Abstract
We report a case of a recurrent clear cell meningioma (ccm) in the frontal lobe of the brain of a 67-year-old man. The patient developed three recurrences: at 3, 10, and 12 years after his initial surgery. Histopathology observations revealed a grade 2 ccm with positivity for vimentin and epithelial membrane antigen. Expression of E-cadherin was positive only in the primary tumour and in the first available recurrence. Fluorescence in situ hybridization analyses demonstrated 1p and 14q deletions within the last recurrence. Multiplex ligation-dependent probe amplification studies revealed a heterozygous partial NF2 gene deletion, which progressed to total loss in the last recurrence. The last recurrence showed homozygous deletions in CDKN2A and CDKN2B. The RASSF1 gene was hypermethylated during tumour evolution.
In this report, we show the genetic alterations of a primary ccm and its recurrences to elucidate their relationships with the changes involved in the progression of this rare neoplasm.
Keywords: Clear cell meningioma, recurrence, intracranial disease, non-pediatric disease, genetics, NF2, tumour suppressor genes, molecular progression
INTRODUCTION
Clear cell meningioma (ccm) classified as grade 2 is a rare, potentially aggressive entity that represents about 0.2% of all meningiomas, with most cases being described in young women1–5. Surgical resection and postoperative radiotherapy are the standard treatment for primary disease and local recurrences.
The genesis of meningioma is associated with loss of genetic material on chromosome 22. Monosomy of that chromosome is the most common genetic alteration in meningioma and is linked to mutations of the NF2 gene, located in 22q12.2, which codes for the tumour suppressor protein merlin6. Losses of 1p and alterations in chromosome 14 are present in many atypical meningiomas and have been associated with tumour progression7,8.
Here, we report a case of grade 2 ccm that arose in the right frontal lobe of a 67-year-old man and recurred three times. Histologic and genetic studies of the primary tumour and its recurrences showed changes in NF2 and other tumour suppressor genes. We analyzed the evolution and implications of the various genetic aberrations present in this neoplasm with the aim of elucidating the genetic changes involved in the progression of ccm.
CASE DESCRIPTION
A 67-year-old man presented with a mass (12 cm3) in the right frontal lobe, which was histologically diagnosed as a grade 2 ccm. The tumour, resected in a surgical procedure, was sporadic, and the patient did not show any feature of NF2 disease. A control magnetic resonance imaging exam 3 years after the initial surgery revealed a recurrence of the tumour in the same location, which was treated with radiosurgery (15 Gy). No material from the recurrence could be saved. Another recurrence (37 cm3), called the “first recurrence” in the present work, was resected 7 years after the second surgery. The frontal lesion again recurred (9 cm3) 2 years later, and it (here called the “second recurrence”) was resected. In all surgeries, the resection was maximal, and neither chemotherapy nor radiotherapy was applied. To date, further control exams have not shown new recurrences.
Pathology Examination
Tumour sections were stained with hematoxylin and eosin. Antibodies against vimentin, epithelial membrane antigen, CD34, E-cadherin, and glial fibrillary acidic protein were assayed and valued as negative or positive. A Ki-67/MIB-1 antibody was used to estimate the proliferation index by determining the percentage of immunopositive nuclei. Results were defined as low proliferation (≤1% stained nuclei), moderate proliferation (1%–5%), or high proliferation (≥5%)9. All antibodies were purchased from Dako (Dako Diagnostics, Barcelona, Spain).
Fluorescence In Situ Hybridization
The fluorescence in situ hybridization (fish) probes used were LSI-1p36/LSI-1q25 (red and green signals respectively) and IGH/CCND1-DF to detect loss of chromosome 14, with chromosome 11 acting as a control (Vysis: Abbott Molecular, Madrid, Spain). One signal or less per chromosome with respect to the control signal was considered to be a deletion, and a 2:2 ratio was considered normal8.
Multiplex Ligation-Dependent Probe Amplification
Biopsy punches from selected areas of paraffin blocks from each tumour sample were used for dna extraction with the QIAmp DNA FFPE tissue kit (Qiagen, Valencia, CA, U.S.A.). For multiplex ligation-dependent probe amplification (mlpa) assays, salsa mlpa probemix P044-B1-NF2 and MS-MLPA (mlpa and methylation-specific mlpa) and probemix ME001-C2 tumour suppressor 1 kits were used (MRC-Holland, Amsterdam, Netherlands). Losses in 1p34 and 14q13 were detected with reference probes 14839-L16547 and 01063-L00061 included in the P044-B1-NF2 kit. All probe sequences, gene loci, and chromosome locations can be found at http://www.mlpa.com. Values between 0.7 and 1.3 were considered to be the normal genetic dose. Results between 0.7 and 0.3 were interpreted as heterozygous deletion. Results below 0.3 were interpreted as homozygous deletion10.
Statistical Analysis
Data are expressed as means with standard error. Statistical differences were determined using the Student t-test for dual comparisons. Data were analyzed in the GraphPad Prism software application (version 6: GraphPad Software, San Diego, CA, U.S.A.). Significance was accepted at p < 0.05.
RESULTS
Pathology
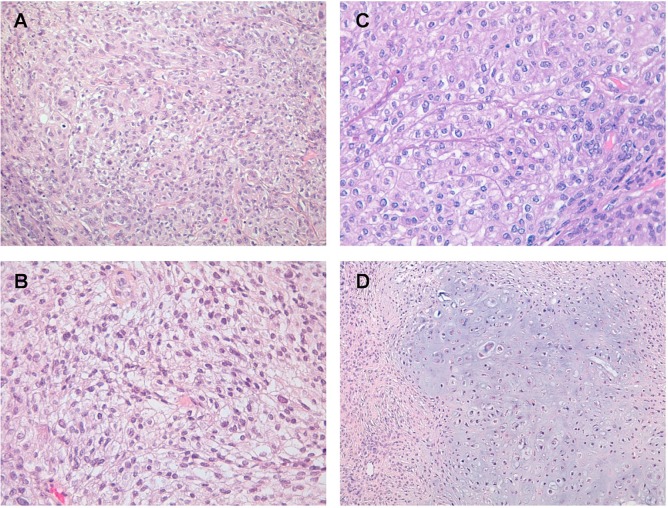
The primary tumour, diagnosed as ccm, had moderate cellularity and was composed of sheets of polygonal cells with small round monomorphic nuclei and clear cytoplasm [Figure 1(A)]. Increase of cellularity, pleomorphism, nucleolar prominence, and focal micro-necrosis were observed in the recurrences [Figure 1(B,C)]. Additionally, the last recurrence showed chondroid areas [Figure 1(D)].
FIGURE 1.
Histopathologic pattern of the reported clear cell meningioma: (A) primary tumour; (B) first recurrence; (C) second recurrence; (D) chondroid area in the second recurrence. Hematoxylin and eosin stain, 40× original magnification.
Immunohistochemistry
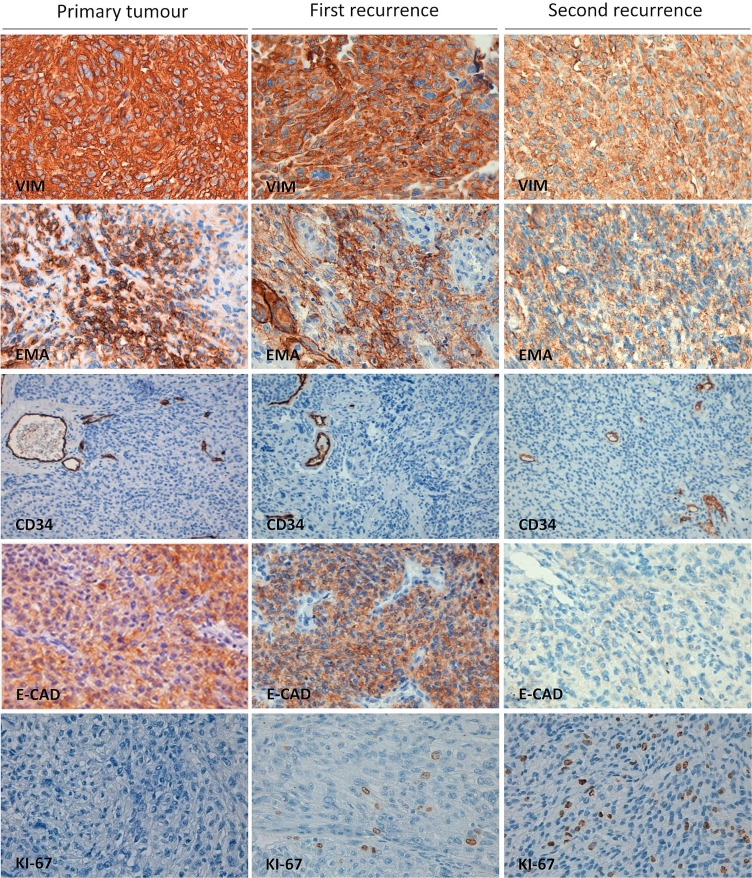
Vimentin and epithelial membrane antigen expression were strongly positive in all samples (Figure 2). In addition, expression of the glial fibrillary acidic protein was negative in both the original tumour and the two recurrences (data not shown). In the various samples, CD34 revealed regular (no hyperplastic) vascularity. Expression of E-cadherin showed important changes: It was expressed in the primary tumour and in the first recurrence, but was absent in the last recurrence. With respect to Ki-67 expression, the primary tumour presented a low proliferation index, the first recurrence was assessed as moderate, and the last recurrence had a high proliferation index (Figure 2).
FIGURE 2.
Immunohistochemical stains. Left column: Primary clear cell meningioma tumour. Middle column: First recurrence. Right column: Second (last) recurrence. VIM = vimentin; EMA = epithelial membrane antigen; E-CAD = E-cadherin. Each micrograph is representative from various areas of the same recurrence (40× original magnification).
FISH
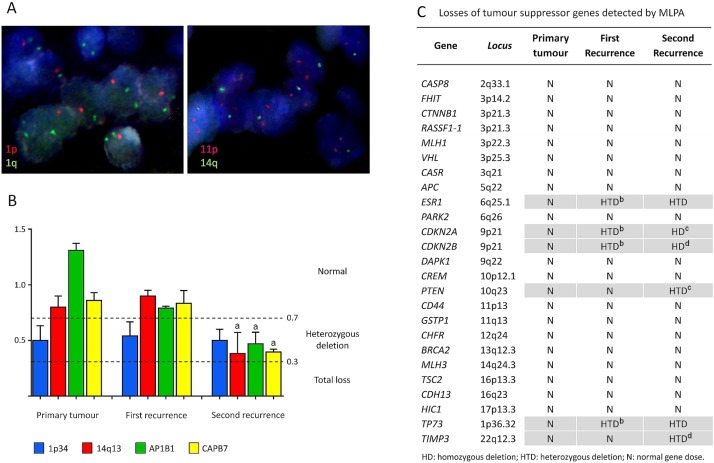
All samples underwent fish studies for chromosomes 1p and 14q, but the quality of the primary tumour and first recurrence samples was suboptimal, being unsuitable for fish. Analysis of the second recurrence revealed partial losses of chromosomes 1p and 14q [Figure 3(A)].
FIGURE 3.
(A) Fluorescence in situ hybridization in paraffin-embedded tissue sections from the second recurrence of the clear cell meningioma. Left panel: One red signal indicates the loss of 1p36 region; 1q25 region (two green signals) acts as control. Right panel: One green signal evidences the loss of one chromosome 14q; two red signals indicate the presence of chromosome 11 (control). (B) Multiplex ligation-dependent probe amplification (MLPA) study of chromosome 1p34, chromosome 14q13, AP1B1, and CABP7 in primary tumour, first recurrence, and second recurrence. Values between 0.7 and 1.3 correspond to normal gene doses. Values between 0.3 and 0.7 correspond to heterozygous deletion. Values below 0.3 correspond to total loss. Results are expressed as mean ± standard error of the mean, from independent experiments performed on three fragments of each tumour. ap < 0.05 with respect to first-recurrence results. (C) Losses of tumour suppressor genes detected by MLPA. N = normal gene dose; HTD = heterozygous deletion; HD = homozygous deletion. Results from each gene—mean ± standard error of the mean—are obtained from independent experiments performed on three fragments of each tumour. bp < 0.05 with respect to primary tumour results. cp < 0.05 and dp < 0.01 with respect to first recurrence results.
MLPA
Integrity of the NF2 exons was studied by mlpa. The primary tumour and the first recurrence presented a heterozygous deletion of 15 exons from among the 17 total exons (exons 2–16); in the second recurrence, the entire codifying region of NF2 was deleted. The AP1B1 and CABP7 genes that flank NF2 were also deleted in the last recurrence [Figure 3(B)]. In the three samples studied, 1p34 was lost. On the other hand, chromosome 14q13 was in the normal range in the primary tumour and the first recurrence, but lost in the last recurrence [Figure 3(A,B)].
In addition to NF2, other tumour suppressor genes were studied [Figure 3(C)]. Heterozygous and homozygous losses of CDKN2A/2B were found within the first and the second recurrences respectively. Both recurrences presented ESR1 and TP73 heterozygous deletions. Moreover, the last recurrence also presented heterozygous deletions in PTEN and TIMP3. Study of the methylation status of tumour suppressor genes showed hypermethylation of RASSF1 in the second recurrence, with TP73 proving negative in every sample.
DISCUSSION
Although ccm has been considered to occur mainly in the pediatric age range, the literature describes a considerable number of cases of intracranial ccm in the middle-aged and elderly population (Table i). Tumour aggressiveness and recurrence rates are frequently greater than those seen with benign grade 1 meningiomas17,26. The second recurrence in our patient with ccm showed areas of chondroid differentiation. Some hypotheses explain the local differentiation as a reversible change in which some meningeal cells are replaced by chondroid cells27, although the reason that such metaplasia occurs in specific tumours remains unclear.
TABLE I.
Brief literature review of cases of intracranial clear cell meningioma in patients more than 45 years of age
| Reference | Patient characteristics | ||
|---|---|---|---|
|
| |||
| Age (years) | Sex | Tumour location | |
| Shiraishi, 199111 | 49 | Female | Left frontal |
| Pimentel et al., 199812 | 61 | Male | Convexity |
| Kuzeyli et al., 200313 | 52 | Female | Right frontal and right occipital regions |
| 50 | Male | Left temporal fossa | |
| Jain et al., 200714 | 47 | Male | Left Sphenoid wing |
| 65 | Female | Left tentorium | |
| Tena-Suck et al., 20074 | 62 | Female | Right parasagittal |
| 45 | Female | Right frontal lobe | |
| 62 | Female | Right frontal lobe | |
| Cassereau et al., 200815 | 50 | Male | Right lateral ventricle |
| Pizzoni et al., 200816 | 66 | Female | Olfactory groove |
| Ohba et al., 200817 | 60 | Male | Foramen magnum |
| 60 | Male | Right posterior clinoid | |
| Prayson et al., 200818 | 58 | Male | Left sphenorbital |
| 72 | Male | Left frontal | |
| 76 | Male | Right frontal | |
| 56 | Male | Left cavernous sinus | |
| 80 | Female | Left frontal lobe | |
| 49 | Male | Left cavernous sinus | |
| 69 | Female | Left cavernous sinus | |
| 70 | Male | Right frontal lobe | |
| 72 | Female | Right frontal lobe | |
| 45 | Male | Right temporal lobe | |
| 79 | Male | Right clinoidal | |
| Tong-tong et al., 201019 | 65 | Male | Right frontal |
| Chen et al., 201120 | 62 | Male | Right parietal lobe |
| 56 | Female | Left hypoglossal canal | |
| 63 | Male | Left tuberculum sellae | |
| Chen et al., 201121 | 79 | Male | Parasagittal |
| 77 | Male | Parasagittal | |
| 71 | Female | Convexity | |
| 56 | Male | Frontal base | |
| 69 | Female | Tentorium | |
| 56 | Male | Parasagittal | |
| 59 | Male | Parasagittal | |
| 47 | Male | Parasagittal | |
| 88 | Male | Parasagittal | |
| Hori et al., 201222 | 65 | Male | Left frontal lobe |
| Wang et al., 201423 | 56 | Male | Left tuberculum sellae |
| 53 | Female | Left hypoglossal canal | |
| 62 | Male | Right parietal lobe | |
| Li et al., 201624 | 50 | Female | Right parietal lobe |
| 63 | Female | Left temporal lobe | |
| 77 | Male | Left parietal lobe | |
| 65 | Female | Left frontal lobe | |
| 64 | Male | Parafalx | |
| 48 | Female | Foramen magnum | |
| Yin et al., 201625 | 55 | Male | Intrasellar |
| Present case | 67 | Male | Right frontal lobe |
Loss of E-cadherin expression with tumour progression suggests that ccm cells would have lost cellular junctions, which would be directly related to the biologic behaviour of tumour cells28.
Genetically, the most important abnormality in meningioma is the loss of heterozygosity of tumour suppressor gene NF229,30. In the primary tumour and the first recurrence, 15 exons were deleted; in the last recurrence, every NF2 exon was lost, showing that loss of NF2 exons can progress during ccm progression. Furthermore, to our knowledge, no studies have considered the status of the AP1B1 and CABP7 genes in meningioma. Those genes, adjacent to NF2, also were found to be deleted, in heterozygosis, in the last recurrence of our patient’s ccm. According to those results, we suggest that progressive deletions in NF2 carry other associated deletions that involve different regions in chromosome 36.
The most frequent progression-associated genetic aberration in meningioma is the total or partial deletion of chromosome 1p31. Del(1p) was evidenced in the primary tumour by mlpa, but loss of chromosome 14q, homozygous deletion of CDKN2A/2B and heterozygous deletions of PTEN and TIMP3 were evidenced only in the last recurrence. Those findings are associated with tumour progression. PTEN and TIMP3 have been identified as candidates implicated in the progression of malignant meningiomas26,32. Heterozygous deletions of ESR1 and TP73 in the first recurrence suggest that they are earlier changes during the acquisition of aggressiveness.
Some earlier studies described TP73 hypermethylation as the mechanism involved in the suppression of its expression29, but other studies claim that TP73 expression could be higher in anaplastic meningiomas, but lower because of hypermethylation in low-grade meningiomas33. In the present case, TP73 was not methylated in any tumour. ESR1 was deleted in heterozygosis in the recurrences, but its relationship with malignant progression remains unclear34. Hypermethylation in RASSF1 occurs in 63% of atypical meningiomas35. In our case, we found hypermethylation in the last recurrence, thus indicating epigenetic differences along the evolutionary path of the tumour.
SUMMARY
Here, we describe the genetic changes occurring during the evolution of a ccm, through the primary tumour and two recurrences. A progressing heterozygous loss of NF2 exons, together with deletions of 1p and 14q, was detected related with progression of the meningioma. AP1B1 and CABP7 deletions are described here for the first time in ccm. Additionally, deletions of CDKN2A/2B and suppression of RASSF1 function were evidenced and should be considered factors involved in the advancement of the tumour and important with respect to the therapy for this neoplasm.
ACKNOWLEDGMENTS
Informed consent of the patient was obtained, and approval for the study reported here was given by the Institutional Ethics Committee of the University of Valencia and Clinic Hospital of Valencia. This work was supported by grants PROMETEOII/2015/007 from Generalitat Valenciana and PI14/01669 from the Instituto de Salud Carlos III and the Spanish Ministerio de Economía y Competitividad. We acknowledge the Fundación Investigación Hospital Clínico de Valencia and the Instituto de Investigación Sanitaria.
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Deb P, Datta SG. An unusual case of clear cell meningioma. J Can Res Ther. 2009;5:324–7. doi: 10.4103/0973-1482.59902. [DOI] [PubMed] [Google Scholar]
- 2.Li Z, Zhang Y, Wang E, et al. Intracranial clear cell meningioma in two children with blood relations: two case reports and literature review. Childs Nerv Syst. 2012;28:2143–51. doi: 10.1007/s00381-012-1840-7. [DOI] [PubMed] [Google Scholar]
- 3.Kane AJ, Sughrue ME, Rutkowski MJ, et al. Anatomic location is a risk factor for atypical and malignant meningiomas. Cancer. 2011;117:1272–8. doi: 10.1002/cncr.25591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tena-Suck ML, Salinas-Lara C, Gómez C, Bojórquez DR. Frontotemporal clear cell meningioma. Report of 3 cases. Ann Diagn Pathol. 2007;11:182–9. doi: 10.1016/j.anndiagpath.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Zhang H, Ma L, Wang YB, et al. Intracranial clear cell meningiomas: study on clinical features and predictors of recurrence. World Neurosurg. 2017;97:693–700.e11. doi: 10.1016/j.wneu.2016.10.010. [DOI] [PubMed] [Google Scholar]
- 6.Wellenreuther R, Kraus JA, Lenartz D, et al. Analysis of the neurofibromatosis 2 gene reveals molecular variants of meningioma. Am J Pathol. 1995;146:827–32. [PMC free article] [PubMed] [Google Scholar]
- 7.López-Ginés C, Cerdá-Nicolás M, Gil-Benso R, et al. Association of loss of 1p and alterations of chromosome 14 in meningioma progression. Cancer Genet Cytogenet. 2004;148:123–8. doi: 10.1016/S0165-4608(03)00279-6. [DOI] [PubMed] [Google Scholar]
- 8.López-Ginés C, Cerdá-Nicolás M, Gil-Benso R, Barcia-Salorio JL, Llombart-Bosch A. Loss of 1p in recurrent meningiomas: a comparative study in successive recurrences by cytogenetics and fluorescence in situ hybridization. Cancer Genet Cytogenet. 2001;125:119–24. doi: 10.1016/S0165-4608(00)00365-4. [DOI] [PubMed] [Google Scholar]
- 9.Barberá S, San Miguel T, Gil-Benso R, et al. Genetic changes with prognostic value in histologically benign meningiomas. Clin Neuropathol. 2013;32:311–17. doi: 10.5414/NP300580. [DOI] [PubMed] [Google Scholar]
- 10.González JR, Carrasco JL, Armengol L, et al. Probe-specific mixed-model approach to detect copy number differences using multiplex ligation-dependent probe amplification (mlpa) BMC Bioinformatics. 2008;9:261. doi: 10.1186/1471-2105-9-261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shiraishi K. Glycogen-rich meningioma. Case report and short review. Neurosurg Rev. 1991;14:61–4. doi: 10.1007/BF00338195. [DOI] [PubMed] [Google Scholar]
- 12.Pimentel J, Fernandes A, Pinto AE, et al. Clear cell meningioma variant and clinical aggressiveness. Clin Neuropathol. 1998;17:141–6. [PubMed] [Google Scholar]
- 13.Kuzeyli K, Cakir E, Usul H, et al. Clear cell meningioma: case report and literature review. J Clin Neurosci. 2003;10:264–6. doi: 10.1016/S0967-5868(02)00287-4. [DOI] [PubMed] [Google Scholar]
- 14.Jain D, Sharma MC, Sarkar C, et al. Clear cell meningioma, an uncommon variant of meningioma: a clinicopathologic study of nine cases. J Neurooncol. 2007;81:315–21. doi: 10.1007/s11060-006-9237-7. [DOI] [PubMed] [Google Scholar]
- 15.Cassereau J, Lavigne C, Michalak-Provost S, Ghali A, Dubas F, Fournier HD. An intraventricular clear cell meningioma revealed by an inflammatory syndrome in a male adult: a case report. Clin Neurol Neurosurg. 2008;110:743–6. doi: 10.1016/j.clineuro.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Pizzoni C, Sarandria C, Pierangeli E. Clear-cell meningioma of the anterior cranial fossa. Case report and review of the literature. J Neurosurg Sci. 2009;53:113–17. [PubMed] [Google Scholar]
- 17.Ohba S, Sasaki H, Kimura T, Ikeda E, Kawase T. Clear cell meningiomas: three case reports with genetic characterization and review of the literature. Neurosurgery. 2010;67:E870–1. doi: 10.1227/01.NEU.0000374857.06732.CD. [DOI] [PubMed] [Google Scholar]
- 18.Prayson RA, Chamberlain WA, Angelov L. Clear cell meningioma: a clinicopathologic study of 18 tumors and examination of the use of CD10, CA9, and RCC antibodies to distinguish between clear cell meningioma and metastatic clear cell renal cell carcinoma. Appl Immunohistochem Mol Morphol. 2010;18:422–8. doi: 10.1097/PAI.0b013e3181dd35d2. [DOI] [PubMed] [Google Scholar]
- 19.Tong-Tong W, Li-Juan B, Zhi L, Yang L, Bo-Ning L, Quan H. Clear cell meningioma with anaplastic features: case report and review of literature. Pathol Res Pract. 2010;206:349–54. doi: 10.1016/j.prp.2009.06.015. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Li XM, Chen YC, et al. Intracranial clear cell meningioma: a clinicopathologic study of 15 cases. Acta Neurochir (Wien) 2011;153:1769–80. doi: 10.1007/s00701-011-1052-z. [DOI] [PubMed] [Google Scholar]
- 21.Chen HK, Wu YT, Lin YJ, Lin JW. Clear cell meningioma with frequent chordoid features and aggressive behavior: a clinicopathologic study of ten cases at a single institution. J Neurooncol. 2011;103:551–9. doi: 10.1007/s11060-010-0418-z. [DOI] [PubMed] [Google Scholar]
- 22.Hori S, Hayashi N, Ishizawa S, et al. Clear cell meningioma with histologically aggressive appearance and clinically aggressive behavior: a case report. Neuropathology. 2012;32:415–19. doi: 10.1111/j.1440-1789.2011.01272.x. [DOI] [PubMed] [Google Scholar]
- 23.Wang XQ, Huang MZ, Zhang H, et al. Clear cell meningioma: clinical features, ct, and mr imaging findings in 23 patients. J Comput Assist Tomogr. 2014;38:200–8. doi: 10.1097/RCT.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 24.Li P, Yang Z, Wang Z, et al. Clinical features of clear cell meningioma: a retrospective study of 36 cases among 10,529 patients in a single institution. Acta Neurochir (Wien) 2016;158:67–76. doi: 10.1007/s00701-015-2635-x. [DOI] [PubMed] [Google Scholar]
- 25.Yin S, Zhou P, Li Q, Jiang S. Intrasellar clear cell meningioma mimicking invasive pituitary adenoma: a case report and review of the literature. Turk Neurosurg. 2015;25:976–9. doi: 10.5137/1019-5149.JTN.11847-14.1. [DOI] [PubMed] [Google Scholar]
- 26.Alexiou GA, Markoula S, Gogou P, Kyritsis AP. Genetic and molecular alterations in meningiomas. Clin Neurol Neurosurg. 2011;113:261–7. doi: 10.1016/j.clineuro.2010.12.007. [DOI] [PubMed] [Google Scholar]
- 27.Becker C, Kuchelmeister K, Richter HP, Schachenmayr W. Metaplastic meningioma with cartilaginous differentiation [German] Pathologe. 1999;20:335–9. doi: 10.1007/s002920050367. [DOI] [PubMed] [Google Scholar]
- 28.Zhou K, Wang G, Wang Y, Jin H, Yang S, Liu C. The potential involvement of E-cadherin and beta-catenins in meningioma. PloS One. 2010;5:e11231. doi: 10.1371/journal.pone.0011231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choy W, Kim W, Nagasawa D, et al. The molecular genetics and tumor pathogenesis of meningiomas and the future directions of meningioma treatments. Neurosurg Focus. 2011;30:E6. doi: 10.3171/2011.2.FOCUS1116. [DOI] [PubMed] [Google Scholar]
- 30.Riemenschneider MJ, Perry A, Reifenberger G. Histological classification and molecular genetics of meningiomas. Lancet Neurol. 2006;5:1045–54. doi: 10.1016/S1474-4422(06)70625-1. [DOI] [PubMed] [Google Scholar]
- 31.Shibuya M. Pathology and molecular genetics of meningioma: recent advances. Neurol Med Chir. 2015;55(suppl 1):14–27. doi: 10.2176/nmc.ra.2014-0233. [DOI] [PubMed] [Google Scholar]
- 32.San-Miguel T, Cerdá-Nicolás M, Gil-Benso R, Callaghan RC, Muñoz-Hidalgo L, López-Ginés C. Evolution to malignancy: a genetic stepwise study of tumor suppressor genes loss in a recurrent meningioma. Clin Neuropathol. 2015;34:237–9. doi: 10.5414/NP300839. [DOI] [PubMed] [Google Scholar]
- 33.He S, Pham MH, Pease M, et al. A review of epigenetic and gene expression alterations associated with intracranial meningioma. Neurosurg Focus. 2013;35:E5. doi: 10.3171/2013.10.FOCUS13360. [DOI] [PubMed] [Google Scholar]
- 34.Lomas J, Amiñoso C, González-Gómez P, et al. Methylation status of TP73 in meningiomas. Cancer Genet Cytogenet. 2004;148:148–51. doi: 10.1016/S0165-4608(03)00244-9. [DOI] [PubMed] [Google Scholar]
- 35.Nakane Y, Natsume A, Wakabayashi T, et al. Malignant transformation-related genes in meningiomas: allelic loss on 1p36 and methylation status of p73 and RASSF1A. J Neurosurg. 2007;107:398–404. doi: 10.3171/JNS-07/08/0398. [DOI] [PubMed] [Google Scholar]