Abstract
Introduction
The Rehabilitation and Exercise Oncology model of care (ActivOnco) was established to optimize cancer survivorship through exercise prescription and active lifestyle promotion, providing a transition of care from hospital to community. Patients having any cancer diagnosis, stage of disease, and treatment were eligible for evaluation and exercise prescription upon deterioration of performance status. The team of professionals included hospital-based physiotherapists proactively screening for rehabilitation needs, loss of functional independence, and exercise eligibility, plus exercise specialists in a community-based Wellness Centre to provide follow-up or direct access for post-treatment or non-complex patients.
Methods
From January 2011 to December 2015, the hospital team assessed 1635 patients representing all major cancer sites, and the Wellness Centre team evaluated and prescribed exercise for 1066 participants. Primary interventions provided were education about fatigue management, physical activity promotion, exercise prescription, fracture risk reduction, referral to specialized follow-up services (for example, occupational therapy, lymphedema clinic), and coordination for mobility aids and paratransit services.
Results and Conclusions
Implementation of the ActivOnco model of care showed that exercise alone is not a panacea for all functional deterioration associated with the cancer trajectory and its treatment. However, screening to identify rehabilitation needs combined with exercise prescription can effectively improve the quality of survivorship in cancer patients. Program developments are limited by the cost of human resources, lack of hospital-based physical resources, and lack of public funding, all of which significantly limit the scope and development of appropriate services.
Keywords: Oncology rehabilitation, exercise, models of care, survivorship, quality of life
INTRODUCTION
Advances in cancer treatment have prolonged survival, significantly increasing the number of people living with physical and psychosocial morbidities related to cancer and its treatment. In 2004, the cost to the Canadian economy was estimated to be $4.2 billion in direct expenditures, with an additional $12.9 billion in indirect expenditures, including premature mortality and disability1. In Quebec alone, the economic burden of cancer was estimated to be $4.2 billion in 20132. Cancer rehabilitation might improve functional outcomes and return-to-work potential for cancer survivors, thereby reducing burden on the economy3.
Cancer rehabilitation services and exercise prescription have been shown to enhance the quality of survivorship by providing targeted interventions to reduce impairments and the short- and long-term side effects of treatment, allowing for adaptation to daily activities and life roles4–11. Evidence includes a substantial number of peer-reviewed articles on the benefits of exercise alone and greatly supports the use of rehabilitation interventions in various cancer populations12. Systematic analysis of that research shows a positive effect at all stages of disease for reducing fatigue13–15; improving tolerance to treatment16–18; significantly improving time to recovery19 and sleep20; and enhancing immune function21, cardiovascular function15, and health-related quality of life18,22. Reports of positive outcomes are mounting23, and it has become imperative to develop models of care that systematically include rehabilitation and exercise prescription.
In a recent study conducted by Canestraro et al.24, 20 cancer rehabilitation sites were identified in Canada, and the authors concluded that the needs of patients were not being met by current services. Of the identified programs, 50% were located in hospital outpatient departments and were limited in scope largely to specific tumour sites. Great variability in the cancer-specific exercise programs available in Canada has also been reported, with little standardization of the services provided and a wide range of medical and allied care professionals coordinating and implementing the programs24–29. Some link directly to cancer centres25,26,29; others have evolved primarily in a community setting27,28 and focus on the post-treatment population. Human resources include either a physiotherapist25,29 or an exercise specialist26,27, but few have both28. Significant variability in eligibility criteria and duration and content of programs is also observed in Canada. Santa Mina et al.26 concluded that exercise programs are an exception, and not the norm, for cancer care.
The limited availability of cancer rehabilitation services and exercise prescription is not unique to Canada. Stubblefield et al.11 identified the scarcity and high variability of programs available within the United States, including within comprehensive cancer centres. Some European countries have incorporated cancer rehabilitation services at a national level, although, once again, content and structure significantly vary from country to country30. The Korean Academy of Physical Medicine conducted a study of cancer rehabilitation practice and barriers, concluding that neither standardization nor adequate infrastructure was available to implement services to the general cancer population31. Most authors have concluded that, given the prevalence of cancer and the functional morbidities associated with treatment, it is time to move forward to develop integrated models of care that incorporate triage, decision-making, and service delivery.
ActivOnco MODEL OF CARE
In 2008, Hope and Cope established the Rehabilitation and Exercise Oncology Program (ActivOnco) at the Segal Cancer Centre within the Jewish General Hospital, Montreal, Quebec. The initiative began in 2007 with the building of Hope and Cope’s community-based and privately funded Wellness Centre in close proximity to the Jewish General Hospital. The centre offers a diverse set of programs and activities aimed at improving quality of life for patients and their caregivers. In addition, patients have access to a well-equipped gymnasium and a broad variety of exercise classes staffed by volunteers and overseen by exercise specialists.
During development of the program, with direct access to the community-based Wellness Centre, it became evident that many patients needed greater screening for exercise prescription and risk management before joining physical activity programs. Post-chemotherapy toxicities, hematologic changes, neuropathy, cardiac autonomic insufficiency, radiation fibrosis, bone metastasis, and skeletal fragility necessitated professional guidance and supervision of programs. In response to that need, a hospital-based group of physiotherapists with specialized training in oncology were progressively introduced to various populations of cancer patients to screen for exercise eligibility and rehabilitation needs. During treatment, the objectives were to maintain performance status (ps), prevent the development of treatment-related disability, and reduce the number of medical crises caused by functional decline. Post-treatment, the principal objectives were to reduce timelines for functional recovery and to enhance overall wellness. Throughout the development process, the principal mandate and focus of this unique ActivOnco model of care remained exercise prescription and promotion, and when possible, it aimed to provide patients with a seamless transition from hospital to the community.
Successful implementation of the program relied on the multidisciplinary team’s awareness and advocacy of the many benefits of exercise for their patients. To guide appropriate referrals, clinical and scientific overviews of the value of rehabilitation and exercise interventions were provided to departments responsible for specific tumour sites. Table i lists the guidelines for referral. All patients were eligible for an initial evaluation and exercise prescription, particularly if their ps had deteriorated.
TABLE I.
Guidelines for referral
|
MET = metabolic equivalent of task; VAS = visual analog scale.
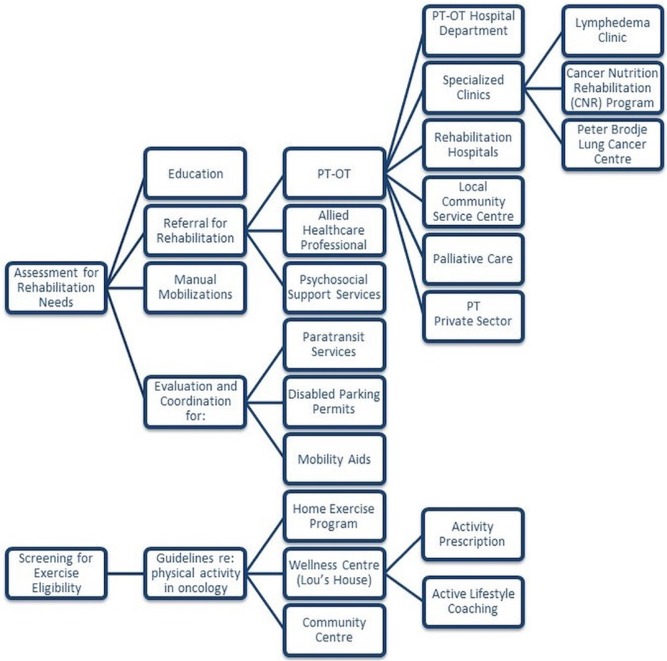
Figure 1 depicts the model of care that was developed, and the triage and referral pathways followed by our patients. The human resources required to effectively screen and implement this model of care included a clinical director (physiotherapist), 4 staff physiotherapists, and 3 kinesiologists.
FIGURE 1.
Model of care and referral pathway. PT = physiotherapy; OT = occupational therapy.
Evaluation and Screening
A comprehensive review of each patient’s medical history and treatment status was followed by a physical exam and functional performance evaluation. Outcome measures used during the patient assessment included, but were not limited to, the Brief Fatigue Inventory, a physical activity profile using weekly met–hours (metabolic equivalent of task hours), the Brief Pain Inventory, the Edmonton Symptom Assessment System, and Disabilities of the Arm, Shoulder and Hand. Specific evaluations were structured by the patient’s diagnosis, stage of disease, and referral objectives. The assessments were designed to detect treatment-related musculoskeletal and neurologic issues; limitations related to skeletal fragility or bone metastases; loss of mobility, balance, and strength; and functional or activity limitations.
Triage and Referral Pathways
Patients were categorized into the complex or non-complex group depending on disease- and treatment-related side effects, as well as factors that interfered with the ability to exercise.
The Non-complex Patient
Non-complex patients included those who were diagnosed at earlier stages, who had nonrecurrent or nonmetastatic disease, who had received standard treatment protocols, and who were experiencing no significant side effects related to treatment. The rehabilitation needs of this subset of patients were minimal, allowing for emphasis on exercise promotion and healthy lifestyle maintenance.
Non-complex patients were referred to the Wellness Centre, where care was transferred to one of the team’s kinesiologists. The patient’s individual goals and barriers were identified, and strategies were devised to overcome the barriers. The kinesiologist reinforced education provided by the physiotherapist and provided more-specific exercise guidelines and a personalized program.
The Complex Patient
Most service requisitions were for complex cases referred to the program by members of the multidisciplinary oncology team. The side effects of aggressive treatment protocols and extensive or reconstructive surgeries were prevalent, affecting physical capacities and the ability to maintain activities of daily living.
Physical presentations of the complex patients included, but were not limited to, significant fatigue, pain, peripheral neuropathies, radiation fibrosis, axillary web syndrome, and scar tissue impeding performance. Specific guidelines on exercise and progressions for general physical activity were provided by the physiotherapist in consultation with the oncology team about the safety of the interventions. However, when patient needs exceeded the program’s mandate, they were referred to rehabilitation specialists within the public or private sectors. Notwithstanding, the latter patients were also referred to the Wellness Centre for activity prescription and guidance. In addition, the team initiated requests for disabled parking permits, paratransit transport services, and procurement of mobility aids when necessary.
Exercise Prescription
Programs were based on recommendations provided by the American College of Sports Medicine roundtable on exercise guidelines for cancer survivors12 and included components of flexibility, cardiovascular, and resistance training whenever possible. In addition, given the evidence concerning improved survivorship and quality of life in cancer patients associated with increased levels of energy expenditure (met–hours/week)12,32, exercise prescriptions focused primarily on increasing baseline levels of physical activity. More specifically, patients were asked to choose daily activities in which they normally participated and were advised to increase either the intensity or frequency of those physical activities. Programs were personalized to include a combination of home exercise, Wellness Centre–based training, or participation in exercise classes, depending on the status of their disease and treatments at any given time.
Given the health status fluidity of the survivors, patient re-assessments were scheduled every 3 months, at which time the kinesiologist discussed the patient’s compliance and barriers, re-assessed physical functioning, and established new goals. In the event that patients were, because of time constraints or geographic location, unable to attend the Wellness Centre for their physical activity, recommendations were provided for use of the most accessible community-based exercise facilities available, and communication was established with trainers as needed. Patients were also offered the opportunity to participate in diverse psychosocial programs offered through Hope and Cope and its Wellness Centre.
Risk Management and Communication
Patients were re-assessed when significant changes to either their treatment status (for example, stem-cell transplantation, radiation, chemotherapy protocols) or deterioration in disease status occurred. The physiotherapist had access to each patient’s disease and treatment status through medical records, multidisciplinary meetings, tumour boards, and communication with medical and allied health care professionals. Functional status evaluations were recorded electronically for access by all medical personnel. The team’s hospital-based physiotherapists communicated directly with Wellness Centre–based kinesiologists to supplement reports on changes in the physical condition of patients. The kinesiologists were in communication with the physio therapists about exercise tolerance and the development of exercise or non-exercise-related problems necessitating medical overview by the oncology team.
Additional precautions were taken to review files of patients with metastatic disease and to discuss the management of those with bone disease. A physical activity card system was implemented at the Wellness Centre to alert all exercise class instructors of any specific risks associated with exercise for individual patients. Continuous staff mentoring and education were essential to ensuring consistency in case management.
Education
Education was an integral part of the interventions provided through this model of care. The education provided to patients included fatigue management, activity modifications to improve functional status and to perform activities of daily living, postural correction, gait training, and transfer training. Essential counselling on reducing the risk of fractures and falls in patients with bone fragility or metastasis was provided. Pain management strategies, lymphedema awareness and risk reduction, and protocols to manage sensory neuropathies, adherent scars, and sensitive skin were provided.
RESULTS OF MODEL IMPLEMENTATION
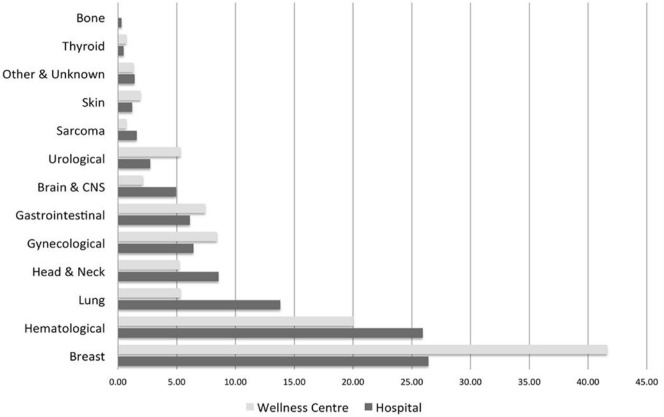
In a review of demographics between January 2011 and December 2015, the hospital team found that 1635 patients spanning all major cancer sites had been assessed, with an average of 5.8 follow-up visits, and the Wellness Centre team had evaluated and prescribed exercise for 1066 participants. The demographics of the hospital population did not necessarily reflect patient needs but rather the recognition and commitment of each tumour site team to the value of the program. Differences in the demographics of the hospital and Wellness Centre populations are the result of differences in case complexity (Figure 2).
FIGURE 2.
ActivOnco demographics 2011–2015. CNS = central nervous system.
In reviewing the outcomes of this model of care, the volume and diversity of the cancer patient population screened with respect to diagnosis, stage of disease, stage and type of treatment, age, and comorbidities made it difficult to globally assess the effects of the services provided. A 2-month representative sample (1 June–31 July 2013) was therefore chosen to illustrate in greater detail the activities provided by the ActivOnco program, as well as the relative proportions of education, exercise, and rehabilitation services delivered to improve functional status. In addition, two brief retrospective reviews of select population outcomes (young adults, multiple myeloma) that were conducted during implementation of this model of care are included to illustrate the benefits of this program.
Patient Demographics
The hospital team evaluated 75 new patients during the period of interest and gave follow-up care to an additional 159 patients. Median age at diagnosis of the patients referred to the program during that period was 52 years (standard deviation: 15.5 years). The cohort was 65% female, and 52% of the patients were seen while undergoing treatment. More than one third of the patients (35.5%) were living with advanced disease or metastatic cancer, and 16% had bone metastases. Primary cancer diagnoses in the new-intake patients were hematologic (26.7%), breast (24%), head and neck (18.7%), and gastrointestinal (10.7%). However, in accounting for hospital team activities overall, including patients seen in follow-up, the proportion of patients changed to include increased numbers of patients with head and neck (17.5%) and lung (11.1%) cancer. Those patients had greater rehabilitation needs and required more intense supervision. More than half the patients with metastatic disease (57.7%) had been diagnosed with lung primaries.
Referral Sources
Referrals were received from a variety of sources within the hospital. Most referrals (71%) came from members of the multidisciplinary team (nurse coordinators, nutritionists, and social workers), including a significant number of direct referrals by treating oncologists (35%). An additional 15% of intake patients were self-referred for screening and exercise eligibility; the remaining 14% were referred by a staff member or volunteer from Hope and Cope psychosocial services.
Symptom Profile and Physical Presentation
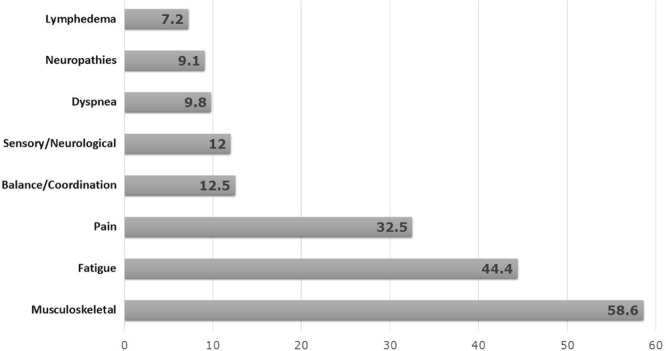
Per Figure 3, 44.4% of the 234 patients subjectively reported fatigue (mean score: 5.6 on a 0–10 visual analog scale), 32.5% reported pain (mean score: 4.6 on a 0–10 visual analog scale), 9.8% had symptoms of dyspnea, and 9% had symptoms of peripheral neuropathy. Upon physical examination, more than half the patients (58.6%) had musculoskeletal issues, including loss of movement because of surgical interventions, radiation fibrosis, muscular weakness associated with neck dissection, or submuscular breast implants. In addition, 12.5% of the patients showed deficits in balance or coordination; 12% had signs of sensory or neurologic deficits; and 7% presented with lymphedema. Notably, some of the impairments observed were related to comorbidities that preceded cancer and were further exacerbated by treatment.
FIGURE 3.
Symptom profile and physical presentation.
Interventions and Referral Pathway
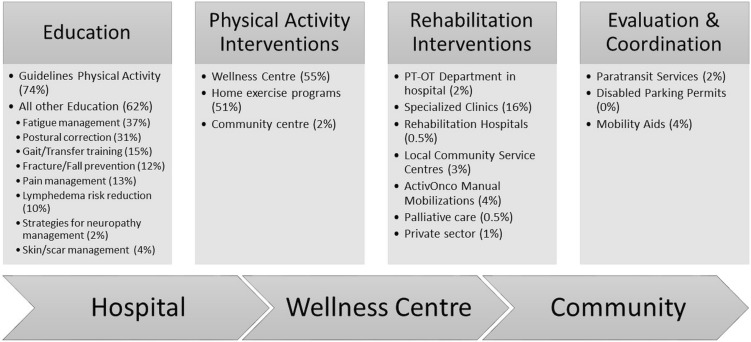
Primary interventions were education, physical activity promotion, and referral to appropriate follow-up services. During the period of interest, other educational guidelines—including transfer training, fall prevention, scar management, lymphedema risk reduction, and strategies for management of neuropathies—were provided to 62% of the patients (Figure 4). Referral to rehabilitation specialists within the private and public sectors was required for 27% of the population. A small percentage of patients required evaluation for and coordination of paratransit services, and procurement and recommendations for mobility aids. The volumes of specific interventions align with the ActivOnco data measured throughout the program’s operational implementation.
FIGURE 4.
Interventions and referral pathways, 1 June to 31 July 2013. PT = physiotherapy; OT = occupational therapy.
Select Population Outcomes
In 2010, we conducted a review of 97 young adults (18–45 years of age) with central nervous system, gastrointestinal, breast, sarcoma, and testicular cancers consecutively recruited to the program. Despite aggressive treatment and metastatic disease in 65% of that population, participants were capable of increasing their deliberate physical activity levels to a weekly average of 18.6 met–hours from 8.2 met–hours, a finding that represented a significant increase in physical activity (p = 0.01) with no adverse effects reported. Moreover, fatigue severity and impact (Brief Fatigue Inventory) remained stable in exercisers. Non-exercisers decreased their weekly physical activity levels to below 3 met–hours, which is essentially bedridden33.
To identify factors that contribute to compliance versus noncompliance with our model of exercise prescription, we retrospectively conducted, in 2014, a review of 41 patients with multiple myeloma34. Despite the fact that 81% of the patients had bone lesions and were undergoing active treatment, overall compliance with exercise prescription was 71%, mean levels of physical activity increased significantly (p < 0.001), and fatigue severity was reduced (p = 0.003). Noncompliance increased with the incidence of skeletal-related events, including pathologic fracture, spinal cord compression, and radiation for stabilization of bone lesions. However, it was noted that many patients at high risk could exercise safely with appropriate guidance.
DISCUSSION
Many integrated models of care have been developed for the management of chronic disease by linking primary medical services to either hospital-, home-, or community-based rehabilitation services35. The elements of those programs include a team approach, inter-professional trust, communication, decision-making, and professional service delivery. However, despite improved survivorship, few models of care have been implemented to provide services to the general population of cancer patients shown to develop chronic dysfunction associated with the disease and its treatment.
The infrastructure developed within the ActivOnco model of care with respect to systematic screening, identification of rehabilitation needs and exercise capacity, triage and decision-making, education, communication, and provision of exercise prescription is relatively unique in oncology. Implementation of this combined hospital- and community-based program, with specialized teams of both physiotherapists and kinesiologists, allowed for screening and evaluation of the incidence of functional disability in a general cancer population and the feasibility of providing exercise prescription for all cancer patients.
The inclusiveness of the model of care was its greatest strength: patients with all cancer diagnoses, stages of disease, and treatment statuses were eligible for participation. The program benefited from ease of access to medical charts and interaction with the medical team for constant updates on the medical and treatment statuses of patients. Participants were encouraged to partake in physical activity based on individual goals and preferences, whether in a group or individual setting. Allowing participants to have input on their wellness plan increased the chance of successful lifestyle alterations.
Integrated models of care require inter-professional trust and timely referrals for patients in need of rehabilitation interventions31,35; the education of the medical teams was therefore an essential component of our program. Notwithstanding the quality and quantity of evidence concerning improved recovery and survival with rehabilitation and exercise interventions, the benefits of cancer rehabilitation remained peripheral to the standard of care provided by many members of the oncology team. The proactive participation of our hospital team of physiotherapists in multidisciplinary clinics and rounds was essential, given the focus of medical interventions on cure of disease without sensitization to treatment-induced dysfunction. Requests for rehabilitation interventions were commonly delayed and triggered by significant loss of ps.
Patients with metastatic disease, those in the geriatric age range, and those with comorbidities were at greatest risk for functional decline with treatment36–38. The cancer-specific populations requiring the greatest follow-up and supervision during exercise implementation were those with bone disease (multiple myeloma), bone metastasis (breast, lung, and thyroid cancers, and melanoma), or skeletal fragility related to treatment (breast, ovarian, and prostate cancers). Those patients received specific physical activity guidelines and education for fracture prevention, including location and nature of their lesions, plus overall specific education for injury prevention with activities of daily living and work. Individualized exercise programs were provided to maintain ps and prevent skeletal-related events. Within the period of interest for our analysis, 39.4% of patients with bone metastasis exercised at the Wellness Centre and an additional 26% were given home programs. In our previously summarized studies, patients with spinal metastases were successfully treated with low-level cardiovascular and resistance training, improving their quality of life despite advanced cancer33,34.
Limitations
Exercise alone is not a panacea for all physical inactivity related to cancer treatment. Bottlenecks in the transfer of patients who required rehabilitation services (27%) and the lack of oncology-specialized community resources were a significant impediment to the maintenance of our mandate to primarily provide activity guidance and referral. Our hospital team of physiotherapists was constantly under pressure to provide basic rehabilitation interventions, including manual mobilizations to prevent the development of frozen shoulder in breast cancer patients or to reduce the effects of radiation fibrosis in head-and-neck cancer patients; and sensory stimulation and balance and gait training to prevent falls in elderly patients or total deconditioning in hematologic cancer patients undergoing stem-cell transplantation or highly toxic chemotherapy regimens.
Unfortunately, the lack of systemic funding is a major barrier to the development and sustainability of cancer rehabilitation programs across Canada24,39 and internationally11,30,31. ActivOnco is a program offered by Hope and Cope, a nonprofit organization relying on private donations, targeted fundraising events, and grants for research within the context of the program. The recurrent human resource costs are difficult to maintain and the lack of assistive funding from either the Oncology or the Physiotherapy department speaks to the difficulties associated with the development of rehabilitation services for cancer patients within the context of reduced hospital funding. The prevention and management of potential long-term dysfunction and disability and the loss of vitality to families and society of many principal breadwinners because of an inability to return to work and normal activities associated with cancer and its treatment constitute a tremendous cost to society and must be factored into strategic plans for health care reform.
CONCLUSIONS
For rehabilitation and exercise prescription to become an integral part of cancer care, funding must be prioritized, models of care must be developed, and services must be standardized, becoming evidenced-based. Research in cancer rehabilitation is growing exponentially23; however, the integration of research into clinical practice is far from reality11,24,38. Most research on physical activity and rehabilitation within the context of cancer survivorship has focused on homogenous groups with very specific interventions11,40. The reality is that cancer patients are a heterogeneous group, having varying treatment approaches and symptom profiles, and a holistic approach to rehabilitation is therefore warranted11. Hospital-based rehabilitation services and community-based exercise facilities must be prepared with specialized education, communication strategies, and clinical tools for the management of cancer patients.
ACKNOWLEDGMENTS
The authors sincerely thank Hope and Cope for providing the resources needed to develop and maintain ActivOnco, Susan Wener for generous donations and persistent advocacy for this model of care, and the cure Foundation, which supported the program’s initiation and research based on its infrastructure.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Mirolla M. The Cost of Chronic Disease in Canada. Ottawa, ON: The Chronic Disease Prevention Alliance of Canada; 2004. [Google Scholar]
- 2.Boucher P, Langlois PE, on behalf of the Coalition Priorité Cancer au Québec . Les coûts économiques du cancer au Québec en 2013. Montreal, QC: Coalition Priorité Cancer au Québec; 2014. [Available online at: http://en.calameo.com/read/0012859279adcfde6a198?authid=pIlthvR5cjdR; cited 5 July 2016] [Google Scholar]
- 3.Silver JK, Baima J, Newman R, Galantino ML, Shockney LD. Cancer rehabilitation may improve function in survivors and decrease the economic burden of cancer to individuals and society. Work. 2013;46:455–72. doi: 10.3233/WOR-131755. [DOI] [PubMed] [Google Scholar]
- 4.Egan MY, McEwen S, Sikora L, Chasen M, Fitch M, Eldred S. Rehabilitation following cancer treatment. Disabil Rehabil. 2013;35:2245–58. doi: 10.3109/09638288.2013.774441. [DOI] [PubMed] [Google Scholar]
- 5.Alfano CM, Ganz PA, Rowland JH, Hahn EE. Cancer survivorship and cancer rehabilitation: revitalizing the link. J Clin Oncol. 2012;30:904–6. doi: 10.1200/JCO.2011.37.1674. [DOI] [PubMed] [Google Scholar]
- 6.Alfano CM, Cheville AL, Mustian K. Developing high-quality cancer rehabilitation programs: a timely need. Am Soc Clin Oncol Educ Book. 2016;35:241–9. doi: 10.14694/EDBK_156164. [DOI] [PubMed] [Google Scholar]
- 7.Mewes JC, Steuten LM, Ijzerman MJ, van Harten WH. Effectiveness of multidimensional cancer survivor rehabilitation and cost-effectiveness of cancer rehabilitation in general: a systematic review. Oncologist. 2012;17:1581–93. doi: 10.1634/theoncologist.2012-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott DA, Mills M, Black A, et al. Multidimensional rehabilitation programmes for adult cancer survivors. Cochrane Database Syst Rev. 2013:CD007730. doi: 10.1002/14651858.CD007730.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silver JK, Baima J, Mayer RS. Impairment-driven cancer rehabilitation: an essential component of quality care and survivorship. CA Cancer J Clin. 2013;63:295–317. doi: 10.3322/caac.21186. [DOI] [PubMed] [Google Scholar]
- 10.Stubblefield MD, Schmitz KH, Ness KK. Physical functioning and rehabilitation for the cancer survivor. Semin Oncol. 2013;40:784–95. doi: 10.1053/j.seminoncol.2013.09.008. [DOI] [PubMed] [Google Scholar]
- 11.Stubblefield MD, Hubbard G, Cheville A, Koch U, Schmitz KH, Dalton SO. Current perspectives and emerging issues on cancer rehabilitation. Cancer. 2013;119(suppl 11):2170–8. doi: 10.1002/cncr.28059. [DOI] [PubMed] [Google Scholar]
- 12.Schmitz KH, Courneya KS, Matthews C, et al. on behalf of the American College of Sports Medicine American College of Sports Medicine roundtable on exercise guidelines for cancer survivors. Med Sci Sports Exerc. 2010;42:1409–26. doi: 10.1249/MSS.0b013e3181e0c112. [Erratum in: Med Sci Sports Exerc 2011;43:195] [DOI] [PubMed] [Google Scholar]
- 13.Andersen C, Rørth M, Ejlertsen B, et al. The effects of a sixweek supervised multimodal exercise intervention during chemotherapy on cancer-related fatigue. Eur J Oncol Nurs. 2013;17:331–9. doi: 10.1016/j.ejon.2012.09.003. [DOI] [PubMed] [Google Scholar]
- 14.Cramp F, Daniel J. Exercise for the management of cancerrelated fatigue in adults. Cochrane Database Syst Rev. 2008:CD006145. doi: 10.1002/14651858.CD006145.pub2. [DOI] [PubMed] [Google Scholar]
- 15.Schneider CM, Hsieh CC, Sprod LK, Carter SD, Hayward R. Effects of supervised exercise training on cardiopulmonary function and fatigue in breast cancer survivors during and after treatment. Cancer. 2007;110:918–25. doi: 10.1002/cncr.22862. [DOI] [PubMed] [Google Scholar]
- 16.Marques-Aleixo I, Santos-Alves E, Mariani D, et al. Physical exercise prior and during treatment reduces sub-chronic doxorubicin-induced mitochondrial toxicity and oxidative stress. Mitochondrion. 2015;20:22–33. doi: 10.1016/j.mito.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Cormie P, Galvão DA, Spry N, et al. Can supervised exercise prevent treatment toxicity in patients with prostate cancer initiating androgen-deprivation therapy: a randomised controlled trial. BJU Int. 2015;115:256–66. doi: 10.1111/bju.12646. [DOI] [PubMed] [Google Scholar]
- 18.Streckmann F, Kneis S, Leifert JA, et al. Exercise program improves therapy-related side-effects and quality of life in lymphoma patients undergoing therapy. Ann Oncol. 2014;25:493–9. doi: 10.1093/annonc/mdt568. [DOI] [PubMed] [Google Scholar]
- 19.Dimeo F, Fetscher S, Lange W, Mertelsmann R, Keul J. Effects of aerobic exercise on the physical performance and incidence of treatment-related complications after high-dose chemotherapy. Blood. 1997;90:3390–4. [PubMed] [Google Scholar]
- 20.Cheville AL, Kollasch J, Vandenberg J, et al. A home-based exercise program to improve function, fatigue, and sleep quality in patients with stage iv lung and colorectal cancer: a randomized controlled trial. J Pain Symptom Manage. 2013;45:811–21. doi: 10.1016/j.jpainsymman.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hutnick NA, Williams NI, Kraemer WJ, et al. Exercise and lymphocyte activation following chemotherapy for breast cancer. Med Sci Sports Exerc. 2005;37:1827–35. doi: 10.1249/01.mss.0000175857.84936.1a. [DOI] [PubMed] [Google Scholar]
- 22.Burnham TR, Wilcox A. Effects of exercise on physiological and psychological variables in cancer survivors. Med Sci Sports Exerc. 2002;34:1863–7. doi: 10.1097/00005768-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 23.Ugolini D, Neri M, Cesario A, et al. Scientific production in cancer rehabilitation grows higher: a bibliometric analysis. Support Care Cancer. 2012;20:1629–38. doi: 10.1007/s00520-011-1253-2. [DOI] [PubMed] [Google Scholar]
- 24.Canestraro A, Nakhle A, Stack M, et al. Oncology rehabilitation provision and practice patterns across Canada. Physiother Can. 2013;65:94–102. doi: 10.3138/ptc.2011-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Segal R, Evans W, Johnson D, et al. Oncology rehabilitation program at the Ottawa Regional Cancer Centre: program description. CMAJ. 1999;161:282–5. [PMC free article] [PubMed] [Google Scholar]
- 26.Santa Mina D, Alibhai SM, Matthew AG, et al. Exercise in clinical cancer care: a call to action and program development description. Curr Oncol. 2012;19:e136–44. doi: 10.3747/co.19.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Noble M, Russell C, Kraemer L, Sharratt M. uw well-fit: the impact of supervised exercise programs on physical capacity and quality of life in individuals receiving treatment for cancer. Support Care Cancer. 2012;20:865–73. doi: 10.1007/s00520-011-1175-z. [DOI] [PubMed] [Google Scholar]
- 28.Cheifetz O, Park Dorsay J, Hladysh G, MacDermid J, Serediuk F, Woodhouse LJ. CanWell: meeting the psychosocial and exercise needs of cancer survivors by translating evidence into practice. Psychooncology. 2014;23:204–15. doi: 10.1002/pon.3389. [DOI] [PubMed] [Google Scholar]
- 29.Chasen M, Feldstain A, Gravelle D, MacDonald N, Pereira J. An interprofessional palliative care oncology rehabilitation program: effects on function and predictors of program completion. Curr Oncol. 2013;20:301–9. doi: 10.3747/co.20.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hellbom M, Bergelt C, Bergenmar M, et al. Cancer rehabilitation: a Nordic and European perspective. Acta Oncol. 2011;50:179–86. doi: 10.3109/0284186X.2010.533194. [DOI] [PubMed] [Google Scholar]
- 31.Yang EJ, Chung SH, Jeon JY, et al. Current practice and barriers in cancer rehabilitation: perspectives of Korean physiatrists. Cancer Res Treat. 2015;47:370–8. doi: 10.4143/crt.2014.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Clague J, Bernstein L. Physical activity and cancer. Curr Oncol Rep. 2012;14:550–8. doi: 10.1007/s11912-012-0265-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dalzell M, Shallwani S, Kavan P, et al. Activity levels and fatigue related to exercise compliance in young adults with cancer [abstract e19555] J Clin Oncol. 2010;28 doi: 10.1200/jco.2010.28.15_suppl.e19555. [Available online at: http://meetinglibrary.asco.org/content/53415-74; cited 9 April 2017] [DOI] [Google Scholar]
- 34.Shallwani S, Dalzell M, Sateren W, O’Brien S. Exercise compliance among patients with multiple myeloma undergoing chemotherapy: a retrospective study. Supportive Care Cancer. 2015;23:3081–8. doi: 10.1007/s00520-015-2680-2. [DOI] [PubMed] [Google Scholar]
- 35.McColl MA, Shortt S, Godwin M, et al. Models for integrating rehabilitation and primary care: a scoping study. Arch Phys Med Rehabil. 2009;90:1523–31. doi: 10.1016/j.apmr.2009.03.017. [DOI] [PubMed] [Google Scholar]
- 36.Pergolotti M, Deal AM, Lavery J, Reeve BB, Muss HB. The prevalence of potentially modifiable functional deficits and the subsequent use of occupational and physical therapy by older adults with cancer. J Geriatr Oncol. 2015;6:194–201. doi: 10.1016/j.jgo.2015.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balducci L, Fossa SD. Rehabilitation of older cancer patients. Acta Oncol. 2013;52:233–8. doi: 10.3109/0284186X.2012.744142. [DOI] [PubMed] [Google Scholar]
- 38.Cheville A. Rehabilitation of patients with advanced cancer. Cancer. 2001;92(suppl 4):1039–48. doi: 10.1002/1097-0142(20010815)92:4+<1039::AID-CNCR1417>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 39.Mina DS, Petrella A, Currie KL, et al. Enablers and barriers in delivery of a cancer exercise program: the Canadian experience. Curr Oncol. 2015;22:374–84. doi: 10.3747/co.22.2650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Phillips SM, Alfano CM, Perna FM, Glasgow RE. Accelerating translation of physical activity and cancer survivorship research into practice: recommendations for a more integrated and collaborative approach. Cancer Epidemiol Biomarkers Prev. 2014;23:687–99. doi: 10.1158/1055-9965.EPI-13-1355. [DOI] [PMC free article] [PubMed] [Google Scholar]