Abstract
Acute myeloid leukemia (AML) is defined as ≥20% myeloblasts, representing a change from original guidelines where ≤30% blasts were considered as myelodysplastic syndromes (MDS), and 20–29% blasts classified as refractory anemia with excess blasts in transformation (RAEB-T). Whether the diagnostic bone marrow blast percentage has current value with regards to patient prognostication or identification of optimal treatment strategies is unclear. We retrospectively studied 1652 treatment-naïve adults with MDS or AML and ≥10% blasts from January 2000 to April 2014. Patients with 20–29% blasts were more similar to MDS patients in terms of advanced age, increased frequency of poor-risk cytogenetics, lower WBC count, and less frequent NPM1 and FLT3-ITD mutations. Median overall survival of MDS and RAEB-T were similar, 16.0 and 16.0 months, compared to 13.5 months for AML with ≥30% blasts (P =0.045). Multivariate analysis showed inferior survival with increased age (HR 1.81 age 60–69, HR 2.68 age ≥70, P < 0.0005); poor-risk cytogenetics (HR 2.25, P < 0.0005); therapy-related disease (HR 1.44, P < 0.0005); and markers of proliferative disease including WBC ≥25 × 109/L (HR 1.35, P = 0.0003), elevated LDH count (HR 1.24, P =0.0015), and peripheral blasts (HR 1.25, P =0.004). Among younger patients (≤60 years), intensive AML-type therapy resulted in similar outcomes regardless of blast percentage, suggesting this to be optimal therapy in this context. Among older patients (≥70 years), patients with 20–29% blasts had similar outcomes to patients with <20% blasts, and better than those with ≥30% blasts. In addition, among older patients, epigenetic therapy provided at least equivalent outcome to intensive chemotherapy.
Introduction
Within myeloid malignancies, the bone marrow blast cutpoint of 20% to distinguish myelodysplastic syndrome (MDS) from acute myeloid leukemia (AML) remains a subject of debate. In the original French-American-British (FAB) MDS classification system established by pathologists in the 1970s based on bone marrow morphologic assessment, MDS categories included refractory anemia with excess blasts (RAEB; 5–20% marrow myeloblasts), and refractory anemia with excess blasts in transformation (RAEB-T; 21–30% marrow myeloblasts) [1]. Greater than 30% blasts defined AML. Stemming from this FAB system and incorporating the degree of peripheral cytopenias and karyotype information, the International Prognostic Scoring System (IPSS) and the Revised International Scoring System (R-IPSS) were developed. They remain the most commonly applied risk models for the evaluation and treatment of patients with MDS, notably including patients with up to 30% marrow blasts [2,3]. In the 2012 revised R-IPSS scoring system, patients with blasts 11–30% are considered to have the same “risk” and are grouped together for prognostication purposes.
In 2001, the World Health Organization (WHO) Classification of myeloid neoplasms was developed, and differed from the FAB Classification in that AML was defined as ≥20% myeloblasts [4]. This updated definition was based on several lines of evidence. First was the identification that in subsets of patients treated on CALGB cooperative group trials for AML, who on subsequent central review were reclassified to have MDS with <30% blasts, these patients with “MDS” appeared to respond as well as patients with AML to standard AML therapy, with no difference in remission or overall survival rates [5]. A study by Estey et al. demonstrated that the outcome of RAEB, RAEB-T, and/or AML treated with high-dose cytarabine containing AML regimens (once controlled for covariates such as age, performance status and cytogenetics by multivariate analysis) was irrespective of the blast count at diagnosis, with the conclusion that patients with RAEB-T in particular may benefit from AML-type chemotherapy [6].
These studies continue to shape the practice of MDS and AML therapy to date, but notably the median age of patients treated in these two studies were 53 and 59 years, respectively. In practice, both AML and MDS are diseases of advanced age, with a median age at diagnosis of 70 years [7–10]. In comparison to younger patients (arbitrarily defined as <65 or <60 years), complete remission (CR) rates in elderly patients with AML are substantially lower (10–40%), the median survival less than one year, and the 5-year survival rate less than 10% [11–13]. The reasons for the worse outcomes in elderly patients are multifactorial, including poor tolerance to intensive chemotherapy, an increased incidence of comorbidities and compromised organ functions, poor performance status, increased rate of therapy-related disease and/or intrinsically higher risk leukemia features, such as poor risk cytogenetics (i.e., chromosome 5, 7 and complex karyotype) and TP53 mutations [7,11,14–16]. The impact of proliferative disease (such as degree of leukocytosis or peripheral blast percentage at diagnosis) among elderly patients is furthermore not well defined.
With particular attention to the overlap of 20–30% myeloblasts between the WHO and the FAB classification systems, we sought to address whether the percent of blasts and degree of proliferation in newly diagnosed patients with myeloid malignancies impacts clinical outcome, and whether this effect is age-dependent. A secondary aim was to evaluate outcome based on particular treatment strategies, such as intensive induction chemotherapy or hypomethylating agent therapy. This may help answer two important questions in clinical practice: (1) whether younger patients (i.e., ≤60 years) with 10–20% blasts should be treated with AML-type intensive chemotherapy or treated as MDS; and (2) whether some older patients (i.e., ≥70 years) with ≥30% blasts have an AML that is more indolent and best treated with MDS-type hypomethylating agent strategies.
Methods
All adult patients treated at the University of Texas MD Anderson Cancer Center from January 2000 to April 2014 with a diagnosis of untreated AML or MDS and ≥10% blasts were reviewed (Table I). Patients previously treated for a non-myeloid malignancy were included, and were classified as treatment-related per WHO guidelines. Patients with acute promyelocytic leukemia (APL) and favorable risk cytogenetics were excluded from the analysis. Cytogenetics were classified utilizing the global MD Anderson model as diploid/isolated-Y, poor-risk (complex ≥3 abnormalities or chromosome 7 abnormalities), or other intermediate karyotype [17]. The study was conducted in compliance with institutional guidelines. Informed consent was obtained in accordance with the Declaration of Helsinki. Treatment received varied by diagnosis, patient age, and treatment period. For this study, therapies were classified into [1] intensive induction therapy (IC), defined as conventional “7 +3” induction regimens or high-dose (≥1 g/m2 daily) cytarabine-containing chemotherapy regimens, [2] hypomethylating agent (HMA)-based chemotherapy, and [3] other, including low-dose intensity therapy incorporating lower dose cytarabine-based regimens, and clinical trials not incorporating either IC or HMA-based therapy. A table of the treatments is provided in Supporting Information Table 1 and summarized by age in Table II. Cytogenetic analysis was performed at diagnosis in all patients. FLT3, KRAS, NRAS, and NPM1 mutational status was recorded when available.
TABLE I.
Baseline Clinicopathologic Characteristics No. (%) by Bone Marrow Blast %.
| Characterisitics | 10–19 | 20–29 | 30+ | Total | P value | |
|---|---|---|---|---|---|---|
| No | 263 | 230 | 1159 | 1652 | ||
| Gender | Female | 101 (38) | 99 (43) | 541 (47) | 741 (45) | |
| Age | (Continuous) | 65 (17–90) | 65 (18–85) | 63 (17–92) | 64 (17–92) | 0.04 |
| <60 | 70 (26) | 68 (30) | 497 (43) | 635 (38) | ||
| 60–69 | 90 (34) | 78 (34) | 302 (26) | 470 (28) | <0.001 | |
| ≥70 | 103 (39) | 84 (37) | 360 (31) | 547 (33) | ||
| WBC (x 109/L) | ≥25 | 18 (7) | 20 (9) | 240 (21) | 278 (17) | <0.001 |
| Serum LDH (IU/L) | ≥600 | 117(45) | 101(45) | 762(67) | 980(59) | <0.001 |
| Peripheral blasts | Present | 131 (50) | 152 (66) | 953 (82) | 1236 (75) | <0.001 |
| Induction therapy | HMA | 166 (63) | 61 (27) | 205 (18) | 432 (26) | |
| IC | 50 (19) | 102 (44) | 684 (59) | 836 (51) | <0.001 | |
| Other | 47 (18) | 67 (29) | 270 (23) | 384 (23) | ||
| FLT3-ITD | Negative | 258 (98) | 216 (94) | 908 (78) | 1382 (84) | |
| Positive | 5 (2) | 14 (6) | 251 (22) | 270 (16) | <0.001 | |
| NPM1 | Negative | 109 (92) | 138 (94) | 492 (71) | 739 (77) | |
| Positive | 9 (8) | 9 (6) | 201 (29) | 219 (23) | <0.001 | |
| KRAS or NRAS | Negative | 224 (93) | 175 (90) | 852 (87) | 1251 (89) | |
| Positive | 16 (7) | 20 (10) | 125 (13) | 161 (11) | 0.02 | |
| Cytogenetics | Diploid/-Y | 113 (45) | 100 (47) | 571 (51) | 784 (49) | |
| Intermediate | 46 (18) | 38 (17) | 285 (25) | 369 (23) | <0.001 | |
| Complex or 27/7q- | 92 (37) | 81 (37) | 270 (24) | 443 (28) | ||
| Therapy Related Response | Yes | 55 (21) | 42 (18) | 184 (16) | 281 (17) | 0.13 |
| CR/CRp | 134 (51) | 136 (59) | 746 (64) | 1016 (62) | ||
| NR | 108 (41) | 71 (31) | 301 (26) | 480 (29) | <0.001 | |
| Induction Death | 21 (8) | 23 (10) | 112 (10) | 156 (9) |
TABLE II.
Therapy Received by Age No on Therapy (%).
| Age (years) | No. | Intensive | Hypomethylating | Other low intensity |
|---|---|---|---|---|
| <60 | 630 | 592 (94) | 40 (6) | 3 (0.005) |
| 60–69 | 470 | 167 (35) | 217 (46) | 86 (18) |
| ≥ 70 | 547 | 77 (14) | 294 (54) | 176 (32) |
Response categories were assessed using the modified International Working Group (IWG) criteria [18,19]. Induction mortality was defined as 8-week mortality [11]. Patient characteristics are summarized by median (range) values for continuous variables and frequency (percentage) for categorical values. Categorical variables were compared using the χ2 or Fisher’s exact test, and continuous variables using the Wilcoxon’s rank sum test. Survival was evaluated by the Kaplan–Meier method, with differences compared between groups using the log-rank test. Overall survival was measured as the time from MD Anderson diagnosis until death from any cause or date of last follow-up (censored). The predictive effects of patient and disease characteristics on survival were examined using univariate and multivariate Cox proportional hazards models. Analyses were performed using SAS 9.0 and Statistica software, version 12.0 (College Station, TX).
Results
For this analysis and manuscript clarity, we hereafter utilize the FAB classification system and refer to MDS as 10–19% myeloblasts, RAEB-T as 20–29% blasts, and AML ≥30% myeloblasts. A total of 1652 patients were analyzed. These included 263 patients with 10–19% bone marrow blasts, 230 patients with 20–29% blasts, and 1159 patients with ≥30% blasts. The median age for the entire cohort was 64 years (range 17–92); 55% of patients were male. Clinical and disease-specific characteristics for the entire study group and by disease at diagnosis are provided in Table I.
Pretreatment associations
Statistically significant differences between most clinicopathologic characteristics by FAB classification were identified (Table I). The frequency of therapy-related disease was 17% and did not vary by FAB diagnosis. One-third of patients were ≥70 years; a higher proportion of older patients was observed in the RAEB-T (37%) and MDS (39%) categories compared to AML (31%) (P <0.001). Nearly half of all patients had diploid or isolated-Y cytogenetics at diagnosis (n =784, 49%). A complex karyotype or abnormalities involving chromosome 7 were present in 28%, with a higher incidence of poor-risk cytogenetics in the MDS and RAEB-T versus AML categories (37% and 37 versus 24%, respectively, P <0.001).
A minority of patients with MDS (7%) and RAEB-T (9%) had a peripheral white blood cell (WBC) count ≥25 × 109/L at the time of diagnosis, compared to 21% of patients with AML (P <0.001). Differences in the incidences of the presence of peripheral blasts (MDS 50%, RAEB-T 66%, AML 82%; P <0.001) and elevated LDH (MDS 45%, RAEB-T 45%, AML 67%; P =0.001) were similarly noted.
Molecular profiling was performed including FLT3 internal tandem duplication (FLT3-ITD) in all patients (n =1652), NPM1 in 58% (n =958), and NRAS or KRAS in 85% (n =1412). Patients with AML had a higher frequency of these mutations compared to either MDS or RAEB-T. Specifically, FLT3-ITD mutations were present in 2% of MDS, 6% of RAEB-T, and 22% of AML (P <0.001). NPM1 mutations were present in 8% of MDS, 6% of RAEB-T, and 29% of AML (P <0.001). Thus, the frequency of both FLT3 and NPM1 molecular events among patients with 20–29% blasts resembled those with <20% blasts. KRAS or NRAS mutations occurred in 7% of MDS, 10% of RAEB-T, and 13% of AML (P =0.02).
Overall, 51% of patients (n =836) received IC, 26% (n =432) HMA-based, and 23% (n =384) other therapies. Patients with MDS were more likely to receive HMA-based therapy (63%) compared to patients with RAEB-T (27%) or AML (18%), and were less likely to receive IC-based regimens (MDS-19% vs. RAEB-T–44% vs. AML-59%). By age, 94 and 6% of patients <60 years received IC and HMA-based therapy, respectively. This compares to 35 and 46% in patients 60–69 years of age, and 14 and 54% in patients ≥70 years (Table II).
Prognostic factors for survival in the total study group
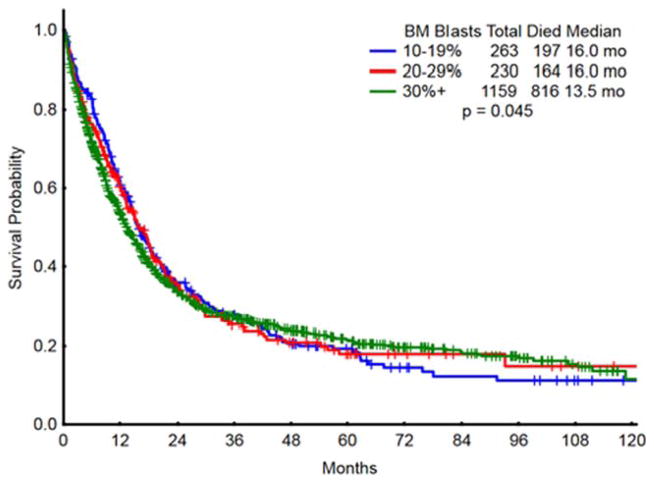
With a median follow-up of 38 months, overall survival was 16.0 months in MDS, 16.0 months in RAEB-T, and 13.5 months in AML (P =0.045) (Table III; Fig. 1).
TABLE III.
Prognostic Factors Associated with Overall Survival by Univariate Analysis in the Total Study Group.
| Characteristic | Overall | Median survival (months) | P value |
|---|---|---|---|
| BM blasts | 10–19 | 16.0 | |
| 20–29 | 16.0 | ||
| 30+ | 13.5 | 0.045 | |
| Age | <60 | 22.5 | |
| 60–69 | 13.5 | ||
| ≥70 | 9.2 | <0.001 | |
| WBC (x109/l) | <25 | 15.1 | |
| ≥25 | 9.6 | 0.002 | |
| Treatment | HMA | 11.9 | |
| IC | 18.4 | ||
| Other | 8.7 | <0.001 | |
| FLT3-ITD | Neg | 14.6 | |
| Pos | 13.1 | 0.73 | |
| Cytogenetics | Diploid/-Y | 19.4 | |
| Other Intermediate | 13.8 | ||
| Complex or 27 | 7.5 | <0.001 | |
| Therapy related | No | 15.8 | |
| Yes | 8.2 | <0.001 | |
| LDH (IU/l) | <600 | 16.7 | |
| ≥600 | 12 | <0.001 | |
| PB blasts | None | 19.5 | |
| Present | 12 | <0.001 | |
| NPM1 | Negative | 12.9 | |
| Positive | 19.4 | <0.001 | |
| RAS | Negative | 13.5 | |
| Positive | 13.5 | 0.77 |
Figure 1.
Survival in the study group by percent of bone marrow blasts. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Pretreatment patient and disease characteristics associated with differences in outcome by univariate analysis are detailed in Table III. Survival was not affected by the presence of either FLT3-ITD or RAS mutations. By multivariate analysis, older age, WBC count ≥25 × 109/L, poor-risk cytogenetics, therapy-related AML, elevated LDH and the presence of peripheral blasts were significantly associated with inferior survival (Table IV). Notably, the blast percentage at diagnosis (MDS versus RAEB-T versus AML) was not an independent factor for survival after accounting for the other independent prognostic factors in multivariate analysis (Table IV).
TABLE IV.
Multivariate Analysis Results for Survival in the Total Study Group.
| Variable | Hazard ratio | 95% Confidence interval | P value |
|---|---|---|---|
| Poor risk cytogenetics | 2.3 | 1.99–2.66 | <0.0005 |
| Other intermediate cytogenetics | 1.34 | 1.15–1.56 | <0.0005 |
| Therapy-related AML | 1.45 | 1.24–1.69 | <0.0005 |
| Age 60–69 years | 1.81 | 1.55–2.11 | <0.0005 |
| Age ≥70 years | 2.68 | 2.29–3.14 | <0.0005 |
| Marrow blasts 20–29% | 1.03 | 0.82–1.29 | 0.78 |
| Marrow blasts >30% | 1.17 | 0.97–1.4 | 0.085 |
| Intensive chemotherapy | 1.0 | 0.84–1.2 | 0.93 |
| “Other” treatment | 1.18 | 1.01–1.38 | 0.034 |
| WBC | 1.35 | 1.15–1.59 | <0.0005 |
| LDH ≥600 IU/l | 1.24 | 1.09–1.42 | 0.0015 |
| Peripheral blasts | 1.25 | 1.07–1.45 | 0.004 |
There was a strong association between the type of chemotherapy and age. Among younger patients (age ≤60 years), 592/630 (94%) received IC. Among older patients, 294/547 (54%) received HMA and 470/547 (86%) received HMA or other low-intensity therapies. Such strong confounding associations may mask the benefit of particular therapy (IC vs HMA) in the total population, and may explain why the type of therapy was not identified as prognostic by multivariate analysis within the total study group.
Prognostic factors for survival in younger and older patients
Because of the strong and confounding association between age and therapy, we conducted prognostic factor analyses in the age subsets (younger, older) to define better the effects of blasts percent and of therapy. We divided patients into three age groups (<60, 60–69, and ≥70 years) and repeated the survival analyses (Tables (V–VIII)). Univariate results are displayed in Table V and multivariate analyses in Table (VI–VIII).
TABLE V.
Univariate Analysis of Prognostic Factors Associated with Survival by Age.
| Category | Characteristics | Median OS
|
P value | Median OS
|
P value | Median OS
|
P value |
|---|---|---|---|---|---|---|---|
| Age <60 (n =635) | Age 60–69 (n =470) | Age 70+ (n =537) | |||||
| BM blasts | 10–19 | 39 | 15 | 15 | |||
| 20–29 | 18 | 21 | 9 | ||||
| 30+ | 24 | 0.98 | 11 | 0.006 | 7 | <0.001 | |
| WBC (×109/l) | <25 | 26 | 14 | 9 | |||
| ≥25 | 14 | <0.001 | 9 | 0.07 | 6 | 0.097 | |
| Treatment | HMA | 15 | 14 | 10 | |||
| IC | 23 | 16 | 5 | ||||
| Other | 28 | 0.45 | 9 | 0.16 | 8 | <0.001 | |
| Cytogenetics | Diploid/-Y | 40 | 18 | 15 | |||
| Other intermediate | 20 | 15 | 9 | ||||
| Complex or 27 | 11 | <0.001 | 8 | <0.001 | 4 | <0.001 | |
| Therapy-related | No | 27 | 16 | 10 | |||
| Yes | 13 | <0.001 | 9 | <0.001 | 4 | <0.001 | |
| LDH (IU/l) | <600 | 28 | 17 | 12 | |||
| 600+ | 19 | 0.056 | 10 | <0.001 | 6 | 0.002 | |
| PB blasts | None | 31 | 24 | 15 | |||
| Present | 20 | 0.086 | 11 | <0.001 | 7 | 0.002 | |
| FLT3-ITD | Negative | 25 | 14 | 10 | |||
| Positive | 15 | 0.019 | 14 | 0.97 | 10 | 0.83 | |
| NPM1 | Negative | 17 | 13 | 9 | |||
| Positive | 61 | 0.002 | 16 | 0.07 | 9 | 0.11 | |
| RAS | Negative | 22 | 14 | 9 | |||
| Positive | 20 | 0.74 | 9 | 0.18 | 8 | 0.37 |
TABLE VIII.
Multivariate Analysis for Survival in Patients Age ≥70 Years.
| HR | 95% CI | P value | |
|---|---|---|---|
| Poor risk cytogenetics | 2.49 | 1.98–3.14 | <0.0005 |
| Other intermediate cytogenetics | 1.85 | 1.44–2.37 | <0.0005 |
| Therapy-related | 1.30 | 1.02–1.65 | 0.031 |
| LDH | 1.42 | 1.17–1.72 | 0.0005 |
| Marrow blasts 20–29% | 1.18 | 0.85–1.64 | 0.32 |
| Marrow blasts >30% | 1.29 | 0.98–1.68 | 0.067 |
| WBC ≥25 (×109/l) | 1.13 | 0.85–1.50 | 0.41 |
| PB blasts | 1.11 | 0.87–1.41 | 0.39 |
| Intensive chemotherapy | 1.13 | 0.85–1.50 | 0.41 |
| Other therapy | 1.19 | 0.96–1.47 | 0.11 |
TABLE VI.
Multivariate for Survival in Patients <60 Years.
| HR | 95% CI | P value | |
|---|---|---|---|
| Poor risk cytogenetics | 2.33 | 1.81–2.99 | 0.0005 |
| Therapy-related | 1.56 | 1.16–2.1 | <0.0005 |
| WBC ≥25 (×109/l) | 1.63 | 1.26–2.1 | 0.0002 |
| FLT3-ITD+ | 1.54 | 1.18–2.0 | 0.002 |
| Other intermediate cytogenetics | 1.23 | 0.93–1.61 | 0.14 |
| LDH | 1.15 | 0.88–1.49 | 0.30 |
| Peripheral blasts | 0.93 | 0.70–1.26 | 0.67 |
Molecular characteristics were noticeably significant only among younger patients (<60 years), with FLT3-ITD mutations conferring an inferior survival of 15 versus 25 months (P =0.019) and NPM1 mutations conferring an improved survival of 61 versus 17 months (P =0.002) (Tables V and VII). This survival impact was not observed among older patients ≥60 years (Table V). Cytogenetics and therapy-related status were the only variables that maintained significance in all age cohorts (Table VI–VIII).
TABLE VII.
Multivariate Analysis for Survival in Patients Age 60–69 Years.
| HR | 95% CI | P value | |
|---|---|---|---|
| Poor risk cytogenetics | 2.18 | 1.72–2.76 | <0.0005 |
| Therapy-related | 1.55 | 1.19–2.02 | 0.001 |
| Peripheral blasts | 1.54 | 1.18–2.0 | 0.001 |
| LDH | 1.34 | 1.07–1.67 | 0.010 |
| Marrow blasts | 0.72 | 0.49–1.05 | 0.08 |
| 20–29% | |||
| Marrow blasts ≥30% | 1.05 | 0.79–1.41 | 0.72 |
| WBC ≥25 (×109/l) | 1.22 | 0.90–1.66 | 0.20 |
| Other intermediate cytogenetics | 1.04 | 0.78–1.40 | 0.77 |
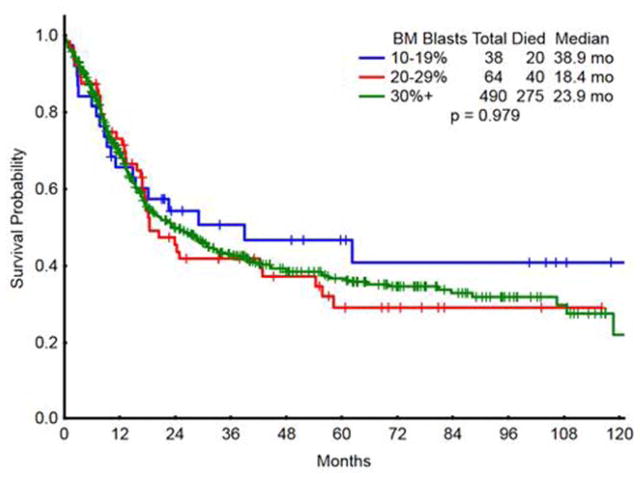
Among younger patients (<60 years), outcome was similar in IC-treated patients independent of BM blast percentage at diagnosis (Fig. 2). Median survivals by blasts 10–19, 20–29, and ≥30% were 39, 18, and 24 months, respectively (P =0.98; Table V). This suggests that in all younger patients with ≥10% blasts, regardless of classification of MDS or AML, intensive chemotherapy should be considered. Only 33 patients <60 years with 10–29% blasts received HMA therapy; median survival was 20 months, versus 39 months in the patients with 10–29% blasts who received IC, which was not statistically significant (P =0.51). The small number of patients precluded meaningful conclusions about the comparative benefit of HMA to IC in younger patients with 10–29% blasts.
Figure 2.
Survival in young patients(<60 years) treated with intensive chemotherapy according to bone marrow blasts. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Older patients were noted to have equivalent or better outcome with HMA therapy (Table V). Among patients >70 years, the median survival was 10 months with HMA and 5 months with IC (Table V). By multivariate analysis, IC was associated with a hazard ratio of 1.13 (P =0.41; Table VIII) suggesting HMAs to be at least equivalent to IC among older patients.
Among older patients >70 years the median survival with blasts 10–19% was 15 months, versus 9 months with blasts 20–29% and 7 months with ≥30% blasts. However, among patients 60–69 years old, the median survivals with blasts 10–19, 20–29, and ≥30% were 15, 21, and 11 months respectively. This suggests that the cut-off blasts of 20–29% should be a stratification risk factor in studies of novel agents in older patients, since it is not clear whether patients with 20–29% blasts behave like MDS or AML.
Of interest, markers of proliferative disease (high WBC, peripheral blasts, LDH) remained independent prognostic factors for survival in the multivariate analyses in older patients (Table VI–VIII), again indicating their importance in stratifying patients on future therapeutic trials in MDS and AML.
Discussion
The separation of MDS and AML by blast percentage remains an arbitrary distinction, with the original FAB classification defining >30% blasts as AML, the current WHO guidelines defining ≥20% blasts as AML, and the National Comprehensive Cancer Network (NCCN) endorsing both systems [20]. The importance of these cut-points in relationship to other factors including patient age and measures of proliferative disease is ill-defined. Yet this separation is important, as currently patients with “RAEB-T” (20–29% blasts) are treated most often like AML, with intensive chemotherapy regimens favored over HMA-based regimens, despite the predominantly elderly RAEB-T patient population and the increased toxicity with high dose cytarabine-based combinations. Furthermore, among older patients with AML (currently defined as ≥20% bone marrow blasts), no accepted treatment algorithms or standards of care exist. We therefore sought to analyze a large cohort of adults with newly diagnosed MDS, RAEB-T, and AML to investigate whether the RAEB-T classification was more comparable to MDS or AML, and whether other characteristics, most specifically age, molecular characteristics, degree of proliferation, and treatment strategy impacted patient survival within these diagnostic entities.
Several findings from this analysis are significant and may guide therapy in clinical practice. First, the analysis demonstrated that patients with RAEB-T more closely resembled patients with MDS in relation to both clinicopathologic and molecular characteristics: advanced age at diagnosis, increased presence of poor-risk cytogenetics, less proliferative disease and decreased frequency of FLT3-ITD and NPM1 mutations.
Second, the most consistently significant variables impacting patient survival were the well-established prognostic variables of age at diagnosis, cytogenetics and therapy-related disease status. The independent importance of therapy-related disease status suggests additional factors are at play in therapy-related myeloid disorders over and above the increased frequency of poor-risk cytogenetics, likely at least partly due to the development of TP53 mutations which were not annotated in our historical cohort [21,22]. Inferior survival in the entire study population was also significantly associated with markers of proliferative disease, including WBC ≥25 × 109/L, the presence of circulating blasts, and/or elevated LDH (≥600 IU/L) at diagnosis, suggesting these standard clinical variables are not only prognostic, but provide equivalently useful information at the time of diagnosis as the bone marrow blast percentage.
Third, it was only among the younger patients that FLT3-ITD and NPM1 mutations impacted overall survival. The median survival was 15 months versus 25 months depending on the presence versus absence of FLT3-ITD (P =0.019), and the median survival was 61 months versus 17 months with the presence versus absence of NPM1 mutation (P =0.002). Molecular mutations in FLT3-ITD and NPM1 are now routinely analyzed as part of the current WHO guidelines for risk stratification of patients at diagnosis. The survival impact of these mutations in elderly patients has not been fully characterized. Mutational status of NPM1 and FLT3-ITD in particular have been reported to be of less prognostic importance in elderly populations [23–25] a conclusion substantiated in our analysis. The presence of RAS mutations did not affect patient survival in any analyzed cohort.
Fourth, outcomes based on treatment strategy provided insights into potential clinical practice. Intensive-chemotherapy based-treatment strategies are appropriate for younger patients regardless of the blasts percentage at AML diagnosis, suggesting this to be an appropriate strategy in all patients with blasts are ≥10%. However, among older patients, HMA therapy was associated with similar or improved outcomes compared with intensive chemotherapy approaches. In addition, older patients with ≥20–29% blasts seemed to behave more like patients with <20% blasts rather than patients with >30% blasts in terms of clinical and pathologic characteristics.
In summary, through a detailed evaluation of a large study group of patients with myeloid malignancy, we conclude that patients with 20–29% bone marrow blasts at the time of diagnosis have clinicopathologic features more similar to MDS than AML. Among younger patients, intensive chemotherapy provided similar benefits regardless of the percent of blasts (10–19% vs. 20–29% vs. ≥30%). Among older patients, the 20% blasts cutoff should be considered as a stratification variable in studies evaluating novel therapies in AML and MDS. As “standard of care”, epigenetic therapy in older patients with AML offers at least equivalent benefits to intensive chemotherapy.
Acknowledgments
Contract grant sponsor: The MD Anderson Cancer Center Support Grant (CCSG); Contract grant number: CA01667.
Footnotes
Disclosure: None.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the acute leukaemias. French-American-British (FAB) cooperative group. Br J Haematol. 1976;33:451–458. doi: 10.1111/j.1365-2141.1976.tb03563.x. [DOI] [PubMed] [Google Scholar]
- 2.Greenberg P, Cox C, LeBeau MM, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. Epub 1997/03/15. eng. [PubMed] [Google Scholar]
- 3.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood. 2012;120:2454–2465. doi: 10.1182/blood-2012-03-420489. Epub 2012/06/29. eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vardiman JW, Harris NL, Brunning RD. The World Health Organization (WHO) classification of the myeloid neoplasms. Blood. 2002;100:2292–2302. doi: 10.1182/blood-2002-04-1199. [DOI] [PubMed] [Google Scholar]
- 5.Bernstein SH, Brunetto VL, Davey FR, et al. Acute myeloid leukemia-type chemotherapy for newly diagnosed patients without antecedent cytopenias having myelodysplastic syndrome as defined by French-American-British criteria: a Cancer and Leukemia Group B Study. J Clin Oncol. 1996;14:2486–2494. doi: 10.1200/JCO.1996.14.9.2486. [DOI] [PubMed] [Google Scholar]
- 6.Estey E, Thall P, Beran M, et al. Effect of diagnosis (refractory anemia with excess blasts, refractory anemia with excess blasts in transformation, or acute myeloid leukemia [AML]) on outcome of AML-type chemotherapy. Blood. 1997;90:2969–2977. [PubMed] [Google Scholar]
- 7.Estey E, Dohner H. Acute myeloid leukaemia. Lancet. 2006;368:1894–1907. doi: 10.1016/S0140-6736(06)69780-8. [DOI] [PubMed] [Google Scholar]
- 8.Phekoo KJ, Richards MA, Moller H, et al. The incidence and outcome of myeloid malignancies in 2,112 adult patients in southeast England. Haematologica. 2006;91:1400–1404. [PubMed] [Google Scholar]
- 9.Thein MS, Ershler WB, Jemal A, et al. Outcome of older patients with acute myeloid leukemia. Cancer. 2013;119:2720–2727. doi: 10.1002/cncr.28129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Derolf AR, Kristinsson SY, Andersson TM, et al. Improved patient survival for acute myeloid leukemia: A population-based study of 9729 patients diagnosed in Sweden between 1973 and 2005. Blood. 2009;113:3666–3672. doi: 10.1182/blood-2008-09-179341. [DOI] [PubMed] [Google Scholar]
- 11.Kantarjian H, O’Brien S, Cortes J, et al. Results of intensive chemotherapy in 998 patients age 65 years or older with acute myeloid leukemia or high-risk myelodysplastic syndrome: Predictive prognostic models for outcome. Cancer. 2006;106:10908. doi: 10.1002/cncr.21723. [DOI] [PubMed] [Google Scholar]
- 12.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107:3481–3485. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roboz GJ. Current treatment of acute myeloid leukemia. Curr Opin Oncol. 2012;24:711–719. doi: 10.1097/CCO.0b013e328358f62d. [DOI] [PubMed] [Google Scholar]
- 14.Frohling S, Schlenk RF, Kayser S, et al. Cytogenetics and age are major determinants of outcome in intensively treated acute myeloid leukemia patients older than 60 years: Results from AMLSG trial AML HD98-B. Blood. 2006;108:3280–3288. doi: 10.1182/blood-2006-04-014324. [DOI] [PubMed] [Google Scholar]
- 15.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: Recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115:453–474. doi: 10.1182/blood-2009-07-235358. [DOI] [PubMed] [Google Scholar]
- 16.Rucker FG, Schlenk RF, Bullinger L, et al. TP53 alterations in acute myeloid leukemia with complex karyotype correlate with specific copy number alterations, monosomal karyotype, and dismal outcome. Blood. 2012;119:2114–2121. doi: 10.1182/blood-2011-08-375758. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, O’Brien S, Ravandi F, et al. Proposal for a new risk model in myelodysplastic syndrome that accounts for events not considered in the original International Prognostic Scoring System. Cancer. 2008;113:1351–1361. doi: 10.1002/cncr.23697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood. 2006;108:419–425. doi: 10.1182/blood-2005-10-4149. [DOI] [PubMed] [Google Scholar]
- 19.Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21:4642–4649. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- 20.Myelodysplastic syndromes. NCCN Clinical Practice Guidelines on Oncology [Internet] 2011 doi: 10.6004/jnccn.2011.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cleven AH, Nardi V, Ok CY, et al. High p53 protein expression in therapy-related myeloid neoplasms is associated with adverse karyotype and poor outcome. Mod Pathol. 2014:2541–2846. doi: 10.1038/modpathol.2014.153. [DOI] [PubMed] [Google Scholar]
- 22.Wong TN, Ramsingh G, Young AL, et al. Role of TP53 mutations in the origin and evolution of therapy-related acute myeloid leukaemia. Nature. 2014 doi: 10.1038/nature13968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Whitman SP, Maharry K, Radmacher MD, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: A Cancer and Leukemia Group B study. Blood. 2010;116:3622–3626. doi: 10.1182/blood-2010-05-283648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Andersson A, Johansson B, Lassen C, et al. Clinical impact of internal tandem duplications and activating point mutations in FLT3 in acute myeloid leukemia in elderly patients. Eur J Haematol. 2004;72:307–313. doi: 10.1111/j.1600-0609.2004.00225.x. [DOI] [PubMed] [Google Scholar]
- 25.Rollig C, Thiede C, Gramatzki M, et al. A novel prognostic model in elderly patients with acute myeloid leukemia: results of 909 patients entered into the prospective AML96 trial. Blood. 2010;116:971–978. doi: 10.1182/blood-2010-01-267302. [DOI] [PubMed] [Google Scholar]