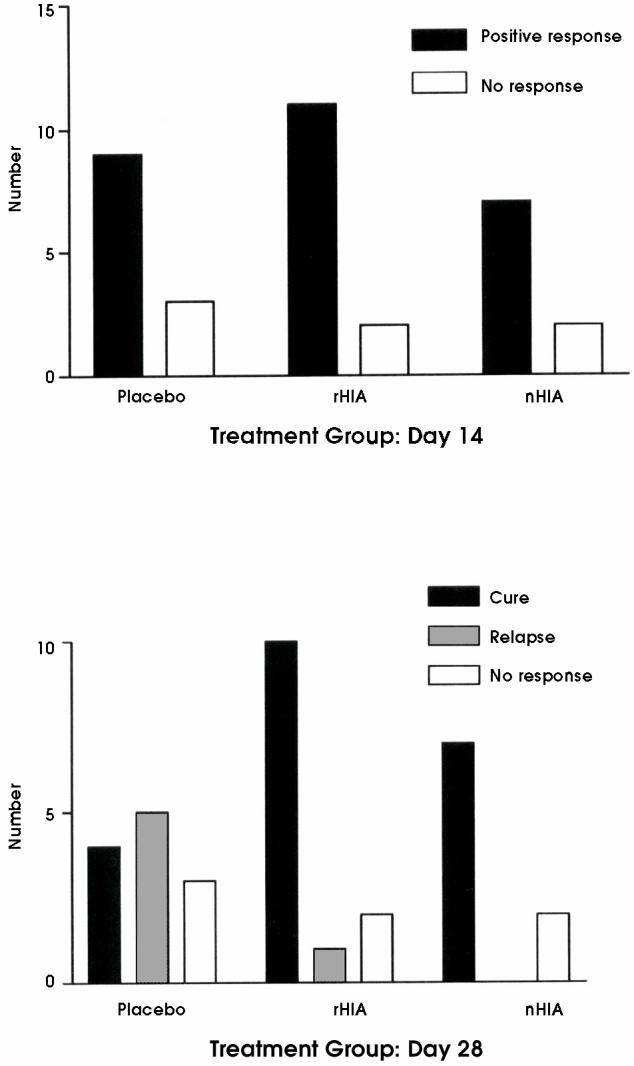
Response rate at day 14 (top) and relapse rate at day 28 (bottom) in young standardbreds with inflammatory airway disease receiving either placebo, oral recombinant interferon alpha (rHIA), or oral natural interferon alpha (nHIA). The response rate was not significantly different between groups (Chi-square: 0.018, 2 df; P = 0.91), but there was a significant difference in the relapse rate between placebo and interferon treated groups (Chi-square: 8.88, 2 df; P = 0.012).
Bronchoalveolar lavage fluid was obtained from all horses on day 0 and day 14. Total cell counts in BAL fluid samples (Table 4) collected on day 0 did not differ significantly between the placebo and the combined interferon groups. On day 14, the interferon-treated horses had significantly lower total BAL fluid cell counts compared with the placebo group. The number of lymphocytes and macrophages were also significantly lower in the interferon-treated group at day 14 than in the placebo-treated group. The neutrophil numbers showed a similar trend, although this was not statistically significant. The percentage of each cell type did not differ significantly between the groups either before or after treatment (Table 5).
Table 4.
Effect of oral low-dose human interferon alpha on bronchoalveolar lavage cell counts at day 14. Data presented as mean (standard error of the mean)
| Cell type | Day | Placebo (109 cells/L) (n = 10) | Human interferon alpha (109 cells/L) (n = 18) | P-value |
|---|---|---|---|---|
| Total cell count | 1 | 0.42 (0.06) | 0.51 (0.12) | 0.6 |
| 14 | 0.64 (0.16) | 0.32 (0.05) | 0.018 | |
| Neutrophils | 1 | 0.047 (0.26) | 0.18 (0.10) | 0.37 |
| 14 | 0.054 (0.017) | 0.024 (0.008) | 0.074 | |
| Lymphocytes | 1 | 0.14 (0.026) | 0.15 (0.024) | 0.99 |
| 14 | 0.26 (0.082) | 0.13 (0.021) | 0.047 | |
| Macrophages | 1 | 0.22 (0.043) | 0.18 (0.02) | 0.38 |
| 14 | 0.31 (0.075) | 0.16 (0.026) | 0.023 |
There were no significant abnormalities noted on CBC count or serum biochemical analyses in any horses during the trial (data not shown).
Discussion
Chronic cough and mucoid-to-mucopurulent nasal discharge are common clinical findings in racehorses with airway inflammation (1,2,4). Cytologic examination of BAL fluid in older horses with inflammatory airway disease often reveals mixed inflammation with a high total count of nucleated cells, a mildly increased neutrophil percentage (usually 10% to 15% of total cells, but with reported ranges of 4% to 17%), and increased lymphocyte and macrophage counts (1,2,5). While some authors have identified occasional horses with eosinophilia or increased mast cells in the BAL fluid (2), none of the horses presented for this study had increased eosinophils or mast cells in BAL fluid. An unaffected control population was unavailable at the same time as the patient population; however, when similar protocols to collect BAL are used, typical total cell counts reported in healthy horses are 0.15 to 0.3 × 109 nucleated cells/L (2,7). Thus, compared with typical literature values, the study horses exhibited an increased total cell count at the time of enrollment (Table 4). The percent neutrophils in BAL fluid from healthy horses is typically around 4% to 5% (2,8). There was a considerable range in our patient population, with the majority of our horses (68%) having a BAL neutrophil percentage of less than 10%. The average was 14%, consistent with previous reports of inflammatory airway disease in horses (1,2,8). These results confirm the presence of mixed inflammatory response in the airways of the study population of young standardbreds with a chronic cough and nasal discharge, although some horses, as previously reported, have neutrophil differential counts overlapping with the normal range (2,5,7). The majority of horses had a history consistent with a viral-type respiratory illness; however, the horses were not examined at the time of the initial event and, because of the time of enrollment relative to the initial reported episode of respiratory disease (a minimum of 2 wk and up to 5 mo), serological confirmation or virus isolation to confirm an exact diagnosis of the nature of the previous respiratory illness was not carried out. By excluding any horses with fever, abnormal CBC count, or intracellular bacteria in the BAL fluid, the chances of including horses with bacterial or other active infectious processes in the study were minimized. Thus, the clinical presentation of the standardbreds enrolled in this trial was consistent with IAD.
The results of the double-blind, placebo-controlled clinical trial reported here demonstrate a beneficial effect from treatment with low-dose, oral interferon. The selection of interferon dose and duration of treatment was based on previously published work (5,6). There were 2 important observations in this previous work: doses of natural interferon greater than 50 U nHIA were less effective than were lower doses in producing persistent changes in BAL fluid. The changes in BAL fluid observed at 8 d were reversed at 15 d when 150 U nHIA was administered. Moore et al (5) reported no overall change in a cumulative bronchoscopic examination score on day 15. Therefore, we elected to use a low dose of HIA and to assess BAL changes at 2 wk, as this would give a better indication of a persistent response than evaluation at 6 d. By doing 2 BAL analyses the costs of the trial were reduced and the invasive procedures on the horses were minimized in order to encourage participation by owners. The horses in the Moore et al (5,6) study group were identified on a primary complaint of poor exercise performance, as opposed to persistent cough, which was the primary presenting complaint used to identify study subjects in the trial reported here. In order to have an assessment of short- or long-term clinical response based on the presenting clinical complaint, we followed not only laboratory changes, but also clinical signs, and included a prolonged follow-up period.
Although the objective of this study was to evaluate the long-term efficacy and safety of HIA for IAD associated with a persistent cough in young standardbreds, rest is commonly recommended for any equine athlete with airway disease and should, therefore, be part of any treatment protocol. Further, it was anticipated that a rest period would reduce variability in response that might be associated with different training levels. Thus, all horses enrolled in the study were required to rest for 1 wk during treatment.
All 3 groups experienced a significant decline in cough and nasal discharge scores in the first 2 wk. There was a trend towards a more rapid decrease in the interferon-treated groups (Figure 1), but a larger sample size would have been required to determine if there was truly a difference in the rate of response. The key point, however, is that all 3 groups had similar clinical responses in the first 2 wk, and the response rate was not different between the treatment groups. This is probably attributable to the beneficial effects of rest in all 2 groups. Despite the initial response rates, over the following 2 wk as horses returned to normal activity, there was a recurrence of clinical signs in some horses. The rate of relapse or return of clinical signs in the subsequent 2 wk was significantly higher in the placebotreated group (55%) than in the interferon-treated groups (less than 10% in both groups). Only those horses that remained free of clinical signs on day 28 were considered to have been cured. Overall, approximately 80% in each of the interferon-treated groups compared with 35% in the placebo group were considered to have been cured. The significant difference in relapse rates supports the hypothesis of a significant beneficial clinical effect of interferon alpha in IAD.
The objective assessment of differences in the cellular composition of BAL fluid collected on day 14 between the interferon-treated groups and the placebo group confirmed an effect of interferon at the level of the respiratory system. The total cell count, with decreases in all 3 cell types, was significantly less in the interferontreated horses compared with the placebo-treated horses treated on day 14. The total neutrophil count and the percentage of neutrophils in the BAL fluid of the interferon-treated groups appeared to decrease from day 0 to day 14 (Tables 4 and 5), but there was considerable variability, and the change was not statistically significant. The changes in BAL fluid cellular composition were similar to those previously reported by Moore et al (6) and reflect the mixed inflammatory response associated with IAD. An additional assessment of BAL at day 6 of the trial might have provided further objective evidence of a quicker response in the interferon-treated horses; however, this BAL was not included for the reasons noted above.
Horses that did not respond by day 14 to the 1st treatment with either placebo or HIA, or that relapsed prior to day 28, were subsequently treated with rHIA in an open label fashion. To maintain blinding of the trial, neither owners nor the attending veterinarian were aware of the initial treatment used. All horses that were subsequently treated (for the 1st or 2nd time) with HIA became symptom free and remained so for the duration of the 4-month monitoring period. These horses were not included in the results or statistical analysis of the trial presented here, but the subsequent response strengthens the suggestion that interferon is an effective treatment for IAD in standardbreds.
There are over 22 genes that code for different alpha-interferons, 1 of the major subgroups of interferons (9). Interferon alpha is used therapeutically in human viral, neoplastic, and immune-mediated disorders. It is thought that orally administered interferons have systemic effects as a result of cell to cell transfer of the interferon’s effects rather than systemic distribution of the interferon (10). Interferon alpha could reduce inflammation via elimination of persistent viral infections, modulation of an allergic response, or attenuation of proximal mediators of inflammation. Although interferons from 1 species may have activity in another species, this is not always the case and significant heterogeneity in interspecies exists. Natural preparations contain many different types of alpha-interferons, so that greater species cross-reactivity may be anticipated compared with that obtained with recombinant interferons (9,11). Therefore, it was expected that use of natural interferons would have a higher likelihood of therapeutic success. However, we did not identify any significant differences between the 2 types of interferon. A larger study population would likely be required to identify any differences between preparations.
Seven of the horses that were excluded from the final analysis because of the presence of intracellular bacteria in the BAL fluid had received treatment with oral interferon in a blinded fashion. Only 1 of these 7 horses responded to treatment with oral interferon alone; the other horses required additional therapies. This highlights the importance of ruling out an underlying bacterial infection prior to diagnosing and treating IAD.
The presence of a Pneumocystis-like organism is of interest as the identification of Pneumocystis organisms in BAL fluid has been reported only in severely debilitated or immunosuppressed and typically immature horses (12–14). Studies to further characterize this organism and its association with airway disease in horses are on-going.
In conclusion, the results of our study demonstrate that IAD responds initially to rest, but clinical signs will return in the majority of horses unless other treatment is instituted. Treatment with oral low-dose interferon for 5 d in combination with rest appears to be an effective and inexpensive therapeutic option. While nearly 80% of the horses responded to 1 treatment course with either oral nHIA or oral rHIA, a 2nd treatment course in horses that failed to respond resulted in a 100% success rate. Oral rHIA or nHIA appears to be a useful adjunct to rest in horses with IAD. CVJ
Footnotes
Support: This work was supported by a grant from the Sir James Dunn Animal Welfare Centre, Atlantic Veterinary College, University of Prince Edward Island.
References
- 1.Moore BR. Lower respiratory tract disease. Vet Clin North Am Equine Pract. 1996;12:457–472. doi: 10.1016/s0749-0739(17)30267-5. [DOI] [PubMed] [Google Scholar]
- 2.Moore BR, Krakowka S, Robertson JT, Cummins JM. Cytologic evaluation of bronchoalveolar lavage fluid obtained from Standardbred racehorses with inflammatory airway disease. Am J Vet Res. 1995;56:562–567. [PubMed] [Google Scholar]
- 3.MacKay RJ. What’s new with inhalant therapies for inflammatory airway disease in horses? Compend Contin Educ Pract Vet. 1999;21:353–355. [Google Scholar]
- 4.Couetil LL, Rosenthal FS, DeNicola DB, Chilcoat CD. Clinical signs, evaluation of bronchoalveolar lavage fluid, and assessment of pulmonary function in horses with inflammatory respiratory disease. Am J Vet Res. 2001;62:538–546. doi: 10.2460/ajvr.2001.62.538. [DOI] [PubMed] [Google Scholar]
- 5.Moore BR, Krakowka S, Cummins JM, Robertson JT. Changes in airway inflammatory cell populations in Standardbred racehorses after interferon-alpha administration. Vet Immunol Immunopathol. 1996;49:347–358. doi: 10.1016/0165-2427(95)05480-4. [DOI] [PubMed] [Google Scholar]
- 6.Moore BR, Krakowka S, Mcvey DS, Cummins JM, Robertson JT. Inflammatory markers in bronchoalveolar lavage fluid of Standardbred racehorses with inflammatory airway disease: response to interferon-alpha. Equine Vet J. 1997;29:142–147. doi: 10.1111/j.2042-3306.1997.tb01656.x. [DOI] [PubMed] [Google Scholar]
- 7.Derksen FJ, Brown CM, Sonea I, Darien BJ, Robinson NE. Comparison of transtracheal aspirate and bronchoalveolar lavage cytology in 50 horses with chronic lung disease. Equine Vet J. 1989;21:23–26. doi: 10.1111/j.2042-3306.1989.tb02084.x. [DOI] [PubMed] [Google Scholar]
- 8.Viel L. Lower airway inflammation in young performance horses. In: Robinson NE, ed. Current Therapy in Equine Medicine, 4th ed. Philadelphia: WB Saunders, 1997:426–428.
- 9.Mannering GJ, Deloria LB. The pharmacology and toxicology of interferons: an overview. Annu Rev Pharmacol Toxicol. 1986;26:777–781. doi: 10.1146/annurev.pa.26.040186.002323. [DOI] [PubMed] [Google Scholar]
- 10.Fleischmann WR, Koren S, Fleischmann CM. Orally administered interferons exert their white blood cell suppressive effects via a novel mechanism. Proc Soc Exp Biol Med. 1992;201:200–207. doi: 10.3181/00379727-201-43499. [DOI] [PubMed] [Google Scholar]
- 11.Moore BR. Clinical applications of interferons in large animal medicine. J Am Vet Med Assoc. 1996;208:1711–1715. [PubMed] [Google Scholar]
- 12.Perron Lepage MF, Gerber V, Suter MM. A case of interstitial pneumonia associated with Pneumocystis carinii in a foal. Vet Pathol. 1999;36:621–624. doi: 10.1354/vp.36-6-621. [DOI] [PubMed] [Google Scholar]
- 13.Flaminio MJ, Rush BR, Cox JH, Moore WE. CD4+ and CD8+ T-lymphocytopenia in a filly with Pneumocystis carinii pneumonia. Aust Vet J. 1998;76:399–402. doi: 10.1111/j.1751-0813.1998.tb12387.x. [DOI] [PubMed] [Google Scholar]
- 14.Ewing PJ, Cowell RL, Tyler RD, MacAllister CG, Meinkoth JH. Pneumocystis carinii pneumonia in foals. J Am Vet Med Assoc. 1994;204:929–933. [PubMed] [Google Scholar]