Abstract
The sodium-activated potassium channel Slack (Kcnt1, Slo2.2) is highly expressed in dorsal root ganglion neurons where it regulates neuronal firing. Several studies have implicated the Slack channel in pain processing, but the precise mechanism or the levels within the sensory pathway where channels are involved remain unclear. Here, we furthered the behavioral characterization of Slack channel knockout mice and for the first time examined the role of Slack channels in the superficial, pain-processing lamina of the dorsal horn. We performed whole-cell recordings from spinal cord slices to examine the intrinsic and synaptic properties of putative inhibitory and excitatory lamina II interneurons. Slack channel deletion altered intrinsic properties and synaptic drive to favor an overall enhanced excitatory tone. We measured the amplitudes and paired pulse ratio of paired excitatory post-synaptic currents at primary afferent synapses evoked by electrical stimulation of the dorsal root entry zone. We found a substantial decrease in the paired pulse ratio at synapses in Slack deleted neurons compared to wildtype, indicating increased presynaptic release from primary afferents. Corroborating these data, plantar test showed Slack knockout mice have an enhanced nociceptive responsiveness to localized thermal stimuli compared to wildtype mice. Our findings suggest that Slack channels regulate synaptic transmission within the spinal cord dorsal horn and by doing so establishes the threshold for thermal nociception.
Keywords: Neurotransmission, potassium channels, slice physiology, spinal cord, thermal hyperalgesia
Introduction
The precise molecular mechanism underlying the processing and transmission of nociceptive signals to the brain via the dorsal root ganglion (DRG) and spinal cord dorsal horn (DH) remain unclear. As a result, treatment options are limited, and a significant portion of the population suffering from one of the many types of pain is faced with inadequate analgesia or debilitating side effects. The superficial DH consists of a heterogeneous neuronal network that processes sensory signals before they are relayed to the brain. Identifying key proteins involved in regulating the flow of pain-related signals at primary afferent synapses, and subsequent processing within the DH is a key step in identifying potential targets for novel therapeutic strategies.
Slack (Kcnt1, Slo2.2) channels are large conductance sodium-activated potassium (KNa) channels expressed throughout the central and peripheral nervous systems.1,2 High levels of expression in DRG and downstream spinal cord DH, along with a functional role regulating neuronal firing, have made them of interest in the study of pain.3–7 Functionally, they are thought to control neuronal firing rates and patterns by promoting adaptation and afterhyperpolarization.8,9 Studies in Slack knockout (KO) mice have been focused primarily on electrophysiological analysis of the DRG and behavioral paradigms. Several reports indicate that cultured DRGs lacking Slack channels are hyperexcitable,4,7 and behavioral phenotypes suggest an anti-nociceptive role for Slack in neuropathic pain.6 The involvement of Slack channels in regulating synaptic function in the superficial, pain-processing lamina of the DH is unclear. The superficial DH receives nociceptive inputs from DRG neurons, which make connection with second-order sensory neurons, forming the first central synapse along the pain pathway. Within the DH, inhibitory and excitatory interneurons modulate the signal before projection neurons relay the information to the brain.10–12 Previous studies indicate that Slack channels are located on primary afferents terminating in the superficial DH.6 To date, electrophysiological analyses of Slack channels have focused on the peripheral sensory neurons in the DRG, leaving the channels contribution to the first central synapse and DH network unknown. To this end, we performed whole-cell recordings from transverse spinal cord slices to investigate the role of Slack channels in the superficial lamina of the DH.
Materials and methods
Animals
Wildtype (WT) and Slack KO mice were housed at the University at Buffalo Laboratory Animal Facility on a 12/12 light/dark cycle with free access to food and water. All experimental procedures were in accordance with the guideline of the National Institute of Health and were approved by the University at Buffalo Institutional Animal Care Use Committee.
Slice preparation
Under isoflurane anesthesia, mice (P15–P32) were decapitated, and a ventral laminectomy was performed to access the spinal cord. The lumbar portion was dissected and embedded in low-gelling temp agarose (Sigma Aldrich, St. Louis, MO) and bathed in ice-cold modified artificial cerebrospinal fluid (ACSF) of the following composition (in mM): 110 choline chloride, 2.5 KCl, 0.5 CaCl2, 7 MgSO4, 1.25 NaH2PO4, 26.2 NaHCO3, 11.6 sodium L-ascorbate, 3.1 sodium pyruvate, and 25 glucose. Transverse slices of the lumbar spinal cord were obtained using a vibrating blade microtome (Lancer series 1000; Leica Biosystems, St. Louis, MO). Slices were incubated for at least 45 min at 35℃ to 37℃ in regular ACSF of the following composition (in mM): 119 NaCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 15 glucose, and 1.0 sodium L-ascorbate and continuously bubbled with 95% O2 to 5% CO2. After incubation, slices were left for 1 h to recover before being transferred to the recording chamber (Warner Instruments, Hamden, CT) fixed to an upright microscope. The slices were continuously perfused (2–3 ml/min) with room temperature (∼22℃) ACSF saturated with 95% O2 to 5% CO2.
Electrophysiological recordings
Neurons within the translucent band that delineates lamina II (substantia gelatinosa) were visualized with a 40X water-immersion lens and equipped with differential interference contrast and infrared optical system. Whole-cell recordings were obtained with patch electrodes (3–5 MΩ) filled with internal solution containing (in mM): 120 potassium gluconate, 10 KCl, 10 sodium phosphocreatine, 10 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1 MgCl2, 1 ethylene glycol-bis(2-amino-ethylether)-N,N, N′, N′-tetra-acetic acid, 2 Na2-ATP, and 0.25 Na-GTP, at a pH of 7.3 and osmolarity of 280 to 290 mosmol−1. Excitatory post-synaptic currents (EPSCs) were evoked using a stimulating electrode filled with ACSF and placed in the dorsal root entry zone (DREZ), located by the remaining dorsal roots. The duration (100–200 µs) and intensity (5–99 V) of the stimulus were adjusted to 75% of the maximum amplitude of EPSCs. EPSCs were evoked every 20 s. In some experiments, a paired stimulus was delivered at inter-stimulus interval of 40 ms to determine the paired pulse ratio (PPR). Membrane currents were amplified with a Multiclamp 700B amplifier (Molecular Devices, Union City, CA), digitized with Digidata 1440 and collected using pCLAMP 10 software (Molecular Devices, Union City, CA). All chemicals used for ACSF preparation were purchased from Fisher Scientific (Pittsburgh, PA). Picrotoxin and GYKI 54266 (4-(8-Methyl-9H-1,3-dioxolo[4,5-h][2,3]benzodiazepin-5-yl)-benzenamine hydrochloride) were purchased from Tocris Cookson (Ellisville, MO, USA). Loxapine succinate (LOX) was purchased from Sigma Aldrich.
Hargreaves (plantar) test for thermal nociception
Baseline thermal nociception was measured using the Hargreaves Apparatus by Ugo Basile (Varese, Italy). Mice (6–10 weeks) underwent two days of habituation followed by three days of measurements. On days 1 and 2 (habituation), mice spent 30 min in homecages acclimatizing to the testing room and were then transferred to testing chambers for 1 h. On days 3 through 5, mice underwent testing. An infrared stimulus (IR 40) was delivered through the plexiglass floor to the plantar surface of the hind paw, and the latency to withdrawal was measured automatically. For each subject, three to six measurements per hind paw were taken and used to compute the average latency to withdraw (seconds). A maximum IR exposure time of 15 s was set to ensure no tissue damage occurred, and at least 5 min was left between measurements taken from the same mouse.
Dynamic plantar test for mechanical nociception
Baseline mechanical nociception was measured using the Dynamic Plantar Aesthesiometer by Ugo Basile (Verese, Italy). Mice (4–8 weeks) underwent two days of habituation (described above) followed by one to two days of baseline measurements. A Von Frey–type 0.5 mm filament was applied to the hind paw with a max force of 5 g occurring over a 2-s period. The latency to withdraw (seconds) and force applied at the time of withdrawal (grams) were automatically recorded. For each subject, 3 to 6 measurements per hind paw were taken and used to compute the average latency and force.
Data analysis
MiniAnalysis software (Synaptosoft, Decatur, GA) was used to analyze spontaneous EPSCs. Clampfit 10.2 software (Molecular Devices) was used to analyze evoked EPSCs and current clamp recordings. The amplitude of evoked EPSCs was calculated as the difference between the average current during the 2 ms window at peak amplitude and the average baseline current taken 5 ms before the stimulus artifact. For each cell, recordings were normalized to a baseline of 10 min prior to drug application. PPR was calculated by dividing the amplitude of the second EPSC by the amplitude of the first (PPR = EPSC2/EPSC1). For each cell, the PPR was calculated during baseline and peak effect following drug application. Graphs were plotted, and statistics computed using Origin 8.0 software (Microcal Software, Northampton, MA, USA) and GraphPad Prism 6 software (GraphPad Software Inc., La Jolla, CA, USA). Results are expressed as mean +/− SEM. A two-way analysis of variance with multiple comparisons and bonferroni post hoc test were used to compare propensity to fire between KO and WT in tonic and delayed firing neurons. All other comparisons were made using a paired or unpaired student t test. P < 0.05 was considered significant.
Results
All studied neurons were classified as putatively inhibitory or excitatory based on previously described and characterized firing phenotypes.13,14 Tonic firing (Figure 1(a,i)) or initial bursting (Figure 1(a,ii)) neurons were considered to be inhibitory, and delayed (Figure 1(b,i)) or single spiking (Figure 1(b,ii)) neurons were considered excitatory (Figure 1). To assess whether Slack channel deletion alters neuronal excitability, we measured the propensity to fire of tonic (inhibitory) and delayed (excitatory) firing neurons in response to various magnitudes of current injected for 600 ms (Figure 2). The percentage of each firing class encountered was consistent between genotypes. For WT, 64% were tonic firing and 36% delayed, while 63% were tonic and 36% delayed in KO tissue. In tonic firing neurons, Slack channel deletion significantly decreased the propensity to fire at all magnitudes of current injections except 90 pA (Figure 2(a) to (c)). In delayed firing neurons, Slack deletion increased the propensity to fire at lower current injection levels, reaching significance at 50 and 70 pA (Figure 2(d) to (f)). Resting membrane potential differed between WT (−47.05 +/− 1.97 mV) and KO (−53.35 +/− 2.34 mV) tonic firing neurons (p = 0.045), but not WT (−58.42 +/− 2.34) and KO (−57.5 +/− 2.40) delayed firing neurons.
Figure 1.
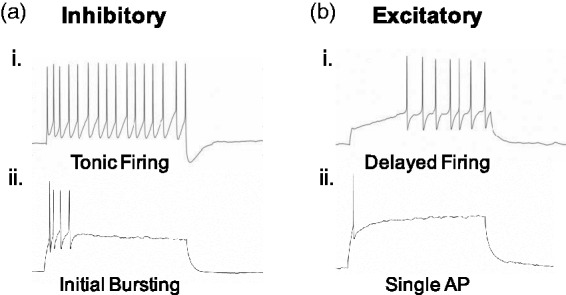
Classification as inhibitory or excitatory based on previously established firing phenotypes. (a) Tonic firing (i) and initial bursting (ii) neurons are considered to be inhibitory. (b) Delayed firing (i) and neurons that fire a single action potential (ii) are considered to be excitatory.
Figure 2.
Slack deletion alters intrinsic excitability of dorsal horn neurons. (a) to (c) Input output curve (a) and histogram (b) showing the average propensity to fire of tonic firing WT (n = 25) and KO (n = 19) neurons. (c) Representative traces from tonic firing neurons. (d) to (f) Same parameters as (a) to (c) illustrating comparison between delayed firing WT (n = 14) and KO (n = 11) neurons.
WT: wildtype; KO: knockout.
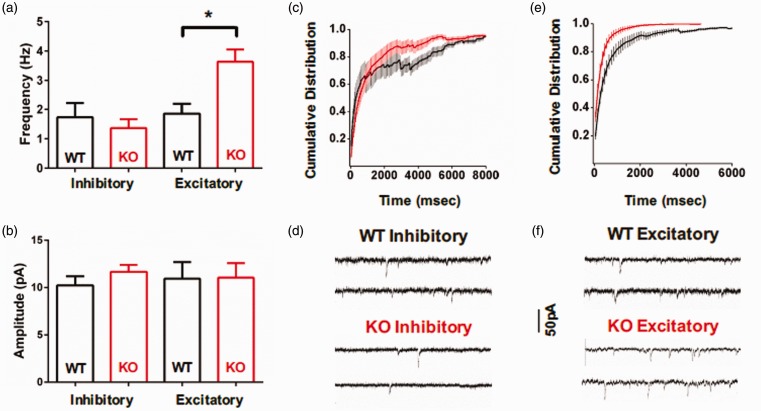
A balance between excitatory and inhibitory tone within superficial DH network is required for normal processing of sensory information.12 We determined whether Slack channels impacted optimum network synaptic drive by measuring glutamatergic spontaneous EPSCs onto putative excitatory and inhibitory neurons. In Slack KO mice, the frequency of spontaneous EPSCs was significantly increased in excitatory (67% delayed firing, 33% single action potential (AP)) neurons (Figure 3(a), (e), (f)) and a slight but insignificant decrease in inhibitory (93% tonic firing, 7% initial bursting) neurons (Figure 3(a), (c), (d)) without affecting amplitude (Figure 3(b)). The two-fold frequency increase in excitatory neurons is clearly illustrated by a leftward shift in the inter-event interval cumulative distribution (Figure 3(e)).
Figure 3.
Slack deletion increases the frequency of spontaneous EPSCs in putative excitatory neurons. Average baseline frequency (a) and amplitude (b) of spontaneous EPSCs for inhibitory WT (n = 15) and KO (n = 10) neurons, and excitatory WT (n = 9) and KO (n = 9) neurons. Cumulative distribution of the inter-event interval for inhibitory (c) and excitatory (e) WT (black) and KO (red) neurons. Representative traces WT and KO inhibitory (d) and excitatory (f) neurons.
WT: wildtype; KO: knockout.
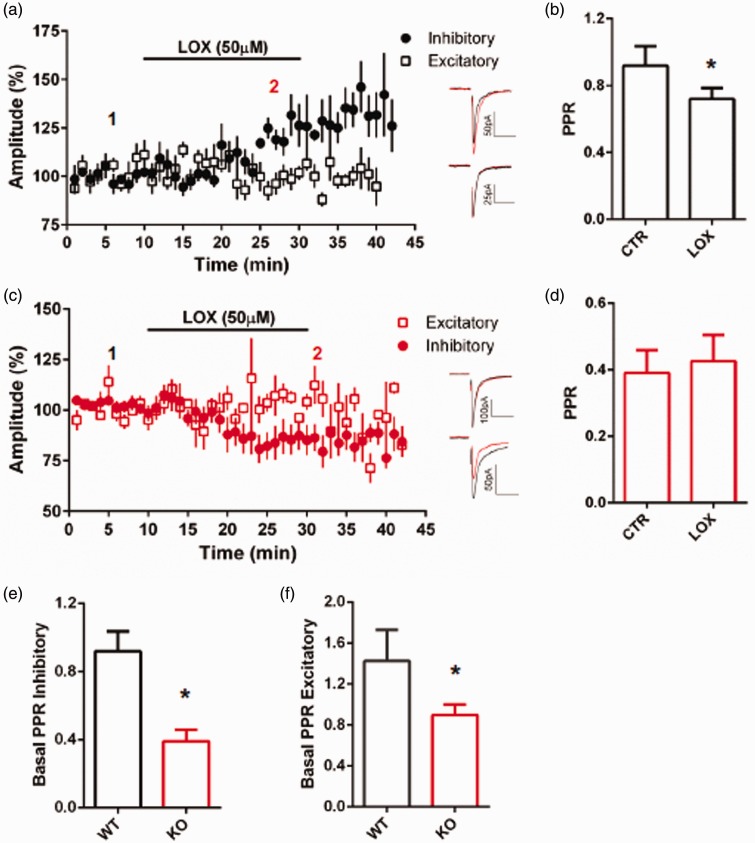
To investigate the contribution of Slack channels to regulating transmission at primary afferent synapses, we measured the amplitude and PPR of paired EPSCs evoked by electrical stimulation of the DREZ. In WT tissue, bath application of the non-selective Slack channel opener LOX15 (50 µM) potentiated the amplitude of evoked EPSCs onto inhibitory (83% tonic firing, 17% initial bursting) neurons but had no effect on excitatory (60% delayed, 40% single AP) neurons (Figure 4(a)). This potentiation was accompanied by a decrease in the PPR (Figure 4(b)), indicating increased presynaptic release from primary afferents. Remarkably, in the absence of Slack, LOX decreased the amplitude of evoked EPSCs in inhibitory (86% tonic firing, 14% initial bursting) neurons and again had no effect on excitatory (80% delayed firing, 20% single AP) neurons (Figure 4(c)). The LOX-mediated inhibition was not accompanied by an altered PPR (Figure 4(d)). Taken together, the data indicate that Slack channels are involved in regulating primary afferent synapses onto inhibitory neurons, and LOX has Slack-dependent and -independent effects. Under control conditions prior to the application of LOX, the PPR significantly differed in WT and KO tissue (Figure 4(e) and (f)). The reduced PPR in KO tissue indicates altered basal release properties result from Slack channel deletion. In the absence of Slack, there is an increased probability of glutamate release from primary afferents.
Figure 4.
Slack deletion alters synaptic responses to LOX in inhibitory but not excitatory neurons. (a) Average EPSC amplitude in response to LOX (50 µM) for inhibitory (circles) and excitatory (squares) WT neurons. Inset representative traces before (1, black) and after (2, red) LOX for inhibitory (above) and excitatory (below) neurons, p < 0.01. (b) PPR for WT inhibitory neurons before and after LOX. (c) Same parameters as (a) for Slack KO, p < 0.01. Inset representative traces before (1, black) and after (2, red) LOX for excitatory (above) and inhibitory (below). (d) PPR for Slack KO inhibitory neurons before and after LOX (p < 0.05). (e) Basal PPR for WT and KO inhibitory neurons. (f) Basal PPR for WT and KO excitatory neurons (p < 0.05).
WT: wildtype; KO: knockout; LOX: loxapine.
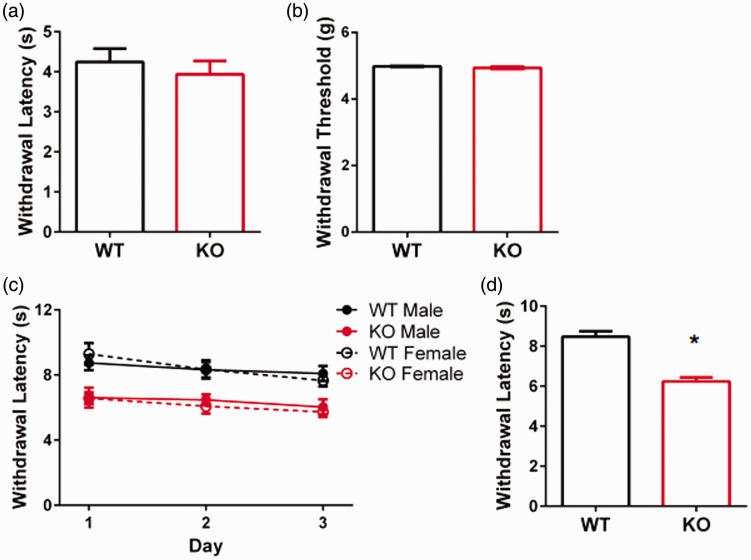
Recent reports indicate that Slack KO mice do not have altered baseline mechanical or thermal nociception.6,7 In agreement with previous reports, we found that baseline mechanical measurements did not differ in KO mice (Figure 5(a) and (b)). Thermal nociception of Slack KO mice was determined previously using the hot plate assay which is believed to reflect supraspinal pain processing.16,17 In contrast and despite the supraspinal notion, Huang et al.5 did show that intrathecal injection of siRNA to knockdown Slack protein significantly reduced 50℃ hot plate latencies. To firmly assess thermal nociception in the Slack KO mouse, we performed the plantar test with the Hargreaves Apparatus that allows for the delivery of a localized heat stimulus to hind paws and prior acclimatization to the testing chamber. This assay more accurately measures nociception at the spinal level. We found that Slack deletion significantly decreased withdrawal latencies in both male and female mice (Figure 5(c) and (d)), indicating that Slack channels contribute to the spinal processing of thermal nociceptive information.
Figure 5.
Baseline nociception behavior in wildtype and slack knockout mice. Latency (a) and threshold (b) to withdraw hind paws from a mechanical stimulus in WT (n = 5) and KO (n = 6) mice. (c) Latency to withdrawal paw from a thermal stimulus (IR: 40) on three consecutive days of testing in WT male (n = 6), WT female (n = 4), KO male (n = 6), and KO female (n = 8) mice. (d) Average withdrawal latencies from C combined for WT (n = 10) and KO (n = 14) mice, p < 0.001.
WT: wildtype; KO: knockout.
Discussion
It is known that pain models can cause long-term changes to neurotransmission and neuronal excitability of specific neuronal populations within the spinal cord DH, commonly seen is a reduction in inhibitory tone and increase in excitation,18 changes that are believed to underlie central sensitization and allow the pain to persist long after healing.19 Likewise, deletion of proteins, such as ion channels, involved in regulating DH neurotransmission, and network excitability can cause similar changes producing pro-nociceptive phenotypes.20 Here, we characterized the basal effects of Slack deletion on synaptic transmission in the superficial DH and nociceptive behaviors. In addition, we investigate the effect of the Slack channel opener and potential analgesic LOX in the pain-processing lamina of the DH.
We show that in the absence of Slack, synaptic drive onto putative excitatory lamina II interneurons is significantly increased, shown by a two-fold increase in the frequency of spontaneous EPSCs. No change was observed in putative inhibitory interneurons, suggesting a change in network excitability favoring excitation. Lamina II excitatory interneurons receive glutamatergic input from primary afferents, as well as, inhibitory and excitatory local interneurons that could be responsible for the increased synaptic drive. Interestingly, the intrinsic properties were also altered in such a way that promotes excitation and decreases inhibition. In the absence of Slack, inhibitory (tonic firing) neurons fire less, and excitatory (delayed firing) neurons fire more APs than WT. While the data indicate an overall network shift toward excitation, it should be noted that this study does not provide insight into how Slack deletion affects inhibitory post-synaptic currents, an equally important determinant of network excitability in the DH.
Lu et al.6 report that Slack channels are localized to isolectin B4+ central terminals in the superficial DH. To date, there is no immunohistochemical evidence suggesting Slack channels are present on DH interneurons, which leads us to conclude that differences in afferent input and resultant developmental changes are responsible for the observed altered intrinsic and synaptic excitability. High levels of Slack expression in the DRG support the idea that Slack channels are positioned at the terminals of DRG sensory neurons to influence the flow of sensory information from the peripheral to the central nervous system.20 Moreover, the basal PPR of evoked EPSCs was significantly reduced in KO tissue, indicating increased neurotransmitter release from primary afferent terminals. This supports the idea that Slack channels are positioned presynaptically and act to control or limit basal transmitter release.
A lack of Slack-specific pharmacological tools has limited progress in the field, and investigations have largely focused on the use of Slack KO mice. However, when used in combination with Slack KO mice, non-specific openers have the potential to offer insight into channel function. For example, Lu et al.6 show that LOX ameliorates neuropathic pain in a Slack-dependent manner. LOX and its metabolite amoxapine, a tricyclic antidepressant, open Slack channels.15 Also, amoxapine has been shown to potentiate the analgesic effects of morphine, an effect thought to be mediated at the spinal level.21 Noteworthy, a Phase 1 clinical trial is currently investigating the tolerability and efficacy of LOX in patients with neuropathic pain.22
The potentiation and inhibition of evoked EPSCs in WT and KO tissue, respectively, reflect the actions of LOX at primary afferent synapses, a key connection point where unprocessed sensory information is received by the spinal cord. The fact that DREZ stimulation activates many fibers and various signalling systems must be taken into account. The resultant evoked EPSC is influenced by the pre- and post-synaptic mechanisms and activation of various signaling systems, all converging on the cell in question. Precise molecular and cellular mechanisms underlying the therapeutic efficacy of LOX remain unclear, but in addition to being a Slack channel opener, it is known to influence dopaminergic, serotonergic, muscarinic, adrenergic, and histamine systems.23,24 For these reasons, we cannot accurately discern the Slack-specific contribution to the LOX-mediated modulation of primary afferent synapses onto inhibitory neurons. More specific Slack channel pharmacological tools are needed. Nonetheless, an increase in the activity of inhibitory lamina II interneurons is consistent with the proposed efficacy of LOX as an analgesic.22
It is possible that, like BK channels, Slack channels exert negative control over neurotransmitter release under basal conditions,25 aligning with the decreased basal PPR in the absence of Slack. However, it remains unclear why potentiation of channel activity with LOX results in a decrease in PPR similar to that seen basally in Slack KO tissue. The Slack channel’s role in development is not well understood; perhaps, in the absence of Slack channels, compensatory changes to other channels or G protein-coupled receptor signaling systems occur. These possibilities, along with the action of LOX at multiple receptors known to be present in the DH, make it particularly difficult discern pharmacologically the contribution of Slack by directly comparing responses in WT and KO tissue. Developmental changes resulting from enhanced basal afferent tone may be responsible for the altered intrinsic properties we observed. Furthermore, the crosstalk between excitatory and inhibitory interneurons, essential for maintaining optimal network excitability, may also be impacted in such a way that the processing of sensory information is altered.
Lastly, we examined basal thermal and mechanical nociception. In agreement with previous reports,6 we saw no difference in responses to mechanical stimuli between WT and KO mice. To date, thermal nociception in Slack KO mice has been assessed using the hotplate assay, and no differences between WT and Slack KO have been reported.6,7 Using the plantar test, we observed a prominent and significant decrease in the latency to withdraw hind paws from a localized infrared thermal stimulus in both male and female mice, indicating that Slack channels regulate the thermal threshold for nociception. It is possible that this phenotypic difference was not previously detected by the hotplate because it reflects supraspinal nociceptive processing,16,17 or due to the tests limitations, which include lack of habituation, increased animal handling, and stimulation of all body surfaces in contact with the plate.26
Our finding suggests that Slack channels play a role in regulating synaptic transmission at the level of the DH, a key part of the sensory pathway that has been overlooked in studies addressing the channels role in nociceptive processing or more broadly synaptic function. This work reflects the role of Slack channels during the basal state and lays a foundation for forming hypotheses regarding regulation in various models of pain, which involve long-term changes in synaptic strength and arrangement. This report furthers the overall characterization of Slack KO mice with a focus on the pain-processing lamina of the DH and uncovers a baseline behavioral phenotype not previously reported. In addition, it sheds light on how LOX, a clinically approved drug on trial for its potential to treat pain, affects neurotransmission in the DH.
Acknowledgment
The authors thank Dr. Julie Kauer and Dr. Michelle Kloc at Brown University for providing instruction on spinal cord slice preparation.
Author Contributions
KME conducted experiments and data analysis and wrote the draft of the manuscript. ABh, SH-D, and KME oversaw experimentation and experimental design and edited the manuscript. KP helped run behavioral nociception assays and edited the manuscript. ABa, PR, and RL provided the Slack knockout mice and edited the manuscript.
Declaration of Conflicting Interests
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The author(s) confirm that they have read Molecular Pain’s position on ethical publication and affirm that this communication is consistent with those guidelines.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institutes of Health, Grant NS078184 (to ABh).
References
- 1.Bhattacharjee A, Kaczmarek LK. For K+ channels, Na+ is the new Ca2+. Trends Neurosci 2005; 28: 422–428. [DOI] [PubMed] [Google Scholar]
- 2.Rizzi S, Knaus HG, Schwarzer C. Differential distribution of the sodium-activated slick and slack in mouse brain. J Comp Neurol 2016; 524: 2093–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamsett TJ, Picchione KE, Bhattacharjee A. NAD+ activates KNa channels in dorsal root ganglion neurons. J Neurosci 2009; 29: 5127–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nuwer MO, Picchione KE, Bhattacharjee A. PKA-induced internalization of slack KNa channels produces dorsal root ganglion neuron hyperexcitability. J Neurosci 2010; 30: 14165–14172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang F, Wang X, Ostertag EM, et al. TMEM16C facilitates Na(+)-activated K+ currents in rat sensory neurons and regulates pain processing. Nat Neurosci 2013; 16: 1284–1290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R, Bausch AE, Kallenborn-Gerhardt W, et al. Slack channels expressed in sensory neurons control neuropathic pain in mice. J Neurosci 2015; 35: 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Espinosa PL, Wu J, Yang C, et al. Knockout of Slo2.2 enhances itch, abolishes KNa current, and increases action potential firing frequency in DRG neurons. Elife 2015; 4: e10013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franceschetti S, Lavazza T, Curia G, et al. Na+-activated K+ current contributes to postexcitatoryhyperpolarization in neocortical intrinsically bursting neurons. J Neurophysiol 2003; 89: 2101–2111. [DOI] [PubMed] [Google Scholar]
- 9.Wallen P, Robertson B, Cangiano L, et al. Sodium-dependent potassium channels of a Slack-like subtype contribute to the slow afterhyperpolarization in lamprey spinal neurons. J Physiol 2007; 585: 75–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Basbaum AI, Bautista DM, Scherrer G, et al. Cellular and molecular mechanisms of pain. Cell 2009; 139: 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Todd AJ, Spike RC. The localization of classical transmitters and neuropeptides within neurons in laminae I-III of the mammalian spinal dorsal horn. Prog Neurobiol 1993; 41: 609–645. [DOI] [PubMed] [Google Scholar]
- 12.Todd AJ. Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 2010; 11: 823–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Punnakkal P, von Schoultz C, Haenraets K, et al. Morphological, biophysical and synaptic properties of glutamatergic neurons of the mouse spinal dorsal horn. J Physiol 2014; 592: 759–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smith KM, Boyle KA, Madden JF, et al. Functional heterogeneity of calretinin-expressing neurons in the mouse superficial dorsal horn: implications for spinal pain processing. J Physiol 2015; 593: 4319–4339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biton B, Sethuramanujam S, Picchione KE, et al. The antipsychotic drug loxapine is an opener of the sodium-activated potassium channel slack (Slo2.2). J Pharmacol Exp Ther 2012; 340: 706–715. [DOI] [PubMed] [Google Scholar]
- 16.Caggiula AR, Epstein LH, Perkins KA, et al. Different methods of assessing nicotine-induced antinociception may engage different neuronal mechanisms. Psychopharmacology 1995; 122: 301–306. [DOI] [PubMed] [Google Scholar]
- 17.Rubinstein M, Mogil JS, Japón M, et al. Absence of opioid stress-induced analgesia in mice lacking beta-endorphin by site-directed mutagenesis. Proc Natl Acad Sci U S A 1996; 93: 3995–4000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen Y, Derkach VA, Smith PA. Loss of Ca2+-permeable AMPA receptors in synapses of tonic firing substantia gelatinosa neurons in the chronic constriction injury of neuropathic pain. Exp Neurol 2016; 279: 168–177. [DOI] [PubMed] [Google Scholar]
- 19.Balasubramanyan S, Stemkowski PL, Stebbing MJ, et al. Sciatic chronic constriction injury produces cell type specific changes in electrophysiological properties of substantia gelatinosa neurons. J Neurophysiol 2006; 96: 579–590. [DOI] [PubMed] [Google Scholar]
- 20.Waxman SG, Zamponi GW. Regulating excitability of peripheral afferents: emerging ion channel targets. Nat Neurosci 2014; 17: 153–163. [DOI] [PubMed] [Google Scholar]
- 21.Hamon M, Gozlan H, Bourgoin S, et al. Opioid receptors and neuropeptides in the CNS in rats treated chronically with amoxapine or amitriptyline. Neuropharmacology 1987; 26: 531–539. [DOI] [PubMed] [Google Scholar]
- 22.National Institute of Health; University of Witten/Herdecke. Tolerability and analgesic efficacy of loxapine in patients with refractory, chemotherapy-induced neuropathic pain (LOX2015PILOT). Bethesda: National Library of Medicine, https://clinicaltrials.gov/ct2/show/NCT02820519 NLM Identifier: NCT02820519 (2016, accessed 13 June 2017).
- 23.Chakrabarti A, Bagnall A, Chue P, et al. Loxapine for schizophrenia. Cochrane Database Syst Rev 2007; 4: CD001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Popovic D, Nuss P, Vieta E. Revisiting loxapine: a systemic review. Ann Gen Psychiatry 2015; 14: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Griguoli M, Sgritta M, Cherubini E. Presynaptic BK channels control transmitter release: physiological relevance and potential therapeutic implications. J Physiol 2016; 594: 3489–3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banik RK, Kabadi RA. A modified Hargreaves method for assessing threshold temperatures for heat nociception. J Neurosci Methods 2013; 219: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]