Abstract
Autoimmune encephalitides may account for epilepsies of so far unknown cause. These “autoimmune epilepsies” may respond well to immunotherapy. More than a dozen autoantibodies have been found with this constellation; therefore, broad autoantibody testing of serum-CSF pairs offers the best diagnostic yield. Several particular features raise the suspicion of an autoimmune cause in otherwise unexplained seizure disorders.
Commentary
One of the most important challenges of scientific and clinical epileptology is the elucidation of the causes of seizure disorders: in a general sense and on the individual patient level. In recent years, neuroimmunology has contributed to these efforts by identifying immunoglobulin G (IgG) antibodies against neural antigens in patients with so far unrecognized autoimmune encephalitides, which often lead to recurrent seizures. This review article does not try to give a comprehensive review of what has been achieved in this field. The authors of this review article, two neurologists with long-lasting expertise in clinical epileptology, one of whom has particular interest in the diagnosis of neural antibodies, try to give a clinically meaningful overview and useful management suggestions for autoimmune encephalitides with predominant epileptic features, that is, the “autoimmune epilepsies” (1).
Some Basic Facts
In the first decade of this century, IgG autoantibodies against proteins on the surfaces of neurons were identified as markers and pathogens in autoimmune encephalitides that in approximately 80% of cases are accompanied by repetitive focal seizures (1). Apart from those surface targets, intracellular antigens (most of them first identified in the 1980s and 1990s) account for a relevant, albeit smaller number of antibody associated encephalitides: the onconeural antibodies–those potentially occurring with seizures are directed against Hu, Ma, CV2, amphiphysin, Delta/Notch-like EGF-related receptor (previously: Tr), or Sox1 and those against glutamic acid decarboxylase (GAD). For an overview of autoantibodies with epileptologic relevance, see Figure 1.
Figure 1.
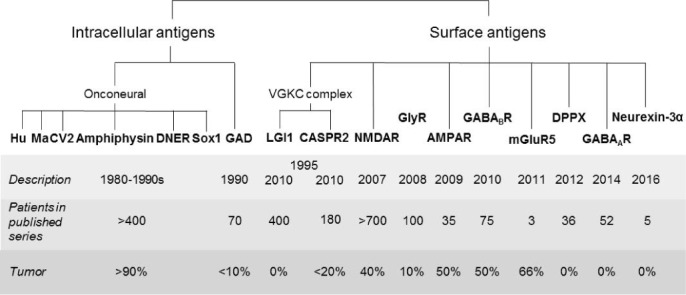
Autoantigens in autoimmune encephalitides with seizures.
From an epileptologic viewpoint, a highlight was the discovery of a new seizure type, faciobrachial dystonic seizures (FBDS), by a team of neuroimmunologists and epileptologists based on the demonstration of antibodies against the voltage-gated potassium channel (VGKC) complex; the antigenic target was later identified as Leucine-rich, glioma inactivated 1 (LGI1) (2, 3), an element of the VGKC complex.
The pathophysiology of antibody-defined autoimmune encephalitides, including their epilepsy-related aspects, has been studied by in vitro and in vivo experiments. The results suggest that the antibodies against surface antigens contribute directly to the disease processes. This issue has been reviewed by others recently (4).
Testing for autoantibodies has been incorporated into practical neurology and epilepsy because many tests have been shown to be reliable and specific for distinct phenotypes. The key technique for the demonstration of antibodies against the N-methyl-D-aspartate receptor (NMDAR), LGI1, contactin-associated protein-like 2 (CASPR2), the gamma-amino-butyric B receptor (GABABR), and other surface antigens is the incubation of patient's diluted serum or CSF (undiluted or mildly diluted) with human embryonic kidney (HEK) cells transfected with the antigens of interest. Potential antibody binding is then visualized with a “secondary” antibody against human IgG carrying a fluorochrome or other chemicals permitting the detection of a surface staining of the HEK cells under the microscope (5).
Other techniques include testing for autoantigen binding on rodent brain, immunoblotting, or radioimmunoprecipitation. All investigations follow the same principle: binding of autoantibodies to the matrix is detected by an anti-human IgG antibody. Formal requirements for making antibody diagnoses are given in Table 1.
TABLE 1.
Formal requirements for making antibody diagnoses

Antibody-Negative Autoimmune Epilepsy
Some patients may have phenotypes typical of autoimmune epilepsy (e.g., an acute, unexplained epilepsy onset with accompanying neuropsychiatric features; elevated cell count or intrathecal IgG synthesis demonstrated by CSF studies; or inflammatory brain lesions according to neuroimaging or histopathology) in the absence of a known autoantibody despite extensive testing. These patients may benefit from immunotherapy (8). In the absence of a reliable biomarker, it is difficult to gain systematic and general insights into these patients' diseases. It can be hoped that detection of novel antibodies or other encephalitis markers will decrease the number of patients in this group.
Which Epilepsy Patients Should be Tested, and How?
The answer to this “deontological” question is only in part a biological–medical one. It rather depends on how one weighs the costs of the diagnostic evaluations, the likelihood of having a positive result, and the risks of immunotherapies in antibody-positive cases. For some of these issues, only limited data are available. There are, however, certainly clinical circumstances in which a positive test result is more likely than in others. A general suggestion for the identification of autoimmune encephalitides prior to autoantibody diagnostics has recently been published (9). Features suggestive of autoimmune epilepsies are summarized in Table 2. In recent years, several authors have recommended to test CSF and serum at the same time (11–13). They have done so because in some instances, antibodies may not be detectable in both materials, for example, NMDAR antibodies not always in serum (14) and LGI1 antibodies not always in CSF (12, 15). Exceptions from the general suggestion to test CSF-serum pairs may be young females with an encephalopathic presentation suggestive of anti-NMDAR encephalitis (CSF sufficient) or faciobrachial dystonic seizures (serum for LGI1 antibody testing usually sufficient). If the results of those specific tests are negative, there may be need for subsequent more extensive testing.
TABLE 2.
Features of patients with seizures and increased likelihood of having autoimmune epilepsy (modified from [10])
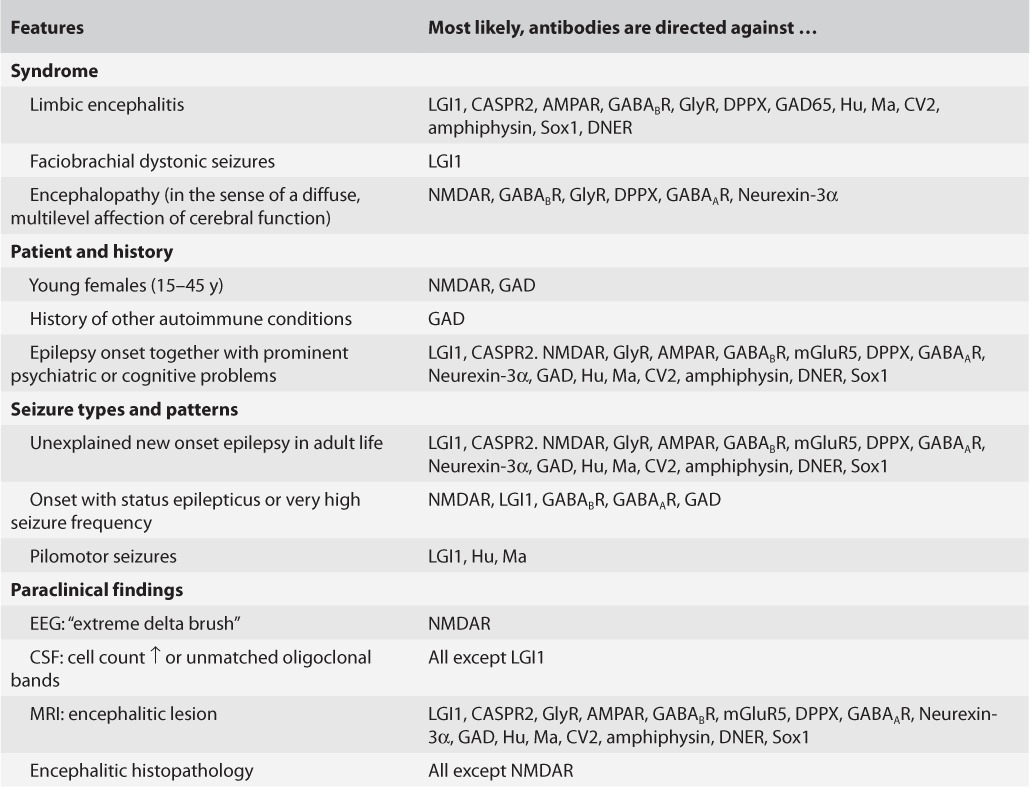
Similarly, the selection of antibodies to be tested for should probably not be too small. The phenotypic features of autoimmune epilepsy rarely permit a targeted testing for a specific antibody, except for a typical presentation of anti-NMDAR encephalitis and the faciobrachial dystonic seizures seen with anti-LGI1 antibodies. In most other cases, a broader approach should be taken to avoid time consuming and expensive sequential testing for single antibodies. One strategy is the use of rodent brain as a “general” assay to select for samples containing any antibody before subtyping it by cell-based assays (taking into account the staining pattern on rodent brain) as suggested by the Philadelphia and Barcelona groups (16). Another approach is the use of multivalent panels of specific tests in form of commercially available mosaics of cell-based assays presenting surface antigens plus multivalent immunoblots with intracellular antigens (17).
What Does a “Positive Result” Mean?
The clinician is most interested in antibodies with two characteristics: 1) confirmed specificity for distinct syndromes (two criteria fulfilled: the antibodies do not or only exceptionally occur in persons with other or without neurological disease; and, the syndromic specificity has been found in more than one series), and 2) predictive potency for responsiveness to immunologic therapy.
Using these criteria, not all neural autoantibodies have the same “value.” Here, we propose a simple dichotomy of “high-rank antibodies” (reproducibly specific and treatment responsive) and “grey cases” (not fulfilling both criteria) (Table 3). In some cases, the site of antibody detection (blood or CSF) makes the difference between the two categories. In the absence of comparative studies across all antibodies, this classification has a certain degree of arbitrariness, and may require refinement with future data.
TABLE 3.
“High-rank antibodies” versus “grey cases” according to the published case series (N ≥ 10 where available)
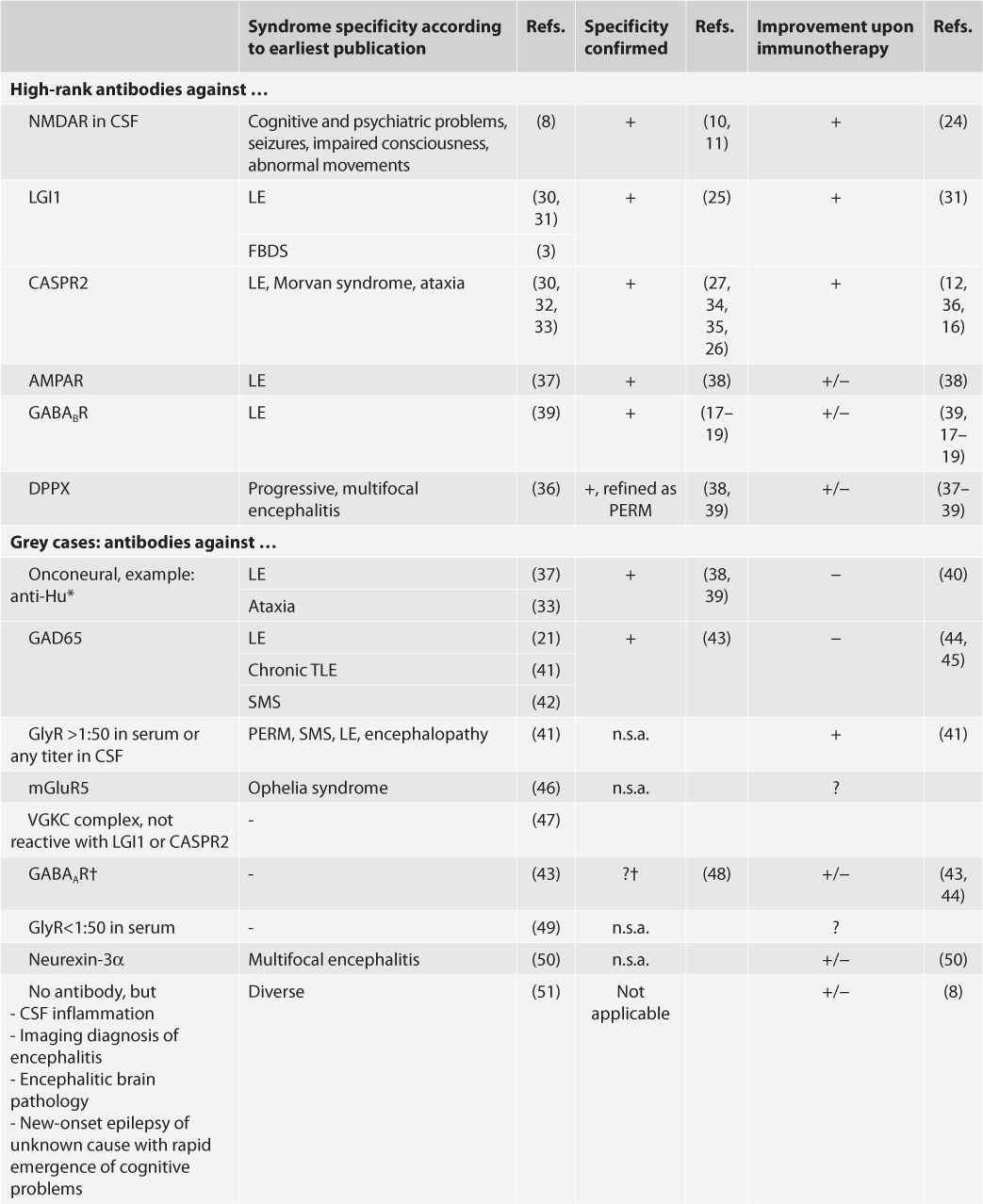
TABLE 3. Continued.
“High-rank antibodies” versus “grey cases” according to the published case series (N ≥ 10 where available)
Applying these categories, the percentage of epilepsy patients with “high rank antibodies” in series of patients without encephalitic features is less than 5 percent, with a larger proportion of “grey cases” of less clear specificity (18–21). The proportion of positive antibodies goes up with inclusion of patients clinically suggestive of having an encephalitis as a cause of their recurrent seizures, but again, most series contain “grey cases” of unknown significance (22, 23). To conclude, autoimmune epilepsy is rare in the whole epilepsy population unless features of Table 2 are considered. In anti-NMDAR encephalitis, monosymptomatic cases account for only 1 percent in the largest available series (“seizures only” being just a part of this fraction) (24). Even if one concedes some sampling bias in favor of more severely (polysymptomatically) affected patients, this seems to be characteristic for many antibodies. In contrast, there may be prolonged disease periods with “seizures only” with LGI1 antibodies (18% [25]). One or more “seizure(s) only” as a presenting symptom are frequent with CASPR2 antibodies (24% [26] to 72% [27]) and in male patients with anti-NMDAR encephalitis (27% [28] to 62% [29]); in these patients, other CNS symptoms evolved within 1 to 17 days (29, 27) emphasizing again the importance of antibody testing especially in patients with complex phenotypes.
Which Treatment to Choose?
The conditions associated with antibodies against surface antigens often have a favorable prognosis with immunotherapy. From an epilepsy standpoint, this is particularly striking with FBDS. They hardly respond to antiepileptic drugs, and these medications surprisingly often cause allergic reactions in this condition. On the other hand, FBDS respond well to steroids (55). In the absence of prospective, let alone randomized trials, therapeutic recommendations have relied on retrospective evaluations. The most influential one comes from the experience in anti-NMDAR encephalitis (56), which suggested the sequence of first line therapies (steroids, intravenous immunoglobulin, or apheresis therapy–or combinations of these interventions) and second line therapies (cyclophosphamide and/or rituximab). There are no evidence-based dosing recommendations. Recently, rituximab (57, 58) and apheresis techniques (59, 60) have received increasing attention.
It is to be expected that local preferences, individual patient features (i.e., special risk situations), reimbursement issues, and personal experience of treating physicians will influence the therapeutic sequence. A suggested sequence of interventions in cases with “high-rank antibodies” is given in Figure 2. In addition to prednisolone, an apheresis technique may be used to reduce the antibody burden rapidly. Prior to the oral prednisolone, a course of three to five pulses of 500- to 1000-mg methylprednisolone may be given on consecutive days. For the “grey cases,” a similar approach has been suggested recently (8).
Figure 2.
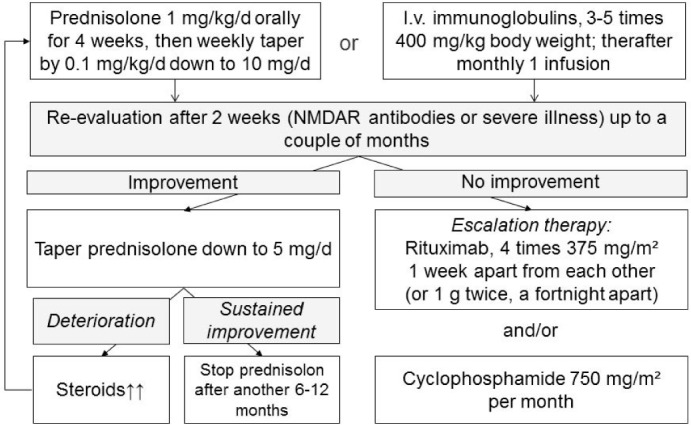
Suggestion for a treatment pathway in patients with “high-rank antibodies.” This treatment pathway is used in the author's (CGB) institution. It is based on previous recommendations (56).
Summary, Final Remarks
Autoimmunity by antibodies is causative for a small proportion of epilepsies. The identification of such cases is, nevertheless, of importance because many of the affected patients have a favorable prognosis with immunotherapy. Neural autoantibodies can be specifically diagnosed by established techniques. Clinical challenges include identifying which patients should undergo antibody testing and delineating the most appropriate therapeutic interventions. Preliminary, readily applicable suggestions exist, and will continue to be refined with increased knowledge over time.
Conflicts of interest
CGB gave scientific advice to Eisai (Frankfurt, Germany) and UCB (Monheim, Germany), undertook industry-funded travel with support of Eisai (Frankfurt, Germany), UCB (Monheim, Germany), Desitin (Hamburg, Germany), and Grifols (Frankfurt, Germany), obtained honoraria for speaking engagements from Eisai (Frankfurt, Germany), UCB (Monheim, Germany), Desitin (Hamburg, Germany), diamed (Köln, Germany), Fresenius Medical Care (Bad Homburg, Germany), and Biogen (Ismaning, Germany). He received research support from diamed (Köln, Germany) and Fresenius Medical Care (Bad Homburg, Germany). He is a consultant to the Laboratory Krone, Bad Salzuflen, Germany, regarding neural antibodies and therapeutic drug monitoring for antiepileptic drugs.
MH received speaker's honoraria and/or consultancy fees from Cyberonics, Desitin, Eisai, GlaxoSmithKline, Janssen-Cilag, Novartis, UCB and Viropharma.
References
- 1. Irani SR, Bien CG, Lang B.. Autoimmune epilepsies. Curr Opin Neurol 2011; 24: 146– 153. [DOI] [PubMed] [Google Scholar]
- 2. Irani SR, Buckley C, Vincent A, Cockerell OC, Rudge P, Johnson MR, Smith S.. Immunotherapy-responsive seizure-like episodes with potassium channel antibodies. Neurology 2008; 71: 1647– 1648. [DOI] [PubMed] [Google Scholar]
- 3. Irani SR, Michell AW, Lang B, Pettingill P, Waters P, Johnson MR, Schott JM, Armstrong RJ, A SZ, Bleasel A, Somerville ER, Smith SM, Vincent A.. Faciobrachial dystonic seizures precede Lgi1 antibody limbic encephalitis. Ann Neurol 2011; 69: 892– 900. [DOI] [PubMed] [Google Scholar]
- 4. Seebohm G, Piccini I, Strutz-Seebohm N.. Paving the way to understand autoantibody-mediated epilepsy on the molecular level. Front Neurol 2015; 6: 149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dalmau J, Tüzün E, Wu HY, Masjuan J, Rossi JE, Voloschin A, Baehring JM, Shimazaki H, Koide R, King D, Mason W, Sansing LH, Dichter MA, Rosenfeld MR, Lynch DR.. Paraneoplastic anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 2007; 61: 25– 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Graus F, Delattre JY, Antoine JC, Dalmau J, Giometto B, Grisold W, Honnorat J, Sillevis Smitt P, Vedeler C, Verschuuren JJ, Vincent A, Voltz R.. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004; 75: 1135– 1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. van Coevorden-Hameete MH, Titulaer MJ, Schreurs MW, de Graaff E, Sillevis Smitt PA, Hoogenraad CC.. Detection and characterization of autoantibodies to neuronal cell-surface antigens in the central nervous system. Front Mol Neurosci 2016; 9: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lee WJ, Lee ST, Byun JI, Sunwoo JS, Kim TJ, Lim JA, Moon J, Lee HS, Shin YW, Lee KJ, Kim S, Jung KH, Jung KY, Chu K, Lee SK.. Rituximab treatment for autoimmune limbic encephalitis in an institutional cohort. Neurology 2016; 86: 1683– 1691. [DOI] [PubMed] [Google Scholar]
- 9. Graus F, Titulaer MJ, Balu R, Benseler S, Bien CG, Cellucci T, Cortese I, Dale RC, Gelfand JM, Geschwind M, Glaser CA, Honnorat J, Hoftberger R, Iizuka T, Irani SR, Lancaster E, Leypoldt F, Pruss H, Rae-Grant A, Reindl M, Rosenfeld MR, Rostasy K, Saiz A, Venkatesan A, Vincent A, Wandinger KP, Waters P, Dalmau J.. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol 2016; 15: 391– 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bien CG. Value of autoantibodies for prediction of treatment response in patients with autoimmune epilepsy: review of the literature and suggestions for clinical management. Epilepsia 2013; 54( suppl 2): 48– 55. [DOI] [PubMed] [Google Scholar]
- 11. McKeon A, Lennon VA.. NMDAR encephalitis: which specimens, and the value of values. Lancet Neurol 2014; 13: 133– 135. [DOI] [PubMed] [Google Scholar]
- 12. Leypoldt F, Armangue T, Dalmau J.. Autoimmune encephalopathies. Ann N Y Acad Sci 2015; 1338: 94– 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Panzer JA, Dale RC.. Anti-AMPA receptor encephalitis: the family of glutamatergic autoencephalitides further expands. Neurology 2015; 84: 2390– 2391. [DOI] [PubMed] [Google Scholar]
- 14. Gresa-Arribas N, Titulaer MJ, Torrents A, Aguilar E, McCracken L, Leypoldt F, Gleichman AJ, Balice-Gordon R, Rosenfeld MR, Lynch D, Graus F, Dalmau J.. Antibody titres at diagnosis and during follow-up of anti-NMDA receptor encephalitis: a retrospective study. Lancet Neurol 2014; 13: 167– 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Leypoldt F, Wandinger KP, Bien CG, Dalmau J.. Autoimmune encephalitis. Eur Neurol Rev 2013; 8: 31– 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Höftberger R, Dalmau J, Graus F.. Clinical neuropathology practice guide 5-2012: updated guideline for the diagnosis of antineuronal antibodies. Clin Neuropathol 2012; 31: 337– 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Dahm L, Ott C, Steiner J, Stepniak B, Teegen B, Saschenbrecker S, Hammer C, Borowski K, Begemann M, Lemke S, Rentzsch K, Probst C, Martens H, Wienands J, Spalletta G, Weissenborn K, Stocker W, Ehrenreich H.. Seroprevalence of autoantibodies against brain antigens in health and disease. Ann Neurol 2014; 76: 82– 94. [DOI] [PubMed] [Google Scholar]
- 18. Brenner T, Sills GJ, Hart Y, Howell S, Waters P, Brodie MJ, Vincent A, Lang B.. Prevalence of neurologic autoantibodies in cohorts of patients with new and established epilepsy. Epilepsia 2013; 54: 1028– 1035. [DOI] [PubMed] [Google Scholar]
- 19. Suleiman J, Brilot F, Lang B, Vincent A, Dale RC.. Autoimmune epilepsy in children: case series and proposed guidelines for identification. Epilepsia 2013; 54: 1036– 1045. [DOI] [PubMed] [Google Scholar]
- 20. Ekizoglu E, Tuzun E, Woodhall M, Lang B, Jacobson L, Icoz S, Bebek N, Gurses C, Gokyigit A, Waters P, Vincent A, Baykan B.. Investigation of neuronal autoantibodies in two different focal epilepsy syndromes. Epilepsia 2014; 55: 414– 422. [DOI] [PubMed] [Google Scholar]
- 21. Borusiak P, Bettendorf U, Wiegand G, Bast T, Kluger G, Philippi H, Munstermann D, Bien CG.. Autoantibodies to neuronal antigens in children with focal epilepsy and no prima facie signs of encephalitis. Eur J Paediatr Neurol 2016; 20: 573– 579. [DOI] [PubMed] [Google Scholar]
- 22. Hacohen Y, Wright S, Waters P, Agrawal S, Carr L, Cross H, De Sousa C, Devile C, Fallon P, Gupta R, Hedderly T, Hughes E, Kerr T, Lascelles K, Lin JP, Philip S, Pohl K, Prabahkar P, Smith M, Williams R, Clarke A, Hemingway C, Wassmer E, Vincent A, Lim MJ.. Paediatric autoimmune encephalopathies: clinical features, laboratory investigations and outcomes in patients with or without antibodies to known central nervous system autoantigens. J Neurol Neurosurg Psychiatry 2013; 84: 748– 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bektas O, Jacobson L, Tutkak H, Karagol S, Lang B, Clover L, Vincent A, Deda G.. Epilepsy and autoimmunity in pediatric patients. Neuropediatrics 2015; 46: 13– 19. [DOI] [PubMed] [Google Scholar]
- 24. Titulaer MJ, McCracken L, Gabilondo I, Armangue T, Glaser C, Iizuka T, Honig LS, Benseler SM, Kawachi I, Martinez-Hernandez E, Aguilar E, Gresa-Arribas N, Ryan-Florance N, Torrents A, Saiz A, Rosenfeld MR, Balice-Gordon R, Graus F, Dalmau J.. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol 2013; 12: 157– 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Arino H, Armangue T, Petit-Pedrol M, Sabater L, Martinez-Hernandez E, Hara M, Lancaster E, Saiz A, Dalmau J, Graus F.. Anti-LGI1-associated cognitive impairment: presentation and long-term outcome. Neurology 2016; 87: 759– 765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. van Sonderen A, Arino H, Petit-Pedrol M, Leypoldt F, Kortvelyessy P, Wandinger KP, Lancaster E, Wirtz PW, Schreurs MW, Sillevis Smitt PA, Graus F, Dalmau J, Titulaer MJ.. The clinical spectrum of Caspr2 antibody-associated disease. Neurology 2016; 87: 521– 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Joubert B, Saint-Martin M, Noraz N, Picard G, Rogemond V, Ducray F, Desestret V, Psimaras D, Delattre JY, Antoine JC, Honnorat J.. Characterization of a subtype of autoimmune encephalitis with anti-contactin-associated protein-like 2 antibodies in the cerebro-spinal fluid, prominent limbic symptoms, and seizures. JAMA Neurol 2016; 73: 1115– 1124. [DOI] [PubMed] [Google Scholar]
- 28. Titulaer MJ, Dalmau J.. Seizures as first symptom of anti-NMDA receptor encephalitis are more common in men. Neurology 2014; 82: 550– 551. [DOI] [PubMed] [Google Scholar]
- 29. Viaccoz A, Desestret V, Ducray F, Picard G, Cavillon G, Rogemond V, Antoine JC, Delattre JY, Honnorat J.. Clinical specificities of adult male patients with NMDA receptor antibodies encephalitis. Neurology 2014; 82: 556– 563. [DOI] [PubMed] [Google Scholar]
- 30. Irani SR, Alexander S, Waters P, Kleopa KA, Pettingill P, Zuliani L, Peles E, Buckley C, Lang B, Vincent A.. Antibodies to Kv1 potassium channel-complex proteins leucine-rich, glioma inactivated 1 protein and contactin-associated protein-2 in limbic encephalitis, Morvan's syndrome and acquired neuromyotonia. Brain 2010; 133: 2734– 2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lai M, Huijbers MG, Lancaster E, Graus F, Bataller L, Balice-Gordon R, Cowell JK, Dalmau J.. Investigation of LGI1 as the antigen in limbic encephalitis previously attributed to potassium channels: a case series. Lancet Neurol 2010; 9: 776– 785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Lancaster E, Huijbers MG, Bar V, Boronat A, Wong A, Martinez-Hernandez E, Wilson C, Jacobs D, Lai M, Walker RW, Graus F, Bataller L, Illa I, Markx S, Strauss KA, Peles E, Scherer SS, Dalmau J.. Investigations of Caspr2, an autoantigen of encephalitis and neuromyotonia. Ann Neurol 2011; 69: 303– 311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Becker EB, Zuliani L, Pettingill R, Lang B, Waters P, Dulneva A, Sobott F, Wardle M, Graus F, Bataller L, Robertson NP, Vincent A.. Contactin-associated protein-2 antibodies in non-paraneoplastic cerebellar ataxia. J Neurol Neurosurg Psychiatry 2012; 83: 437– 440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bien CG, Mirzadjanova Z, Baumgartner C, Dogan Onugoren M, Grunwald T, Holtkamp M, Isenmann S, Kermer P, Melzer N, Naumann M, Riepe M, Schäbitz WR, von Oertzen TJ, von Podewils F, H. R, May TW.. Anti-contactin-associated protein-2 encephalitis: relevance of antibody titres, presentation and outcome [published onlines ahead of print October 27, 2016]. Eur J Neurol doi: 10.1111/ene.13180. [DOI] [PubMed] [Google Scholar]
- 35. Bien CG. Contactin-Associated Protein-like 2 Antibodies: Tackling the Issue of Syndrome Diversity. JAMA Neurol 2016; 73: 1058– 1059. [DOI] [PubMed] [Google Scholar]
- 36. Boronat A, Gelfand JM, Gresa-Arribas N, Jeong HY, Walsh M, Roberts K, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R, Graus F, Rudy B, Dalmau J.. Encephalitis and antibodies to dipeptidyl-peptidase-like protein-6, a subunit of Kv4.2 potassium channels. Ann Neurol 2013; 73: 120– 128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Alamowitch S, Graus F, Uchuya M, Rene R, Bescansa E, Delattre JY.. Limbic encephalitis and small cell lung cancer. Clinical and immunological features. Brain 1997; 120( pt 6): 923– 928. [DOI] [PubMed] [Google Scholar]
- 38. Graus F, Keime-Guibert F, Rene R, Benyahia B, Ribalta T, Ascaso C, Escaramis G, Delattre JY.. Anti-Hu-associated paraneoplastic encephalomyelitis: analysis of 200 patients. Brain 2001; 124: 1138– 1148. [DOI] [PubMed] [Google Scholar]
- 39. Sillevis Smitt P, Grefkens J, De Leeuw B, Van Den BM, Van Putten W, Hooijkaas H, Vecht C.. Survival and outcome in 73 anti-Hu positive patients with paraneoplastic encephalomyelitis/sensory neuronopathy. J Neurol 2002; 249: 745– 753. [DOI] [PubMed] [Google Scholar]
- 40. Keime-Guibert F, Graus F, Fleury A, Rene R, Honnorat J, Broet P, Delattre JY.. Treatment of paraneoplastic neurological syndromes with antineuronal antibodies (anti-Hu, anti-Yo) with a combination of immunoglobulins, cyclophosphamide, and methylprednisolone. J Neurol Neurosurg Psychiatry 2000; 68: 479– 482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liimatainen S, Peltola M, Sabater L, Fallah M, Kharazmi E, Haapala AM, Dastidar P, Knip M, Saiz A, Peltola J.. Clinical significance of glutamic acid decarboxylase antibodies in patients with epilepsy. Epilepsia 2010; 51: 760– 767. [DOI] [PubMed] [Google Scholar]
- 42. Butler MH, Solimena M, Dirkx R Jr, Hayday A, De Camilli P.. Identification of a dominant epitope of glutamic acid decarboxylase (GAD-65) recognized by autoantibodies in stiff-man syndrome. J Exp Med 1993; 178: 2097– 2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Saiz A, Blanco Y, Sabater L, Gonzalez F, Bataller L, Casamitjana R, Ramio-Torrenta L, Graus F.. Spectrum of neurological syndromes associated with glutamic acid decarboxylase antibodies: diagnostic clues for this association. Brain 2008; 131: 2553– 2563. [DOI] [PubMed] [Google Scholar]
- 44. Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG.. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol 2010; 67: 470– 478. [DOI] [PubMed] [Google Scholar]
- 45. Malter MP, Frisch C, Zeitler H, Surges R, Urbach H, Helmstaedter C, Elger CE, Bien CG.. Treatment of immune-mediated temporal lobe epilepsy with GAD antibodies. Seizure 2015; 30: 57– 63. [DOI] [PubMed] [Google Scholar]
- 46. Lancaster E, Martinez-Hernandez E, Titulaer MJ, Boulos M, Weaver S, Antoine JC, Liebers E, Kornblum C, Bien CG, Honnorat J, Wong S, Xu J, Contractor A, Balice-Gordon R, Dalmau J.. Antibodies to metabotropic glutamate receptor 5 in the Ophelia syndrome. Neurology 2011; 77: 1698– 1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. van Sonderen A, Schreurs MW, de Bruijn MA, Boukhrissi S, Nagtzaam MM, Hulsenboom ES, Enting RH, Thijs RD, Wirtz PW, Sillevis Smitt PA, Titulaer MJ.. The relevance of VGKC positivity in the absence of LGI1 and Caspr2 antibodies. Neurology 2016; 86: 1692– 1699. [DOI] [PubMed] [Google Scholar]
- 48. Pettingill P, Kramer HB, Coebergh JA, Pettingill R, Maxwell S, Nibber A, Malaspina A, Jacob A, Irani SR, Buckley C, Beeson D, Lang B, Waters P, Vincent A.. Antibodies to GABAA receptor alpha1 and gamma2 subunits: clinical and serologic characterization. Neurology 2015; 84: 1233– 1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Carvajal-Gonzalez A, Leite MI, Waters P, Woodhall M, Coutinho E, Balint B, Lang B, Pettingill P, Carr A, Sheerin UM, Press R, Lunn MP, Lim M, Maddison P, Meinck HM, Vandenberghe W, Vincent A.. Glycine receptor antibodies in PERM and related syndromes: characteristics, clinical features and outcomes. Brain 2014; 137: 2178– 2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gresa-Arribas N, Planaguma J, Petit-Pedrol M, Kawachi I, Katada S, Glaser CA, Simabukuro MM, Armangue T, Martinez-Hernandez E, Graus F, Dalmau J.. Human neurexin-3a antibodies associate with encephalitis and alter synapse development. Neurology 2016; 86: 2235– 2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bien CG, Schulze-Bonhage A, Deckert M, Urbach H, Helmstaedter C, Grunwald T, Schaller C, Elger CE.. Limbic encephalitis not associated with neoplasm as a cause of temporal lobe epilepsy. Neurology 2000; 55: 1823– 1828. [DOI] [PubMed] [Google Scholar]
- 52. Dalmau J, Rosenfeld MR.. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008; 7: 327– 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ohkawa T, Satake S, Yokoi N, Miyazaki Y, Ohshita T, Sobue G, Takashima H, Watanabe O, Fukata Y, Fukata M.. Identification and characterization of GABA(A) receptor autoantibodies in autoimmune encephalitis. J Neurosci 2014; 34: 8151– 8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Petit-Pedrol M, Armangue T, Peng X, Bataller L, Cellucci T, Davis R, McCracken L, Martinez-Hernandez E, Mason WP, Kruer MC, Ritacco DG, Grisold W, Meaney BF, Alcala C, Sillevis-Smitt P, Titulaer MJ, Balice-Gordon R, Graus F, Dalmau J.. Encephalitis with refractory seizures, status epilepticus, and antibodies to the GABAA receptor: a case series, characterisation of the antigen, and analysis of the effects of antibodies. Lancet Neurol 2014; 13: 276– 286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Irani SR, Stagg CJ, Schott JM, Rosenthal CR, Schneider SA, Pettingill P, Pettingill R, Waters P, Thomas A, Voets NL, Cardoso MJ, Cash DM, Manning EN, Lang B, Smith SJ, Vincent A, Johnson MR.. Faciobrachial dystonic seizures: the influence of immunotherapy on seizure control and prevention of cognitive impairment in a broadening phenotype. Brain 2013; 136: 3151– 3162. [DOI] [PubMed] [Google Scholar]
- 56. Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R.. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol 2011; 10: 63– 74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ishiura H, Matsuda S, Higashihara M, Hasegawa M, Hida A, Hanajima R, Yamamoto T, Shimizu J, Dalmau J, Tsuji S.. Response of anti-NMDA receptor encephalitis without tumor to immunotherapy including rituximab. Neurology 2008; 71: 1921– 1923. [DOI] [PubMed] [Google Scholar]
- 58. Irani SR, Gelfand JM, Bettcher BM, Singhal NS, Geschwind MD.. Effect of rituximab in patients with leucine-rich, glioma-inactivated 1 antibody-associated encephalopathy. JAMA Neurol 2014; 71: 896– 900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. DeSena AD, Noland DK, Matevosyan K, King K, Phillips L, Qureshi SS, Greenberg BM, Graves D.. Intravenous methylprednisolone versus therapeutic plasma exchange for treatment of anti-n-methyl-d-aspartate receptor antibody encephalitis: a retrospective review. J Clin Apher 2015; 30: 212– 216. [DOI] [PubMed] [Google Scholar]
- 60. Dogan Onugoren M, Golombeck KS, Bien C, Abu-Tair M, Brand M, Bulla-Hellwig M, Lohmann H, Munstermann D, Pavenstadt H, Tholking G, Valentin R, Wiendl H, Melzer N, Bien CG.. Immunoadsorption therapy in autoimmune encephalitides. Neurol Neuroimmunol Neuroinflamm 2016; 3: e207. [DOI] [PMC free article] [PubMed] [Google Scholar]


